Abstract
Genetic data support the notion that polymorphisms in members of the matrix metalloproteinase (MMP) family of genes play an important role in extracellular matrix remodeling and contribute to the pathogenesis of vascular disease. To identify novel genetic markers for diabetic nephropathy (DN), we examined the relationship between MMP gene polymorphisms and DN in the Genetics of Kidneys in Diabetes (GoKinD) population. Genotypic data from the Genetic Association Information Network (GAIN) type 1 DN project were analyzed for associations across 21 MMP genes in 1,705 individual with type 1 diabetes, including 885 normoalbuminuric control subjects and 820 advanced DN case subjects. In total, we investigated the role of 1,283 SNPs (198 genotyped SNPs and 1,085 imputed SNPs) mapping to the MMP genes. We identified associations at several correlated SNPs across a 29.2 kb interval on chromosome 11q at the MMP-3/MMP-12 locus. The strongest associations occurred at 2 highly-correlated SNPs, rs610950 (OR = 0.50, P = 1.6×10−5) and rs1277718 (OR = 0.50, P = 2.1×10−5). Further examination of this locus identified 17 SNPs (2 genotyped SNPs and 15 imputed SNPs) in complete linkage disequilibrium associated with DN (P-values < 2.5×10−4), including a non-synonymous SNP (rs652438, Asn357Ser) located in exon 8 of MMP-12 that significantly reduced the risk of DN among carriers of the serine substitution relative to homozygous carriers of asparagine (OR = 0.51; 95% CI = 0.37–0.71, P = 6.2×10−5). Taken together, our study suggests that genetic variations within the MMP-3/MMP-12 locus influence susceptibility of DN in type 1 diabetes.
Keywords: Diabetic nephropathy, type 1 diabetes, end-stage renal disease, matrix metalloproteinase, genetic association
1. INTRODUCTION
Diabetic nephropathy (DN) is a common late complication of type 1 diabetes, which leads to end-stage renal disease (ESRD) and is responsible for excess mortality due to cardiovascular complications [1, 2]. Poor glycemic control is the most important risk factor for DN [3], however, epidemiological [4] and familial [5, 6] studies suggest that genetic susceptibility play significant roles in the development of this complication.
A hallmark of DN is an accumulation of extracellular matrix (ECM) in the kidney, which is manifested as mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis [7, 8]. The presence of these morphological lesions correlates with renal function decline and the development of ESRD. Matrix metalloproteinases (MMPs), a family of zinc-dependent proteinases that degrade extracellular matrix and connective tissue proteins [9], are the major regulators of the accumulation of ECM in the glomeruli, tubules and interstitium. Currently, there are more than 20 known members of the MMP family [10]. In order to maintain the structural and functional integrity of the kidney, the regulation of ECM synthesis and degradation is essential. The role of these genes in disease pathogenesis is supported by recent genetic and functional data demonstrating that common polymorphisms in members of the MMP family play an important role in ECM remodeling and predispose individuals to cardiovascular disease [11–16]. Similarly, alterations in the structure and expression of MMPs through similar mechanisms may contribute to aberrant ECM turnover in the kidney, leading to both glomerular and tubular fibrosis and the manifestation of nephropathy in diabetes [17].
To investigate whether genetic polymorphisms in MMP genes are associated with advanced DN, we examined these loci in genome-wide association data in patients with type 1 diabetes from the Genetic of Kidneys in Diabetes (GoKinD) collection [18]. In the present study, we illustrate the utility of this dataset in identifying genes implicated in the pathogenesis of DN through the comprehensive and targeted investigation of genes involved in this candidate pathway.
2. MATERIALS AND METHODS
2.1. Study populations
A detailed description of the GoKinD collection has been published [19]. Briefly, subjects for the GoKinD collection were recruited through the George Washington University (GWU) Biostatistics Center and the Section on Genetics and Epidemiology at the Joslin Diabetes Center (JDC). Subjects enrolled in GoKinD had type 1 diabetes diagnosed before age 31, began insulin treatment within one year of their diagnosis, and were between 18 and 59 years of age at the time of enrollment. DN cases had either persistent proteinuria, defined by a urinary albumin to creatinine ratio (ACR) ≥ 300 μg/mg in two of the last three measurements taken at least one month apart, or ESRD (dialysis or renal transplant). Controls had type 1 diabetes for at least 15 years and normoalbuminuria, defined by an ACR < 20 μg/mg in two of the last three measurements taken at least one month apart (if a third measurement was required, a value < 40 μg/mg was necessary for inclusion), without ever having been treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and were not being treated with anti-hypertensive medication at the time of their recruitment into the study. A total of 1,705 individuals (885 controls and 820 DN cases, including 284 with proteinuria and 536 with ESRD) of European ancestry were included in the current study (for further details see Pezzolesi et al.)[18].
