Abstract
The prevalence of opioid abuse and dependence has been on the rise in just the past few years. Animal studies indicate that extended access to heroin produces escalation of intake over time, whereas stable intake is observed under limited-access conditions. Escalation of drug intake has been suggested to model the transition from controlled drug use to compulsive drug seeking and taking. Here, we directly compared the pattern of heroin intake in animals with varying periods of heroin access. Food intake was also monitored over the course of escalation. Rats were allowed to lever press on a fixed-ratio 1 schedule of reinforcement to receive intravenous infusions of heroin for 1, 6, 12, or 23 h per day for 14 sessions. The results showed that heroin intake in the 12 and 23 h groups markedly increased over time, whereas heroin intake in the 1 h group was stable. The 6 h group showed a significant but modest escalation of intake. Total heroin intake was similar in the 12 and 23 h groups, but the rate of heroin self-administration was two-fold higher in the 12 h group compared with the 23 h group. Food intake decreased over sessions only in the 12 h group. The 12 and 23 h groups showed marked physical signs of naloxone-precipitated withdrawal. These findings suggest that 12 h heroin access per day may be the optimal access time for producing escalation of heroin intake. The advantages of this model and the potential relevance for studying drug addiction are discussed.
Keywords: Heroin self-administration, opioid dependence, drug addiction, escalation, naloxone-precipitated withdrawal
1. Introduction
Opioid abuse and dependence are major public health problems. According to the Substance Abuse and Mental Health Services Administration (Substance Abuse & Mental Health Services Administration, 2010), the number of people dependent on or abusing pain relievers increased between 2002 (1.5 million) and 2009 (1.9 million), and the number of people classified as dependent on or abusing heroin increased 87% in just the past 2 years. Very recently, Nutt et al. used a multicriteria decision analysis and reported that heroin is the second most harmful psychoactive drug, behind only alcohol (Nutt et al., 2010). Therefore, a major need exists for research into the neurobiology of opioid addiction with the hope of developing better strategies for the treatment of opioid dependence. Treatment options to date are largely limited to substitution-therapy with the use of long-lasting opioid drugs, such as methadone and buprenorphine, or limited use of α2-adrenergic agonists, such as clonidine (Cami et al., 1985, Kleber et al., 1987), which all have pronounced adverse effects.
Animal models of opiate dependence are crucial for elucidating the etiology of addictive behaviors. Although numerous published procedures exist that produce stable levels of heroin self-administration by rodents, it has also been shown that models of extended access to heroin produces escalation of the intake over time compared with animals maintained on a limited-access schedule (Ahmed et al., 2000, Chen et al., 2006, Lenoir and Ahmed, 2008, McNamara et al., 2010, Sim-Selley et al., 2000). Importantly, escalation of drug intake has been suggested to model the transition from controlled drug use to compulsive drug seeking, a hallmark of drug addiction (Ahmed and Koob, 1998). This is further supported by evidence that passive induction of an opioid-dependent state increases the rate of subsequent heroin escalation (Walker et al., 2003). Moreover, animals that demonstrate escalation of intake show greater perseverative responding during extinction trials and increased sensitivity to stress- and heroin-induced reinstatement (Ahmed et al., 2000, Lenoir and Ahmed, 2007, 2008). Finally, rats that exhibit steady heroin intake show lowered intracranial self-stimulation (ICSS) thresholds associated with reward upon heroin administration, whereas escalating rats show marked elevations in ICSS thresholds, indicating a diminished impact of heroin's rewarding effects (Kenny et al., 2006).
Various laboratories, including our own, have published escalation effects and the lack thereof in groups of rodents with 1–23 h access per day. The present study directly compared the escalation patterns of heroin self-administration in animals with identical operant conditions and varying periods of heroin access to determine the effects of oscillating cycles of heroin access and abstinence on self-administration patterns. The pattern of food intake was also monitored over the course of escalation to determine whether variable access to heroin impacts food intake over time. Finally, a subset of animals was subjected to a naloxone-precipitated withdrawal test to evaluate signs of physical opiate dependence. Our hypothesis was that increased heroin access, interposed with periods of forced abstinence, results in reliable escalation of heroin intake and the development of physical dependence.
