Abstract
Previous studies have shown that both αA- and αB-crystallins bind Cu2+, suppress the formation of Cu2+-mediated active oxygen species, and protect ascorbic acid from oxidation by Cu2+. αA- and αB-crystallins are small heat shock proteins with molecular chaperone activity. In the present study we show that the mini-αA-crystallin, a peptide consisting of residues 71–88 of αA-crystallin, prevents copper-induced oxidation of ascorbic acid. Evaluation of binding of copper to mini-αA-crystallin showed that each molecule of mini-αA crystallin binds one copper molecule. Isothermal titration calorimetry and nanospray mass spectrometry revealed dissociation constants of 10.72 μM and 9.9 μM, respectively. bis-ANS interaction with mini-αA-crystallin was reduced after binding of Cu2+, suggesting that the same amino acids are interacting with these two ligands. Circular dichroism spectrometry showed that copper binding to mini-αA crystallin peptide affects its secondary structure. Substitution of the His residue in mini-αA-crystallin with Ala abolished the redox suppression activity of the peptide. During the Cu2+-induced ascorbic acid oxidation assay, the deletion mutant, αAΔ70-77, showed about 75% loss of ascorbic acid protection compared to the wild type αA –crystallin. This difference indicates that 70–77 region is the primary Cu2+ binding site(s) in human native full-size αA-crystallin. The role of the chaperone site in Cu2+ binding in native αA-crystallin was confirmed by the significant loss of chaperone activity by the peptide following Cu2+ binding.
Keywords: crystallin, lens, chaperone, Cu2+ binding, electrospray ionization mass spectrometry, ascorbic acid
Introduction
αA-Crystallin is one of the abundant proteins in the mammalian lens and belongs to the family of small heat shock proteins (sHSP) [1]. In addition to its refractive role, αA-crystallin, like other sHSP members, exhibits chaperone-like function that is believed to be involved in maintaining lens transparency [2–5]. Studies of the critical residues responsible for the chaperone-like activity of αA-crystallin have revealed that R116 [6], R49 [7], R21 [8], and F71 [9,10] are critical for chaperone function. Other studies have reported that the C-terminal region [11–13] and the SRLFDQFFG sequence motif [14] are critical for chaperone-like function. There is a general agreement that the hydrophobic regions play a role in chaperone-like activity of αA-crystallin [15]. Our hydrophobic site-specific reagent (bis-ANS) binding study suggested that the chaperone site in human αA-crystallin is residues 70–88, which was subsequently confirmed by the demonstration of inhibited aggregation activity in a synthetic peptide consisting of αA70-88 residues (KFVIFLDVKHFSPEDLTVK) [16]. We called this peptide “mini-chaperone” or “mini-αA-crystallin” [16]. The mini-αA-crystallin sequence is highly conserved among several sHSPs. During homology modeling studies, this region aligns to β3 and β4 region in the α-crystallin domain of sHSP16.5 [17]. Mini-αA peptide functions like a molecular chaperone by preventing the aggregation and precipitation of denaturing substrate proteins caused by oxidative, thermal, and chemical denaturing agents [16,18,19].
Copper is present in micromolar concentration [3–10 μM] in lens tissue and it is mostly bound to the lens protein [20–23]. Ortwerth et al. [22] reported that lens proteins tightly bind Cu2+ ions and suppress Cu2+-mediated generation of reactive oxygen species (ROS), as well as the oxidation of ascorbic acid. This was further confirmed in a study which showed that both αA- and αB- crystallins are involved in redox silencing [24]. Under some conditions Cu2+ interaction with α-crystallin was found to modulate the chaperone activity [25–27]. However, none of the studies carried out thus far have pinpointed the specific Cu2+ binding sequence in human αA-crystallin. The present study was undertaken to determine whether mini-αA-crystallin binds Cu2+ and inhibits copper-induced oxidation of ascorbic acid, like native α-crystallin or its subunits. We show that the αA-crystallin chaperone site is also a Cu2+ binding site in αA-crystallin and the αA70-88 sequence is sufficient to suppress Cu2+-induced oxidation of ascorbic acid.
Materials and methods
Reagents
Mini-αA-crystallin [DFVIFLDVKHFSPEDLTVK] and the alanine analog [DFVIFLDVKAFSPEDLTVK] were supplied by GenScript Corporation, Piscataway, NJ, USA. The purity of the peptides was >95% as determined by high-performance liquid chromatography (HPLC) and mass spectroscopy. Dry peptides were weighed on a microbalance and dissolved in HPLC-grade water, and fractions of solutions were used to determine the concentration of peptides by amino acid analysis. Copper sulfate solution from Pierce protein assay kit was used as the source of Cu2+. The actual content of copper and peptide were determined by flame photometry and amino acid analysis at the Experimental Station Chemical Laboratories, University of Missouri, Columbia.
Ascorbic acid oxidation
A 100 mM stock solution of ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) was prepared in HPLC-grade water. For the oxidation experiments, 500 μM of ascorbic acid was prepared in Chelex-treated phosphate buffer (50 mM, pH7.2) in the presence and absence of Cu2+. The assays were carried out at 25°C. In other experiments, ascorbic acid (500 μM) and Cu2+ (4 μM) were incubated (25°C) in the presence and absence of mini-αA-crystallin. The absorbance of ascorbic acid was measured at 260 nm, using 1 cm cell path in spectrophotometer, as described earlier [22].
Circular dichroism spectroscopy
Far- ultraviolet (UV) circular dichroism (CD) spectra of the peptide in the presence and absence of 1 μM of copper was recorded using a JASCO J-815 spectropolarimeter (Easton, MD, USA). Mini-αA-crystallin (0.1mg/ml) was prepared in 10 mM phosphate buffer, pH 7.2. Far-UV CD spectra were recorded at 25°C. All of the reported spectra were the cumulative average of 6 scans after subtraction of the buffer blank.
