Abstract
The sternohyoid (SH) and geniohyoid (GH) are antagonist strap-muscles that are active during a number of different behaviors, including sucking, intraoral transport, swallowing, breathing, and extension/flexion of the neck. Because these muscles have served different functions through the evolutionary history of vertebrates, it is quite likely they will have complex patterns of electrical activity and muscle fiber contraction. Different regions of the sternohyoid exhibit different contraction and activity patterns during a swallow. We examined the dynamics of the sternohyoid and geniohyoid muscles during an unrestrained, and vigorous head-shake behavior in an animal model of human head, neck and hyolingual movement. A gentle touch to infant pig ears elicited a head shake of several head revolutions. Using sonomicrometry and intramuscular EMG we measured regional (within) muscle strain and activity in SH and GH. We found that EMG was consistent across three regions (anterior, belly and posterior) of each muscle. Changes in muscle length however, were more complex. In the SH, mid-belly length-change occurred out of phase with the anterior and posterior end-regions, but with a zero-lag timing; the anterior region shortened prior to the posterior. In the GH, the anterior region shortened prior to, and out of phase with the mid-belly and posterior regions. Head-shaking is a relatively simple reflex behavior, yet the underlying patterns of muscle length-dynamics and EMG activity are not. The regional complexity in SH and GH, similar to regionalization of SH during swallowing, suggests that these ‘simple hyoid strap muscles’ are more complex than textbooks often suggest.
INTRODUCTION
The supra and infra-hyoid musculatures are active during various mammalian head and neck movements (Thompson, '41; Brodie, '50; Doty and Bosma, '56; Forsberg et al., '85; Thexton et al., '98; Thexton et al., 2007; Konow et al., 2010). Behaviors relying on this hyoid musculature include suckling, drinking, eating, vomiting, breathing, and extension/flexion of the neck (Forsberg et al., '85; van Lunteren et al., '87; Thexton et al., '98; Lang et al., 2002). The evolutionary rationale for the form and function of the hyoid musculature in mammals is that the tasks of these muscles are not ones of strength, as is true of the muscles of mastication (Hiiemae and Crompton 1985). Their role is to move the hyoid precisely over short distances. As such, these muscles are referred to as classic parallel fibered strap muscles (Hildebrand and Goslow 2001). Several muscles in particular, sternohyoid (SH) and geniohyoid (GH) are antagonist strap-muscles that are active during many of these movements and are important for moving and stabilizing the hyoid bone. Yet, little is known about fascicle strain heterogeneity in the hyolaryngeal musculature, and specifically whether the simple architecture of these muscles translates into homogeneous patterns of activity and length change.
Most studies of fascicle strain heterogeneity have examined vertebrate limb muscles. For example, the turkey lateral gastrocnemius shows superficial to deep regional fascicle strain heterogeneity during ramp-lengthening (Roberts and Azizi 2010), while the medial gastrocnemius in guinea fowl shows proximodistal fascicle strain heterogeneity during running (Higham and Biewener 2008). Much of this work examines muscles that are biarticular and have pinnate fibers (Azizi et al. 2008; Azizi and Roberts 2009; Brainerd and Azizi 2005). Such muscles generate energy to power movement. Some non-pinnate muscles that have broad insertions, including the pigeon pectoralis, show complex fascicle strain heterogeneity (Soman et al. 2005). Here, heterogeneous fascicle strain over the cross-section of the muscle may be an anatomical avenue of streamlining the range of lengths across which the fascicles contract. There are other highly parallel-fibred limb muscles with heterogeneous fascicle strain along their proximodistal axis, such as the toad semimenbranosus (Ahn et al. 2003) and the human biceps brachii (Pappas et al. 2002).
There are few examples of data on muscle fascicle strain for oropharyngeal or hyolaryngeal muscles (Konow et al. 2010; van Lunteren and Dick 2000; van Lunteren et al. 1989; van Lunteren 1987a; 1987b). van Lunteren and colleagues suggest that the relationship between muscle length and function is complex during respiration, particularly in geniohyoid and sternohyoid. They found the geniohyoid to be lengthening while electrically active.
Recent empircal work found that different regions of the sternohyoid have different contraction patterns. The anterior, mid-belly, and posterior regions exhibit different contraction and lengthening patterns during a swallow (Konow et al., 2010). When length change is measured for different regions within the same muscle, different regions (mid-belly vs. ends) change length at different times. Further, when infant pigs swallow, muscle activity in the sternohyoid is most strongly correlated with shortening contraction of the mid-belly region. The belly shortens more than the end-regions, and this suggests that movements at the ends of the muscle are most likely influenced by antagonist muscles during swallowing (Konow et al., 2010). The patterns of EMG activity and strain in the sternohyoid and geniohyoid, however, have not been analyzed during other behaviors than swallowing.
Here, we examine the dynamics of the sternohyoid and geniohyoid muscles during a natural, unrestrained, and vigorous head-shake behavior in an animal model of head, neck and hyolingual movement. Determining the muscle activity and length dynamics relationships during a cyclical, non-oral behavior that incorporates all the hyoid musculature permits us to compare EMG activity and determine relationships of length changes within and among muscles. Whether the same regionalization exists in this behavior as during feeding behaviors is not known. Therefore, we measured sternohyoid and geniohyoid regional activity and length dynamics, (i.e. length-changes), to test the hypothesis that muscle activity and muscle length dynamics (lengthening/shortening) would be equivalent in all three regions during head-shakes. Specifically, our hypothesis was that, given the regional differences seen in these muscles during feeding behaviors and respiration, there would be differences or asynchrony between regional activity and length change in the sternohyoid and geniohyoid during a vigorous head-shake, so that inter-regional activity and length changes would occur asynchronously.
MATERIALS AND METHODS
Animals
The experiments were conducted at Johns Hopkins University and were approved under ACUC #SW07M14. The subjects were eight piglets, from the Tom Morris Farm (Reisterstown, MD). Their body weights were 5–6 kg at an age of 10–16 days. Details about data collection were given in (Konow et al., 2009) and the methods are therefore reviewed briefly here.
