Abstract
This study investigated the systemic and microvascular hemodynamic changes related to increased nitric oxide (NO) availability following significant hemorrhage, made available by administration of NO releasing nanoparticles (NO-nps). Hemodynamic responses to hemorrhagic shock were studied in the hamster window chamber. Acute hemorrhage was induced by arterial controlled bleeding of 50% of blood volume, and the resulting hemodynamic parameters were followed over 90 min. Exogenous NO was administered in the form of NO-nps (5 mg/kg. suspended in 50 µl saline) 10 min following induced hemorrhage. Control groups received equal dose of NO free nanoparticles (Control-nps) and Vehicle solution. Animals treated with NO-nps partially maintained systemic and microvascular function during hypovolemic shock compared to animals treated with Control-nps or the Vehicle (50 µl saline). The continuous NO released by the NO-nps reverted arteriolar vasoconstriction, partially recovered both functional capillary density and microvascular blood flows. Additionally, NO supplementation post hemorrhage prevented cardiac decompensation, and thereby maintained and stabilized the heart rate. Paradoxically, the peripheral vasodilation induced by the NO-nps did not decrease blood pressure, and combined with NO’s effects on vascular resistance, NO-nps promoted intravascular pressure redistribution and blood flow, avoiding tissue ischemia. Therefore, by increasing NO availability with NO-nps during hypovolemic shock, it is possible that cardiac stability and microvascular perfusion can be preserved, ultimately increasing survivability and local tissue viability, and reducing hemorrhagic shock sequelae. The relevance, stability, and efficacy of exogenous NO therapy in the form of NO-nps will potentially facilitate the intended use in battlefield and trauma situations.
Keywords: Microcirculation, hemorrhage, glutathione, nitrosoglutathione, functional capillary density
INTRODUCTION
Hemorrhagic shock accounts for about 50% of battlefield deaths in conventional warfare 1. Early, aggressive, high volume resuscitation was widely accepted and most practiced during the Vietnam War 2. However, metabolic benefits of this approach were only demonstrated in controlled hemorrhage animal models, and when implemented in humans, showed severe deleterious effects including pulmonary failure and re-bleeding 3, 4. Small volume resuscitation can be considered a model to improve the efficiency of fluid therapy by changing the composition and infusion regimens 5. Even following trauma, blood transfusion produces inflammatory responses, potentially causing more morbidity than the original insult 6.
Nitric oxide (NO) plays several major roles in human physiology. NO is a neurotransmitter, a macrophage-derived host-defense molecule, an inhibitor of platelet aggregation and endothelium adhesion molecule expression, an antioxidant, cardiac chronotropic, and a potent vasodilator.7 Our hypotheses to explain the hemorrhagic shock induced cardiovascular collapse are that the production of NO is impaired by 1) drastic reduction on endothelial shear stress (due to low blood pressure, cardiac output, circulating volume and blood viscosity)8; 2) free radical formation/accumulation, uncoupling the endothelial NO synthase (NOS) 9; and 3) hypoxia, as oxygen is a substrate for NO production 10. Thus, restoration of NO bioavailability impaired during hemorrhagic shock will reduce many hemorrhagic shock induced complications. Since endothelial synthesized NO is the major source of NO for the vasculature11, we proposed an intraluminal source, such NO releasing nanoparticles (NO-nps). NO-nps retains NO in a stable form when dry, releases NO upon exposure to moisture, and are stable over long-term dry storage. Unlike most NO generating materials which rely on chemical decomposition or enzymatic catalysis, the NO-nps utilize a thermally driven redox reaction involving nitrite to generate the NO 12, 13. NO-np half-life in vitro was found to be approximately 4 hours at 37°C and pH 7.4, and one mg of powder is capable of releasing 0.32 µmols of NO 13. When suspended at lower pH levels, the total mount of NO released did not change, but faster rates of release were measured. In vivo, circulating NO-np produced a decrease in blood pressure, microvascuslar vasodilatation and increased blood flow in a dose dependent fashion. Maximal decrease in pressure in animals treated with NO-np was measured at 90 min after infusion and lasted 3h after infusion. Exhaled NO increased after infusion of NO-np, maximal exhaled NO levels were measured 1h after infusion and maintained over a period of 2h; after that exhaled NO decreased. Importantly, one mg of NONOates releases 30 times more than the NO-np 13.
The objectives of this study were: i) to determine any protective effect of exogenous NO released from NO-nps during hypovolemic shock; ii) to define the basic mechanisms through which exogenous NO restore microvascular function in the absence of volume resuscitation. To achieve these objectives, we subjected our experimental hamster model to a hemorrhage of 50% of blood volume (BV). Ten minutes following hemorrhage, animals were treated with 5 mg/kg of NO-nps or NO free nanoparticles (Control-nps), suspended in 50 ul of normal saline. Systemic and microvascular parameters were studied up to 90 min after hemorrhage.
METHODS
Synthesis of NO-nps and Control-nps
The synthesis of NO-nps was recently reported 12. Briefly, a hydrogel/glass composite was synthesized using a mixture of tetramethylorthosilicate, polyethylene glycol, chitosan, glucose, and sodium nitrite in a 0.5 M sodium phosphate buffer (pH 7). As previously described, nitrite is reduced to NO within the matrix as a result of the glasys properties of the composite, which allow for redox reactions initiated, with thermally generated electrons from glucose 12. The composite is dried using a lyophilizer and crushed using planetary ball mill, resulting in nanoparticles containing NO. Once exposed to an aqueous environment, the hydrogel allows for an opening of the water channels inside the particles, facilitating the release of the trapped NO over extended time periods. Control-nps, free of NO inside, were produced identically, but without nitrite.