Fluorescence spectroscopy
The relative hydrophobicity of mini-αA-crystallin was measured using the hydrophobic dye, 1,1′-bi(4-anilino) naphthalene-5,′-disulfonic acid (bis-ANS) (Molecular Probes, Inc., Eugene, OR, USA). A stock solution of the dye (14.8 μM) was prepared in 95% ethanol. Peptides were titrated with increasing concentrations of copper in the range of 0–100 μM. Ten μl of bis-ANS stock solution was added to 15μM of mini-αA in 1 ml of 50 mM PO4 buffer at pH 7.2. The mixture was incubated at 37°C for 20 min. The interaction of bis-ANS with mini-αA-crystallin in the absence and presence of copper was examined by recording the emission spectra between 450 to 600 nm. The samples were excited at 385 nm in a Jasco spectrofluorimeter FP-750. Emission at 490 nm was used to calculate F0−F/F0 values. Analysis of the saturation binding curve at one site was performed using the equation f=bmax X abs(x)/(KD+abs(x)), as described earlier [28], where bmax is the maximum binding (maximum extent of quenching), x is the Cu2+ concentration, and f is the fluorescence quenching at a given concentration of Cu2+. KD value was obtained from a semi log plot.
Isothermal titration calorimetric assay
Isothermal titration calorimetry was performed in a VP-ITC (Microcal, Inc., Northampton, MA, USA). Mini-αA-crystallin was prepared in 10 mM cholamine chloride buffer containing 100 mM NaCl (pH 7.4). Copper sulfate, 1.0 mM, was prepared in the same buffer. For each titration, the sample cell (1.41 mL) contained mini-αA, and the buret contained the Cu2+ solution. Following thermal equilbration, injections of titrant, 10 μL, were made at 4- min intervals. The titration protocol included a 2 μl-pre-injection, the heat from which was neglected during the subsequent analysis. The data were analyzed with a single-site model, using the Origin-based software supplied with the instrument.
Analysis of mini-αA-crystallin–copper binding by mass spectrometry
Peptide and Cu2+ were prepared in 5 mM of ammonium acetate buffer (pH 6.3). The final reaction solution was prepared using the same buffer at 10 μM-concentration of mini-αA-crystallin and different concentrations of Cu2+. The pH was recorded after the addition of Cu2+ ion and mini-αA-crystallin. The samples were analyzed in the positive ion mode by static nanospray mass spectrometry (MS) on an Agilent 6520 QTOF mass spectrometer at the University of Missouri Proteomics Center. The resulting spectra were subjected to the Agilent resolved isotope deconvolution software program (Agilent, Santa Clara, CA, USA).
Effects of Cu2+ on mini-αA-crystallin chaperone-like activity
To test whether copper binding to mini-αA-crystallin modulates chaperone-like activity, mini-αA-crystallin was saturated with 1 mM CuSO4 solution, and the peptide–Cu2+ complex was isolated by Sephadex-G20 gel filtration chromatography. Protein aggregation assays were performed using 75 μg of citrate synthase (CS) in 1 ml of 40 mM HEPES-KOH buffer (pH 7.0) in the presence of the mini-αA-crystallin–Cu2+ complex (50 μg) or mini-αA-crystallin alone (50 μg). The extent of aggregation of CS was monitored at 360 nm up to 1 h, as described earlier [10].
αAΔ70-77 region in copper binding
To test whether this region is the only copper binding region in αA-crystallin, a deletion mutant αAΔ70-77 was created by site-directed mutagenesis, and the recombinant protein was purified [10]. Purified protein was used in the copper-induced ascorbic acid oxidation assay, as described above. Oxidation assays were carried out using 100 μg of recombinant protein αAΔ70-77 or wild-type αA-crystallin in the presence and absence of Cu2+ (5 μM).
Results
Inhibition of Cu2+-mediated ascorbic acid oxidation by mini-αA-crystallin
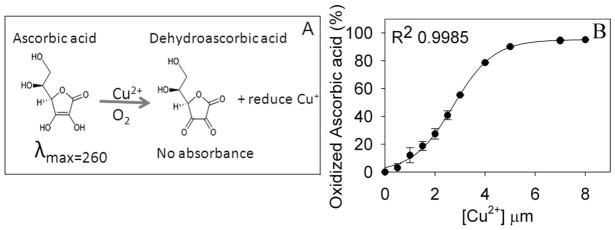
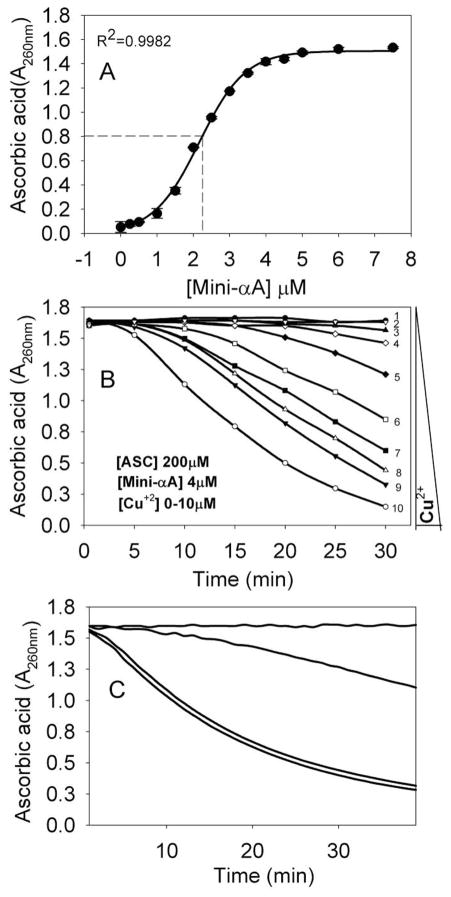
Ascorbic acid is readily oxidized in the presence of Cu2+ ion (Fig. 1A). In the presence of air/oxygen and Cu2+, ascorbic acid is converted to dehydroascorbic acid, with the generation of reduced copper ion. Our baseline experiment of the rate of ascorbic acid oxidation at increasing concentrations of Cu2+ revealed that in about 30 min, 500 μM of ascorbic acid was completely oxidized by 4 μM Cu2+ (Fig. 1B). In another experiment, the effects of Cu2+ (4 μM) on oxidation of ascorbic acid (500 μM) was determined in the presence of different concentrations of mini-αA-crystallin (Fig. 2A). The amount of ascorbic acid remaining in the reaction mixture that contained both Cu2+ and mini-αA-crystallin showed a sigmoidal profile (Fig. 2A), similar to the profile observed with increasing concentrations of Cu2+ and a fixed amount of ascorbic acid (Fig. 1B). A mini-αA-crystallin concentration of 2 μM reduced 50% the amount of ascorbic acid oxidation induced by 4 μM of copper (Fig. 2A), suggesting that mini-αA-crystallin may bind copper and render it unavailable for ascorbic acid oxidation. A reverse titration, with a fixed concentration of mini-αA- and varying concentrations of Cu2+, led to complete suppression of ascorbic acid oxidation when the concentration of the peptide in the reaction mixture reached that of Cu2+ (Fig. 2B). The protection of ascorbic acid by the peptide was nearly lost when the His in mini-αA-crystallin was substituted with Ala (Fig 2C). The inability of Ala-substituted mini-αA- to prevent the Cu2+-mediated oxidation of ascorbic acid indicates that the His residue in the peptide is essential for its redox suppression activity.