Instrumentation of muscles
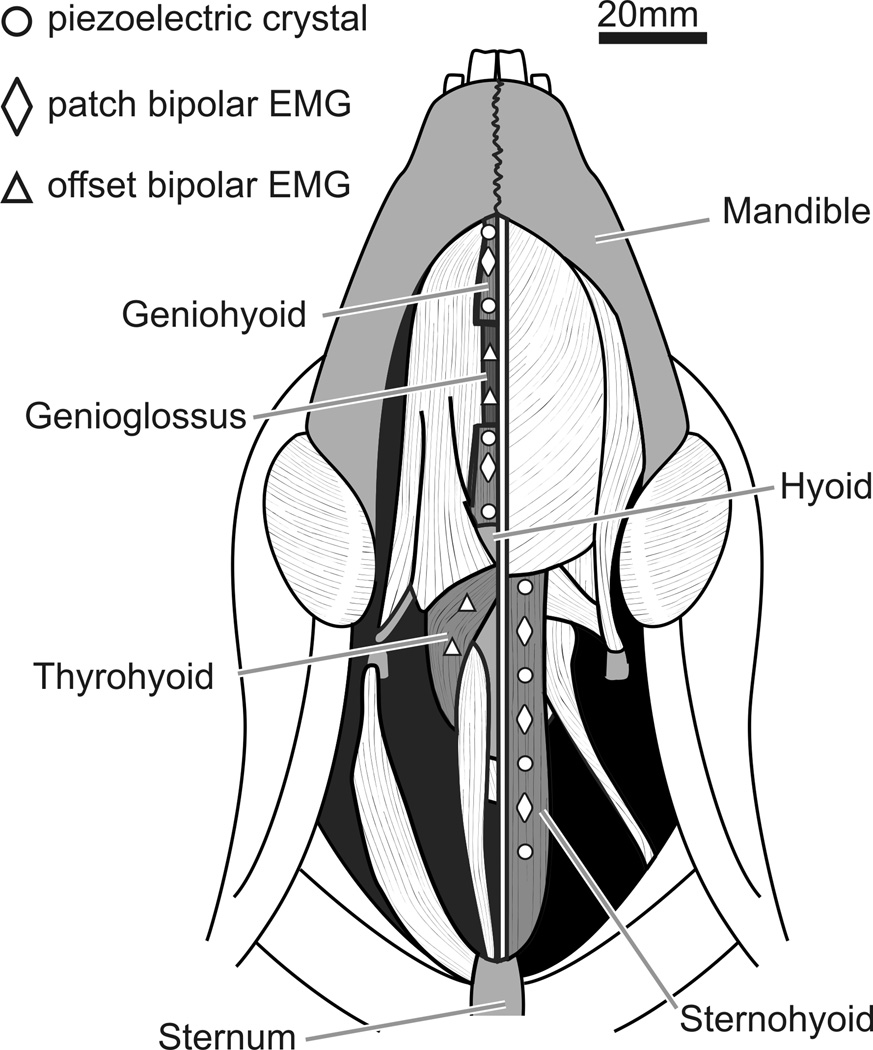
The piglets were placed under anesthesia by 5% Isoflurane inhaled through a face mask. They were intubated, and aseptic conditions were maintained through the surgery. Only one muscle, the sternohyoid or geniohyoid, was studied for length dynamics per animal. Figure 1 show the EMG electrode and sonomicrometry crystal placements.
Figure 1.
We did two sets of parallel studies, the first set of four experimental animals (C, D, M, S), focused on the sternohyoid and the second animals (T, U, V, W) on the geniohyoid. For each set, the focal muscle was divided into three areas, anterior, middle and posterior by four 2-mm piezoelectric crystal (Sonometric Corp, London, ON). Between each pair of crystals, bipolar EMG patch electrodes were attached to the surface The crystals were placed approximately 17 mm apart (Fig. 1). The crystals emit ultrasound energy across the distance they are placed. Therefore, changes in length can be recorded. For the sternohyoid, of the sternohyoid between each pair of crystals (Loeb and Gans, '86). In order to avoid any damaged muscle resulting from the crystal insertion, the electrodes were placed 3–4 mm away from the line of the crystals (Fig. 1). The sternohyoid, genioglossus, geniohyoid, and thyrohyoid were carefully exposed using blunt dissection (Sack, '82).
We placed EMG electrodes in additional muscles that might be involved in the behaviors under study, or be antagonists to the sternohyoid or geniohyoid: the middle of the genioglossus, close to the origin of the thyrohyoid, and the geniohyoid‥ EMG patch electrodes were placed on the surface of the geniohyoid, between the three crystal pairs. These four animals were additionally instrumented with fine wire bipolar electrodes in the thyrohyoid and sternohyoid (Fig. 1). Before surgery, the sonomicrometry crystals had a skin button connector attached (Sonomicrometrics, London, ON), and the electrode wires had a microconnector attached (Glen-Air, Glendale, CA).
Recording procedures
The wires from the crystals and electrodes exited out the ventral surface of the neck through a submandibular incision. The extra-cutaneous wires were carefully folded and bandaged with Vetwrap (3M, MN), and the connectors were sutured to the bandage. The piglets recovered from surgery within 3–5 hours, were fully alert and standing prior to feeding. Data was collected over a 36–48 hour period, during which the pigs were fed and data was recorded every 3–4 hours. We recorded length changes from the sonomicrometry crystals and EMG recordings from all the experimental muscles (Fig. 2) for swallowing, suckling, vocalization, and head-shaking.
Figure 2.
The piglet suckled from a baby bottle with a pig nipple (Nasco, Fort Atkinson, WI) while standing in a pet carrier. Several times during an experiment, we elicited a head-shake by gently brushing inside one of the ears of the pig with a cotton swab. The pig reliably responded with vigorous side-to-side lateral head movement. After the experimental period, the pigs were placed in a deep plane of inhalant anesthesia, and euthanized with veterinary euthanasia solution (IC). A post-mortem dissection was conducted to verify electrode and crystal position.
A PC running Powerlab 16/30 recorded the data in LabChart v.6.1.3 (AD instruments, Colorado Springs, CO). The EMG signals were amplified 1000 times with a MA-300 EMG system (Motion Lab Systems Inc, LA) and a high pass filter of 25 hertz. The inputs to Powerlab, from the EMG amplifiers and sonomicrometer outputs, were digitized at 10 KHz. The sonomicrometer output was 3 crystal pair distances of recorded at 508 hertz with a transmit pulse of 220 ms and inhibit delay set between 2.2–2.6 mm to condition the signals.
Head-shake identification
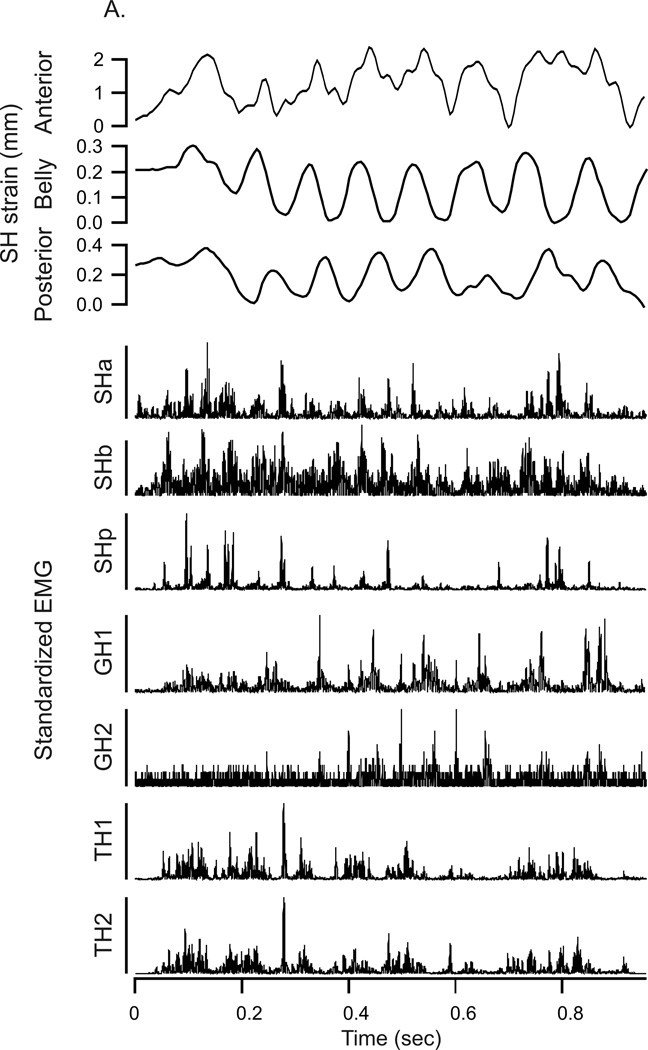
Data sequences were selected with clear shakes, containing several revolutions of the head. The quality of a sequence was determined by the maximum number of channels recording data without artifacts or noise for all behaviors. From each data sequence, the head-shakes were identified and found to be characterized by several short cycles of EMG activity across all 6 muscles and length changes in all the sonomicrometric channels.