2. Material and Methods
2.1. Subjects
Adult male Wistar rats (n = 38; Charles River, Raleigh, NC), weighing between 225–275 g at the beginning of the experiments, were housed in groups of 2–3 per cage in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights on at 18:00 h) with ad libitum access to food and water. The animals were allowed to acclimate to the animal facilities for at least 7 days before surgery. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.2. Surgery
Rats were anesthetized with isoflurane (1.5–2.5%) and prepared with chronic intravenous silastic catheters (Dow Corning, Midland, MI) into the right jugular vein (Caine and Koob, 1993). The catheter was secured to the vein with suture thread and passed subcutaneously to exit dorsally on the animal's back. After surgery, the catheters were flushed daily with 0.2 ml of a sterile antibiotic solution containing heparinized (30 USP units/ml) saline and the antibiotic Cefazolin. Rats were allowed to recover for 7 days before behavioral testing.
2.3. Self-administration chambers
Self-administration sessions were conducted in standard operant chambers (Med Associates, St. Albans, VT). The chambers (30.5 cm × 24.1 cm × 29.2 cm) were located in a dimly lit room and individually enclosed in wooden cubicles fitted with a ventilation fan that also screened external noise. Each operant chamber had two opaque panels as the right and left walls and two clear Plexiglas panels as the front and back walls. The floor consisted of 6 mm diameter steel bars spaced 15 mm apart. Two retractable levers (2 cm × 4 cm × 0.3 cm) were mounted 7 cm above the grid floor on the right operant panel. A white light diode was mounted 8.5 cm above each lever. A 1.1 W miniature light bulb synchronized to the vivarium light/dark cycle illuminated the chamber. A spring-covered Tygon tube connected the animal's catheter through a fluid swivel to a syringe containing a heroin solution. The syringe was placed inside a syringe pump (Razel) that was placed outside and above the chamber. A food dispenser with a trap door (3 cm × 4 cm) positioned 4 cm above the grid floor equidistant between the two levers delivered food pellets (45 mg) upon nosepokes on a fixed-ratio 3 (FR3) schedule of reinforcement. A nosepoke hole (1 cm diameter) equipped with an infrared beam activated water delivery (0.1 ml, FR1) into a drinking cup placed on the left side of the back wall, 5 cm above the grid floor. A computer controlled the delivery of fluids, presentation of visual stimuli and recorded behavioral data. Similar procedures were previously described in Caine et al. (1993), Zorrilla et al. (2005), and Chen et al. (2006).
2.4. Self-administration procedure
All behavioral tests were conducted during the dark phase of the light/dark cycle, 5 to 7 days per week. All rats were trained to press one of the two levers (the active lever) on a FR1 schedule (each response resulted in fluid delivery) to obtain 0.1 ml (over 4 s) of heroin (60 μg/kg/infusion) in 1 h sessions. Reinforced responses were followed by a 20 s timeout period, in which a cue-light (above the active lever) was turned on and lever presses did not result in additional injections. Presses on the other lever (inactive lever) had no programmed consequences. No food restriction was used to establish operant responding. During the acquisition of heroin self-administration, food and water were not available to the rats while in the test chambers. After the acquisition of heroin self-administration (8 consecutive days of 1 h sessions), rats were split into four groups matched for lever press in the last three sessions of the acquisition phase and were given varying access periods of heroin self-administration for 14 sessions: 1, 6, 12, and 23 h. In this phase, all groups were allowed to nosepoke for food (cat# 5TUM, 45 mg pellets, TestDiet, Richmond, IN) on an FR3 schedule and water (0.1 ml) on an FR1 schedule while in the test chambers. “Chew toys” (i.e., wood dice) were placed in the operant chamber to prevent biting the catheter and drug lines caused by stereotyped behavior, manifested as chewing, produced by heroin, as previously observed (Chen et al., 2006).