Fig. 1.
(A) Schematic representation of oxidation pathway of the ascorbic acid. (B) Standard curve of Cu2+-induced oxidation of ascorbic acid. Cu2+-induced oxidation of ascorbic acid was determined as described under methods by following 260 nm absorbance.
Fig. 2.
Mini-αA-crystallin inhibition of Cu2+-induced oxidation of ascorbic acid. (A) Effect of different concentrations of mini-αA-crystallin on 4μM Cu2+-induced oxidation of ascorbic acid (500 μM). The residual ascorbic acid was measured at 260 nm after 30 min. Non-linear regression analysis of these data points shows that the effective concentration (EC 50) of mini-αA-crystallin is 2.2 μM.
(B) Effect of 4 μM mini-αA on different concentrations of Cu2+- induced oxidation of 500 μM ascorbic acid during 30 minutes. 1) without Cu2+; 2) + 1 μM Cu2+; 3) + 2 μM Cu2+; 4) + 3 μM Cu2+; 5) + 4 μM Cu2+; 6) + 5 μM Cu2+; 7) + 6 μM Cu2+; 8) + 8 μM Cu2+; 9) + 10 μM Cu2+ and 10) 10 μM Cu2+ without mini-αA-
C) Effect of His-substituted mini-αA-crystallin on Cu2+-induced oxidation of ascorbic acid. The assays were performed using ascorbic acid (500 μM) and Cu2+ (4 μM) in presence of 4 μM mini-αA- or mini-αA–H79A. 1) without Cu2+ and mini-αA-; 2) + mini-αA + Cu2+; 3) + mini-αA-H79A + Cu2+ and 4) + Cu2+.
Effect of copper on bis-ANS binding to mini-αA-crystallin
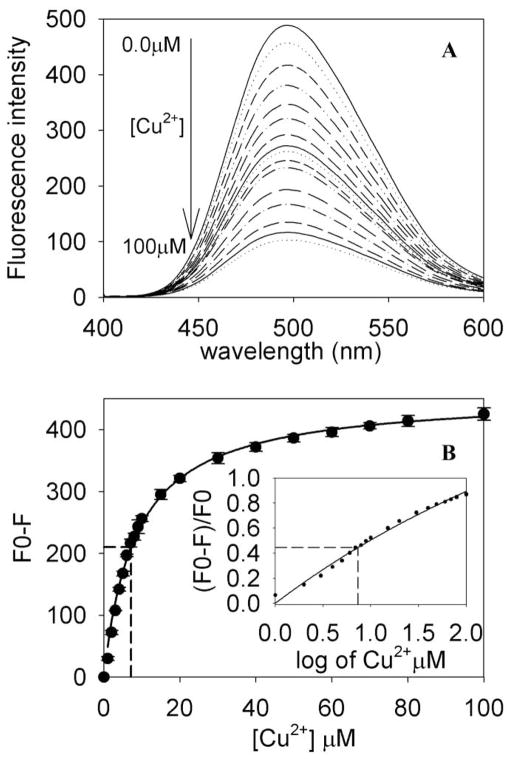
Bis-ANS binds to hydrophobic proteins and peptides, and this binding leads to a several fold enhancement of bis-ANS fluorescence, with a blue shift in the emission maximum. Earlier we reported that mini-αA-crystallin interacts with bis-ANS [16] and that the maximum emission of the complex is around 490 nm. The present study revealed that prior binding of Cu2+ to mini-αA -crystallin decreased bis-ANS binding, as shown in Fig. 3A. Titration of mini-αA (15 μM) with increasing concentrations of Cu2+ (1–100 μM) decreased the bis-ANS fluorescence intensity in a concentration-dependent manner (Fig. 3A). The extent of fluorescence quenching was calculated using F0−F/F0, which gave a hyperbolic plot that showed the fluorescence intensity of mini-αA- plotted against Cu2+ concentration. The plot demonstrated over 98% saturation of Cu2+ binding sites in mini-αA at 100 μM Cu2+ (Fig. 3B). The extent of fluorescence quenching by Cu2+ was used to determine binding constants, as described earlier [28, 29]. The analysis of the semi-log plot of the titration data gave a KD value of 8.4 × 10−6 M for the binding of Cu2+ to mini-αA-crystallin (Fig. 3B inset). The control peptide αA1-14, showed no changes in bis-ANS fluorescence intensity in the presence and absence of Cu2+.
Fig. 3.