Animal Preparation
Investigations were performed in 50 – 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal skinfold chamber window. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster chamber window model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere 14. Catheters were tunneled under the skin and exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion Criteria
Animals were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic Hct > 45%, and arterial oxygen partial pressure (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under a ×650 magnification did not reveal signs of edema or bleeding.
Acute hemorrhage protocol
Acute hemorrhage was induced by withdrawing 50% of estimated total blood volume (BV) via the carotid artery catheter within 5 min. Total BV was estimated as 7% of body weight. Animals were followed over 90 min after hemorrhage induction. The animals were categorized as non-survivors and euthanized earlier if at any time during the protocol their MAP fell below 30 mmHg for more than 10 minutes.
NO synthase during hemorrhagic shock
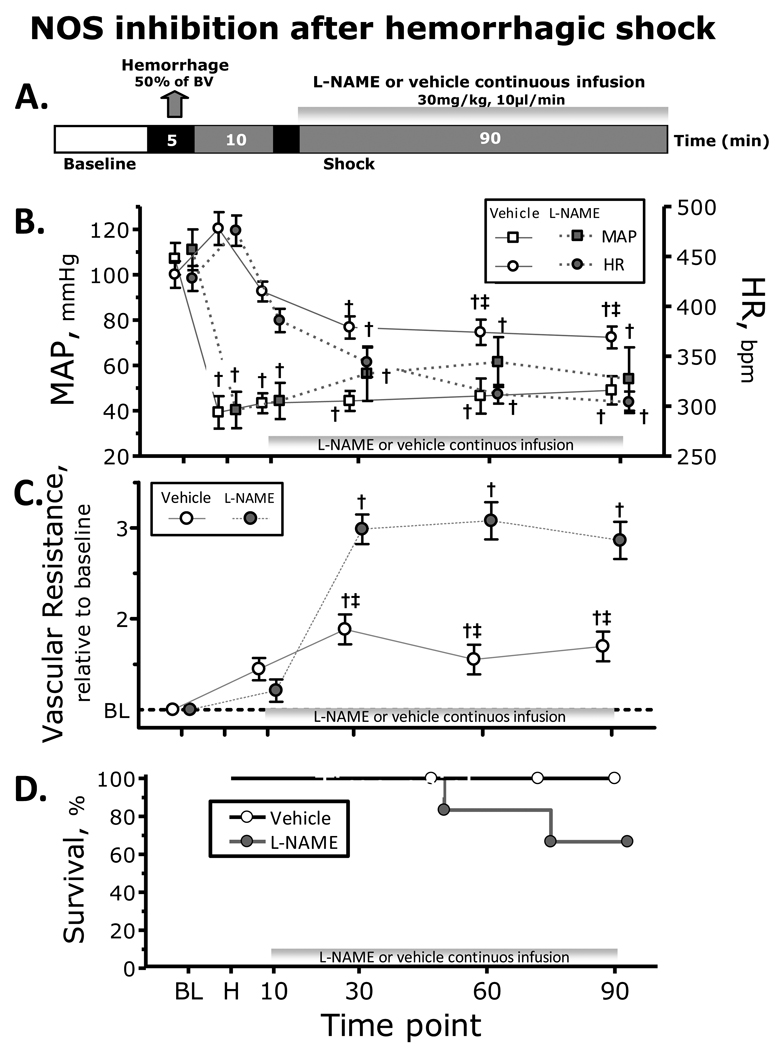
NOS inhibition was induced by continuous infusion of L-NAME (N-nitro-L-arginine methyl ester, 30mg/kg, 10µl/min; Sigma-Aldrich. St. Louis, MO). Vehicle groups received saline at identical rate. L-NAME and vehicle infusion started 10 min after the end of the hemorrhage. Figure 1A presents the experimental protocol.
Figure 1.
Nitric oxide (NO) synthase (NOS) inhibition during hemorrhagic shock. A. Shock protocol. Hemorrhagic shock was induced withdrawal of 50% of the estimated blood volume (7% body weight) and NOS inhibition with L-NAME was started 10 min after the end of the hemorrhage. B. NOS inhibition effects on mean arterial pressure (MAP) and heart rate (HR) during the hemorrhagic shock. C. NOS inhibition effects on vascular resistance during the hemorrhagic shock. D. NOS inhibition effects on survivability during the hemorrhagic shock. †, P<0.05 compared to baseline; ‡, P<0.05 compared to Vehicle
Experimental groups for NO supplementation
Nanoparticles were infused 10 min after the hemorrhage, suspended in 50µl deoxygenated saline, and infused via the jugular vein (100 µl/min). Post-hemorrhage, animals were randomly divided 15 into three groups: 1) NO-nps, 5 mg/kg of NO-nps; 2) Control-nps, 5 mg/kg of NO free nanoparticles; 3) Vehicle, 50µl deoxygenated saline. Eighteen (n = 18) animals were entered into the study, and assigned to the following experimental groups: NO-nps (n = 6); Control-nps (n = 6); and Vehicle (n = 6).
Systemic Parameters
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically from a single drop of blood (Hemocue, Stockholm, Sweden).
Blood Chemistry and Biophysical Properties
Arterial blood was collected in heparinized glass capillaries (0.05 ml) and immediately analyzed for PaO2, PaCO2 and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA). The comparatively low PaO2 and high PaCO2 of these animals is a consequence of their adaptation to a fossorial environment.
Methemoglobin Measurement
Methemoglobin was established according to Winterbourn 16. Calibration is ensured using standard levels at 5.2%, 2.6% and 1.2% MetHb (RNA Medical, Bayer Diagnostics Medfield, MA).
Plasma nitrite/nitrate
Blood samples were collected from carotid artery, and centrifuged to separate RBCs and plasma. Plasma proteins were removed by adding equal volume of methanol, and centrifuged at 15000 rpm for 10 min. Concentrations of NOx in the supernatant were measured with a NOx analyzer (ENO-20; Eicom, Kyoto, Japan). This analyzer combines Griess method and high-performance liquid chromatography.