2.5. Measurement of naloxone-precipitated withdrawal
To directly evaluate whether and to what extent extended access to heroin produces signs of opiate dependence, naloxone-precipitated withdrawal scores were measured using a modified Gellert and Holtzman (1978) scale of somatic opiate withdrawal (Schulteis et al., 1999). Rats from the 1, 12, and 23 h groups were given a challenge dose of naloxone (1 mg/kg, s.c.) and immediately placed in a 1 ft3 Plexiglas box for the observation of somatic opiate withdrawal. Two classes of withdrawal signs were measured for 10 min: graded signs (body weight loss in 60 min, escape attempts, wet dog shakes, abdominal constrictions) and checked signs (defecation/diarrhea, teeth chattering, swallowing movements, salivation, ptosis, penile erection/ejaculation/grooming, hyperirritability upon touch, abnormal posture). Each withdrawal sign was assigned a multiplier based on a weighted measure of somatic opiate withdrawal (Schulteis et al., 1999).
2.6. Statistical analysis
All data are expressed as means and standard errors of the mean (SEM). Data were analyzed using analysis of variance (ANOVA) with or without repeated measures, with Group (1, 6, 12, and 23 h) as the between-subjects factor and Session as the within-subjects factor. When appropriate, post hoc comparisons were performed using the Fisher's Least Significance Difference test. The probability for a Type 1 error for all significance testing was set at p < 0.05.
3. Results
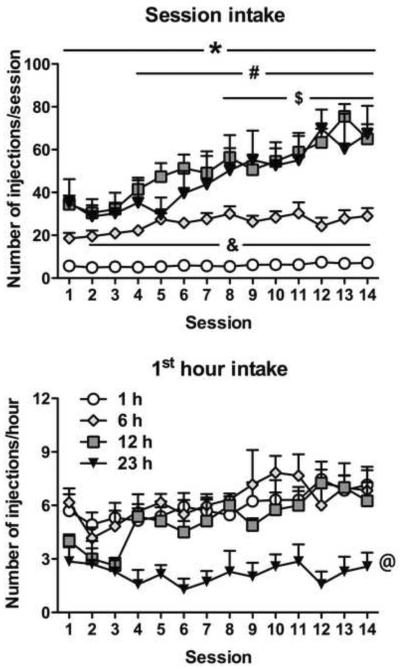
Rats with daily heroin access for 12 and 23 h, and 6 h at a lesser degree, showed escalation of intake over time. Indeed, the ANOVA revealed significant effects of Group (F3,40 = 27.2, p < 0.0001), Session (F13,520 = 19.8, p < 0.0001) and a significant Group × Session interaction (F39,520 = 5.3, p < 0.0001). For the 12 h group, the post hoc comparisons indicated that heroin intake significantly increased above the level of session 1, beginning with session 4 onward (p < 0.001). In the 23 h group, a significant effect was observed from session 8 onward (p < 0.05) compared with session 1. In the 6 h group, a significant effect was observed for sessions 5, 7, 8, 10, 11, 13, and 14 compared with session 1 (p < 0.05). Heroin intake in the 1 h group was stable, and no significant effects were observed across sessions compared with session 1.
Rats with 6 h access to heroin received more infusions compared with rats with 1 h access from session 2 onward (p < 0.05). Rats with 12 h access received more heroin infusions across all sessions compared with the 1 h group (p < 0.01) and from session 4 onward compared with the 6 h group (p < 0.01). Rats with 23 h access received more heroin infusions across all sessions compared with the 1 h group (p < 0.01) and from session 8 onward compared with the 6 h group (p < 0.05). Rats with 12 and 23 h access did not significantly differ (Fig. 1, top panel). Regarding the first hour of heroin self-administration, the ANOVA revealed an overall effect of Group (F3,36 = 8.0, p < 0.001), with the 23 h group showing lower levels of heroin self-administration compared with all other groups (p < 0.01) (Fig. 1, bottom panel). The ANOVA also revealed an overall effect of Session (F13,468 = 4.3, p < 0.0001), indicating a pattern of escalation in intake across sessions, regardless of group.
Figure 1. Prolonged periods of drug access result in significantly increased heroin intake over time.