Effects of Cu2+ binding on Bis-ANS fluorescence of mini-αA-crystallin. (A) Quenching of mini-αA bound Bis-ANS fluorescence by Cu2+ binding. (B) Bis-ANS fluorescence intensity in the presence of mini-αA-crystallin and increasing concentrations of Cu2+. The extent of bis-ANS quenching (F0-F) is calculated where F0 and F emission maxima at 490nm are shown in the absence and presence of Cu2+. The inset shows semi log plot of F0-F/F0 vs Cu2+ concentration.
Mass spectrometric analysis of Cu2+ binding to mini-αA-crystallin
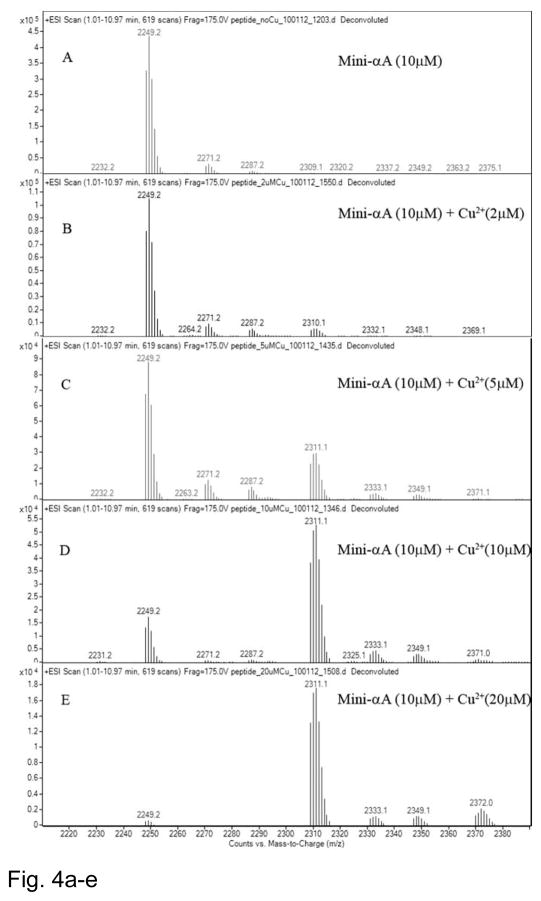
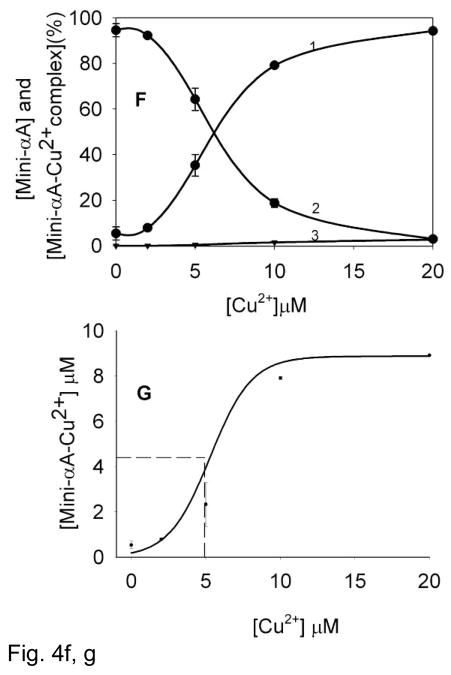
Mass spectrometric analysis allows direct measurement of Cu2+ ions bound to the mini-αA-crystallin in addition to the speciation information. Nanospray QTOF mass spectra were recorded for mini-αA-crystallin (10 μM) in the absence and presence of different concentrations of Cu2+ at pH 6.3. The deconvoluted mass spectrum of mini-αA- without Cu2+ showed one major peak at m/z 2249, corresponding to mini-αA peptide mass (Fig. 4A). The profile also showed several additional minor peaks that corresponded to Na+ or K+ adducts of the peptide; these were subtracted prior to the final data analysis. A higher mass shift to m/z 2309, equivalent to binding of one Cu2+ molecule was observed only after the addition of Cu2+, thus indicating that there is one Cu2+ binding site in mini-αA-crystallin. The Profiles showed a shift in peak mass, corresponding to one Cu2+ addition from a reaction mixture containing 2 to 20 μM copper (Figs. 4B through 4E). Significant gains in peak intensity resulted from binding of one Cu2+ to the peptide (Figs. 4C, 4D, 4E). A 1:1 ratio of copper (10 μM) to mini-αA-crystallin (10 μM) showed a major peak (79%) with one Cu2+ and a small peak (21%) of free peptide (Fig. 4D). With a 1:2 ratio of mini-αA- and Cu2+, the peak shift corresponded to one Cu2+ binding to 94.3% of the peptide. Binding of two Cu2+ per 1.5% and 2.8 % of the total peptide was observed when mini-αA-to-Cu2+ratio of 1:1 and 1:2 was used (Figs. 4D and 4E).
Fig. 4.
Mass spectra of 10μM of mini-αA-crystallin in 5 mM of ammonium acetate buffer pH 6.3, with and without Cu2+. A) mini-αA without Cu2+; B) + 2 μM Cu2+; C) + 5 μM Cu2+; D) + 10 μM Cu2+ and E) + 20 μM Cu2+.
F) Estimation of peptide-Cu2+ complex concentrations after mass spectrometric analysis. 1) amount of one Cu2+ –mini-αA-crystallin complex; 2) free mini-αA-crystallin and 3) two Cu2+ –mini-αA complex.
G) The binding of Cu2+ ions to 10 μM of mini-αA-crystallin at pH 6.3 as a function of the concentration of total Cu2+ added. KD= 5.1 μM can be derived from the half-maximal value in curve.
The dissociation constant for Cu2+ binding to the peptide was determined from the peak intensity of mini-αA-crystallin alone or mini-αA–Cu2+ complex, as described by Whittal et al. [30]. The sum of bound Cu2+ was calculated from the sum of mini-αA–Cu2+ complex, assuming that the total amount of bound Cu2+ is equal to mini-αA–Cu2+ complexes or the concentration of free peptide is equal to the concentration of mini-αA–Cu2+ complex minus the total amount of mini-αA (Table 1). From the data shown in Figs 4F and 4G we estimated that at a Cu2+ concentration of ~5 μM, 50% of the mini-αA-crystallin binds with one Cu2+. The data also reveal that mini-αA-crystallin has one Cu2+ binding site that exhibits a KD value 5.1×10−6.