Microvascular Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The animals were given 20 min to adjust to the change in the tube environment before measurements. The tissue image was projected onto a charge-coupled device camera (COHU 4815) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. The same sites of study were followed throughout the experiment so that comparisons could be made directly to baseline levels.
Microhemodynamics
Arteriolar and venular blood flow velocities were measured on-line by using the photodiode cross-correlation method (Velocity Tracker Model 102B, Vista Electronics, San Diego, CA) 17. Measured centerline velocity was corrected according to vessel size to obtain mean RBC velocity (V) 18. Video image-shearing method was used to measure vessel diameter (D) 19. Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. This calculation assumes a parabolic velocity profile and has been found to be applicable to tubes of 15 – 80 µm internal diameters and for Hcts in the range of 6 – 60% 18. Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Peripheral vascular resistance (PVR) was calculated in terms of pressure (MAP) divide by local blood flow (Q), by mathematically using the Hagen-Poiseuille equation. PVR relative to baseline was calculated by ratio of MAP and Q normalized to baseline. Peripheral vascular hindrance was calculated by normalizing PVR by blood viscosity normalized to baseline, to eliminate viscous resistance component from the Poiseuille equation. Wall shear stress (WSS) was defined by WSS = WSR × η, where WSR is the wall shear rate given by 8V × D−1, and η is blood viscosity.
Functional Capillary Density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least one RBC in a 45s period in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. FCD (cm−1), i.e., total length of RBC perfused capillaries divided by the area of the microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. Changes in FCD from baseline were used as indictors of capillary perfusion.
Data analysis
Results are presented as mean standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Data between groups was analyzed using two-way ANOVA nonparametric repeated measurements, and, when appropriate, post hoc analyses were performed using Bonferroni tests. The Grubbs' method was used to assess closeness for all measured parameters values at baseline. Microhemodynamic measurements were compared to baseline levels obtained before the experimental procedure, for more robust statistics for small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P < 0.05.
RESULTS
NOS inhibition during hemorrhagic shock
Twelve hamsters were used to establish effects of NO inhibition (L-NAME: n=6; 68.4 ± 8.2 g; and Vehicle: n=6; 66.2 ± 7.1 g). Hemorrhage significantly decreased Hct and Hb in both groups. Changes in MAP and HR post hemorrhage are shown in Figure 1B. MAP decreased after hemorrhage, and NOS inhibition produced an initial recovery in pressure, however some animals were not able to maintain the pressure. HR initially increased after hemorrhage, and NOS inhibition reduced HR compared to baseline and Vehicle. Diameters were constricted after hemorrhage, and NOS inhibition increased vasoconstriction compared to baseline and Vehicle. Microvascular flows and FCD were significantly decreased after hemorrhage compared to baseline in both groups. Peripheral vascular resistance is presented in Figure 1C. Two animals treated with L-NAME did not survive the experimental protocol (47 and 72 minutes), Figure 1D.
NO supplementation during hemorrhagic shock
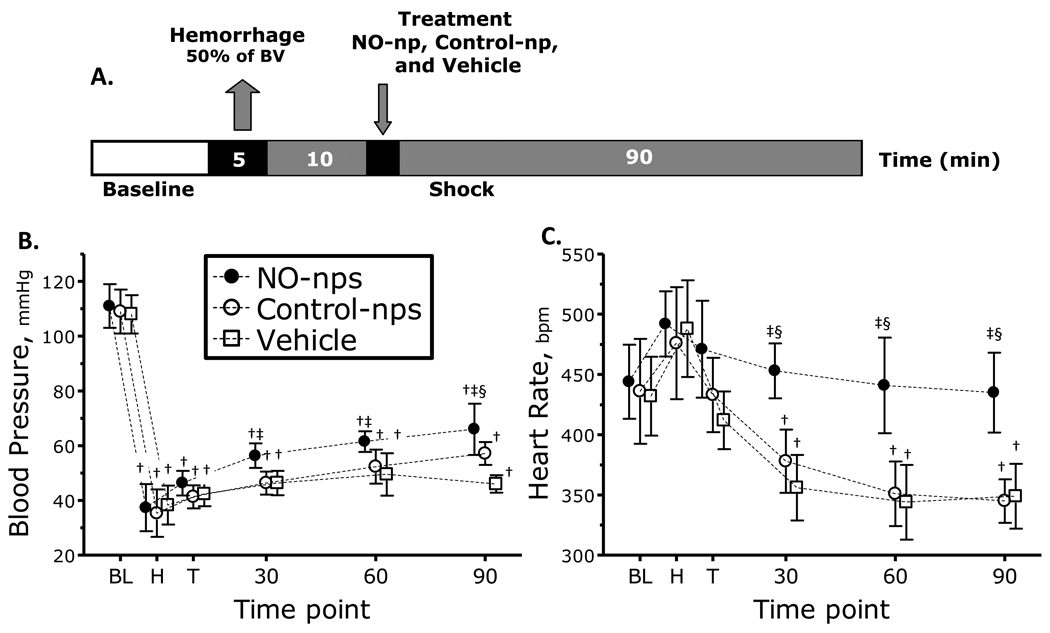
A second group of animals was used to study the role of NO supplementation during hemorrhagic shock, divided in three groups: NO-nps: (n = 6; 66.2 ± 5.1 g); Control-nps: (n = 6; 63.8 ± 4.4 g); and Vehicle: (n = 6; 66.1 ± 4.8 g). Figure 2A presents the experimental protocol. All animals tolerated the experimental protocol. All animals passed Grubbs' test ensuring that parameter at baseline were within a similar population.
Figure 2.