The oscillations observed from the sonomicrometry crystal data consisted of 2–8 revolutions (Fig. 2a) in both the sternohyoid and geniohyoid, and lasted approximately 300 to 800 ms with simultaneous isolated bursts of EMG activity for sternohyoid, geniohyoid, genioglossus and thyrohyoid. Each set of shake oscillations was copied into a separate data file and coded numerically. A total of 152 head-shakes were compiled, 80 for the first four set of pigs, representing sternohyoid sonomicrometry data and 72 for the second set of pigs, representing the geniohyoid sonomicrometry data.
Data extraction
A script was coded in Matlab R2008a (v.7.6.0.3.2.4, The Mathworks, Natick, MA) to process the EMG signals following (German et al., 2009). In brief, EMG signals were rectified and reset integrated to a period of 10ms, with baseline noise subtraction following (Thexton et al., '98). Sonomicrometer signals were left unprocessed, other than a sub-sampling to 100Hz to retain synchronization with the processed EMG signals.
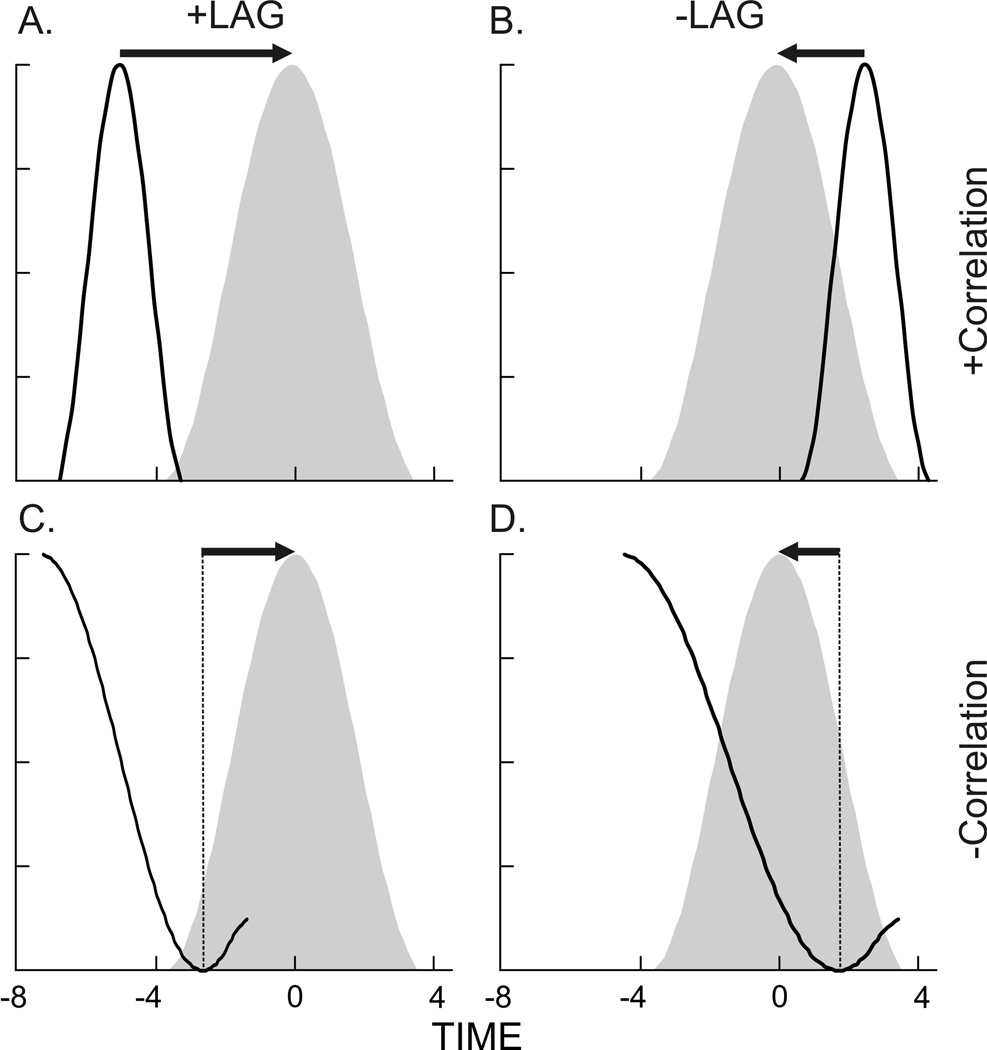
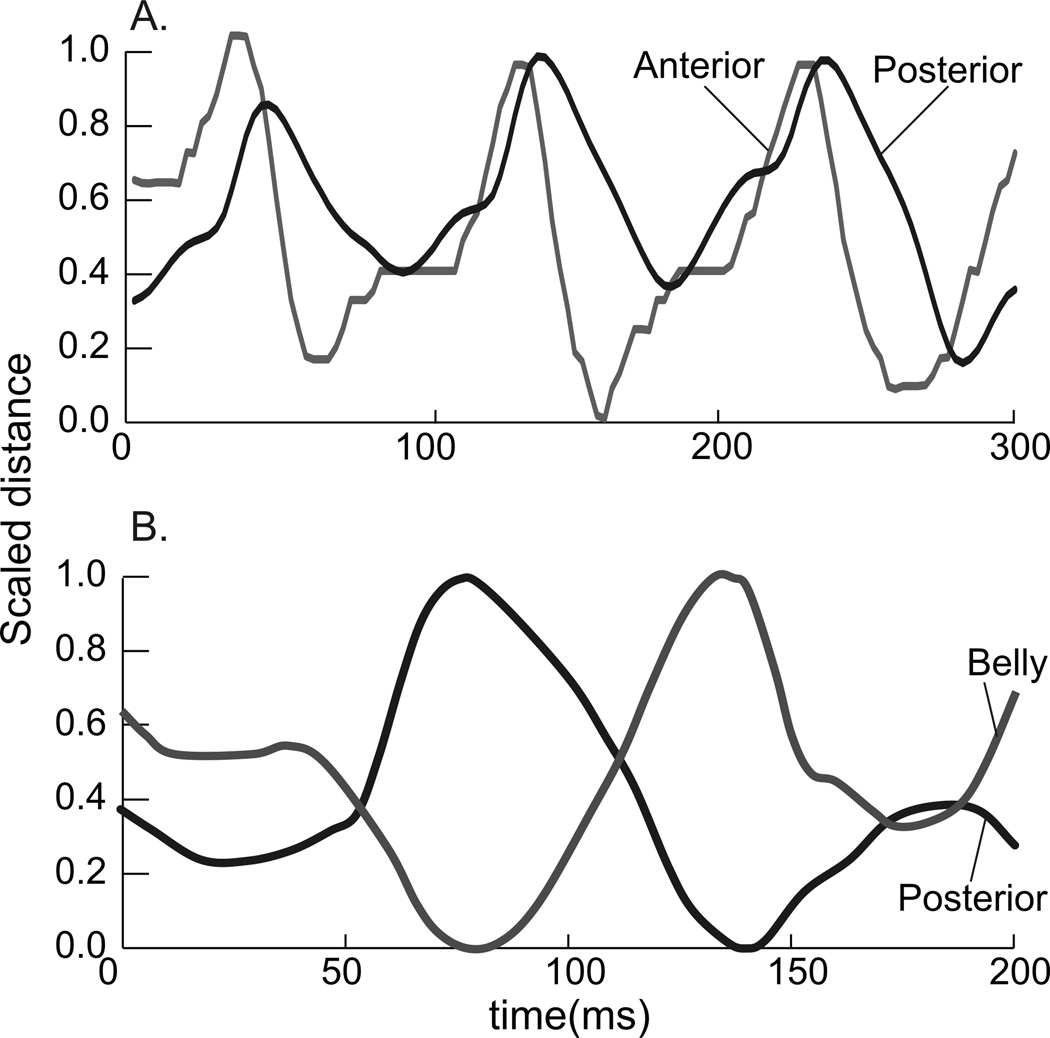
In order to obtain a quantitative measurement and to evaluate synchronization, a cross correlation analysis was run for each shake (Loeb et al., '87; Thexton et al., 2009; Wren et al., 2006). The cross correlation function (CCF) output provides 2 pieces of information. Firstly, it measures the time relationship, or lag, between two sets of time series data (Fig. 2). If a wave from one variable lines up in time with a wave of a second variable, then the lag = 0, and the activity is in synchrony. If there is a positive lag, the response vector must move in a positive direction (to the right) relative to the target vector in order to align (Fig. 2A).