Table 1.
Cu2+ binding data from mass spectrometric analysis mini-αA andCu2+ mixtures at different ratios of mini-αA and Cu2+ The values are average of two independent experiments.
| [Cu2+] μM | Peptide (%) | Peptide + 1 Cu2+ (%) | Peptide + 2 Cu2+ (%) |
|---|---|---|---|
| 0 | 100 | 0 | 0 |
| 2 | 92.2 | 7.8 | 0 |
| 5 | 64.3 | 35.3 | 0.4 |
| 10 | 18.8 | 79.7 | 1.5 |
| 20 | 2.9 | 94.3 | 2.8 |
Effect of Cu2+ binding on chaperone-like activity of mini-αA-crystallin
The effect of prior binding of Cu2+ to mini-αA-crystallin on chaperone-like activity was investigated using CS (Fig. 5). The addition of mini-αA-crystallin efficiently suppressed the CS aggregation. However, in presence of Cu2+, there was a significant increase in the aggregation of denaturing CS. The addition of mini-αA-crystallin suppressed this aggregation but the total aggregation observed in the presence of Cu2+ and mini-αA-crystallin remained slightly higher than that with mini-αA- and CS. The addition of EDTA to chelate Cu2+ augmented the chaperone activity of the peptide, to a level equal to that of the peptide without Cu2+.This also was evident from the overlap of light scattering profile of mini-αA-crystallin in the absence of Cu2+ and in the presence of Cu2+ –EDTA (Fig. 5).
Fig. 5.
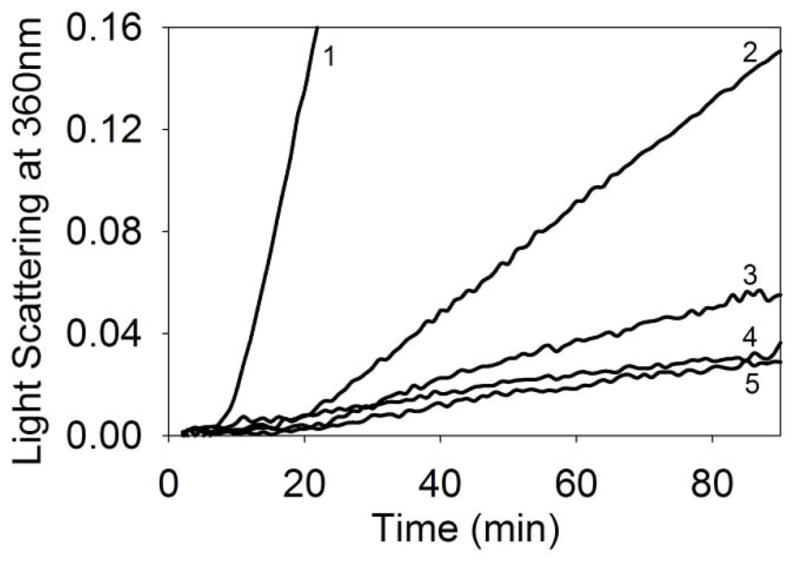
Chaperone-like activity of mini-αA-crystallin in the presence and absence of Cu2+ against aggregating citrate synthase (CS). Light scattering by 75 μg/ml CS was monitored at 360 nm in a spectrophotometer. 1) CS +1 μM Cu2+; 2) CS; 3) CS + 10 μM mini-αA–Cu2+ complex; 4) CS + 10 μM mini-αA and 5) CS + 10 mM mini-αA–Cu2+ complex + 1 mM EDTA.
Selective Cu2+ binding property of mini-αA-crystallin
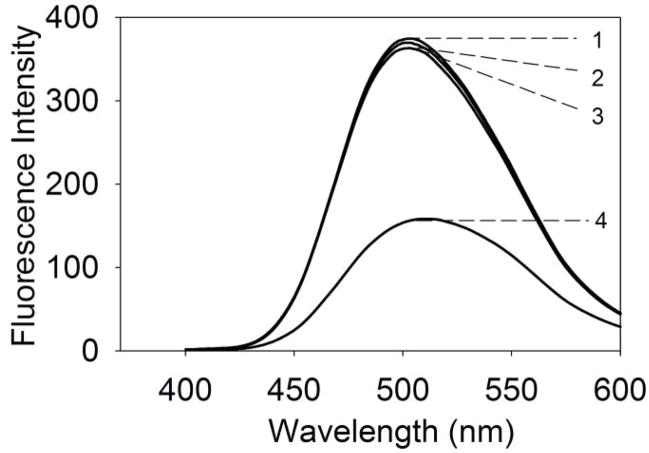
To examine whether Cu2+ binding to mini-αA-crystallin is selective or affected by the presence of other metal ions, Zn2+ (1 mM) or Fe2+ (1 mM) was added to mini-αA-crystallin (50 μg) and set aside for 30 min at room temperature, after which the hydrophobic probe bis-ANS was added. The samples were excited at 385 nm and the emission at 490 nm was recorded. Unlike Cu2+-treated mini-αA-crystallin, Zn2+- and Fe2+-treated mini-αA showed no loss in bis-ANS fluorescence (compare Figs 6 and 3A), suggesting that there is either no binding of metal ions to the peptide at the bis-ANS binding site or the bound metal ions are readily displaced by bis-ANS. However, the addition of Cu2+ to the mini-αA-crystallin sample that contained saturating amounts of Zn2+ or Fe2+ metal ions resulted in a significant decrease in bis-ANS fluorescence values. The net fluorescence was comparable to that observed after the addition of Cu2+ to mini-αA-crystallin. These data suggest that mini-αA-crystallin has a selective Cu2+ binding site that is not affected by the presence of other metal ions Zn2+ or Fe2+ (Fig. 6).
Fig. 6.