All animals had identical pretraining in 1 h sessions to press the active lever for heroin infusions (60 μg/kg/infusion). Animals were given continuous, unlimited access to heroin infusions for 1, 6, 12, or 23 h per day for 14 days. The 1 h access group maintained steady intake over repeated sessions. The 6 h (to a lower extent), 12 h, and 23 h access groups showed escalating patterns of intake across time (see results for details). Overall, the 6, 12, and 23 h groups administered substantially more drug per session (top panel) than the 1 h group. The 12 and 23 h groups administered significantly more heroin per session than the 6 h group, and intake in the 12 and 23 h groups had a similar magnitude. When examining the first hour of drug intake per session (bottom panel), the 23 h group administered fewer heroin infusions than all other groups. Data are expressed as mean ± SEM. n = 5–14 per group. &p < 0.05, 6 h group compared with 1 h group; *p < 0.05, 12 and 23 h groups compared with 1 h group; #p < 0.05, 12 h group compared with 6 h group; $p < 0.05, 23 h group compared with 6 h group; @p < 0.05, 23 h group compared with all other groups (ANOVA followed by Fisher's Least Significance Difference post hoc test).
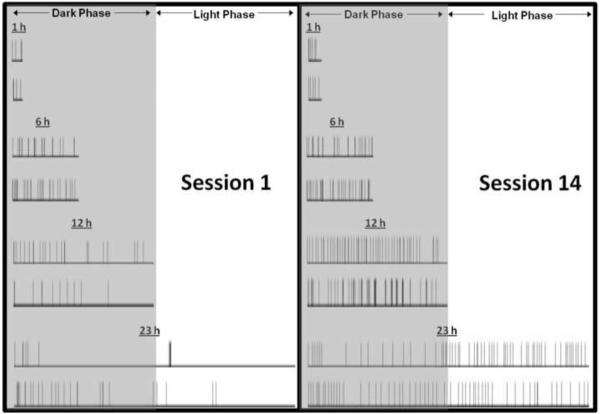
As shown in Fig. 2, when comparing the first and last (14th) heroin self-administration sessions, the 1 and 6 h rats showed an irregular and infrequent heroin intake pattern across the access period. However, the 12 and 23 h rats showed a relatively regular and frequent heroin intake pattern across the access period, decreasing the interresponse interval. As previously reported (Chen et al., 2006), rats with 23 h access expand their intake pattern from primarily during the active, dark phase to evenly throughout the day. This behavior may be necessary as withdrawal effects develop during heroin escalation. Importantly, the 12 h group, while administering similar numbers of infusions compared with the 23 h group, did so at twice the rate. The infusion rate (i.e., average number of infusions during the last three sessions divided by time [h]) was the following: 1 h, 6.6 infusions/h; 6 h, 4.8 infusions/h; 12 h, 5.6 infusions/h; 23 h, 2.5 infusions/h. The total amount of drug intake (i.e., the average of the last three sessions) was the following: 1 h, 0.40 mg/kg/session; 6 h, 1.7 mg/kg/session; 12 h, 4.0 mg/kg/session; 23 h, 3.5 mg/kg/session.
Figure 2. Increasing heroin intake is associated with decreasing interinfusion intervals.
Each row contains representative time-course plots of heroin infusion responses by individual animals from the first (Session 1; left panel) and final (Session 14; right panel) session. The 1 h rats maintained a stable pattern of infusions at distinct intervals. The increased level of intake in the 6, 12, and 23 h groups was associated with an altered pattern of responding that evenly distributed infusions over the access period.
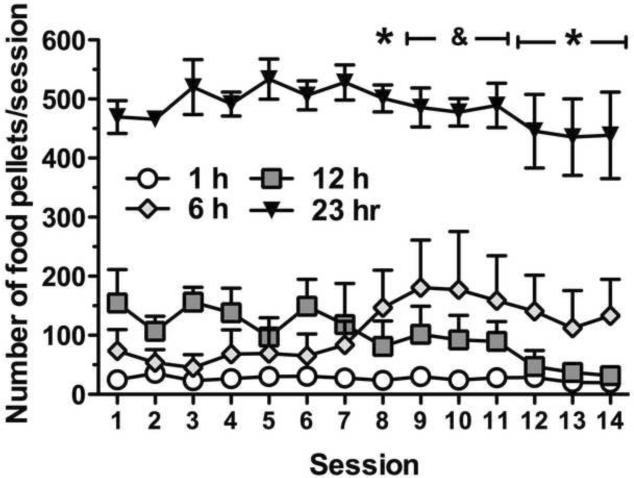
Food intake during escalation of heroin self-administration was compared between groups (Fig. 3). The two-way ANOVA revealed a significant overall effect of Group (F3,26 = 64.7, p < 0.0001) and a significant Group × Session interaction (F39,338 = 1.9, p < 0.01). Post hoc comparisons indicated that food intake decreased across sessions in the 12 h group with a significant effect in session 8 (p < 0.05) and session 12 onward (p < 0.001). In the 6 h group, a significant increase was observed in food intake from session 9 to session 11 (p < 0.05). Food intake was stable across sessions in the 1 and 23 h groups.