The opposite occurs in a negative lag; the response vector moves in a negative direction (to the left) to obtain peak-correlation with the target vector. The second item the CCF indicates is if the vectors are in phase or out of phase. The r-value indicates the degree of correlation between the two wave forms, that is, the pair-wise correlation between two waves at a given lag. A negative r-value indicates an inverse correlation (out of phase waveforms) at a given lag (Fig. 2C, D).
Statistics
Cross-correlation analyses were done in SYSTAT 12 (2009). From this output, the lag with the strongest correlation was statistically determined. All of the identification of shakes in LabCharts, data extraction in Matlab, and assessment of shake quality was done by one person (SEW). We carried out several sub-analyses on the CCF results (also in Systat 12) to test our hypotheses. First, we tested if the lags among crystal pairs were different from zero using a repeated measures linear model, including individual as a factor. Then, we tested if the lags among EMG electrodes were different from zero using repeated measures linear model, including individual as a factor. Lags with a negative correlation were tested separately from lags with a positive correlation. Multiple comparisons included Bonferoni corrections.
RESULTS
General behavior
Animals responded reliably and consistently to a brushing with a cotton swab in their ear with vigorous head shaking. There was regularity in the revolutions, i.e., the individual movements of each shake, from midline, to lateral, then contra-lateral and back to midline (Fig. 3A). In the shake shown in Figure 3, an upward line indicates lengthening of the muscle region. A downward line occurred when the muscle region was shortening. The bottom rows show the vigorous EMG activity during the head-shake behavior.
Figure 3.
Sternohyoid
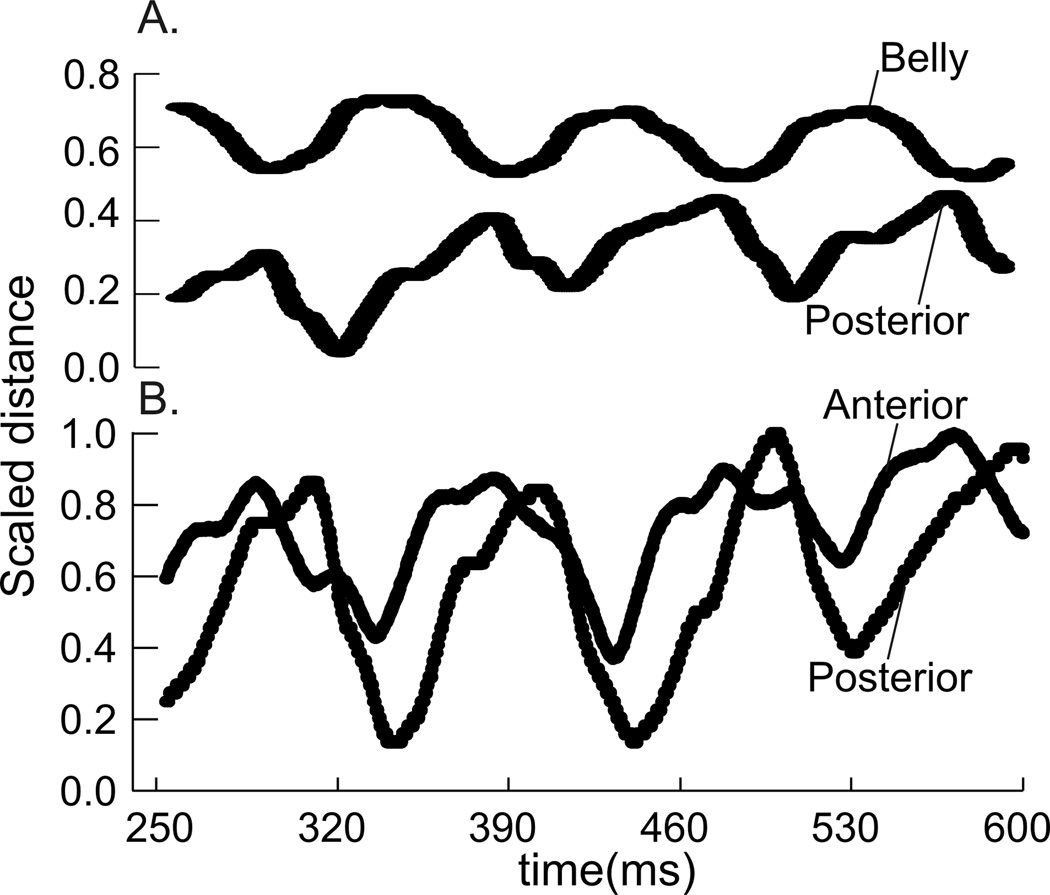
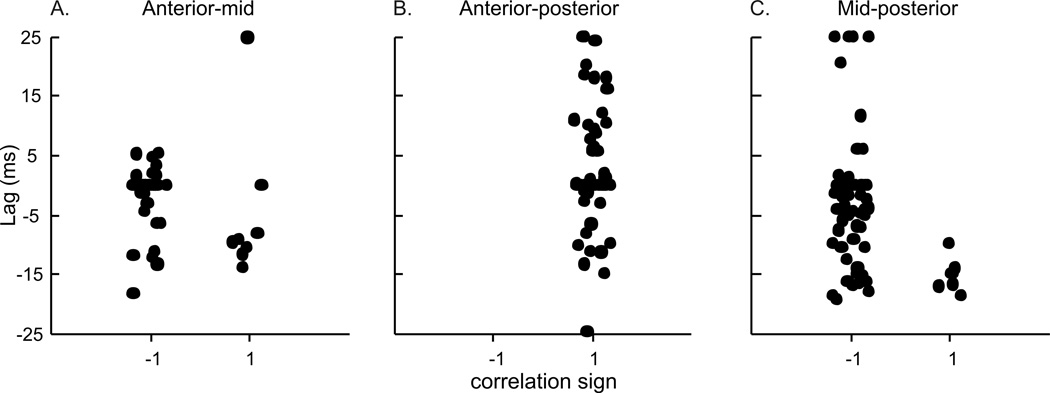
There were no differences in the timing of muscle length-change between the anterior (hyoid) to middle region and between the middle to posterior (sternal) region (Table 1). The majority of the correlations for these two comparisons were negative, indicating an out-of-phase relationship (Fig. 4A, 5A, C). The average lag was zero, indicating that, on average, maximum shortening in one region occurred simultaneously with maximal lengthening in another. However, the wide spread of values, and high variation in the timing lags between these pairs of regions suggests that either region could change length first, but that one was shortening while the other was lengthening. The relationship between anterior and posterior SH consistently returned a positive correlation, with an average lag of 4.02ms (Fig. 4B, 5B). Again, there is large variation in the lag, including both positive and negative lag values. The mean lag of 4.02 ms suggests that length change in the anterior section tends to occur prior to the posterior (sternal) section.
