Abstract
A new labdane-diterpene, viteagnusin I (1), together with 23 known phytoconstituents were isolated from the fruits of Vitex agnus-castus L, and their structures characterized by spectroscopic method (NMR and MS). The known compounds include ten flavonoids, five terpenoids, three neolignans, and four phenolic compounds, as well as one glyceride. Biological evaluation identified apigenin, 3-methylkaempferol, luteolin, and casticin as weak ligands of delta and mu opioid receptors, exhibiting dose-dependent receptor binding.
Keywords: Vitex agnus-castus, labdane diterpene, viteagnusin I, flavonoids, opioid activity
1. Introduction
Vitex agnus-castus L. (Verbenaceae) is a deciduous tree or a large shrub that is native to Europe but also widely distributed in the Southern United States. The fruits of V. agnus-castus (chaste berry, VAC) have a long history (over 2000 years) of use as an herbal medicine. Currently, the fruit extract is used as a dietary supplement for estrogen hormone imbalance which can produce menstrual cycle disorders and premenstrual syndrome (PMS), such as cyclical mastalgia, and corpus luteum insufficiency [1,2] as well as for alleviating menopausal symptoms such as hot flashes [3]. The classes of phytochemicals that have been reported in VAC fruits include essential oils [4,5], flavonoids [6,7], iridoids [8], and diterpenoids [9–11], as well as a diterpene lactam, vitexlactam A [12]. Under investigation for safety and efficacy at the UIC/NIH Center for Botanical Dietary Supplements Research, preliminary screening of a methanolic extract of VAC showed it contained ligands for the estrogen receptor (ER) [3]. Since at that time, there had been no literature reports of estrogenic constituents of VAC, an ER binding assay was used to guide phytochemical fractionation, which led to the identification of linoleic acid as an ER ligand in the MeOH extract [13]. A separate study used ERα or ERβ ligand binding assays to guide fractionation of VAC, which resulted in the isolation of apigenin and its identification as an ERβ- selective phytoestrogen in chaste berry [14].
Other VAC phytoconstituents have been reported to modulate various CNS receptors. In 1999, diterpenoids from a VAC extract (BNO 1095) were found to be dopamine antagonists [15]. However, as the potency of these diterpenoids was weaker than that of the whole VAC extract, the authors proposed possible synergy among the various diterpenoids, or in combination with other constituents in VAC.
A rationale for the choice of opioid receptors as potential targets for VAC phytoconstituents in the present study is summarized as follows: (a) VAC has been shown to be clinically effective against PMS symptoms such as depression, irritability, anxiety, mastalgia, fatigue, and headache [16,17]. Although the extract inhibits prolactin release [18,19] by activating the dopamine D2 receptors in the anterior pituitary, the mechanism of action of this herb is not known. (b) Extracts of VAC have also been reported to have affinity to the opioid μ, δ, or κ receptors (MOR, DOR, KOR, respectively). One study found VAC had high affinity to MOR and KOR, and weak affinity for DOR [20,21]. More recently, in our investigation of two VAC methanol extracts [2], VAC activated MOR in addition to having receptor affinity.
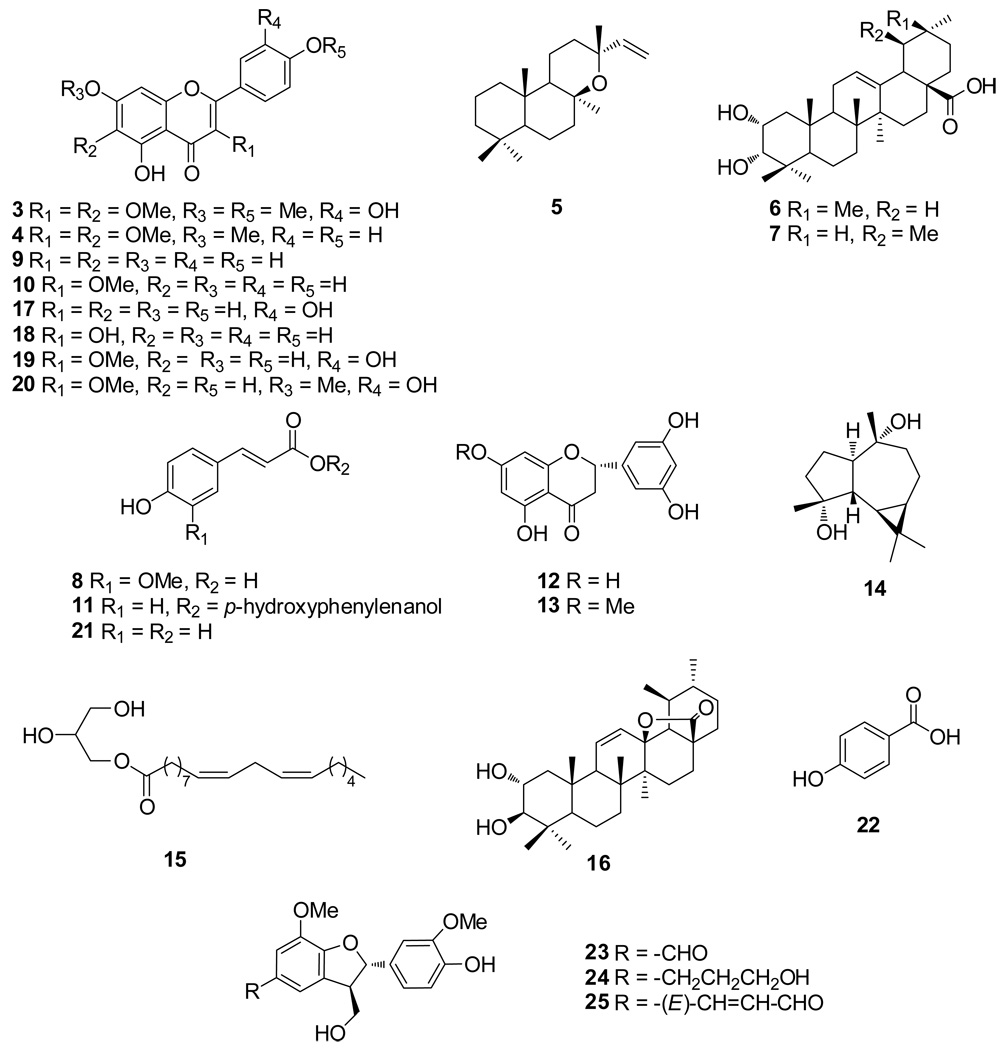