Selective binding of Cu2+ to mini-αA-crystallin to quench bis-ANS flourescence in the presence of Zn2+ or Fe2+. 1) bis-ANS fluorescence spectrum with 10 μM mini-αA; 2) bis-ANS + 10 μM mini-αA- + 1 mM Zn2+; 3) bis-ANS + 10 μM mini-αA- + 1 mM Fe2+; 4. Bis-ANS + 10 μM mini-αA- + 1 mM Cu2+.
Isothermal titration calorimetric assay
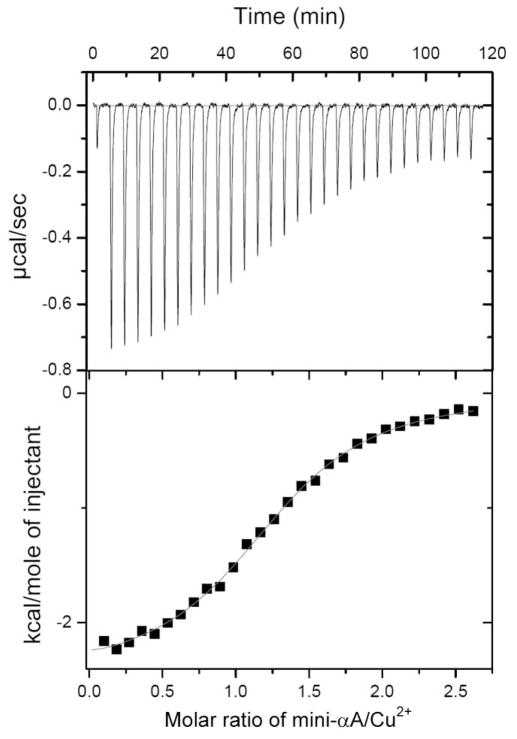
The energetics of Cu2+ binding were examined by isothermal titration calorimetry (Fig. 7). It is apparent from the raw data that the interaction of Cu2+ with mini-αA-crystallin was exothermic under our experimental conditions (Fig 7, top graph). The corresponding integrated data, corrected for the heat of mixing, are presented in the lower panel of Fig. 7. These data can be satisfactorily accommodated by a single-site model, affording estimates for the association constant and apparent binding enthalpy of 1.07 ± 0.06 × 105 M−1 and −2.45 ± 0.03 kcal mol−1, respectively.
Fig. 7.
Calorimetric titration of mini-αA-crystallin with copper. (Top panel) Raw data for 10-μl injections of 1 mM Cu2+ into the reaction cell containing 1.4 ml of 50 μM mini-αA- dissolved in 10 mM cholamine buffer pH 7.4 containing 100 mM NaCl. (Bottom panel) Plot of net heat released as a function of the ration of mini-αA to copper.
Deletion of chaperone-site residues in αA-crystallin affects Cu2+ binding
To demonstrate that the mini-αA sequence is the major copper binding site in human αA-crystallin, we created an αA-crystallin deletion mutant, αAΔ70-77, by site-directed mutagenesis and tested the ability of the purified recombinant proteins to protect against Cu2+-induced oxidation of ascorbic acid. We found that 100 μg of native αA-crystallin is able to protect >90% of ascorbic acid from Cu2+-induced oxidation, whereas 100 μg of the deletion mutant was able to protect <20% of ascorbic acid from Cu2+-mediated oxidation (Fig. 8).
Fig. 8.
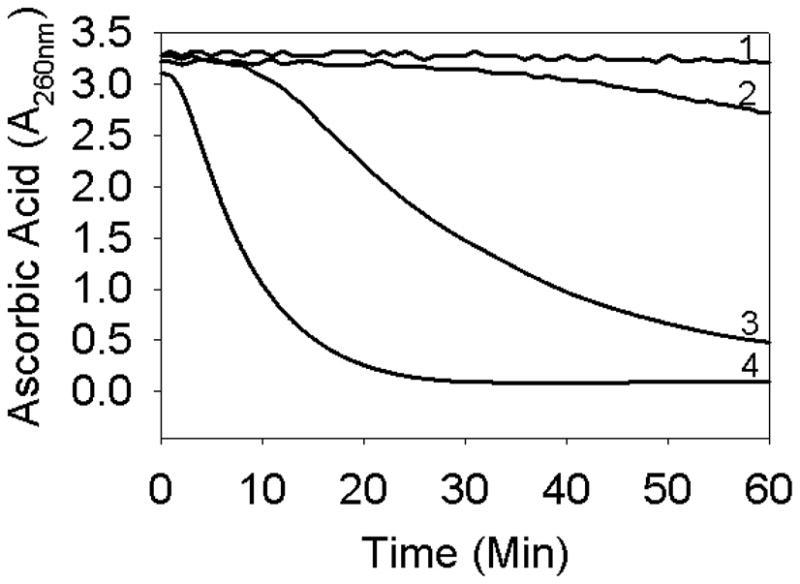
Oxidation of ascorbic acid in the presence of αAΔ70-77 and wild-type αA-crystallin. Ascorbic acid (500 μM) was used in assays. 1) ascorbic acid; 2) + 5 μM Cu2+ + 100 μg αA-wt; 3) + 5 μM Cu2+ + 100 μg αAΔ70-77 and 4) + 5 μM Cu2+.
Discussion
Previous studies have shown that α–crystallin can bind copper and prevent copper- mediated oxidation of ascorbic acid [22,24,31]. Lens α–crystallin also prevents Cu2+-induced inactivation and aggregation of aldose reductase [25]. While these studies confirm the interaction between Cu2+ and lens crystallins and redox suppression of bound Cu2+, no studies have identified specific regions or residues in α-crystallin subunits that are responsible for Cu2+ binding. In a previous study we identified a specific region in crystallin subunits involved in αA-crystallin function [16]. In other studies, we also found evidence of chaperone-like activity in a synthetic peptide KFVIFLDVKHFSPEDLTVK, corresponding to 70–88 amino acids of αA-crystallin, or DFVIFLDVKHFSPEDLTVK (where N-terminal Lys was substituted with Asp) [18,19].