Table 1.
Results of t-tests for SH group. Ho : mean lag = 0
| Regions tested | CCF correlation |
df | mean lag (msec) |
SD (msec) | t-value | p-value | |
|---|---|---|---|---|---|---|---|
| aSH-mSH | Negative | 50 | 0.19 | 10.23 | 0.13 | 0.894 | |
| mSH-pSH | Negative | 64 | −2.07 | 10.62 | −1.57 | 0.122 | |
| aSH-pSH | Positive | 60 | 4.02 | 11.2 | 2.79 | 0.007 | * |
| aSHemg-mSHemg | Positive | 29 | −1.94 | 8.71 | −1.21 | 0.235 | |
| mSHemg-pSHemg | Positive | 22 | 0.23 | 1.77 | 0.62 | 0.540 | |
| aSHemg-pSHemg | Positive | 23 | 2.87 | 7.63 | 1.84 | 0.078 | |
| SHemg-THemg | Positive | 54 | −0.16 | 0.43 | −2.78 | <0.001 | ** |
| SHemg – GHemg | Positive | 42 | 0.01 | 0.19 | 0.23 | 0.82 | |
| SHemg – GGemg | Positive | 64 | −0.23 | 0.45 | −4.05 | <0.001 | ** |
p<.05,
p<.001
Figure 4.
Figure 5.
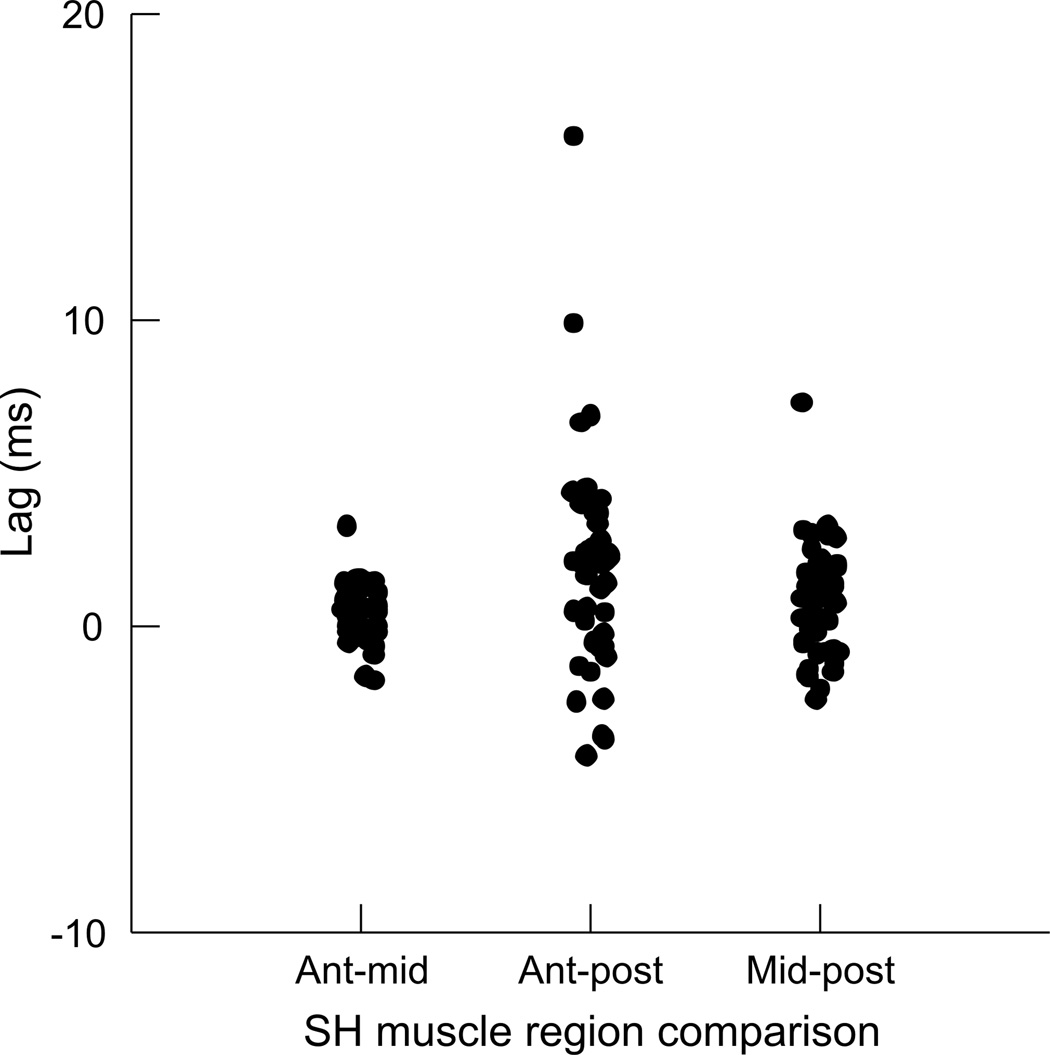
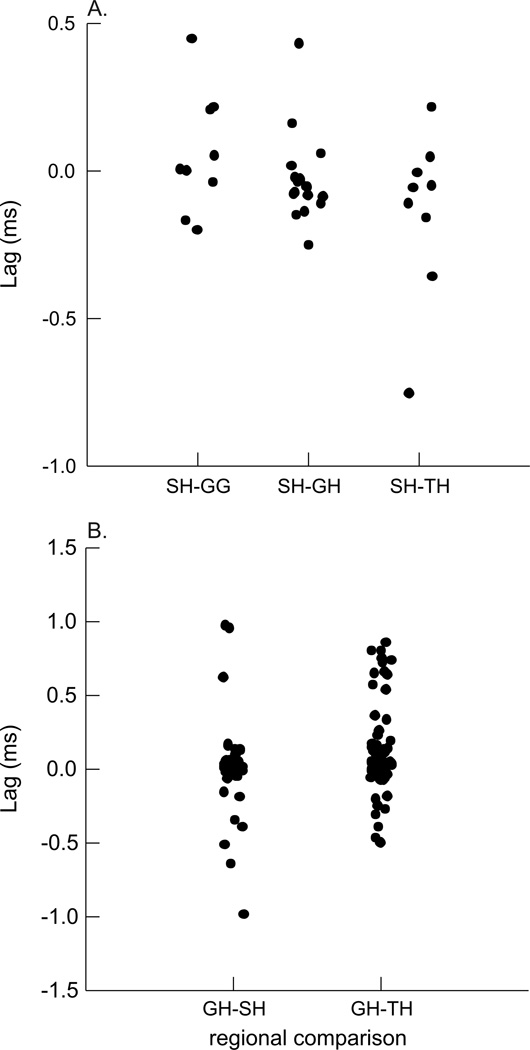
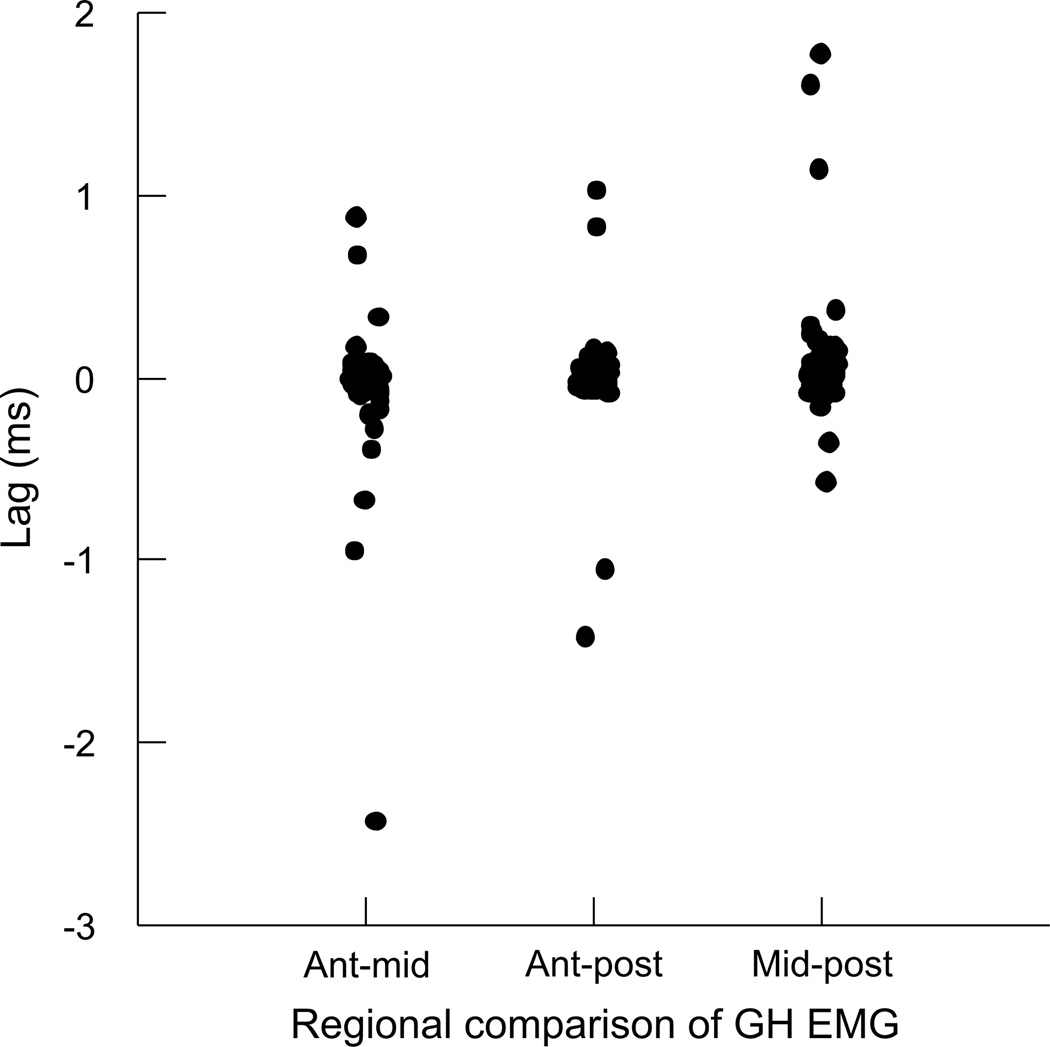
The timing of differences in EMG activity between each pair of regions was not statistically significant (Fig. 6), i.e., the lag was not statistically different from 0.0 (Table 1). When comparing the EMG activity between the SH and other muscles, we found small, but significant lags between both SH and TH and SH and GG. The lag between SH and GH was not statistically different from 0.0 (Table 1, Fig. 7A).
Figure 6.
Figure 7.
Geniohyoid
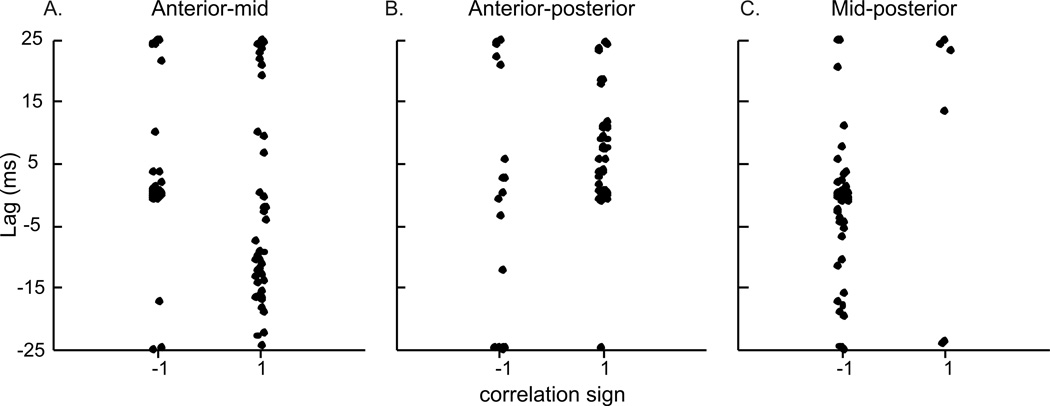