A. Diagram hemorrhagic shock protocol. Hemorrhagic shock was induced withdrawal of 50% of the estimated blood volume (7% body weight). Treatments were administered 10 min after the end of the hemorrhage. B. and C. nitric oxide (NO) supplementation effects on mean arterial pressure (MAP) and heart rate (HR) during hemorrhagic shock. †, P<0.05 compared to baseline; ‡, P<0.05 compared to Vehicle; §, P<0.05 compared to Control-nps. Time points: Bl, baseline; H, after hemorrhage, T, after treatment, and 30, 60 and 90 min after treatment. MAP (mmHg, mean ± SD) for each animal group were as follows: Baseline: Vehicle, 108 ± 7, n = 6; Control-nps, 109 ± 8 n = 6; NO-nps, 111 ± 8, n = 6. n = number of animals. HR (bpm, mean ± SD) at baseline for each animal group was as follows: Vehicle, 432 ± 32; Control-nps, 436 ± 43; NO-nps, 444 ± 31.
Blood gas chemistry
Blood chemistry results are presented in Table 1. Hemorrhage decreased Hct and Hb in all groups. MetHb increased in animals treated with NO-nps compared to Control-nps and Vehicle. Plasma nitrite and nitrate decreased from baseline in animals that received the Vehicle or Control-nps, while animals treated with NO-nps presented and increased compared to baseline and to animals treated with Vehicle and Control-nps. Arterial blood pO2 after hemorrhage increased in all groups compared to baseline. However, animals treated with NO-nps had lower arterial pO2 compared to Vehicle and Control-nps. Arterial blood pCO2 after hemorrhage decreased in all groups compared to baseline, although, animals treated with NO-nps had higher arterial pCO2 compared to Vehicle and Control-nps. Arterial blood pH after hemorrhage decreased in all groups compared to baseline, and animals treated with NO-nps demonstrated a higher arterial pH as compared to Vehicle and Control-np. Blood and plasma viscosity were decreased compared to baseline in all groups. Plasma colloidal osmotic pressure (COP) decreased from baseline in all groups.
Table 1.
Systemic parameters
| Hemorrhagic Shock |
||||||||
|---|---|---|---|---|---|---|---|---|
| 60 min |
90 min |
|||||||
| Baseline | Vehicle | Control-nps | NO-nps | Vehicle | Control-nps | NO-nps | ||
| n | 18 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Hct, % | 49 ± 1 | 29 ± 1† | 30 ± 2† | 29 ± 2† | 27 ± 1† | 28 ± 1† | 27 ± 1† | |
| Total Hb, g/dl | 14.7 ± 0.5 | 9.2 ± 0.6† | 9.4 ± 0.7† | 9.3 ± 0.6† | 8.7 ± 0.4† | 9.0 ± 0.6† | 8.9 ± 0.5† | |
| MetHb, % | - - | - - | - - | 4 ± 2 | - - | - - | 6 ± 2 | |
| Plasma | ||||||||
| NO2−, ηM | 477 ± 64 | 346 ± 62† | 402 ± 63† | 721 ± 126†‡¶ | 369 ± 64† | 345 ± 54† | 818 ± 153†‡¶ | |
| NO3−, µM | 1.5 ± 0.3 | 1.0 ± 0.2† | 0.9 ± 0.3† | 2.1 ± 0.8†‡¶ | 0.8 ± 0.2† | 0.9 ± 0.2† | 2.4 ± 0.7†‡¶ | |
| paO2, mmHg | 58.2 ± 6.3 | 100.6 ± 7.0† | 98.7 ± 6.8† | 71.7 ± 6.5†‡¶ | 97.4 ± 6.8† | 97.0 ± 7.2† | 69.2 ± 6.1†‡¶ | |
| paCO2, mmHg | 53.2 ± 4.7 | 35.4 ± 6.3† | 37.6 ± 6.1† | 44.5 ± 6.8†‡¶ | 36.1 ± 6.0† | 35.6 ± 5.8† | 45.5 ± 6.0†‡¶ | |
| pHa | 7.337 ± 0.022 | 7.230 ± 0.029† | 7.231 ± 0.033† | 7.331 ± 0.026†‡¶ | 7.328 ± 0.024† | 7.327 ± 0.032† | 7.330 ± 0.025†‡¶ | |
| BEa, mmol | 2.9 ± 1.3 | −3.2 ± 1.8† | −2.9 ± 1.5† | 0.2 ± 1.0†‡¶ | −3.4 ± 1.6† | −3.5 ± 1.8† | 0.3 ± 1.4†‡¶ | |
| Viscosity | ||||||||
| Blood*, cP | 4.2 ± 0.2 | 3.1 ± 0.4† | 3.2 ± 0.5† | 3.0 ± 0.3† | ||||
| Plasma*, cP | 1.2 ± 0.1 | 0.9 ± 0.2† | 1.0 ± 0.2† | 1.0 ± 0.2† | ||||
| COP, mmHg | 17.8 ± 2.3 | 13.8 ± 2.4† | 13.6 ± 1.8† | 13.9 ± 1.7† | ||||
Values are means SD. Baseline included all animals. No significant differences were detected between baseline. Hct, hematocrit; Hb, hemoglobin; NO2−, nitrite; NO3−, nitrate; PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; BEa, arterial base excess; COP, colloidal osmotic pressure.
P<0.05 compared to Baseline;
P<0.05 compared to Vehicle;
P<0.05 compared to Control-np.
Changes in MAP and HR post hemorrhage for all groups are shown in Figure 2 B, C. MAP in all groups decreased compared to baseline. Animals treated with NO-nps recovered MAP compared to vehicle within 30 min from the treatment, and after 90 min compared to Control-np. HR in all groups increased after hemorrhage, and after treatment HR dropped in animals treated with Control-nps and Vehicle. However, animals treated with NO-nps maintained HR over the observation time, and higher compared to animals treated with Control-nps and Vehicle.