In order to further detect and isolate additional chemical constituents in chaste berry for different bioassays, a phytochemical investigation was performed on a defatted methanol extract of VAC fruits using a variety of chromatographic techniques, including vacuum liquid chromatography (VLC), gel permeation chromatography, high-speed countercurrent chromatography (HSCCC), and semi-preparative reverse-phase HPLC. As a result of these experiments, 24 compounds were isolated and identified using spectroscopic methods, including 1D and 2D NMR as well as high resolution electrospray mass spectrometry. These compounds included a new labdane-type diterpene named viteagnusin I (1) together with 23 known compounds, including 9 isolates, which were identified for the first time from the genus Vitex, i.e. 3-epi-maslinic acid (6) [22], 3-epi-corosolic acid (7) [22], p-hydroxyphenylethanol-p-coumarate (11) [23], 5,7,3’,5’-tetrahydroxyflavanon (12) [24], 5,3’,5’-trihydroxymethoxylflavanone (13) [25], ilelatifol D (16) [26], 3,7-dimethylquercetin (20) [27], ficusal (23) [28] and balanophonin (25) [29]. Three of the isolates, 3-O-methylkaempferol (10) [30], 3-methylquercetin (19) [31], and vladirol F (24) [32] are reported here for the first time from the species, V. agnus-castus. The other 11 compounds previously reported from V. agnus castus include casticin (3), penduletin (4), 8-epi-manoyl oxide (5), ferulic acid (8), apigenin (9), aromadendrane-4α,10α-diol (14), 1-glyceryl linoleate (15), luteolin (17), kaempferol (18). Herein, the isolation and structural elucidation of the new diterpene 1 is presented.
2. Experimental
2.1. General
NMR spectra were recorded on a Bruker (Billerica, MA) Avance using a 5 mm Quadra Nuclear Probe instrument in CDCl3 and MeOH-d4 at 360 MHz for 1H and 90 MHz for 13C. TMS was used as an internal reference. The off-line data processing carried out on Nuts Pro software (Acorn NMR, Livermore, CA). High-resolution electrospray mass spectra and product ion collision-induced dissociation tandem mass spectra were obtained using a Waters (Milford, MA) Synapt QTOF mass spectrometer. TLC was performed on precoated glass plates (250 µm thickness, silica gel 60 F254; and 200 µm thickness, RP-18 silica gel 60 F254; EM Science, Germany) with visualization by spraying with 5% H2SO4 in 95% ethanol and heating at 120°C for 10 min in addition to UV detection at 254 and 365 nm. Semi-preparative HPLC was carried out using a Waters Delta 600 system with a Waters 996 photodiode array detector, Waters 717 plus autosampler, and Waters Empower Chromatography Manager on a GROM-Sil 120 ODS-4 HE (Watrex International, San Francisco, CA) semi-preparative column (10 µm, 300 × 20 mm), or on a Waters YMC ODS-AQ semi-preparative column (5 µm, 250 × 10 mm) with a flow rate at 6 mL/min or 3 mL/min, respectively. Reversed-phase low-pressure liquid chromatography (LPLC) was carried out on Merck (EM Science; Gibbstown, NJ) Lobar LiChroprep RP-18 columns (300 × 20 mm or 250 × 10 mm) with a Fluid Metering Inc. pump (Soyesset, NY) and a Spectrum Chromatography (Houston, TX) fraction collector . Silica gel (230–400 mesh; Davisil, W.R. Grace) was used for open-column chromatography or vacuum liquid chromatography (VLC). The high-speed CCC apparatus was a Pharma-Tech Research (Baltimore, MD) J-type CCC-1000 ( with 3 × 80 mL coils and equipped with a Lab-Alliance III pump, Shimadzu SPD-10A VP UV-VIS detector, and a Cole-Parmer recorder Model 80807-00, as well as an Isco fraction collector.
