Abstract
In addition to its critical role in normal cell function, growth, and metabolism, zinc is implicated as a major factor in the development and progression of many pathological conditions and diseases. Despite this importance of zinc, many important factors, processes, and mechanisms of the physiology, biochemistry, and molecular biology of zinc remain unknown. Especially important is the unresolved issue regarding the mechanism and process of the trafficking, transport, and reactivity of zinc in cells; especially in mammalian cells. This presentation focuses on the concept that, due to the existence of a negligible pool of free Zn2+ ions in the mammalian cell environment, the trafficking, transport and reactivity of zinc occurs via a direct exchange of zinc from donor Zn-Ligands to acceptor ligands. This Zn exchange process occurs without the requirement for production of free Zn2+ ions. The direct evidence from mammalian cell studies is presented in support of the operation of the direct Zn-Ligand exchange mechanism. The paper also provides important information and conditions that should be considered and employed in the conduct of studies regarding the role and effects of zinc in biological/biomedical research; and in its clinical interpretation and application.
Keywords: zinc transport, zinc trafficking, zinc ligand, ZIP transporters, mitochondria, metallothionein
1. Introduction
Zinc is an essential element for the growth, metabolism and function of all cells. It is a cofactor for over three hundred different enzymes; and it has been demonstrated to play an important role in transcription factors for gene regulation and as an intracellular signaling agent. In addition to its critical role in normal cell function, growth, and metabolism, in recent years zinc has been implicated as a major factor in the development and progression of many pathological conditions and diseases; such as various cancers, Alzheimer disease and other neurological conditions, diabetes, aging process, and numerous other pathological conditions; and the list continues to grow.
Despite the importance of zinc in cellular function and in health and disease, many important factors, processes, and mechanisms of the physiology, biochemistry, and molecular biology of zinc remain unknown. Among the most important is the mechanism and process, of the trafficking, transport, and reactivity of zinc in cells; especially in mammalian cells. This most fundamental relationship is the subject of this paper. It is not our intent to provide an extensive literature review since there are many excellent reviews of the physiology, biochemistry, and molecular biology of zinc and zinc transport [e.g. 1-9]. We will focus on the information directly relevant to the issues of trafficking, transport, and reactivity of zinc in mammalian cells.
Evidence will be presented that, due to the existence of a negligible pool of free Zn2+ ions in the mammalian cellular environment, the trafficking, transport and reactivity of zinc occurs via a direct exchange of zinc from donor ZnLigands to acceptor zinc proteins in the absence of a requirement for free Zn2+ ions, as we proposed in previous reports [10-13] . We hasten to add that others have postulated and conceptualized the same or similar process, as we will discuss below. However, direct evidence for its operation in mammalian cells has been lacking. This report provides the evidence that supports the operational existence of this mechanism for trafficking, transport, and reactivity of zinc in mammalian cells.
2. The forms of zinc in mammalian biological systems (terminology issues)
The first essential point for this presentation and our concept is the recognition as stated in reviews by Vallee [7] that “Biologically, zinc is found to be complexed to organic ligands rather than free in solution as the metallic ion”; and by Vallee and Falchuk [8] that “In biological systems, very little, if any, zinc is free in solution.” This is a major consideration for the concept and the evidence that we will present. To avoid confusion regarding this presentation, clarification and definition of some terminology is required. We will identify extracellular and intracellular total zinc as being comprised of three pools of zinc: 1) “immobile zinc” in which zinc is tightly bound predominantly to proteins, and represents a non-exchangeable, unreactive pool of zinc; 2) “mobile reactive zinc” in which zinc is loosely to moderately bound to ligands (as discussed below, formation constants of log Kf~10 and lower) and is an exchangeable reactive pool of zinc; and 3) “free Zn2+ ion” pool that is a reactive pool of zinc. The free Zn2+ ion pool is negligible in mammalian cells and in extracellular fluid (described below). We do not employ the definition of “free zinc” or “free zinc ion” as components or forms of the exchangeable Zn-Ligand pool. This description is provided in order to avoid confusion with the definition used by Krezel and Maret [14] and others: “Free” zinc has been referred to as “freely available,” “labile,” or “rapidly exchangeable” zinc that is readily bound to chelating agents. Each term is an operational definition and has its limitations. For the lack of a better term, “free” zinc is used in this work, albeit with the understanding that the chemical nature of the ligands of ionic zinc is not known. “Rapidly exchangeable” implies certain kinetic mechanisms. Thus, there are pools of thermodynamically tightly bound zinc with considerable “kinetic lability” in exchange reactions. A prime example is metallothionein”. We do not imply any disagreement with any validity of this definition.