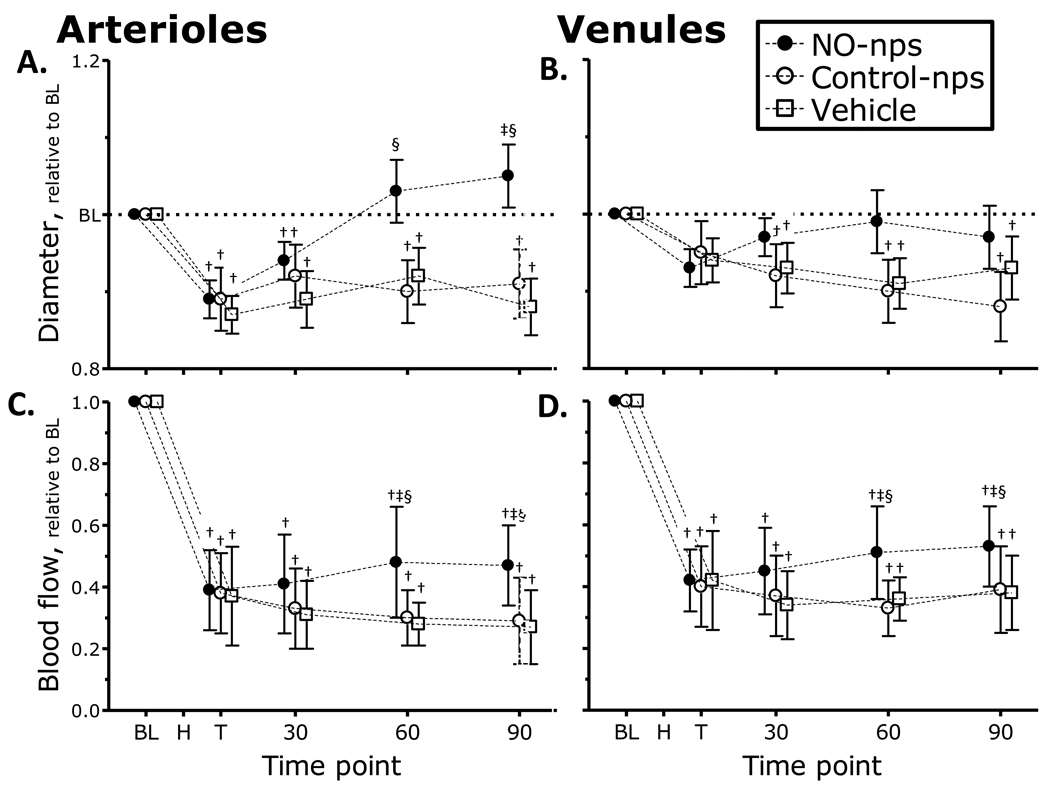
Microvascular diameters and both arteriolar and venular blood flow are presented in Figure 3. Arteriolar and venular diameters (Figure 3 A, B) were significantly constricted from baseline after hemorrhage for all groups. Microvascular flows were significantly decreased after hemorrhage compared to baseline for all groups. Arteriolar diameters remained constricted compared to baseline for Control-nps and Vehicle treated groups. Conversely, animals treated with NO-nps restored arteriolar diameters, and significant dilation was witnessed, compared to Control-nps and Vehicle treated groups. Arteriolar microvascular blood flow (Figure 3 C, D) for all groups remained lower than baseline, although animals treated with NO-nps demonstrated higher arteriolar flow as compared to Control-nps and Vehicle treated animals. Venular diameters were constricted after hemorrhage for all groups compared to baseline, and remained constricted for Control-nps and Vehicle treated groups. Venular diameters in animals treated with NO-nps were not different from baseline. Venular blood flow decreased after hemorrhage compared to baseline, and remained lower than baseline for all groups. NO-nps had significantly higher venular blood flow as compared to Control-nps and Vehicle.
Figure 3.
Nitric oxide (NO) supplementation effects on changes in arteriolar and venular diameter (A. and B.) and blood flow (C. and D.) during hemorrhagic shock. Broken line represents baseline level. Time points: Bl, baseline; H, after hemorrhage, T, after treatment, and 30, 60 and 90 min after treatment. †, P<0.05 compared to baseline; ‡, P<0.05 compared to Vehicle; §, P<0.05 compared to Control-nps. Diameters (µm, mean ± SD) in Figures 3A (arteriolar) and 3B (venular) for each animal group were as follows: Baseline: Vehicle (arterioles (A): 62.7 ± 8.2, n = 26; venules (V): 64.5 ± 6.8, n = 24); Control-nps (A: 60.5 ± 6.8, n = 24; V: 65.7 ± 8.7, n = 27); NO-nps (A: 62.0 ± 7.4, n = 25, V: 64.5 ± 8.2, n = 26). n = number of vessels studied. RBC velocities (mm/s, mean ± SD for each animal group were as follows: Baseline: Vehicle (A: 4.3 ± 1.0, V: 2.3 ± 0.9); Control-nps (A: 4.5 ± 0.8; V: 2.4 ± 1.0); NO-nps (A: 4.3 ± 1.0; V: 2.6 ± 0.7). Calculated flows (nl/s, mean ± SD) in Figures 3C (arteriolar) and 3D (venular) for each animal group were as follows: Baseline: Vehicle (A: 11.7 ± 3.4; V: 6.9 ± 2.2); Control-nps (A: 12.1 ± 3.2; V: 7.1 ± 2.3); NO-nps (A: 12.0 ± 2.8; V: 6.8 ± 2.3).