2.2 Plant material
The ripe fruits of Vitex agnus-castus L. were provided by Naturex (previously PureWorld Botanicals; South Hackensack, NJ) from cultivated material grown in New Mexico (USA). Voucher specimens (BC164) have been deposited at the Field Station PCRPC of the Department of Medicinal Chemistry and Pharmacognosy at the University of Illinois at Chicago.
2.3. Extraction and isolation
Ripe fruits of V. agnus-castus L (VA, 70 kg) were ground and extracted with petroleum ether (30 L) to yield 1,830 g. The Marc was then extracted with 90% methanol (30 L) to yield a defatted methanol extract (1760 g). After in vacuo removal of the methanol, 879 g of the extract was reconstituted with 1 L of 5% aqueous methanol, loaded on a column of Diaion HP-20 (2 kg) pre-moistened with water, and eluted with water (8 L, VA-1: 452 g), 20% methanol (3.5 L, VA-2: 35.8 g), 50% methanol (3.5 L, VA-3: 72.5 g), 75% methanol (3.5 L, VA-4: 93.5 g) and 100% methanol (5 L, VA-5: 145 g) to yield five fractions (VA-01 to VA-05). Fraction VA-05 (125 g) was subjected to CC on silica gel (2 Kg), eluted with a gradient of CHCl3 and acetone (100% CHCl3, 1.25%, 2.5%, 5%, 10%, 20%, 33%, 50%, to 100% acetone, 10 L solvent for each gradient). Finally, the column was washed with 10 L of 100% methanol. A total of 102 fractions were collected at 1,000 mL each. These fractions were further pooled into 13 combined fractions (VA-05-01 to 13) based on similar chemical behavior on normal phase TLC following spraying with 5% H2SO4 and heating at 120 °C for 10 min.
Yellow crystals (3, 5.1 g) obtained from fraction VA-05-04 (20 g) were identified as casticin. Penduletin (4, 230 mg) and 8-epi-manoyl oxide (5, 100 mg) were separated from VA-05-05 (2.12 g) on silica gel VLC (hexane/isopropanol, 15:1, v/v), and purified by isocratic LPLC RP-18 with 80% methanol. The new compound, viteagnusin I (1, 1.4 mg), and the known isolates, 3-epi-maslinic acid (6, 30 mg) and 3-epi-corosolic acid (7, 40 mg) were separated and purified from VA-05-06 by repeated CC and RP-18 LPLC. Fraction VA-05-07 (6.2 g) was loaded onto a silica gel (250 g) VLC column (60 × 300 mm) and eluted with a gradient solvent system to yield 33 sub-fractions as follows: initial solvent was 100% petroleum ether, followed by a gradient to 100% ethyl acetate, then gradually moving to 100% methanol (a volume of 250 mL solvent was used per gradient step). The gradient was accomplished by increasingly adding 20 mL of the more polar solvent for each subsequent step until 100% methanol was reached. Then, the column was washed with 4 × 250 mL of 100% methanol. The resulting 33 sub-fractions were combined into 10 final fractions, VA-05-07-01 to 10, based on the Rf values of major bands observed on normal phase TLC upon visualization by spraying 5% H2SO4 and heating 10 min under 120°C.
Fraction VA-05-07-04 (2.4 g) was loaded on a Sephadex LH-20 column and eluted with methanol to yield two fractions, VA-05-07-04-01 and VA-05-07-04-02. Fraction VA-05-07-04-02 (190 mg) was purified using HPLC on a semi-preparative YMC ODS-AQ column (10 X 250 mm, flow rate: 3 mL/min) to yield ferulic acid (8, 3.0 mg), apigenin (9, 83 mg), 3-O-methylkaempferol (10, 16 mg), p-hydroxyphenylethanol-p-coumarate (11, 31 mg), 5,7,3',5'-tetrahydroxyflavanone (12, 4.3 mg) and 5,3′,5′-trihydroxymethoxyflavanone (13, 23 mg). VA-05-07-04-01 was separated by RP-18 LPLC with 70% methanol to yield 22 sub-fractions, VA-05-07-04-01-A to V. Fraction VA-05-07-04-01-Q was subjected to silica gel VLC column (10 × 200 mm) chromatography, eluting with CHCl3/isopropanol (25:1, v/v) to yield 60 mg of aromadendrane-4α,10α-diol (14). 1-Glyceryl linoleate (15, 20.4 mg) and ilelatifol D (16, 20.1 mg) were purified from VA-05-07-05 (800 mg) by repeated separation on silica gel VLC (20 × 250 mm) with CHCl3/isopropanol in different ratios (10:1 v/v for 15; and 20:1 v/v for 20).