2.2. Genotyping and Imputation
The GoKinD collection was genotyped on the Affymetrix 5.0 500K SNP Array by the GAIN genotyping laboratory at the Eli and Edythe L. Broad Institute (Cambridge, MA). Quality control metrics, including filters for minor allele frequency (MAF) < 0.01, rejection of Hardy-Weinberg assumptions (P ≤ 10−5) and differential rates of missingness were applied to these data, resulting in high-quality genotypic data for 359,193 autosomal single nucleotide polymorphisms (SNPs) [18].
For the present study, genotypic data for all 198 SNPs mapping to 21 MMP loci (MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-11, MMP-12, MMP-13, MMP-14, MMP-15, MMP-16, MMP-17, MMP-19, MMP-20, MMP-24, MMP-25, MMP-26, MMP-27, and MMP-28), including 20 kb of flanking sequence, were extracted from the GoKinD data.
To further enhance the coverage of these data, we recently expanded our analysis of the GoKinD dataset by performing genome-wide imputation (GWI) of un-genotyped SNPs available from the HapMap Project [20]. Briefly, GWI of the GoKinD collection was carried out using data for all autosomal chromosomes from the HapMap Centre d'Etude du Polymorphisme (Utah residents with northern and western European ancestry) (CEU) population (release 22, build 36) and the MACH software program, resulting in an augmented dataset containing genotypes for a total of 2,402,395 SNPs (359,193 SNPs genotyped plus 2,043,202 high-quality imputed SNPs). In total, 1,085 high-quality imputed HapMap SNPs with MAF > 1% and r2 > 0.30 were examined for association with DN in the present study.
One polymorphism, rs3025058, located in MMP-3 was genotyped in the GoKinD collection using TaqMan technology (Applied Biosystems, Foster City, CA) by the Genetics Core of the Diabetes and Endocrinology Research Center at the JDC in accordance with the manufacturer's protocols. DNA samples used for the genotyping of this SNP were obtained through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository (www.niddkrepository.org).
2.3. Statistical Analysis
All SNPs were analyzed using stratified additive tests of association (adjusting for both gender and JDC/GWU strata, as previously described [18]) using the Cochran-Mantel-Haenszel test procedure to calculate combined P-values and odds ratios (ORs). Associations with DN were also assessed under dominant, recessive, and genotypic genetic models. All statistical analyses were performed using PLINK [21]. Evidence of association was considered for SNPs achieving P-values < 2.5×10−4 (198 genotyped SNPs/0.05).
3. RESULTS
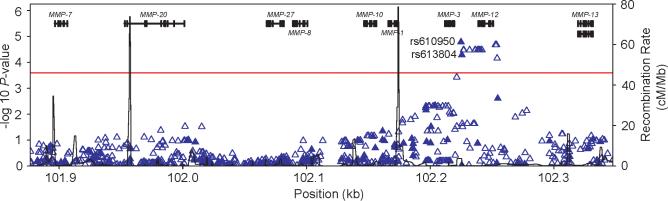
Clinical characteristics of the GoKinD collection used in this analysis have previously been described [18]. One-thousand seven-hundred and five GoKinD samples of European ancestry, including 885 controls (413 from the GWU panel and 472 from the JDC panel) and 820 cases (379 from the GWU panel and 441 from the JDC panel) were examined for genetic associations at 21 MMP genes mapping to 17 distinct chromosomal loci, including; MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-11, MMP-12, MMP-13, MMP-14, MMP-15, MMP-16, MMP-17, MMP-19, MMP-20, MMP-24, MMP-25, MMP-26, MMP-27, and MMP-28. In total, 1,283 SNPs, including 198 genotyped SNPs (Supplementary Table 1) and 1,085 imputed SNPs, spanning 1.3 Mb across these loci were examined for associations with DN. The results are summarized in Table 1. A total of 121 SNPs showed nominal evidence of association with DN (P < 0.05). The strongest associations were observed at 2 highly-correlated SNPs (r2 = 0.99), rs610950 (OR = 0.50, P = 1.6×10−5) and rs1277718 (OR = 0.50, P = 2.1×10−5), that map to a region on chromosome 11q that contains a cluster of 9 MMP genes (Figure 1).