In the present study, using both biophysical and biochemical techniques, we evaluated the copper binding and redox suppression activity of an αA-crystallin peptide (mini-αA-crystallin). The oxidation of ascorbic acid by Cu2+ and its prevention by mini-αA-crystallin was used to measure the ability of the peptide to bind Cu2+ (Figs. 1 and 2). We found that the addition of mini-αA-crystallin to the -ascorbic acid–Cu2+ reaction mixture prevented ascorbic acid oxidation (Fig. 2). However, when the His in the peptide was substituted with Ala, yielding DFVIFLDVKAFSPEDLTVK, the peptide was unable to protect ascorbic acid from Cu2+-induced oxidation. The data suggest that the peptide-mediated suppression of ascorbic acid oxidation by Cu2+ is due to the binding of the metal ions to the peptide, suppressing the redox activity of the bound Cu2+. On the basis of these data, we propose that the previously reported redox suppression activity of α-crystallin resides at the same sequence as the previously identified chaperone activity region. Metal ions are known to coordinate to His residues in metal chelating peptides and proteins [29,30]. Studies of the binding of Cu2+ to peptides derived from α-synuclein and prion proteins and to β-amyloid have shown that His residues in the peptides are involved in metal binding. The absence of redox suppression activity in αA-crystallin that lacked His suggests that His is involved in the binding of Cu2+ to this peptide. Unlike the Cu2+ bound to mini-αA-crystallin, the Cu2+ bound to His-containing β-amyloid peptide stimulated oxidation of ascorbic acid and generated H2O2 [35].
We used isothermal titration calorimetric assays and mass spectrometric methods to investigate the interaction between mini-αA-crystallin and Cu2+. Both methods showed that the peptide interacts with Cu2+ with high affinity. The stoichiometry of the mini-αA–copper complex formation was estimated by nanospray ionization mass spectrometry. The mass spectrometric data demonstrating that mini-αA-crystallin binds to Cu2+ at a 1:1 ratio provide direct evidence for the interaction of mini-αA with Cu2+. However, at higher Cu2+ concentration (1: 2 peptide to Cu2+) we observed that about 2 Cu2+ was bound to 2.8 % of the peptide. On the basis of isothermal titration calorimetric data, the dissociation constant (KD) for Cu2+ interaction with the peptide is 9.3 μM. This value agrees well with the 8.4 μM value obtained by analysis of the quenching of peptide-bound bis-ANS fluorescence by Cu2+ ion. Previously, it was reported that KD(apparent) for αA-crystallin–Cu2+ interactions in glycine buffer is in the range of 5–12 μM [24].
A crystal structure study of truncated αA-crystallin (αAC59-163) reported that three subunits contribute to a zinc binding motif [36]. His 100 and Glu 102 of one subunit and His 107 and His154 of two other subunits were found to be involved in the formation of tetrahedral coordination geometry of Zn2+ binding [36]. A molecular modeling study predicted that residues H 18, Glu 99, H 101, H 119, and Lys 121 in αA-crystallin are coordinating with Cu2+ [26]. However, this prediction has yet to be validated experimentally. To test whether the mini-αA region (residues 70–88) is the only copper binding site in αA-crystallin, we prepared a deletion mutant of α-crystallin (αAΔ70-77), which lacks a portion of the mini-chaperone sequence, and tested the ability of purified recombinant protein to prevent Cu2+-mediated ascorbic acid oxidation. The αAΔ70-77 was soluble and relatively stable with a significant loss in chaperone activity. We found that αAΔ70-77 has a 70% reduced activity against Cu2+-induced oxidation of ascorbic acid as compared to wild-type αA-crystallin. Deletion of the 70–88 region in αA-crystallin produced an unstable aggregation protein that we could not test. These results indicate that the 70–77 region is the major Cu2+ binding site in human αA-crystallin. At this time, due to the lack of crystal structure of wild-type αA-crystallin, we are unable to discuss the relative location of Cu2+ in αA-crystallin oligomer.
There are three nitrogen donor systems involved in the Cu2+ binding to protein/peptide. It has been reported that the imidazolic nitrogen atoms of histidine residues act as principle anchor points for the Cu2+ binding. To some extent, Lys and Glu amino acids may also be considered as amino acids involved in copper binding. Alternatively, at least 2 histidines and one arginine are required to form one copper binding site [33]. However, mini-αA-crystallin has only one histidine and no arginine residues. Therefore it remains to be determined which amino acid residues in the peptide coordinate with copper. It could be possible that the single histidine along with lysine and N-terminal amino group may be involved in copper coordination in mini-αA-crystallin. Mlynarz et al. [34] reported that in short peptides, a single histidine could play the role of an anchoring site using its imidazole side chain nitrogen as a donor atom. Mini-αA-crystallin with single His seems to be fulfilling this criterion. To conclude, we have identified in αA-crystallin, for the first time, the Cu2+ binding region and specific His residue involved in such binding.