VA-05-08 (3 g) was loaded onto a Sephadex LH-20 column and eluted with methanol to yield 4 sub-fractions, VA-05-08-01 to 04. Fraction VA-05-08-03 (76 mg from a total of 800 mg) was purified by HPLC on a YMC ODS-AQ column to yield known flavonoids luteolin (17, 18 mg), kaempferol (18, 6.6 mg), 3-methylquercetin (19, 5 mg), and 3,7-dimethyquercetin (20, 2.3 mg). Fraction VA-05-08-01 (1.76 g) was separated by HSCCC using hexane/ethyl acetate/methanol/water, 6:4:6:4, v/v/v/v) [32] to yield 11 fractions, VA-05-08-01-01 to 11. p-Coumaric acid (21, 0.9 mg) and 4-hydroxybenzoic acid (22, 5 mg) were purified from fraction VA-05-08-01-05 by means of semi-preparative HPLC on a YMC ODS-AQ column. Fraction VA-05-08-01-09 was fractionated by HPLC on a YMC C-18 column to yield three known dihydrobenzo[b]furan neolignans: ficusal (23), vladirol F (24) and balanophonin (25).
Viteagnusin I (1): Colorless syrup. Positive ion HR-ESI-MS m/z 412.2709 (calc. for C22H38NO6, [M+NH4]+, 412.2699; ΔM 2.4 ppm), positive ESI MSMS of m/z 412.271: m/z (relative abundance): 352.249 (15%), 355.222 (90%), 317.211 (50%), 299.201 (100%), and 197.081 (30%). 1H-, 13C-NMR spectral data: see Table 1.
Table 1.
NMR spectroscopic data of compounds 1 and 2 in CDCl3
| Position | 1a | 2b | ||
|---|---|---|---|---|
| δH, mult (J in Hz) | δC (mult) | δH, mult (J in Hz) | δC | |
| 1 | 1.55, 1.47c | 33.57 t | 1.54, 1.45c | 33.7 |
| 2 | 1.64, 1.51c | 18.55 t | 1.64, 1.50c | 18.6 |
| 3 | 1.341, ddd (13.4,3.3,3.3) | 43.50 t | 1.35 br d (13.0) | 43.6 |
| 1.160, ddd (13.4,13.4,3.9) | 1.17 ddd (3.0, 13.0, 13.0) | |||
| 4 | 33.99 s | 34.0 | ||
| 5 | 1.59c | 47.60 d | 1.57c | 47.7 |
| 6 | 5.382, ddd (2.5, 2.5, 2.5) | 69.88 d | 5.39 ddd (2.5, 2.5, 2.5) | 69.9 |
| 7 | 1.57, 1.62c | 35.99 t | 1.56, 1.58c | 36.1 |
| 8 | 2.130, qdd (6.8, 12.5, 5.4) | 32.06 d | 1.94 ddd (5.5, 9.0, 15.5) | 31.9 |
| 9 | 76.97 s | 76.6 | ||
| 10 | 43.67 s | 43.8 | ||
| 11 | 2.004, ddd (14.7, 8.1, 8.5) | 31.29 t | 1.75 m | 31.2 |
| 1.780, ddd (14.7, 4.8, 8.8) | ||||
| 12 | 2.537, br dd (2H, 7.8, 7.4) | 24.31 t | 2.52 m, 2.39 m | 24.5 |
| 13 | 170.31 s | 168.1 | ||
| 14 | 5.855, br dd (2.1, 1.8)d | 117.15 d | 5.87 m | 117.8 |
| 15 | 171.29 s | 170.4 | ||
| 16 | 6.022, br s/md | 99.35 d | 5.65 s | 104.4 |
| 17 | 0.915, d (6.8) | 16.11 q | 0.90 d (7.0) | 16.0 |
| 18 | 1.002, s | 33.57 q | 0.97 s | 33.6 |
| 19 | 0.960, s | 23.66 q | 1.01 s | 23.7 |
| 20 | 1.259, s | 18.95 q | 1.26 s | 19.0 |
| OAc | 2.060, s | 21.94 q | 2.06 s | 21.9 |
| 170.65 s | 170.4 | |||
| OCH3 | 3.57 s | 57.0 | ||
1H and 13C data were recorded at 360 MHz and 90 MHz, respectively.
1H and 13C reported at 500 MHz and 125 MHz, respectively [11].
Chemical shift values were determined by 1H-1H COSY of the heavily overlapped signals.