Table 1.
Summary of associations at 21 MMP genes with advanced DN in the GoKinD collection
| Gene | Chr. | Position (Mb) | Number of SNPs | Strongest Association at Each Locus |
|||
|---|---|---|---|---|---|---|---|
| SNP | Allele (Minor/Major) | P-value | OR (95% CI) | ||||
| MMP-16 | 8 | 89.10–89.42 | 276 | rs13257117 | A/G | 2.3×10−4 | 0.72 (0.61–0.86 |
| MMP-26 | 11 | 4.94–4.99 | 69 | rs11034990 | C/T | 0.03 | 0.83 (0.70–0.99) |
| MMP-7 | 11 | 101.87–101.93 | 70 | rs17098292 | T/C | 0.14 | 0.63 (0.34–1.18) |
| MMP-20 | 11 | 101.93–102.02 | 161 | rs1573954 | T/C | 0.03 | 0.86 (0.75–0.99) |
| MMP-27 | 11 | 102.05–102.09 | 77 | rs12361599 | T/C | 0.43 | 1.07 (0.91–1.25) |
| MMP-8 | 11 | 102.09–102.11 | 50 | rs11602288 | A/G | 0.10 | 0.67 (0.41–1.09) |
| MMP-10 | 11 | 102.13–16 | 60 | rs547561 | C/T | 0.07 | 1.30 (0.98–1.72) |
| MMP-1 | 11 | 102.16–102.19 | 48 | rs502174 | C/A | 0.01 | 1.19 (1.04–1.36) |
| MMP-3 | 11 | 102.20–102.23 | 56 | rs610950 | C/T | 1.6×10−5 | 0.50 (0.36–0.69) |
| MMP-12 | 11 | 102.23–102.27 | 42 | rs1277718 | C/G | 2.1×10−5 | 0.50 (0.36–0.69) |
| MMP-13 | 11 | 102.30–102.35 | 53 | rs12792912 | G/T | 0.06 | 1.14 (1.00–1.31) |
| MMP-19 | 12 | 54.50–54.53 | 10 | rs2046292 | T/G | 0.17 | 0.74 (0.48–1.14) |
| MMP-17 | 12 | 130.86–130.92 | 33 | rs4964933 | T/C | 7.4×10−3 | 1.84 (1.17–2.89) |
| MMP-14 | 14 | 22.36–22.41 | 8 | rs3751488 | A/G | 0.15 | 0.89 (0.76–1.05) |
| MMP-25 | 16 | 3.02–3.07 | 27 | rs12447486 | G/A | 0.05 | 1.16 (1.00–1.34) |
| MMP-2 | 16 | 54.05–54.12 | 93 | rs1005913 | G/T | 0.08 | 0.87 (0.75–1.02) |
| MMP-15 | 16 | 56.61–56.68 | 23 | rs16959642 | C/T | 0.21 | 1.15 (0.93–1.41) |
| MMP-28 | 17 | 31.10–31.17 | 23 | rs4251700 | G/T | 1.3×10−3 | 0.51 (0.34–0.78) |
| MMP-24 | 20 | 33.26–33.35 | 37 | rs619865 | A/G | 0.09 | 0.82 (0.66–1.03) |
| MMP-9 | 20 | 44.06–44.10 | 31 | rs3848723 | G/A | 0.04 | 0.64 (0.41–0.99) |
| MMP-11 | 22 | 22.43–22.48 | 36 | rs11703707 | G/A | 0.20 | 1.44 (0.82–2.51) |
|
| |||||||
| Total | 1,283 | ||||||
The most strongly associated SNPs for each MMP gene in the GoKinD collection are presented. P-values and odds ratios (ORs) for all cases versus controls were calculated using stratified additive tests of association using the Cochran-Mantel-Haenszel method, adjusting for both gender and JDC/GWU strata. Chromosomal locations are in reference to NCBI Build 36.1.
Figure 1.