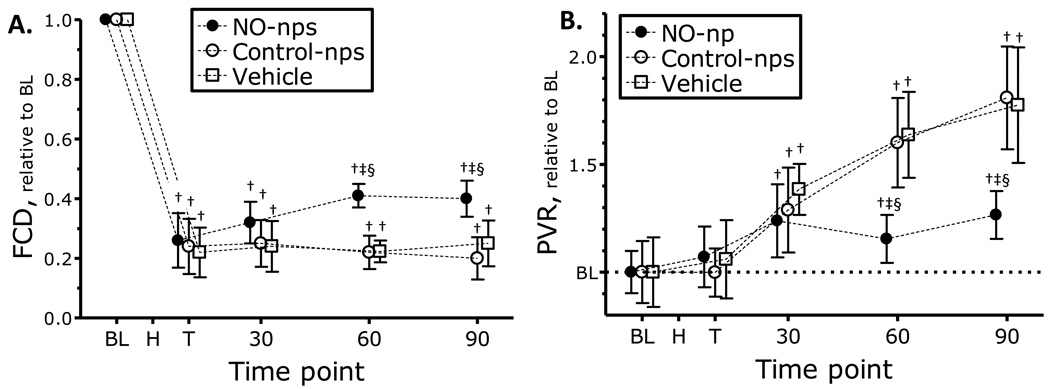
Changes in capillary perfusion during the protocol are presented in Figure 4 A. FCD was significantly reduced after hemorrhage for all groups, at all time points. FCD partially recovered in animals treated with NO-nps compared to Control-nps and Vehicle treated animals.
Figure 4.
A. Nitric oxide (NO) supplementation effects on changes in functional capillary density (FCD) and B. estimated peripheral vascular resistance (PVR) during hemorrhagic shock. Broken line represents baseline level. Estimation of PVR was made using Hagen-Poiseuille equation, with MAP and microvascular blood flow. Time points: Bl, baseline; H, after hemorrhage, T, after treatment, and 30, 60 and 90 min after treatment. †, P<0.05 compared to baseline; ‡, P<0.05 compared to Vehicle; §, P<0.05 compared to Control-nps. FCD (capillaries per cm, mean ± SD) for each animal group were as follows: Baseline: Vehicle, 111 ± 9; Control-nps, 112 ± 11; NO-nps, 109 ± 8.
Estimated PVR are presented in Figure 4 B. PVR increased over the observation time for animals treated with Control-nps and Vehicle, significantly higher when compared to the NO-nps treated group. Calculated hemodynamic parameters are presented in Table 3. Peripheral vascular hindrance, which reflects the contribution of vascular geometry, was increased compared to baseline after hemorrhage independent of the treatment. Arteriolar and venular vessel WSR and WSS decreased after hemorrhage, compared to baseline, and remained decreased, independent of the treatment.
DISCUSSION
The principal finding of the study is that treatment of hemorrhagic shock by NO supplementation via NO-nps prevented cardiovascular collapse, and allowed the animals to maintain superior systemic and microvascular hemodynamic conditions as compared to controls. The importance of providing sustained exogenous NO after hemorrhage was evidenced in this study in the form of maintenance of heart rate, microvascular function, FCD and vascular resistance. Our findings relate to scenarios where an increase in NO bioavailability could potentially alter the course and ultimately survival, until volume resuscitation is available. Our results support the hypothesis that severe hemorrhage induces vascular decompensation associated with low NO availability, and that NO supplementation prevents the risks of profound hemorrhage. As generation of NO in vivo is closely linked to endothelial cell function, local shear stress, and local oxygen conditions, the lack of differences in WSS and Hct among groups, suggests that the differences observed between groups are mostly due to the NO released from NO-np.
Hemorrhagic hypotension leads to a well-characterized sequence of events, and ultimately to vascular decompensation, due to a continuous increase in peripheral vascular resistance. Cardiovascular adaptation to hemorrhagic shock is dynamically controlled by endocrine and local paracrine factors, such as NO, in part to compensate for the sudden hypovolemia 20. NO production keeps the vasculature relaxed, regulating blood pressure and tissue perfusion. The effects of perfusion on gas exchange are one of the most important features of NO supplementation in pulmonary physiology. As changes in the distribution of blood flow to different areas of the lung, and adjustments of vessel diameter in the respective regions of the lung, could rapidly affect gas exchange. NO is a key molecule for fast response, which links alveolar ventilation to local lung perfusion. Hamster's environmental adaptations are responsible for their low arterial PO2, their natural environment are in burrows, either permanently or intermittently, and often demonstrate structural and functional differences from surface dwelling mammals. The marked improvement in gas exchange, decrease in oxygen content and increase in PCO2 seen in the NO-group can be the result of improved ventilation-perfusion, as NO has the singular property of preferentially dilating vascular segments located in ventilated areas 21. Additionally, NO modulates synaptic signaling, cellular defense and mitochondria oxygen utilization 22. Our results indicate that restoring intravascular NO concentrations by replenishing/increasing intravascular NO availability by treatment with NO-nps reduces vascular complications resulting from hemorrhage shock. The exogenous NO released induced by treatment with 5 mg/kg of NO-nps was evidenced by significant vasodilation, metHb formation, and increase in plasma nitrite and nitrate.
In our study, NOS inhibition caused an increase of vascular resistance, decreased heat rate, and increased mortality. Similarly, a clinical study using L-NAME was discontinued because the treatment showed higher mortality 23. Other NOS inhibitors were clinically evaluated to prevent hypotension; with poor results, as they increased ischemia and cardiovascular collapse 24, 25. Several investigations suggest that inducible NOS (iNOS) is responsible in part for producing organ injury after hemorrhage and resuscitation 26–28. Until selective iNOS inhibitors are available, these findings should be analyzed with discretion. Moreover, hypoxia and ischemia during shock initiate tissue adaptive responses through transcriptional activation of several factors, such as HIF-1, which requires NO as a regulatory molecule. Traditionally, NO has been thought to be a signal, exclusively via its stimulation of guanylyl cyclase, inducing an increase in intracellular cGMP levels and, in turn, the allosteric activation of cGMP dependent kinase. Moreover, in addition to the activation of PKG, the cGMP-dependent effects can also be mediated by other proteins whose activities are allosterically modified by cGMP, and via cross-activation of cAMP-dependent kinase. In previous work, we have demonstrated that NO-np vascular effect effects are mediated by guanylyl cyclase, and can be enhanced by phosphodiesterase inhibitors and blocked by irreversible heme-site inhibitors of soluble guanylyl cyclase 13. Thus, acute inhibition of phosphodiesterase activity, by drugs such as sildenafil, combined with NO-np represents an attractive therapeutic strategy to manipulate systemic vascular tone.
