Abstract
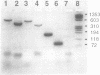
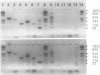
Extrachromosomal circular DNA (eccDNA) generated from chromosomal DNA is found in all mammalian cells and increases with cell stress or aging. Studies of eccDNA structure and mode of formation provide insight into mechanisms of instability of the mammalian genome. Previous studies have suggested that eccDNA is generated through a process involving recombination between repetitive sequences. However, we observed that approximately one half of the small eccDNA fragments cloned from HeLa S3 cells were composed entirely of nonrepetitive or low-copy DNA sequences. We analyzed four of these fragments by polymerase chain reaction and nucleotide sequencing and found that they were complete eccDNAs. We then screened a human genomic library with the eccDNAs to isolate the complementary chromosomal sequences. Comparing the recombination junctions within the eccDNAs with the chromosomal sequences from which they were derived revealed that nonhomologous recombination was involved in their formation. One of the eccDNAs was composed of two separate sequences from different parts of the genome. These results suggest that rejoining of ends of fragmented DNA is responsible for the generation of a substantial portion of the eccDNAs found in HeLa S3 cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertelsen A. H., Humayun M. Z., Karfopoulos S. G., Rush M. G. Molecular characterization of small polydisperse circular deoxyribonucleic acid from an African green monkey cell line. Biochemistry. 1982 Apr 27;21(9):2076–2085. doi: 10.1021/bi00538a015. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores S. C., Sunnerhagen P., Moore T. K., Gaubatz J. W. Characterization of repetitive sequence families in mouse heart small polydisperse circular DNAs: age-related studies. Nucleic Acids Res. 1988 May 11;16(9):3889–3906. doi: 10.1093/nar/16.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Tsuda T., Toda M., Yamagishi H. Transposon-like sequences in extrachromosomal circular DNA from mouse thymocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2072–2076. doi: 10.1073/pnas.82.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubatz J. W. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat Res. 1990 Sep-Nov;237(5-6):271–292. doi: 10.1016/0921-8734(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Hahn P. J. Molecular biology of double-minute chromosomes. Bioessays. 1993 Jul;15(7):477–484. doi: 10.1002/bies.950150707. [DOI] [PubMed] [Google Scholar]
- Iwasato T., Shimizu A., Honjo T., Yamagishi H. Circular DNA is excised by immunoglobulin class switch recombination. Cell. 1990 Jul 13;62(1):143–149. doi: 10.1016/0092-8674(90)90248-d. [DOI] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. Characterization of cloned human alphoid satellite with an unusual monomeric construction: evidence for enrichment in HeLa small polydisperse circular DNA. Nucleic Acids Res. 1985 Feb 11;13(3):1027–1042. doi: 10.1093/nar/13.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Potter S. S. L1 sequences in HeLa extrachromosomal circular DNA: evidence for circularization by homologous recombination. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1989–1993. doi: 10.1073/pnas.82.7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R. A., Wang J. C. A subthreshold level of DNA topoisomerases leads to the excision of yeast rDNA as extrachromosomal rings. Cell. 1989 Jun 16;57(6):975–985. doi: 10.1016/0092-8674(89)90336-x. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Okumura K., Oishi M. A group of repetitive human DNA families that is characterized by extrachromosomal oligomers and restriction-fragment length polymorphism. J Mol Biol. 1987 Feb 20;193(4):591–597. doi: 10.1016/0022-2836(87)90342-1. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Rush M. G. Some extrachromosomal circular DNAs containing the Alu family of dispersed repetitive sequences may be reverse transcripts. J Mol Biol. 1984 Mar 25;174(1):31–40. doi: 10.1016/0022-2836(84)90363-2. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Schindler C. W., Rush M. G. Structure of extrachromosomal circular DNAs containing both the Alu family of dispersed repetitive sequences and other regions of chromosomal DNA. J Mol Biol. 1984 Mar 25;174(1):41–54. doi: 10.1016/0022-2836(84)90364-4. [DOI] [PubMed] [Google Scholar]
- Kunisada T., Yamagishi H., Ogita Z., Kirakawa T., Mitsui Y. Appearance of extrachromosomal circular DNAs during in vivo and in vitro ageing of mammalian cells. Mech Ageing Dev. 1985 Jan;29(1):89–99. doi: 10.1016/0047-6374(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Kunisada T., Yamagishi H. Sequence organization of repetitive sequences enriched in small polydisperse circular DNAs from HeLa cells. J Mol Biol. 1987 Dec 20;198(4):557–565. doi: 10.1016/0022-2836(87)90199-9. [DOI] [PubMed] [Google Scholar]
- McWhinney C., Leffak M. Autonomous replication of a DNA fragment containing the chromosomal replication origin of the human c-myc gene. Nucleic Acids Res. 1990 Mar 11;18(5):1233–1242. doi: 10.1093/nar/18.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Matera A. G., Schmid C. W., Rush M. G. Recombination mediates production of an extrachromosomal circular DNA containing a transposon-like human element, THE-1. Nucleic Acids Res. 1989 Oct 25;17(20):8327–8341. doi: 10.1093/nar/17.20.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Shih A., Rush M., Wong E., Schmid C. W. Cloned extrachromosomal circular DNA copies of the human transposable element THE-1 are related predominantly to a single type of family member. J Mol Biol. 1987 Jul 20;196(2):233–243. doi: 10.1016/0022-2836(87)90687-5. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Yezzi M. J., Young B. R. Recombination events during integration of transfected DNA into normal human cells. Nucleic Acids Res. 1990 May 11;18(9):2733–2738. doi: 10.1093/nar/18.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Kiyama R., Oishi M. Sequence analyses of extrachromosomal Sau3A and related family DNA: analysis of recombination in the excision event. Nucleic Acids Res. 1987 Sep 25;15(18):7477–7489. doi: 10.1093/nar/15.18.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Misra R. Extrachromosomal DNA in eucaryotes. Plasmid. 1985 Nov;14(3):177–191. doi: 10.1016/0147-619x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Schindler C. W., Rush M. G. Discrete size classes of monkey extrachromosomal circular DNA containing the L1 family of long interspersed nucleotide sequences are produced by a general non-sequence specific mechanism. Nucleic Acids Res. 1985 Nov 25;13(22):8247–8258. doi: 10.1093/nar/13.22.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C. W., Rush M. G. The KpnI family of long interspersed nucleotide sequences is present on discrete sizes of circular DNA in monkey (BSC-1) cells. J Mol Biol. 1985 Jan 20;181(2):161–173. doi: 10.1016/0022-2836(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Multiple mechanisms generate extrachromosomal circular DNA in Chinese hamster ovary cells. Nucleic Acids Res. 1986 Apr 25;14(8):3527–3538. doi: 10.1093/nar/14.8.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnerhagen P., Sjöberg R. M., Bjursell G. Increase of extrachromosomal circular DNA in mouse 3T6 cells on perturbation of DNA synthesis: implications for gene amplification. Somat Cell Mol Genet. 1989 Jan;15(1):61–70. doi: 10.1007/BF01534670. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Forseth B., Clare C. N., Hansen K. L., VanDevanter D. Double minutes arise from circular extrachromosomal DNA intermediates which integrate into chromosomal sites in human HL-60 leukemia cells. J Clin Invest. 1990 Jun;85(6):1887–1895. doi: 10.1172/JCI114650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- Wittwer C. T., Garling D. J. Rapid cycle DNA amplification: time and temperature optimization. Biotechniques. 1991 Jan;10(1):76–83. [PubMed] [Google Scholar]