Summary of genome-wide association (GWA) and genome-wide imputation (GWI) results for the chromosome 11q locus. ▲, SNPs genotyped on the Affymetrix array (n = 94); △, imputed SNPs (n = 523). The −log10 P-values calculated using the Cochran-Mantel-Haenszel method (adjusting for gender and GoKinD sub-collection (JDC/GWU)) are shown for the combined GoKinD collection. The horizontal dashed line corresponds to a −log10 P-value = 3.6 (P-value = 2.5×10−4). rs610950 and rs613804 reside at position −5,388 and −6,079, respectively, upstream of MMP-3.
To examine the chromosome 11q locus further, we analyzed all genotyped and imputed SNPs across this 471 kb region. As shown in Figure 1, 17 SNPs (2 genotyped SNPs and 15 imputed SNPs) were associated with DN with P-values < 2.5×10−4 (Table 2). All 17 SNPs are in complete linkage disequilibrium (LD) (r2 = 1.0) and are located in a 29.2 kb interval extending from position 102,224,940 to 102,254,155 and inclusive of the MMP-12 gene. Among these variants, rs652438, located in exon 8 of MMP-12, results in a non-synonymous substitution of asparagine to serine at codon 357 (Asn357Ser). Interestingly, analysis of the rs652438 genotypes revealed that carriers of the serine substitution had a significantly reduced risk of DN relative to homozygous carriers of asparagine (OR = 0.51; 95% CI = 0.37–0.71, P = 6.2×10−5) (Table 3). Association results for all SNPs located in MMP-3 and MMP-12 are provided in Supplementary Table 2. Additionally, the haplotype structure at the MMP-3/MMP-12 locus was investigated to determine whether specific haplotypes at this locus were associated with DN. Analysis of two distinct haplotype blocks across the MMP-3 and MMP-12 region, spanning 43 kb and 22 kb respectively, were not more strongly associated with DN than our single SNP analysis (data not shown).
Table 2.
Summary of SNPs associated with DN in GoKinD collection (P≤2.5×10−4) at the MMP-3/MMP-12 locus
| Risk Allele Frequencies and P values for Controls and Cases by Panel |
P values and ORs for Combined Analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus |
GWU GoKinD |
JDC GoKinD |
||||||||||
| Gene | SNP† | Position | Location | Risk Allele (Non-risk Allele) | Controls (n=413) | Cases (n=379) | P value | Controls (n=472) | Cases (n=441) | P value | P value | OR (95% CI) |
| MMP-3 | rs610950 | 102,224,940 | 5' flanking region | T(C) | 0.93 | 0.97 | 6.8×10−4 | 0.94 | 0.96 | 6.6×10−3 | 1.6×10 −5 | 2.00 (1.45–2.78) |
| rs613804 | 102,225,631 | 5' flanking region | G(A) | 0.93 | 0.96 | 1.9×10−3 | 0.94 | 0.96 | 8.5×10−3 | 5.1×10 −5 | 1.92 (1.39–2.63) | |
| rs629946 | 102,226,906 | 5' flanking region | G(A) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs641857 | 102,227,261 | 5' flanking region | G(T) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| MMP-12 | rs615932 | 102,231,915 | 3' flanking region | G(A) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) |
| rs660727 | 102,236,284 | 3' flanking region | A(G) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs484171 | 102,236,613 | 3' flanking region | A(G) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs686375 | 102,238,444 | 3' flanking region | C(T) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs626729 | 102,238,580 | 3' flanking region | T(C) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs505770 | 102,239,420 | intron 9 | A(T) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs476391 | 102,240,240 | intron 8 | C(G) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs644552 | 102,240,350 | intron 8 | G(A) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs651159 | 102,241,629 | intron 8 exon8 | T(C) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs652438 | 102,241,852 | (Asn357Ser) | T(C) | 0.93 | 0.96 | 1.1×10−3 | 0.94 | 0.96 | 8.5×10−3 | 3.3×10−5 | 1.96 (1.41–2.7) | |
| rs1277718 | 102,252,761 | 5' flanking region | G(C) | 0.93 | 0.97 | 6.8×10−4 | 0.94 | 0.96 | 8.5×10−3 | 2.1×10−5 | 2.00 (1.45–2.78) | |
| rs501371 | 102,253,660 | 5' flanking region | A(G) | 0.93 | 0.97 | 6.8×10−4 | 0.94 | 0.96 | 8.5×10−3 | 2.1×10−5 | 2.00 (1.45–2.78) | |
| rs636648 | 102,254,155 | 5' flanking region | A(G) | 0.93 | 0.96 | 1.7×10−3 | 0.94 | 0.96 | 0.01 | 6.9×10−5 | 1.89 (1.37–2.56) | |
Association results for SNPs in the MMP-3/MMP-12 region associated with DN (P≤2.5×10−4) are presented for the individual GWU and JDC GoKinD panels along with P-values and OR from the combined analysis of these two panels (calculated using the Cochran-Mantel-Haenszel method, adjusting for sex, between case and control subjects within each collection) are presented and P values for each separate collection. Combined P-values and ORs were calculated using the Cochran-Mantel-Haenszel method. SNP positions and gene annotations are in reference to NCBI Build 36.1.