Acknowledgments
This work was supported by National Institutes of Health Grant 5R01EY011981 and a grant-in-aid from Research to Prevent Blindness to the department. The authors thank Sharon Morey for help with preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–156. doi: 10.1016/s0065-3233(01)59004-x. [DOI] [PubMed] [Google Scholar]
- 2.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76(2):145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genetics. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 7.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genetics. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 8.Graw J, Klopp N, Illig T, Preising MN, Lorenz B. Congenital cataract and macular hypoplasia in humans associated with a de novo mutation in CRYAA and compound heterozygous mutations in P. Graefe’s Arch Clin Exp Ophthalmol. 2006;244:912–919. doi: 10.1007/s00417-005-0234-x. [DOI] [PubMed] [Google Scholar]
- 9.Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, Petrash JM, Bhanuprakash Reddy G, Padma T. A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract. Biochim Biophys Acta. 2009;1792:974–981. doi: 10.1016/j.bbadis.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J Biol Chem. 2001;276:47094–47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto L, Emmons T, Horwitz JJ. The C-terminal region of alpha-crystallin: involvement in protection against heat-induced denaturation. Biochem J. 1993;294(Pt 2):435–438. doi: 10.1042/bj2940435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J Biol Chem. 271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 13.Kumarasamy A, Abraham EC. Interaction of C-terminal truncated human alphaA-crystallins with target proteins. PLoS One. 2008;3:e3175. doi: 10.1371/journal.pone.0003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasta SY, Raman B, Ramakrishna T, Rao CM. Role of the conserved SRLFDQFFG region of alpha-crystallin, a small heat shock protein. J Biol Chem. 2003;278:51159–51166. doi: 10.1074/jbc.M307523200. [DOI] [PubMed] [Google Scholar]
- 15.Raman B, Rao CM. Chaperone-like activity and quaternary structure of alpha-crystallin. J Biol Chem. 1994;269:27264–27268. [PubMed] [Google Scholar]
- 16.Sharma KK, Kumar RS, Kkumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in αA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 17.Rehna EA, Singh SK, Dharmalingam K. Functional insights by comparison of modeled structures of 18kDa small heat shock protein and its mutant in. Mycobacterium leprae Bioinformation. 2008;3:230–234. doi: 10.6026/97320630003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar RS, Sharma KK. Chaperone-like activity of a synthetic peptide toward oxidized gamma-crystallin. J Pept Res. 2000;56:157–164. doi: 10.1034/j.1399-3011.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- 19.Sreelakshmi Y, Sharma KK. Interaction of alpha-lactalbumin with mini-alphaA-crystallin. J Protein Chem. 2001;20:123–130. doi: 10.1023/a:1011077307262. [DOI] [PubMed] [Google Scholar]
- 20.Racz P, Ordogh M. Investigations on trace elements in normal and senile cataractous lenses. Activation analysis of copper, zinc, manganese, cobalt, rubidium, scandium, and nickel. Graefe’s Arch Clin Exp Ophthalmol. 1977;204:67–72. doi: 10.1007/BF02387418. [DOI] [PubMed] [Google Scholar]
- 21.Eckhert CD. Elemental concentrations in ocular tissues of various species. Exp Eye Res. 1983;37:639–647. doi: 10.1016/0014-4835(83)90138-0. [DOI] [PubMed] [Google Scholar]
- 22.Ortwerth BJ, James HL. Lens proteins block the copper-mediated formation of reactive oxygen species during glycation reactions in vitro. Biochem Biophys Res Commun. 1999;259:706–710. doi: 10.1006/bbrc.1999.0841. [DOI] [PubMed] [Google Scholar]
- 23.Cook CS, Bentley PJ. Copper exchanges and toxicity in the rabbit lens in vitro. Exp Eye Res. 1986;42:107–116. doi: 10.1016/0014-4835(86)90035-7. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad MF, Singh D, Taiyab A, Ramakrishna T, Raman B, Rao CM. Selective Cu2+ binding, redox silencing, and cytoprotective effects of the small heat shock proteins αA- and αB-crystallin. J Mol Biol. 2008;382:812–824. doi: 10.1016/j.jmb.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 25.Moschini R, Marini I, Malerba M, Cappiello M, Del Corso A, Mura U. Chaperone-like activity of alpha-crystallin toward aldose reductase oxidatively stressed by copper ion. Arch Biochem Biophys. 2006;453:13–17. doi: 10.1016/j.abb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Ganadu ML, Aru M, Mura GM, Coi A, Mlynarz P, Kozlowski H. Effects of divalent metal ions on the alphaB-crystallin chaperone-like activity: spectroscopic evidence for a complex between copper(II) and protein. J Inorg Biochem. 2004;98:1103–1109. doi: 10.1016/j.jinorgbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Biswas A, Das KP. Zn2+ enhances the molecular chaperone function and stability of alpha-crystallin. Biochemistry. 2008;4:804–816. doi: 10.1021/bi7011965. [DOI] [PubMed] [Google Scholar]
- 28.Singh D, Tangirala R, Bakthisaran R, Rao CM. Synergistic effects of metal ion and the pre-senile cataract-causing G98R alphaA-crystallin: self-aggregation propensities and chaperone activity. Mol Vis. 2009;15:2050–2060. [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GS, Murray I, Hosszu LL, Gibbs N, Waltho JP, Clarke AR, Collinge J. Location and properties of metal-binding sites on the human prion protein. Proc Natl Acad Sci U S A. 2001;98:8531–8535. doi: 10.1073/pnas.151038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittal RM, Bail HL, Cohen FE, Burlingame AL, Prusiner SB, Baldwin MA. Copper binding to octarepeat peptides of the prion protein monitored by mass spectrometry. Protein Sci. 2000;9:332–343. doi: 10.1110/ps.9.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moschini RM, Marini I, Malerba M, Cappielllo M, Del Corso A, Mura U. Chaperone-like activity of alpha-crystallin toward aldose reductase oxidatively stressed by copper ion. Arch Biochem Biophys. 2006;453:13–17. doi: 10.1016/j.abb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem. 2004;267:147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- 33.Kowalik-Jankowska T, Ruta-Dolejsz M, Wisniewska K, Lankiewicz L, Kozlowski H, et al. Copper(n) complexation by human and mouse fragments (11–16) of β-amyloid peptide. J Chem Soc Dalton Trans. 2000;4:4511–4519. [Google Scholar]
- 34.Mlynarz P, Valensin D, Kozlowski H, Kowalik-Jankowska T, Otlewski J, Valensin G, Gagelli N. Co-ordination ability towards CuII of the 29-amino acid residue trypsin inhibitor of squash and two of its analogues. J Chem Soci Dalton Trans. 2001;5:645–652. [Google Scholar]
- 35.Dikalov SI, Vitek MP, Mason RP. Cupric-amyloid β oxidation of ascorbate and generation of hudroxy radical. Free Rad Biol Med. 2004;36:340–347. doi: 10.1016/j.freeradbiomed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Laganowsky A, Benesch JlP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]