The signals of the butenolide ring are broadened due to multiple long-range coupling (4JH-16,H-14, 4JH-14,H-12a and 4JH-14,H-12a) and dynamic effects (rotation along the C-11/12 axes) at RT, which overlap the small J-couplings. See text for further discussion.
3. Results and Discussion
The molecular formula of compound 1 was determined to be C22H34O6 based on a high resolution accurate mass measurement by positive ion electrospray mass spectrometry analysis of the ammonium adduct of 1, showing m/z 412.2709 for C22H38NO6 (calc. 412.2699). The degree of unsaturation was 6 on the basis of this formula. The loss of 60.0 (m/z 352.249) mass units from the [M+NH4]+ ion (m/z 412.271) of 1 during product ion tandem mass spectrometry is consistent with the loss of a molecule of acetic acid and indicates the presence of an acetyl group. The 1H NMR spectrum of 1 (Table 1) showed four methyl resonance signals at groups at δ 0.915 (3H, d, 6.8 Hz), 0.960 (3H, s), 1.002 (3H, s) and 1.259 (3H, s), as well as an acetyl signal at δ 2.060 (3H, s). Three methine signals at δ 6.022 (1H, brs), 5.856 (1H, brs) and 5.381 (1H, ddd, 2.5, 2.5, 2.5 Hz) indicated the presence of one acetylated hydroxyl group and two olefinic protons. The 13C NMR spectra showed 22 resonance signals. Based on the data of 13C and DEPT spectra, these signals were five methine signals, including three at down field: one sp2 methine signal at 117.15 ppm (C-14), two oxygenated signals at 99.35 ppm (C-16) and 69.88 ppm (C-6) as well as two at upper field: 47.60 ppm and 32.06 ppm; four methyl signals at 33.57 ppm (C-18), 23.66 ppm (C-19), 18.95 ppm (C-20), 16.11 ppm (C-17); and six methylene signals. Five quaternary signals included one carbonyl signal at 171.29 ppm (C-15), one sp2 quaternary signal at 170.31 ppm (C-13), and a low-field oxygenated of a quaternary carbon signal at 76.98 ppm (C-9). The acetyl group exhibited resonances at 170.65 (s) and 21.94 (q) ppm. All these NMR evidences suggested compound 1 should be an acetylated diterpene. According to the reported diterpenes from Vitex genus in the literature [9–11], 1 should have a labdane skeleton.
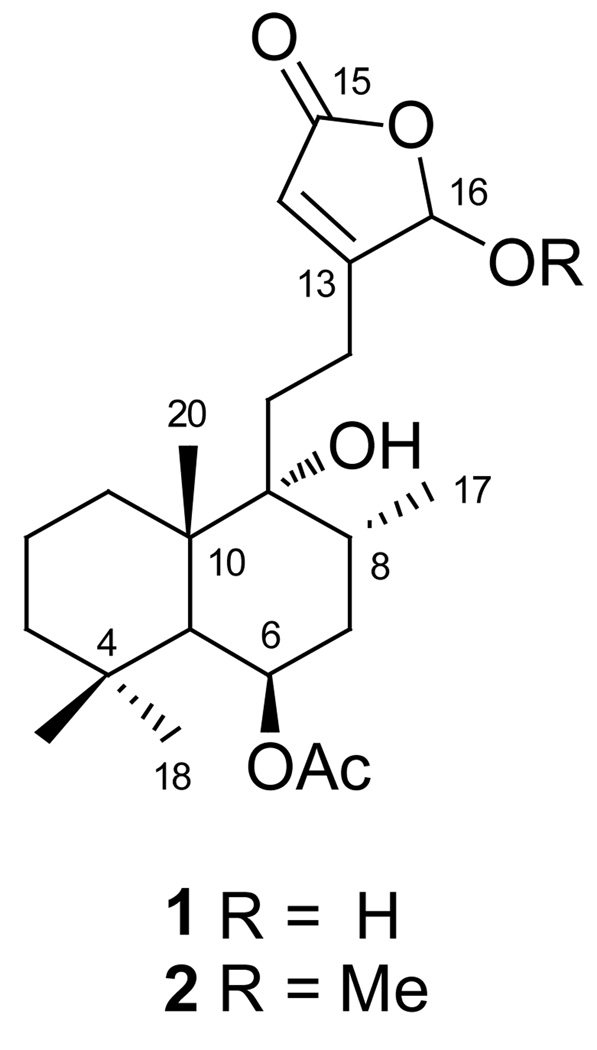
When comparing our data for 1 with data from the literature, the NMR spectroscopic fingerprint (1H and 13C) was similar to that of viteagnusin H (2, Fig 1), a labdane-type diterpene recently isolated from a hexane extract of VAC fruits [11]. However, as the high-resolution mass spectrometric data had indicated, 1 has 22 carbons and contained one CH2 less than 2, which was consistent with the lack of a methoxyl group attached to C-16. Comparing 1H and 13C spectra of 1 with those of 2 indicated that the two spectra were almost identical. The only major difference was at C-16, i.e. the chemical shift of H-16 of 1 shifted to down field 0.372 ppm compared to that of 2, while the chemical shift of C-16 of 1 moved up field 5.05 ppm compared to that of 2. The large difference of carbon chemical shifts could be explained by the deshielding effect of the electron density at the α-position (C-16) caused by the methoxyl group, which replaced the hydroxyl group at C-16. All these date are consistent wiht the presence of a hydroxyl group instead of methoxyl group was attached at the C-16 position in 1.