All SNPs are in complete LD (r2 = 1.0), and were identified through imputation except for rs610950 and rs613804, which were both genotyped on the Affymetrix array and are indicated in bold.
Table 3.
Association analysis of rs652438, a non-synonymous SNP located in exon 8 of MMP-12 (Asn357Ser), in individuals with DN and controls
| Genotype | Cases, n (%) | Controls, n (%) | OR (95% CI) | P value |
|---|---|---|---|---|
| T/T | 763 (93.1) | 772 (87.2) | 1.0 (reference) | --- |
| T/C | 55 (6.7) | 105 (11.9) | 0.53 (0.38–0.75) | 2.0×10−4 |
| C/C | 2 (0.2) | 8 (0.9) | 0.25 (0.05–1.19) | 0.06 |
| T/C C/C | 57 (7.0) | 113 (12.8) | 0.51 (0.37–0.71) | 6.2×10−5 |
Lastly, functional polymorphisms in several MMP genes have recently been shown to predispose to the development of cardiovascular disease, including coronary artery disease, arterial stiffness, and abnormal aortic aneurysm [22]. To investigate whether these polymorphisms also contribute to the susceptibility of DN, genotypic data for 7 previously reported functional polymorphisms were examined in the GoKinD collection (Table 4). Interestingly, one polymorphism, rs3025058, located at position −1612 relative to MMP-3's transcriptional start site was found to be significantly associated with DN (P = 0.02). Contrary to studies of cardiovascular disease, the remaining polymorphisms were not statistically different between case and control subjects from GoKinD.
Table 4.
Summary of association results at functional polymorphisms in MMP genes in the GoKinD collection
| Gene | SNP | Chr. | Position | Alleles (Minor/major) | Location | Reference(s) | Additive | P-value Dominant | Recessive |
|---|---|---|---|---|---|---|---|---|---|
| MMP-2 | rs242866 | 16 | 54,069,038 | A/G | −1575 | Harendza S., et al. (2003) [11] | 0.32 | 0.37 | 0.24 |
| MMP-2 | rs243865* | 16 | 54,069,307 | T/C | −1306 | Price S.J., et al. (2001) [12] | 0.91 | 0.90 | 0.24 |
| MMP-3 | rs3025058 | 11 | 102,221,162 | 5A/6A | −1612 | Ye S., et al. (1996) [13], Ye S., et al. (1999) [36] | 0.02 | 0.08 | 0.02 |
| MMP-7 | rs11568818* | 11 | 101,906,871 | C/T | −181 | Jormsjo S., et al. 2000 [14] | 0.98 | 0.87 | 0.88 |
| MMP-7 | rs11568819* | 11 | 101,906,843 | A/G | −153 | Jormsjo S., et al. 2001 [15] | 0.35 | 0.51 | 1.00 |
| MMP-12 | rs2276109* | 11 | 102,251,001 | C/T | −82 | Jormsjo S., et al. 2000 [14] | 0.76 | 0.77 | 0.92 |
| MMP-13 | rs2252070 | 11 | 102,331,749 | C/T | −77 | Yoon S., et al. 2002 [16] | 0.09 | 0.09 | 0.36 |
P-values were calculated using stratified additive tests of association (adjusting for both gender and JDC/GWU strata) using the Cochran-Mantel-Haenszel test procedure and under both dominant and recessive genetic models. Imputed SNPs are indicated by asterisks. rs243866 and rs2252070 were genotyped on the Affymetrix 5.0 array. rs3025058 was genotyped using a Taqman assay. SNP positions are in reference to NCBI Build 36.1.