Figure 3. Increased responding for heroin infusions corresponds with decreased responding for food pellets in the 12 h group.
Rats were allowed simultaneous access to both food and water while in the self-administration chambers. Food responding was maintained on an FR3 schedule, and dispensers were monitored for uneaten dispensed food at the end of the sessions. Around the same period when the animals in the 12 h group began to significantly escalate their heroin intake, food intake significantly decreased. Data are expressed as mean ± SEM. n = 6–10 per group. *p < 0.05, compared with session 1 responding in the 12 h group; &p < 0.05, compared with session 1 responding in the 6 h group (ANOVA followed by Fisher's Least Significance Difference post hoc test).
Table 1 shows naloxone-precipitated withdrawal scores in the 1, 12, and 23 h groups. One-way ANOVA revealed a significant group effect (F2,21 = 10.8, p < 0.001). Post hoc comparisons indicated that administration of naloxone precipitated significantly higher withdrawal signs in the 12 and 23 h groups compared with the 1 h group (p < 0.01).
Table 1.
Naloxone-precipitated withdrawal scores in 1, 12, and 23 h heroin access rats.
Animals were given a naloxone (1 mg/kg; s.c.) challenge, and the somatic signs of withdrawal were evaluated for 10 min. Withdrawal scores were based on several signs of somatic withdrawal (see Methods).
p < 0.01, significantly different from the 1 h group. n = 5–10.
4. Discussion
In the present study, rats with access to heroin self-administration for 12 or 23 h per day showed a marked escalation in heroin self-administration across 14 sessions. Rats with 6 h access to heroin also showed significant escalation, but it was less robust compared with the 12 and 23 h groups. Animals with access for only 1 h per day showed relatively stable levels of heroin intake. During the first hour of heroin self-administration, rats with 23 h access showed lower levels of heroin intake compared with all other groups, indicating that these animals did not binge in the first hour but rather titrated their heroin dosing throughout the timeline of their session. This was confirmed by the observation that the escalation observed in the 12 and 23 h groups resulted from a steadily increasing frequency of lever presses throughout the session. Moreover, food intake in the 12 h group decreased over sessions, with a pattern that inversely followed the escalation of heroin intake, whereas food intake increased in the 6 h group and did not change in the 1 and 23 h groups. The 12 and 23 h groups also showed robust physical signs of opiate dependence.
The escalation pattern and total amount of heroin intake in the 12 and 23 h groups was quite similar, but the rate of self-administration was higher in the 12 h group. The 12 h access model appears to be advantageous for some reasons. First, the same number of infusions obtained in the 12 and 23 h groups indicates that the plasma concentration of heroin was likely twice as high in the 12 h group because the rate of intake in the 12 h group was double that of the 23 h group. This indicates that the 12 h model may be a more aggressive escalation model than the 23 h model. Second, 12 h animals have repeated cycles of intoxication and forced abstinence, whereas the 23 h animals have minimal periods of imposed abstinence. This persistent, repeated abstinence may be an essential factor in driving the elevated escalation pattern in the 12 h group. The negative emotional state associated with abstinence during dependence has been shown to promote drug taking (Koob, 2009; Sherman et al., 1989). This difference may explain why the 12 h group administered the same amount of heroin as the 23 h group but in half the time. The pattern of heroin intake with 12 h access appears to be more closely related to the human condition (Kaplan, 1992) and therefore may be a more representative, face-valid model of heroin dependence. Confirming this hypothesis, spontaneous withdrawal signs are observed in the 12 h group, including diarrhea, vocalization upon touch, hyperalgesia/allodynia, and abnormal posture, whereas minimal (or absent) signs of withdrawal were observed in the other groups (unpublished observations). However, when given a naloxone challenge, rats with 12 and 23 h heroin access showed marked signs of physical withdrawal.