The timing of length-change between the three pairs of regions in the GH included a large number of both positively and negatively correlated relationships (Table 2; Fig. 9). Despite a large variation in lag values, there was some clustering of values. Approximately half of the anterior (mandibular) to middle distances had a negative relationship with no lag, but the other half had a positive relationship with a mean lag of −12.25ms (Fig. 9A). The anterior to posterior (hyoid) relationship was generally positive, with a significant lag of 6.37ms (Fig. 9B). The middle to posterior relationship was largely negative, but with zero-lag, and high variation around that lag (Fig. 9C). The CCF’s for the EMG activity between all region-pairs returned positive correlations, and lags that were not different from zero (Fig. 10). The CCF for EMG activity of GH relative to TH had a lag of 0.126 ms with positive correlation, while the EMG of GH and SH had no lag and positive correlation (Table 2, Fig. 7b).
Table 2.
Results of t-tests for GH group. Ho : mean lag = 0
| Regions tested | CCF correlation |
df | mean lag (msec) |
SD (msec) | t-value | p-value | |
|---|---|---|---|---|---|---|---|
| aGH-mGH | Positive | 22 | −1 2.25 | 5.97 | −9.838 | <0.001 | ** |
| aGH-mGH | Negative | 18 | 1.19 | 2.65 | 1.953 | 0.067 | |
| aGH-pGH | Negative | 55 | −0.98 | 12.60 | −0.584 | 0.561 | |
| aGH-pGH | Positive | 42 | 6.37 | 7.59 | 5.501 | <0.001 | ** |
| aGHemg-mGHemg | Positive | 69 | −0.05 | 0.34 | −1.272 | 0.207 | |
| mGHemg-pGHemg | Positive | 69 | 0.08 | 0.32 | 2.065 | 0.043 | * |
| aGHemg-pGHemg | Positive | 69 | 0.01 | 0.27 | 0.043 | 0.966 | |
| GHemg-SHemg | Positive | 42 | 0.00 | 0.32 | 0.000 | 0.999 | |
| GHemg-THemg | Positive | 69 | 0.13 | 0.32 | 3.483 | 0.001 | ** |
p<.05
p<.001
Figure 9.