Exogenous NO in the form of NO-nps treatment following severe hemorrhag, partially recovered systemic and microvascular conditions, via reduction of precapillary resistance when compared to animals treated with Control-nps or the Vehicle. Results show a strong correlation between cardiac stability and microvascular flow. Mechanistically, it is expected that intravascular NO supplementation in the form of NO-np reduces MAP by decreasing PVR. Conversely, the experimental results indicate that NO-np increased sustained higher MAP and HR during the observation period. Central cardiac effects could reflect increases in cardiac output mediated by lower arteriolar after-load, cardiac filling and increased preload (venous return), due to NO vasodilatory properties and cardiac chronotropic effects 28. NO is a regulator of cardiac function through indirect vascular-dependent mechanisms and by direct action on the myocardium as a paracrine autacoid involved in autonomic control and contractility 29. This effect appears to be NO concentrations dependent, as physiological levels of NO increases HR via activation of hyperpolarization induced inward current, and is less pronounced at high concentrations of NO 30, 31. The exogenous NO released from the NO-nps maintained central hemodynamic function by preventing rhythm disturbances, implying a protective role of NO on cardiac over-drive and pacing 32. NO has effects in cardiomyocytes, which are cyclic GMP-dependent and other effects that are cyclic GMP-independent 34. Heart muscle cells express the soluble isoform of guanylyl cyclase that catalytically increases intracellular levels of cyclic GMP in response to stimulation by pure NO, or GSNO 34. Moreover, heart rate can directly affect cardiac output, if stroke volume remains constant, therefore results observed with NO-np can be the result of sustaining higher heart rates and potentially, cardiac output, which ultimately increase systemic oxygen delivery. These results show that the principal factor in ensuring hemodynamic restoration by NO-nps is not related to a volume effect since hematocrits did not differ between groups.
Clinically, vasodilators as adjunct therapy to fluid resuscitation were shown to be effective in restoring the microvascular function 35. Nitroglycerin with adequate volume support, has been shown to reinstate capillary flow and restore sublingual microcirculation 36. This work explores the connection between cardiovascular collapse post hemorrhagic shock, and reduced NO bioavailability, by increasing the NO bound to low molecular weight thiols (1 mg/kg nitrosoglutathione, GSNO) prior to the hemorrhage 36. Since GSNO only is stable in vivo at basal conditions for a limited period of time, its application is limited. Therefore, our current study goes beyond previous results with GSNO administrated prior hemorrhage, to a therapy applicable 10 min post the hemorrhage based on NO-np. The most important characteristic to supplement NO post hemorrhage is the intravascular controlled release of moderate levels of NO to mimic physiologically generated NO, a characteristic currently achievable with NO-np. NO donors, such as nitroglycerin, an organic nitrate, and clinically approved, is short acting, involves specific enzymes and reducing agents, and it is not suitable for a controlled and sustained release. Other NO donors, where the NO is bound to a nucleophile, and the NO is liberated after spontaneous decomposition, were not suitable as therapies post hemorrhage. NO-nanoparticles release pure NO, freely soluble in physiological solution, and do not require cofactors to facilitate NO release. The current results indicate that treatment with 5 mg/kg NO-nps, prevented hemorrhagic shock, induced vascular decompensation, and set the proof of the concept that NO supplementation after hemorrhage will reduce the risks of cardiovascular collapse. To date, there are no definitive treatments for addressing massive blood loss on the battlefield, and existing therapies for severe hemorrhage are particularly limited. NO-nps treatment maintains perfusion reducing hemorrhagic shock sequelae, with an infusion volume of less than 2.5% of the shed blood volume. NO-np is the logical progression of the concepts initially defined with GSNO, as GSNO is compromised by thermal decomposition, photolysis and in vivo catalytic decomposition. The reduction in weight therapy provided by NO-np relative to fluid resuscitation can greatly reduce logistical burdens, and the stability of NO-nps for months exposed to air at room temperature, facilitates its application in multiple scenarios.
In conclusion, this study shows that severe hemorrhage induces vascular decompensation, in part, due to low availability of NO during post hemorrhage, and that exogenous NO during this stage can prevent circulatory arrest. Exogenous NO in the from of NO-nps attenuated microvascular complications during hypovolemia, which targets a pivotal protective function by maintaining tissue perfusion, assuring wash-out of metabolic residues and thereby preventing future damage during reperfusion. Mechanistically, partial preservation of microvascular perfusion during hypovolemic shock due to increased NO bioavailability, acts by regulating vascular tone and pressure redistribution, and maintaining capillary pressure and metabolite exchange. Lastly, NO has a fundamental signaling role in cellular function with implications in cardiac chronotropic function, which appears to be jeopardized as hypovolemic shock is established.
Table 2.