4. DISCUSSION
There is mounting evidence to support the role of MMPs in the development and progression of diabetic complications, including diabetic retinopathy [23, 24], diabetic foot ulcers [25, 26], and DN [27]. In the kidney, MMPs produced by mesangial cells account for up to 70% of ECM degradation and turnover during normal matrix remodeling [17]. In DN, dysregulation of MMPs has been shown to contribute to both mesangial expansion and thickening of the tubular basement [27], two hallmarks of DN. Moreover, genetic alterations in a member of this family of proteins (MMP-9) have recently been reported in patients with DN [28, 29]. In this report, we examined whether variants in MMP genes are associated with the risk of DN in patients with type 1 diabetes. Through utilization of GWI data from the GoKinD collection, our comprehensive `candidate pathway' analysis of 21 members of the MMP family expands upon these previous findings and demonstrates that variants at the MMP-3/MMP-12 locus are associated with a reduced risk of DN.
We investigated the role of 1,283 SNPs in 21 MMP genes, including 7 SNPs previously reported to be associated with cardiovascular disease, in individuals from the GoKinD collection. Our analysis identified associations at several correlated SNPs across a 29.2 kb interval on chromosome 11q at the MMP-3/MMP-12 locus, including a non-synonymous substitution (rs652438, Asn357Ser) in exon 8 of MMP-12 that significantly reduced the risk of DN among carriers of the serine substitution. Although rs652438 was not directly genotyped on the Affymetrix array, this SNP is in complete LD (r2=1.0) with 2 SNPs (rs610950 and rs613804) included on this genotyping platform. While it is interesting to speculate that rs652438 may alter the structure and/or function of MMP-12 and contribute to dysregulation of renal ECM metabolism in DN, functional studies are necessary to determine the biological mechanism underlying this association.
Previous studies by Maeda et al. [28] and Hirakawa et al. [29] reported associations between a highly polymorphic (CA)n repeat upstream from the transcriptional initiation site of MMP-9 and diabetic nephropathy in patients with type 2 diabetes. While this variant was not evaluated in the present study, our scan of genetic variants at the MMP-9 locus did not find any evidence of association with nephropathy in the GoKinD type 1 diabetic population (the strongest association at this locus was at rs3848723, P = 0.04, located approximately 20 kb downstream of MMP-9, Table 1). Further investigation is necessary to determine the role of this (CA)n repeat in patients with type 1 diabetic nephropathy.
MMP-12 has recently been shown to play an important role in both the glomerular and tubulointerstitial injury and may contribute to the development and progression of DN. Aberrant expression of MMP-12 has recently been shown to contribute to glomerular injury in mice with progressed nephrotic syndrome [30]. Uchio et al. noted extensive expression of both MMP-12 mRNA and its protein in Institute for Cancer Research-derived glomerulonephrotic mice, with increased expression occurring predominantly in the podocytes. Although further study is required to elucidate the mechanism of podocyte dysfunction in these mice, this study suggests that glomerular expression of MMP-12 contributes to the progression of glomerular basement membrane pathogenesis through its enhanced degradation.
MMP-12 is synthesized and secreted by macrophages, and is essential for macrophage migration [31]. Although DN has primarily been considered a non-immune disease, accumulating data suggest that inflammation may play a role in its development. In addition to being implicated in the development of glomerulosclerosis, macrophages are also associated with the tubulointerstitial changes observed in DN; thereby providing a secondary mechanism for MMP-12's potential contribution to the development and progression of this disease [8, 32, 33]. Additional experiments regarding the mechanisms of its dysfunction in DN are needed.
Functional MMP gene polymorphisms, including rs3025058 at position −1612 in MMP-3 and rs2276109 at position −82 in MMP-12, have recently been shown to be associated with increased susceptibility of cardiovascular disease [22]. In particular, rs3025058, which is located in an interleukin-1 responsive element, has been shown to affect binding of the p65 and p50 subunits of the NKκB transcription factor [34]. As a result of its reduced affinity for the transcriptional repressor p50/p50 homodimer, higher promoter activity is associated with the 5A allele at this polymorphic site.
Although rs2276109 was not significant in our study, we acknowledge that this variant, along with 3 other SNPs previously reported to be associated with cardiovascular disease, was not directly genotyped in our study. Interestingly, and contrary to these studies of cardiovascular disease, the 5A allele of rs3025058 was associated with increased risk of DN. While down-regulation of MMPs in the kidney causes enlargement of the mesangium and tubulointerstitium, resulting in progression of DN, MMP levels tend to increase under inflammatory circumstances due to compensatory effects and both the level and the duration of MMP elevation determine the extent of glomerular damage [35]. This structural damage may induce and accelerate remodeling, resulting in matrix accumulation. Furthermore, as we describe above, inflammatory mechanisms have been implicated as important pathogenic factors in the development of DN. Increased MMP levels as a result of this process may indirectly play an important role in the development of DN through matrix accumulation.