Figure 10.
DISCUSSION
Head-shake muscle activity
During a head-shake, there were rhythmic bursts of activity across all hyoid muscles observed in this study. Contrary to our hypothesis, the EMG of the anterior, middle, and posterior regions in both the sternohyoid and geniohyoid was synchronous, with little variation in lag. The inter-muscular comparisons of EMG highlighted more similarities than differences between hyoid musculature activities. The EMG of the sternohyoid and geniohyoid had zero lag, and were therefore active at the same time; this is not surprising, given that the principal function of these antagonists would be to stabilize the hyoid bone during a vigorous behavior such as a head shake.
There was a statistical difference in EMG between the sternohyoid and thyrohyoid, as well as between the sternohyoid and genioglossus (Table 1). However, the activity lags between pairs of these muscles were in the range of 0.1–0.2 ms, for a revolution that lasted approximately 100 ms. The differences in activity timing of these muscles during a swallow is on the order of 20–50 ms for a behavior that lasts about 400–500 ms (German et al., 2009). It is questionable whether the very small differences in EMG timing make a significant difference in the behavior. Likewise, the difference in timing between EMG of geniohyoid and thyrohyoid is less than 0.15 ms (Table 2). While statistically significant, the small magnitude of differences suggest that in terms of a single head-shake revolution that lasts 100 ms, we are uncertain of their biological meaningfulness.
Head-shake length dynamics
The results from the comparisons of regional length-changes were more complex than those for the EMGs. We found regional differences strain heterogeneity in both ternohyoid and geniohyoid, as well as a high amount of variation in the timing lags among muscle regions. These regional differences are consistent with previous findings for the sternohyoid during swallowing (Konow et al., 2010). This supports our idea that the sternohyoid has complex behavior, even though its architecture is simple (Paniello et al., 2001; Peker et al., 2006). The most consistent results were that the ends of the sternohyoid were lengthening and shortening out-of-phase with the middle of the muscle. Shortening of the belly correlated with the muscle activity in this region, while the ends lengthened during muscle activity. Our interpretation of this pattern is that the muscle was being stretched as the head turns, and the belly was contracting eccentrically to resist this turn and to stabilize the hyoid bone against the pull of the muscles.
For the geniohyoid, strain in the belly and posterior regions were out of phase, while strain in the anterior region was in phase with one or the other of these regions. Given that the length change in the belly and posterior regions are out of phase with each other, if the anterior region is in phase with one region it will be out of phase with the other. There are two potential interpretations of this pattern. First, the anterior region of the GH could be truly random in its behavior with respect its time relationship with the other regions. Sometimes it could be contracting isontoically, and other times actively lengthening or even being stretched by the head turn. Without fluoroscopic visualization, this is hard to resolve. Alternatively, it could be that we are not measuring the "functional units" within this muscle. That is, the end-plates of the portion of the anterior region that is most consistently out of phase with the middle were not aligned with the crystals measuring length change. With more crystals, and histologically guided divisions of the GH, more exact length change phase relationships between the regions could be resolved. It follows that with our larger divisions, the observed length change could sometimes be dominated by a lengthening pool of motor units, and sometimes by a shortening. The existence of multiple pools of motor neurons in the geniohyoid is already well documented (German et al. 2009; Thexton et al. 2009; van Lunteren and Dick 2000).
The timing of changes in regional muscle length was characterized by a high level of variation for all regions in both muscles, complicating the interpretation of these results. For sternohyoid and geniohyoid, the large variation (Fig. 5 and Fig. 9), have an average lag in most cases near zero.
Head movement including shaking
Our results are consistent with some previous studies (Thompson, '41; Thompson and Brodie, '42; Forsberg et al., '85), but not all (Berzin, '95). Previously, head-shaking has only been described in cats (Richmond et al., '92). The cat head-shakes consisted of 1–5 rapid oscillations, usually completed in 100–150 ms, and involving fast alternating movements from one side of the midline to the other. The EMG recordings for cat head-shakes showed large bursts of activity for all the paravertebral muscles (Richmond et al., '92). We found similar large bursts for all of the hyoid musculature under study (Fig. 3a). These findings also support previous research by Forsberg et al. ('85). Using bipolar surface electrodes attached to the skin of the subject, Forsberg et al. ('85) found variation in both the infra- and suprahyoids EMG activity, during different amounts of head extension and flexion (Forsberg et al., '85). Extension and 20° of flexion showed increased activity relative to 5° or 10° of flexion. Interestingly, our results contradict the findings of Berzin ('95), who used needle electrodes inserted into the human neck musculature, and found the sternohyoid to be inactive during neck rotation, flexion, and extension movements (Berzin, '95).
Head-shaking is a cyclical and rapid representation of a head turn. Based on our results, the hyoid musculature, and in particular the sternohyoid and geniohyoid muscles, appears to be active during such a movement. Clearly, kinesiology of the head is principally driven by the paravertebral and sternocleidomastoid muscles (Last, '95). The hyoid musculature is active in maintaining the posture of the head (Thompson, '41; Thompson and Brodie, '42), but the activity of the hyoid musculature during flexion, extension, and lateral movements of the head remains controversial (Brodie, '50; Last, '95; Forsberg et al., '85; Berzin, '95). Our study is consistent with Brodie (‘50), Thompson ('41), and Forsberg et al. ('85) who found that the hyoid musculature shows motor activity during a head turn. Since our research involved regional length-dynamics of the sternohyoid and geniohyoid, we not only quantified electrical activity, but also regional contraction specialization. The anterior to posterior gradient of muscle lengthening during a forceful behavior such as a head shake shows heterogeneity that is surprising for these simple muscles.
Head-shakes compared with swallowing
Some aspects of the complex regional differences in length change of sternohyoid and geniohyoid during head-shakes mirror that of patterns in the sternohyoid during a more quiescent behavior, namely swallowing. The strain patterns in the belly region were negatively correlated with the anterior and posterior regions in swallows occurring early during a feeding session, when the pigs were most hungry (Konow et al., 2009). The belly was contracting at a different time than the end regions of the muscle. During later swallows, when the animal was less hungry, there was a difference between the anterior and posterior regions, with a posterior to anterior delay in muscle lengthening (Konow et al., 2010). This is the opposite of the anterior to posterior delay seen in head-shakes. These data suggest that the sternohyoid displays different lengthening/shortening patterns depending on behavior. The shake behavior involves a more abrupt and intense movement of the head and neck than swallowing, and we speculate that the anterior to posterior delay represents a more immediate response of the anterior region of the muscle closest to the hyoid bone in order to stabilize it.
Similar to our head-shake results, swallowing was homogeneous in EMG activity across the sternohyoid regions. In the swallowing data, length changes in the middle portion of the muscle showed the strongest relationship with peak EMG activity. The motor pattern does not seem to explain all of the dynamics and length changes of the muscle for either swallowing or head shaking behavior. The length of the sternohyoid results not only from its own contraction and relaxation, but also from the contraction and relaxation of its antagonists (i.e. suprahyoids). Therefore, the actions of this muscle include passive stretch and elastic recoil in order to compensate for hyoid actuation by the suprahyoids, and the EMG patterns could be representative of these dynamics, and not pure relaxation/contraction.