Estimated vascular resistance and wall shear rate and stress
| Hemorrhagic Shock 90 min |
||||
|---|---|---|---|---|
| Baseline | Vehicle | Control-nps | NO-nps | |
|
Vascular resistance, relative to baseline |
1.0 | 1.8 ± 0.3† | 1.7 ± 0.4† | 1.3 ± 0.3† |
|
Vascular hindrance relative to baseline |
1.0 | 2.5 ± 0.5† | 2.5 ± 0.6† | 1.7 ± 0.5† |
| Arteriolar | ||||
|
WSR, s-1 WSS, dyn. cm-2 |
706 ± 92 30 ± 4 |
258 ± 57† 8 ± 2† |
254 ± 52† 8 ± 2† |
292 ± 64† 9 ± 2† |
| Venular | ||||
|
WSR, s-1 WSS, dyn. cm-2 |
456 ± 84 20 ± 4 |
196 ± 48† 7 ± 2† |
244 ± 51† 8 ± 2† |
268 ± 46† 8 ± 2† |
Hematocrits are presented in Table 1. WSR, wall shear rate; WSS, wall shear stress.
P<0.05 compared to Baseline;
P<0.05 compared to Vehicle;
P<0.05 compared to Control-np.
ACKNOWLEDGMENTS
This work was supported by Bioengineering Research Partnership grant R24-HL64395, grant R01-HL62354, the FJC, A Foundation of Philanthropic Funds. AJF was supported by research grants from the American Society for Dermatologic Surgery Cutting Edge Program, the La-Roche Posay North American Foundation, and the Women’s Dermatologic Society. The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No conflicts of interest to declare.
REFERENCES
- 1.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]
- 2.Pope AM Institute of Medicine (U.S.) Fluid resuscitation : state of the science for treating combat casualties and civilian injuries. Washington, D.C.: National Academy Press; 1999. [PubMed] [Google Scholar]
- 3.Bacter CR, Canizaro PC, Carrico CJ, Shires GT. Fluid resuscitation of hemorrhagic shock. Postgrad Med. 1970;48:95–99. doi: 10.1080/00325481.1970.11693553. [DOI] [PubMed] [Google Scholar]
- 4.Burris D, Rhee P, Kaufmann C, Pikoulis E, Austin B, Eror A, et al. Controlled resuscitation for uncontrolled hemorrhagic shock. J Trauma. 1999;46:216–223. doi: 10.1097/00005373-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dubick MA, Atkins JL. Small-volume fluid resuscitation for the far-forward combat environment: current concepts. J Trauma. 2003;54:S43–S45. doi: 10.1097/01.TA.0000064514.42470.3B. [DOI] [PubMed] [Google Scholar]
- 6.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand. 1996;40:496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 8.Salazar Vazquez BY, Martini J, Chavez Negrete A, Cabrales P, Tsai AG, Intaglietta M. Microvascular benefits of increasing plasma viscosity and maintaining blood viscosity: counterintuitive experimental findings. Biorheology. 2009;46:167–179. doi: 10.3233/BIR-2009-0539. [DOI] [PubMed] [Google Scholar]
- 9.Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, et al. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 10.Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol. 2009;106:662–667. doi: 10.1152/japplphysiol.91109.2008. [DOI] [PubMed] [Google Scholar]
- 11.Lancaster JR. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Bland JM. Statistics notes: How to randomise. BMJ. 1999;319:703–704. doi: 10.1136/bmj.319.7211.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winterbourn CC. Reaction of superoxide with hemoglobin. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, Florida: CRC Press; 1985. [Google Scholar]
- 17.Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975;10:165–179. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- 18.Lipowsky HH, Zweifach BW. Application of the "two-slit" photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5:309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 20.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305–H318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 21.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 23.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 24.Md S, Moochhala SM, Siew-Yang KL. The role of inducible nitric oxide synthase inhibitor on the arteriolar hyporesponsiveness in hemorrhagic-shocked rats. Life Sci. 2003;73:1825–1834. doi: 10.1016/s0024-3205(03)00510-1. [DOI] [PubMed] [Google Scholar]
- 25.Thiemermann C, Szabo C, Mitchell JA, Vane JR. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banta S, Yokoyama T, Berthiaume F, Yarmush ML. Effects of dehydroepiandrosterone administration on rat hepatic metabolism following thermal injury. J Surg Res. 2005;127:93–105. doi: 10.1016/j.jss.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu T, Tani T, Endo Y, Hanasawa K, Tsuchiya M, Kodama M. Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit Care Med. 2002;30:77–82. doi: 10.1097/00003246-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–18. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Fatehi-Hassanabad Z, Fatehi M. Characterisation of some pharmacological effects of the venom from Vipera lebetina. Toxicon. 2004;43:385–391. doi: 10.1016/j.toxicon.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Kobzik L, Zhao YY, Opel DJ, Liu WD, Kelly RA, et al. Nitric oxide regulation of atrioventricular node excitability. Can J Cardiol. 1997;13:1191–1201. [PubMed] [Google Scholar]
- 31.Herring N, Rigg L, Terrar DA, Paterson DJ. NO-cGMP pathway increases the hyperpolarisation-activated current, I(f), and heart rate during adrenergic stimulation. Cardiovasc Res. 2001;52:446–453. doi: 10.1016/s0008-6363(01)00425-4. [DOI] [PubMed] [Google Scholar]
- 32.Massion PB, Balligand JL. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice. J Physiol. 2003;546:63–75. doi: 10.1113/jphysiol.2002.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khadour FH, O'Brien DW, Fu Y, Armstrong PW, Schulz R. Endothelial nitric oxide synthase increases in left atria of dogs with pacing-induced heart failure. Am J Physiol. 1998;275:H1971–H1978. doi: 10.1152/ajpheart.1998.275.6.H1971. [DOI] [PubMed] [Google Scholar]
- 34.Mayer B, Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Brunner F. A new pathway of nitric oxide/cyclic GMP signaling involving S-nitrosoglutathione. J Biol Chem. 1998;273:3264–3270. doi: 10.1074/jbc.273.6.3264. [DOI] [PubMed] [Google Scholar]
- 35.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 37.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–712. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]