Our study demonstrates the added utility of genome-wide association (GWA) study data in assessing potential disease-associated variants at a priori suspected DN candidate genes. Although the variants identified in the present study were not among the strongest signals detected in the initial GoKinD GWA study, as we recently reported for multiple candidate regions (including the well-studied chromosome 3q region) and the transforming growth factor-β (TGF-β) pathway [20], our MMP pathway-based prioritization led to the identification of biologically-relevant genetic associations at the MMP-3/MMP-12 DN susceptibility locus.
Our investigation is the first to demonstrate a link between genetic variations in MMP-3 and MMP-12 and DN. While the precise mechanisms that underlie these associations remain to be identified, our findings provide important first clues toward disentangling the roles of these genes in the pathogenesis of susceptibility to DN. Firstly, we show that several variants within the MMP-3/MMP-12 locus are associated with DN in two independent GoKinD sub-collections. While intriguing, additional replication of these findings in other collections, especially those from other ethnic groups, is warranted. As well, our investigation centered on patients with type 1 diabetes. Therefore, whether variations at the MMP-3/MMP-12 locus are associated with DN in those with type 2 diabetes remains to be seen.
Secondly, we did not identify any major gene effects on the pathogenesis of DN for polymorphisms located in MMP-3 and MMP-12. Our analysis did, however, identify several low frequency SNPs (with MAF≈0.05) that are associated with nearly a 2-fold change in the odds of developing DN. While several studies are underway to identify polymorphisms that exert a large effect on the susceptibility of this disease, variants such as these that contribute less to disease susceptibility, but perhaps more to its phenotypic variability, are vital to advancing our understanding of the heritability underlying this disease. Finally, we show that carriers of the rare alleles of these polymorphisms have a significantly reduced risk of DN relative to carriers of the major alleles. These findings suggest that `low frequency' variants (those with MAF ≤5%) are likely to play a critical role in the susceptibility of his devastating complication.
Supplementary Material
ACKNOWLEDGEMENTS
Funding support for the GAIN Search for Susceptibility Genes for Diabetic Nephropathy in Type 1 Diabetes (GoKinD study participants) study was provided by the Juvenile Diabetes Research Foundation (JDRF), and the Centers for Disease Control (CDC) (PL 105–33, 106–554, and 107–360 administered by the National Institute of Diabetes and Digestive and Kidney Diseases, NIDDK) and the genotyping of samples was provided through the Genetic Association Information Network (GAIN). The dataset(s) used for the analyses described in this manuscript were obtained from the database of Genotype and Phenotype (dbGaP) found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000018.v1.p1.
We acknowledge grant support from the National Institutes of Health (NIH) (DK58549 and DK77532 to ASK and DK36836 to the Genetics Core of the Diabetes and Endocrinology Research Center at the Joslin Diabetes Center). We also acknowledge the Joslin Diabetes Center's NIH T32 Training Grant (DK007260-31 to MGP) and support from the Juvenile Diabetes Research Foundation (JDRF) (3-2009-397 to JS).
Abbreviations
- GAIN
Genetic Association Information Network
- GoKinD
Genetic of Kidneys in Diabetes
- GWI
genome-wide imputation
- GWU
George Washington University
- JDC
Joslin Diabetes Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DUALITY OF INTEREST The authors declare that there are no dualities of interest associated with this manuscript.