The hyoid musculature is clearly very important during mammalian feeding behaviors and for stabilization of the hyoid bone (Smith, '92). Our understanding of the function and behavior of these muscles remains incomplete. Head-shaking is a relatively simple, reflex behavior, yet the patterns of muscle length dynamics and EMG activity are not. The regional heterogeneity of sternohyoid and geniohyoid strain during this behavior suggests that these muscles are not the simple muscles that textbooks often suggest.
Figure 8.
ACKNOWLEDGEMENTS
We thank G. Kurtz for help with Matlab scripts, A. W. Crompton and A. Thexton for comments and suggestions, and the RAR staff at JHMI for assistance with animal care and surgery. We appreciate the comments of two anonymous reviewers. This work was funded by NIH (DC03604) to RZG.
LITERATURE CITED
- Ahn AN, Monti RJ, Biewener AA. In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J Physiol. 2003;549(Pt 3):877–888. doi: 10.1113/jphysiol.2002.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A. 2008;105(5):1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Roberts TJ. Biaxial strain and variable stiffness in aponeuroses. J Physiol. 2009 doi: 10.1113/jphysiol.2009.173690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzin F. Electromyographic analysis of the sternohyoid muscle and anterior belly of the digastric muscle in head and tongue movements. J Oral Rehabil. 1995;22(11):825–829. doi: 10.1111/j.1365-2842.1995.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Azizi E. Muscle fiber angle, segment bulging and architectural gear ratio in segmented musculature. J Exp Biol. 2005;208(Pt 17):3249–3261. doi: 10.1242/jeb.01770. [DOI] [PubMed] [Google Scholar]
- Brodie AG. Anatomy and physiology of the head and neck musculature. American Journal of Orthodontics. 1950;36:831. doi: 10.1016/0002-9416(50)90038-8. [DOI] [PubMed] [Google Scholar]
- Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- Forsberg C-M, Hellsing E, Linder-Aronson S, Sheikholeslam A. EMG activity in neck and masticatory muscles in relation to extension and flexion of the head. Eur J Orthod. 1985;7(3):177–184. doi: 10.1093/ejo/7.3.177. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–1025. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham TE, Biewener AA. Integration within and between muscles during terrestrial locomotion: effects of incline and speed. J Exp Biol. 2008;211(14):2303–2316. doi: 10.1242/jeb.016139. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM, Crompton AW. Mastication, food transport and swallowing. In: Hildebrand M, Bramble D, Liem K, Wake D, editors. Functional Vertebrate Morphology. Cambridge: University Press; 1985. pp. 262–290. [Google Scholar]
- Hildebrand M, Goslow GE. Analysis of Vertebrate Structure. New York: Wiley; 2001. p. 657. [Google Scholar]
- Konow N, Thexton A, Cromptom AW, German RZ. Regional differences in length change and electromyographic heterogeneity in the sternohyoid muscle during infant mammalian swallowing. J Appl Physiol. 2010;109(2):439–448. doi: 10.1152/japplphysiol.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting and swallowing. Am J Physiol. 2002;283:G529–G536. doi: 10.1152/ajpgi.00062.2002. [DOI] [PubMed] [Google Scholar]
- Last RJ. The muscles of the head and neck in review. International Dental Journal. 1955;5:338. [Google Scholar]
- Loeb G, Gans C. Electromyography for experimentalists. Chicago: University of Chicago Press; 1986. pp. P119–P120. [Google Scholar]
- Loeb GE, Yee WJ, Pratt CA, Chanaud CM, Richmond FJ. Cross-correlation of EMG reveals widespread synchronization of motor units during some slow movements in intact cats. Journal of neuroscience methods. 1987;21(2–4):239–249. doi: 10.1016/0165-0270(87)90119-1. [DOI] [PubMed] [Google Scholar]
- Paniello RC, West SE, Lee P. Laryngeal reinnervation with the hypoglossal nerve. I. Physiology, histochemistry, electromyography, and retrograde labeling in a canine model. Ann Otol Rhinol Laryngol. 2001;110(6):532–542. doi: 10.1177/000348940111000607. [DOI] [PubMed] [Google Scholar]
- Pappas GP, Asakawa DS, Delp SL, Zajac FE, Drace JE. Nonuniform shortening in the biceps brachii during elbow flexion. J Appl Physiol. 2002;92(6):2381–2389. doi: 10.1152/japplphysiol.00843.2001. [DOI] [PubMed] [Google Scholar]
- Peker T, Gulekon N, Turgut BH, Anil A, Karakose M, Mungan T, Danisman N. Observation of the relationship between the shape of skeletal muscles and their nerve distribution patterns: a transparent and microanatomic study. Plast Reconstr Surg. 2006;117(1):165–176. doi: 10.1097/01.prs.0000186539.80555.27. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Thomson DB, Loeb GE. Electromyographic studies of neck muscles in the intact cat. I. Patterns of recruitment underlying posture and movement during natural behaviors. Exp Brain Res. 1992;88(1):41–58. doi: 10.1007/BF02259127. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Azizi EA. The series-elastic shock absorber: tendons attenuate muscle power during eccentric actions. J Appl Physiol:japplphysiol.01272.02009. 2010 doi: 10.1152/japplphysiol.01272.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack WO. Pig anatomy and atlas. Ithaca, NY: 1982. [Google Scholar]
- Smith KK. The evolution of the mammalian pharynx. Zoological Journal of the Linnean Society. 1992;104(4):313–349. [Google Scholar]
- Soman A, Hedrick TL, Biewener AA. Regional patterns of pectoralis fascicle strain in the pigeon Columba livia during level flight. J Exp Biol. 2005;208(Pt 4):771–786. doi: 10.1242/jeb.01432. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. Journal of Experimental Zoology. 1998;280(5):327–343. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102(2):587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol. 2009;101(3):1386–1393. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR. A cephalometric study of the movements of the mandible. Journal of the American Dental Association. 1941;28:705–761. [Google Scholar]
- Thompson JR, Brodie A. Factors in the position of the mandible. Journal of the American Dental Association. 1942;29:925. [Google Scholar]
- van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Respiratoin and Physiology. 2000;124(1):23–33. doi: 10.1016/s0034-5687(00)00182-1. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Haxhiu MA, Cherniack NS. Effects of tracheal airway occlusion on hyoid muscle length and upper. J Appl Physiol. 1989;67(6):2296–2302. doi: 10.1152/jappl.1989.67.6.2296. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Haxhiu MA, Cherniack NS. Mechanical function of hyoid muscles during spontaneous breathing in cats. J Appl Physiol. 1987a;62:582–590. doi: 10.1152/jappl.1987.62.2.582. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Haxhiu MA, Cherniack NS. Relation between upper airway volume and hyoid muscle length. J Appl Physiol. 1987b;63:1443–1449. doi: 10.1152/jappl.1987.63.4.1443. [DOI] [PubMed] [Google Scholar]
- Wren TAL, Patrick Do K, Rethlefsen SA, Healy B. Cross-correlation as a method for comparing dynamic electromyography signals during gait. Journal of Biomechanics. 2006;39(14):2714–2718. doi: 10.1016/j.jbiomech.2005.09.006. [DOI] [PubMed] [Google Scholar]