Compared with previous studies, animals maintained on a 6 h access (Lenoir and Ahmed, 2007; McNamara et al., 2010) model in the present study showed modest escalation and lower total heroin intake. Procedural differences, such as dose, rat strain/substrain, and availability or not of food, may explain these discrepancies. Importantly, using the same experimental procedures in the present study, the 1 h group showed a relatively stable pattern of heroin intake, the 6 h group showed modest escalation, and the 12 and 23 h groups showed a marked escalation of heroin intake. Therefore, prolonged sessions are more likely to induce escalation of heroin self-administration.
Interestingly, Deneau et al. (1969) have provided perhaps the first evidence of escalation of drug intake with extended access in laboratory animals by showing that monkeys having 24 h per day of intravenous morphine or codeine access increased their drug intake over a five weeks period. More recent studies have reported that monkeys withdrawn from extended access to heroin (21 h per day) show increased heroin intake and signs of physical dependence (Negus, 2006).
It is noteworthy that rats are clearly tolerant by the end of the study, reflected by their increased heroin intake. Physical withdrawal signs are also present. Motivational withdrawal, often described as psychological dependence (Kenny et al., 2006) and drug-seeking behavior (Lenoir and Ahmed, 2007) are also showed by animals with extended access to heroin. Finally, escalation of heroin intake is accompanied by disrupted sleep patterns and circadian rhythm (Chen et al., 2006), hypophagia, weight loss and self-injurious gnawing. Most of these features are also observed in human heroin dependence (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV, 2000). Therefore, although mimicking all of the symptoms present in human heroin addiction in animal models is difficult or impossible, the present results support extended access to heroin as a useful model to study some aspects of heroin addiction.
Altogether, the present findings suggest that extended access heroin self-administration with intermittent intervals of abstinence produces reliable escalation of heroin intake, with high rates of intake and minimal risk to the health of the subjects and suggest that the 12 h access model is an interesting tool for the study of the neurobiology of heroin addiction.
Acknowledgements
This is publication number 21017 from The Scripps Research Institute. Research was financially supported by National Institutes of Health grant DA004043 from the National Institute on Drug Abuse and the Pearson Center for Alcoholism and Addiction Research. Training for author JES was provided by National Institute on Alcohol Abuse and Alcoholism grant T32AA007456. The authors would like to thank Michael Arends for proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. American Psychiatric Association; Washington, DC: 2000. Task Force on DSM-IV. [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–6. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Cami J, de Torres S, San L, Sole A, Guerra D, Ugena B. Efficacy of clonidine and of methadone in the rapid detoxification of patients dependent on heroin. Clin Pharmacol Ther. 1985;38:336–41. doi: 10.1038/clpt.1985.182. [DOI] [PubMed] [Google Scholar]
- Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–46. [PubMed] [Google Scholar]
- Kaplan CD. Drug craving and drug use in the daily life of heroin addicts. In: De Vries MW, editor. The Experience of psychopathology : investigating mental disorders in their natural settings. Cambridge University Press; Cambridge ; New York: 1992. p. xvii.p. 429. [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD, Topazian M, Gaspari J, Riordan CE, Kosten T. Clonidine and naltrexone in the outpatient treatment of heroin withdrawal. Am J Drug Alcohol Abuse. 1987;13:1–17. doi: 10.3109/00952998709001497. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–24. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–82. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–65. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–42. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Zinser MC, Sideroff SI, Baker TB. Subjective dimensions of heroin urges: influence of heroin-related and affectively negative stimuli. Addict Behav. 1989;14:611–23. doi: 10.1016/0306-4603(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000;20:4555–62. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse & Mental Health Services Administration . Results from the 2009 National Survey on Drug Use and Health: Volume I. In: Administration SAMHS, editor. Summary of National Findings. Office of Applied Studies; Rockville, MD: 2010. [Google Scholar]
- Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav. 2003;75:349–54. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–67. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]