Fig. 1.
Structures of compounds 1 and 2.
Two spin systems in the 1H-1H COSY spectrum of 1 could clearly be established from low field to high field as δ 5.382 (H-6) to δ 0.915 (H3-17) for the spin system as -CHCHCH2CHCH3-(CH-5 to CH-8 and Me-17), and as δ 5.855 (H-14) through δ 2.537 (H2-12) to δ 2.004 and δ 1.780 (H-11) for the spin system as –CH=C-CH2CH2- (CH-14 to CH2-11). The explanation of the weak correlation across a quaternary carbon (C-13) between H-14 (δ 5.855) and H2-12 (δ 2.537) could be a relay coupling of π-electron via the double bond. In the HMBC spectrum of 1, two sets of cross peaks for δ 5.855 to 171.29 ppm and 99.35 ppm exhibited the presence of an α,β-unsaturated carbonyl structural fragment. The cross peak of δ 6.022 to 171.29 ppm indicated that the α,β-unsaturated carbonyl moiety should be an α,β-unsaturated lactone. The correlations between 170.65 ppm and δ 5.383 as well as 170.65 ppm and δ 2.060 inferred the acetyl group was attached to C-6. The cross peaks of the quaternary carbon 76.97 ppm (C-9) with two methyl groups, Me-20 (δ 1.259) and Me-17 (δ 0.915) inferred C-9 as an oxygenated quaternary carbon.
The relative configuration of H-6 was assigned to the α-position based on 2.5 Hz coupling constants of H-6 with H-5 (J5a,6e = 2.5 Hz) and 2H-7 (J7a,6e and J7e,6e = 2.5 Hz) and, thus, was the same as in 2. The coupling constants of H-8 (J8,7 = 12.5, 5.4 Hz) determined the α-orientation of the C-17 methyl group. Therefore, the structure of compound 1 was consistent with that of 6β-acetoxyl-9α,16-dihydroxy-13(14)-labden-15,16-olide.
The relative stereochemistry of the anomeric center, C-16, could presently not be determined. However the following observations provide evidence for the presence of one predominant C-16 anomer, while the other anomer and the ring-open form are likely to be present in solution as well: (a) the signals for both H-14 and H-16 are both broadened, even when applying Lorentzian-Gaussian resolution enhancement processing; (b) relative to the signal of H-6, the signals for both H-14 and H-16 show reduced integrals of ca. 0.7…0.9:1, depending on the post-acquisition processing parameters applied; (c) the 1H NMR spectrum of 1 exhibits “impurity” signals which, due to sample limitation, were too small to make further assignments; (d) based on preliminary modeling studies, it is very likely that rotation along the C-9/11/12/13 axes affect the five-membered α,β-unsaturated lactone (butenolide) ring and lead to line broadening at RT due to rapid interconversion of conformers; (e) also based on models, the distances between H2-11 and H-16 in the two anomers were estimated to be 2.5 and 2.8 Å for α-OH, and 2.4 and 2.9 Å for β-OH, respectively; which excludes NOE measurements as potential means of generating definitive evidence for anomeric assignment. Finally, the multiple long-range couplings (4JH-16,H-14, 4JH-14,H-12a and 4JH-14,H-12a) present in the butenolide ring are very small and, thus, rather insensitive measures of the relative geometric positions between the 4J coupling partners. While the precise 4J values might potentially be diagnostic for the anomeric configuration, their reliable determination is voided by the aforementioned presence of dynamic line broadening. Separate variable temperature NMR and modeling experiments will be necessary to determine feasibility of such assignments.
Compounds 3–25 (Fig. 2) were identified based on their 1D and 2D NMR; and by comparison of their NMR data with those reported in the literature (see Experimental section).
Fig. 2.
Structures of the 23 phytoconstituents isolated, in addition to 1, from a defatted methanolic extract of Vitex agnus-castus.
All phytoconstituents were biologically evaluated in our laboratory using three bioassay models. Two were opioid receptor assays (DOR and MOR) that indicated the four flavonoids, apigenin, 3-methylkaempferol, luteolin, and casticin had weak dose-dependent binding activity at both receptors [34]. Those isolates with sufficient quantity (3–9, 11–15, 19, and 22) were also evaluated for serotonergic activity using the cAMP serotonergic assay. However, none of the phytoconstituents exhibited substantial activity at the highest test concentration of 40 µg/mL. Evaluation of possible synergistic effects will require further studies involving large-scale re-isolation of the VCA phytoconstituents studied herein.