REFERENCES
- [1].Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- [2].Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–1255. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- [4].Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med. 1985;78:785–794. doi: 10.1016/0002-9343(85)90284-0. [DOI] [PubMed] [Google Scholar]
- [5].Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320:1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- [6].Borch-Johnsen K, Norgaard K, Hommel E, et al. Is diabetic nephropathy an inherited complication? Kidney Int. 1992;41:719–722. doi: 10.1038/ki.1992.112. [DOI] [PubMed] [Google Scholar]
- [7].Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brosius FC., 3rd New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- [10].Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- [11].Harendza S, Lovett DH, Panzer U, Lukacs Z, Kuhnl P, Stahl RA. Linked common polymorphisms in the gelatinase a promoter are associated with diminished transcriptional response to estrogen and genetic fitness. J Biol Chem. 2003;278:20490–20499. doi: 10.1074/jbc.M211536200. [DOI] [PubMed] [Google Scholar]
- [12].Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–7558. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- [13].Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055–13060. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- [14].Jormsjo S, Ye S, Moritz J, et al. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ Res. 2000;86:998–1003. doi: 10.1161/01.res.86.9.998. [DOI] [PubMed] [Google Scholar]
- [15].Jormsjo S, Whatling C, Walter DH, Zeiher AM, Hamsten A, Eriksson P. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2001;21:1834–1839. doi: 10.1161/hq1101.098229. [DOI] [PubMed] [Google Scholar]
- [16].Yoon S, Kuivaniemi H, Gatalica Z, et al. MMP13 promoter polymorphism is associated with atherosclerosis in the abdominal aorta of young black males. Matrix Biol. 2002;21:487–498. doi: 10.1016/s0945-053x(02)00053-7. [DOI] [PubMed] [Google Scholar]
- [17].Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 2009;58:1403–1410. doi: 10.2337/db08-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mueller PW, Rogus JJ, Cleary PA, et al. Genetics of Kidneys in Diabetes (GoKinD) study: a genetics collection available for identifying genetic susceptibility factors for diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. 2006;17:1782–1790. doi: 10.1681/ASN.2005080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pezzolesi MG, Skupien J, Mychaleckyj JC, Warram JH, Krolewski AS. Insights to the genetics of diabetic nephropathy through a genome-wide association study of the GoKinD collection. Semin Nephrol. 2010;30:126–140. doi: 10.1016/j.semnephrol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc Res. 2006;69:636–645. doi: 10.1016/j.cardiores.2005.07.015. [DOI] [PubMed] [Google Scholar]
- [23].Kosano H, Okano T, Katsura Y, et al. ProMMP-9 (92 kDa gelatinase) in vitreous fluid of patients with proliferative diabetic retinopathy. Life Sci. 1999;64:2307–2315. doi: 10.1016/s0024-3205(99)00184-8. [DOI] [PubMed] [Google Scholar]
- [24].Ishizaki E, Takai S, Ueki M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129–134. doi: 10.1016/j.ajo.2005.08.066. [DOI] [PubMed] [Google Scholar]
- [25].Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- [26].Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419–426. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tsilibary EC. Microvascular basement membranes in diabetes mellitus. J Pathol. 2003;200:537–546. doi: 10.1002/path.1439. [DOI] [PubMed] [Google Scholar]
- [28].Maeda S, Haneda M, Guo B, et al. Dinucleotide repeat polymorphism of matrix metalloproteinase-9 gene is associated with diabetic nephropathy. Kidney Int. 2001;60:1428–1434. doi: 10.1046/j.1523-1755.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- [29].Hirakawa S, Lange EM, Colicigno CJ, Freedman BI, Rich SS, Bowden DW. Evaluation of genetic variation and association in the matrix metalloproteinase 9 (MMP9) gene in ESRD patients. Am J Kidney Dis. 2003;42:133–142. doi: 10.1016/s0272-6386(03)00416-5. [DOI] [PubMed] [Google Scholar]
- [30].Uchio K, Sawada K, Manabe N. Expression of macrophage metalloelastase (MMP-12) in podocytes of hereditary nephrotic mice (ICGN strain) J Vet Med Sci. 2009;71:305–312. doi: 10.1292/jvms.71.305. [DOI] [PubMed] [Google Scholar]
- [31].Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824–23829. [PubMed] [Google Scholar]
- [32].Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- [33].Usui HK, Shikata K, Sasaki M, et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes. 2007;56:363–372. doi: 10.2337/db06-0359. [DOI] [PubMed] [Google Scholar]
- [34].Borghaei RC, Rawlings PL, Jr., Javadi M, Woloshin J. NF-kappaB binds to a polymorphic repressor element in the MMP-3 promoter. Biochem Biophys Res Commun. 2004;316:182–188. doi: 10.1016/j.bbrc.2004.02.030. [DOI] [PubMed] [Google Scholar]
- [35].Lenz O, Elliot SJ, Stetler-Stevenson WG. Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol. 2000;11:574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- [36].Ye S, Whatling C, Watkins H, Henney A. Human stromelysin gene promoter activity is modulated by transcription factor ZBP-89. FEBS Lett. 1999;450:268–272. doi: 10.1016/s0014-5793(99)00509-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.