Supplementary Material
Acknowledgments
This research was funded by Grant P50 AT000155 from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), the National Institute of General Medical Sciences (NIGMS), and the Office for Research on Women’s Institutes of Health (ORWH). Contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency.
Appendix A. Supplementary data
Supplementary data associated with this article can be found (NMR spectra of isolated compounds), in the online version, at doi:10.1016/j.fitote.XYZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merz PG, Schrödter A, Rietbrock S, Gorkow C, Löw D. Prolactin secretion and tolerance during treatment with an Agnus castus extract (BP1095E1) - Effect on prolactin secretion. Phytopharmaka in Forschung und Klinischer Anwendung. 1995:93–97. [Google Scholar]
- 2.Webster DE, Lu J, Chen SN, Farnsworth NR, Wang ZJ. Activation of the mu-opiate receptor by Vitex agnus-castus methanol extracts: Implication for its use in PMS. J Ethnopharmacol. 2006;106:216–221. doi: 10.1016/j.jep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Liu JH, Burdette JE, Xu HY, Gu CG, van Breemen RB, Bhat KPL, Booth N, Constantinou AI, Pezzuto JM, Fong HHS, Farnsworth NR, Bolton JL. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen JM, Katsiotis ST. Variation in essential oil yield and composition of Cretan Vitex agnus-castus L. fruits. J Essent Oil Res. 1999;11:599–605. [Google Scholar]
- 5.Senatore F, Napolitano F, Ozcan M. Chemical composition and antibacterial activity of essential oil from fruits of Vitex agnus-castus L. (Verbenaceae) growing in Turkey. J Essent Oil-Bear Plants. 2003;6:185–190. [Google Scholar]
- 6.Wollenweber E, Mann K. Flavonols from fruits of Vitex agnus-castus. Planta Med. 1983;48:126–127. doi: 10.1055/s-2007-969905. [DOI] [PubMed] [Google Scholar]
- 7.Hirobe C, Qiao ZS, Takeya K, Itokawa H. Cytotoxic flavonoids from Vitex agnus-castus. Phytochemistry. 1997;46:521–554. doi: 10.1016/s0031-9422(97)00127-1. [DOI] [PubMed] [Google Scholar]
- 8.Görler K, Öhlke D, Soicke H. Iridoid derivatives from Vitex agnus-castus. Planta Med. 1985;50:530–531. doi: 10.1055/s-2007-969589. [DOI] [PubMed] [Google Scholar]
- 9.Hoberg E, Orjala J, Meier B, Sticher O. Diterpenoids from the fruits of Vitex agnus-castus. Phytochemistry. 1999;52:1555–1558. [Google Scholar]
- 10.Ono M, Yamasaki T, Konoshita M, Ikeda T, Okawa M, Kinjo J, Yoshimitsu H, Nohara T. Five new diterpenoids, viteagnusins A-E, from the fruit of Vitex agnus-castus. Chem Pharm Bull. 2008;56:1621–1624. doi: 10.1248/cpb.56.1621. [DOI] [PubMed] [Google Scholar]
- 11.Ono M, Nagasawa Y, Ikeda T, Tsuchihashi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T. Three new diterpenoids from the fruit of Vitex agnus-castus. Chem Pharm Bull. 2009;57:1132–1135. doi: 10.1248/cpb.57.1132. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Zhang H, Qiu S, Niu X, Santarsiero BD, Mesecar AD, Fong HHS, Farnsworth NR, Sun HD. Vitexlactam A, a novel labdane diterpene lactam from the fruits of Vitex agnuscastus. Tetrahedron Lett. 2002;43:5131–5134. [Google Scholar]
- 13.Liu J, Burdette JE, Sun Y, Deng S, Schlecht SM, Zheng W, Nikolic D, Mahady G, van Breemen RB, Fong HHS, Pezzuto JM, Bolton JL, Farnsworth NR. Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry) Phytomedicine. 2004;11:18–23. doi: 10.1078/0944-7113-00331. [DOI] [PubMed] [Google Scholar]
- 14.Jarry H, Spengler B, Porzel A, Schmidt J, Wuttke W, Christoffel V. Evidence for estrogen receptor beta-selective activity of Vitex agnus-castus and isolated flavones. Planta Med. 2003;69:945–947. doi: 10.1055/s-2003-45105. [DOI] [PubMed] [Google Scholar]
- 15.Christoffel V, Spengler B, Jarry H, Wuttke W. Prolactin inhibiting dopaminergic activity of diterpenes from Vitex agnus-castus, Phytopharmaka V. In: Löw D, Blume H, Dingermann TH, editors. Forschung und Klinische Anwendung. Darmstadt: Steinkopf; 1999. 209-4. [Google Scholar]
- 16.Schellenberg R. Treatment for the premenstrual syndrome with Vitex agnus-castus fruit extract: prospective, randomised, placebo controlled study. BMJ. 2001;322:134–137. doi: 10.1136/bmj.322.7279.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauritzen C, Reuter HD, Repges R, Bohnert KJ, Schmidt U. Treatment of premenstrual tension syndrome with Vitex agnus-castus. Controlled, double-blind study versus pyridoxine. Phytomedicine. 1997;4:183–189. doi: 10.1016/S0944-7113(97)80066-9. [DOI] [PubMed] [Google Scholar]
- 18.Sliutz G, Speiser P, Schultz AM, Spona J, Zeillinger R. Agnus castus extracts inhibit prolactin secretion of rat pituitary cells. Hormone and Metabolic Research. 1993;25:253–255. doi: 10.1055/s-2007-1002090. [DOI] [PubMed] [Google Scholar]
- 19.Jarry H, Leonhardt S, Gorkow C, Wuttke W. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus: direct evidence for a dopaminergic principle by the dopamine receptor assay. Exp. Clin. Endocrinol. 1994;102:448–454. doi: 10.1055/s-0029-1211317. [DOI] [PubMed] [Google Scholar]
- 20.Meier B, Berger D, Hoberg E, Sticher O, Schaffner W. Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine. 2000;7:373–381. doi: 10.1016/S0944-7113(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 21.Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlova-Wuttke D. Chaste tree (Vitex agnus-castus): pharmacology and clinical indications. Phytomedicine. 2003;10:348–357. doi: 10.1078/094471103322004866. [DOI] [PubMed] [Google Scholar]
- 22.Kojima H, Ogura H. Constituents of the Labiate plants. Part 1. Triterpenoids from Prunella vulgaris. Phytochemistry. 1986;25:729–733. [Google Scholar]
- 23.Li Z, Chao Z, Chen K. Isolation and identification of the ethereal constituents of Sargentodoxa cuneata. Shanghai Yike Daxue Xuebao. 1988;15:68–70. [Google Scholar]
- 24.Anthoni U, Rosalba ED, Nielsen PH, Christophersen C. Huazhongilexone is not 3',5,5',7-tetrahydroxyflavanone. Preparation of 3',5'-dimethoxy-5,7-dihydroxyflavanone. Acta Chem Scand. 1998;52:1243–1246. [Google Scholar]
- 25.Lin Y, Long K, Deng Y. Studies on the chemical constituents of the Chinese medicinal plant Blumea balsamifera. Zhongshan Daxue Xuebao, Ziran Kexueban. 1988:77–80. [Google Scholar]
- 26.Nishimura S, Taki M, Takaishi S, Iijima Y, Akiyama T. Structures of 4-aryl-coumarin (neoflavone) dimers isolated from Pistacia chinensis BUNGE and their estrogen-like activity. Chem Pharm Bull. 2000;48:505–508. doi: 10.1248/cpb.48.505. [DOI] [PubMed] [Google Scholar]
- 27.Valesi AG, Rodriguez E, Vander Velde G, Mabry TJ. Methylated flavonols in Larrea cuneifolia. Phytochemistry. 1972;11:2821–2826. [Google Scholar]
- 28.Li Y, Kuo Y. Four new compounds, ficusal, ficusesquilignan A, B, and ficusolide diacetate from the heartwood of Ficus microcarpa. Chem Pharm Bull. 2000;48:1862–1865. doi: 10.1248/cpb.48.1862. [DOI] [PubMed] [Google Scholar]
- 29.Sy L, Brown GD. Coniferaldehyde derivatives from tissue culture of Artemisia annua and Tanacetum parthenium. Phytochemistry. 1999;50:781–785. [Google Scholar]
- 30.Egger K, Tissut M, Wollenweber E. 3-O-Methyl ether of kaempferol and galangin in the oil of Populus nigra buds. Phytochemistry. 1969;8:2425–2426. [Google Scholar]
- 31.Chumbalov TK, Fadeeva OV. Flavonoids of Artemisia transiliensis. Khim Prir Soedin. 1969;5:439. [Google Scholar]
- 32.Tan RX, Jakupovic J, Jia ZJ. Aromatic constituents from Vladimiria souliei. Planta Med. 1990;56:475–477. doi: 10.1055/s-2006-961015. [DOI] [PubMed] [Google Scholar]
- 33.Friesen JB, Pauli GF. G.U.E.S.S. to make generally useful estimations of solvent systems in CCC. J Liq Chrom Relat Technol. 2005;8:2877–2808. [Google Scholar]
- 34.Webster DE. PhD Dissertation. University of Illinois at Chicago; 2008. Botanical, chemical, genetic, and pharmacological studies of Vitex agnus-castus L; p. 260. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.