Our presentation is focused on the “physiological/ kinetic” relevancy of the pools of zinc in the context of delivery of zinc for cellular activities such as transporter function and inhibition of enzyme activity. For discussion purposes, we characterize these as “transient” effects of zinc; as distinguished from the incorporation of zinc into the structural component of proteins such as apoprotein conversion to its structural holoprotein; i.e. a more “intransient” utilization of zinc. There is a danger for a misinterpretation or undue rigidity that must be avoided. These are relative terms in the context of our discussion. To exemplify this distinction up front, the delivery of zinc in the form of a Zn-Ligand to a plasma membrane uptake transporter is transient because the zinc traverses the transporter protein and is not a “permanent” structural component; and the Ligand is recycled. This, in our view, is an important distinction for a discussion of the physiological activities of pools of extracellular and intracellular zinc.
3. The concentration and forms of zinc delivered to mammalian cells in situ
To maintain their cellular concentration of zinc, most mammalian cells obtain their zinc from interstitial fluid (ISF), which is derived as a filtrate of blood plasma. The many reports of the total plasma/serum zinc levels in humans generally range from ~12-17 μM. For example, a large population study [15] reported a range of ~12-14 μM. The distribution of the total zinc in plasma is well represented by the report of Faure et al [16]: total zinc=15.5 μM; protein bound zinc= 98%; ultrafiltrable zinc=2%. The protein bound zinc is comprised mainly of Zn-albumin ~77% and globulin ~20%. Faure et al also characterize albumin+ultrafiltrable pool=80% as the loosely-bound zinc. When converted to concentrations of zinc: Zn-Globulin~3 μM; Zn-Albumin~12 μM; ultrafitrable zinc~0.3 μM; loosely bound zinc~12.4 μM. The ultrafiltrable pool consists of low molecular weight (LMW) organic compounds (such as amino acids, citrate); and we will hereafter refer to this as the ‘LMW pool’. Most importantly, the free Zn2+ ion concentration in plasma is negligible; estimated to ~0.1-1.0 nM [6,17].
These plasma values must be translated to the zinc composition of the ISF. The concentration of the LMW Zn-Ligands will approximate the plasma concentration; i.e. 0.3 μM. The plasma protein concentration is decreased from ~7% plasma proteins to ~2% in ISF; the latter being albumin. The ISF albumin is approximately 40% of its plasma concentration, which translates to ~5 μM Zn-Albumin. Therefore a reasonable estimate of ISF zinc is Zn-Albumin~5 μM; LMWZn-Ligands~0.3 μM; free Zn2+ ions~0.5 nM. This is the pool of zinc that is delivered to the cells as their potential source of zinc donors for the transport of zinc into the cells. As will be described below, this pool of LMW ligands ((log Kf~ 10 and lower) and albumin (log Kf~6-7) exhibit formation constants that are consistent with their potential as Zn donors for plasma membrane uptake transporters. In the case of Zn-Albumin, studies have shown that it can donate Zn for transport [18-21]. Also the ratio of the concentration of the LMW Ligand pool to albumin in ISF is increased by over 100% compared to plasma. This makes it likely that exchange of some zinc from albumin this LMW Zn-Ligand pool will occur for transport into cells. Therefore, a reasonable estimate of Zn-donors in ISF is ~2-4 μM.
4. The cellular pools of zinc
Values for the total cellular zinc content of most mammalian cells range from 0.1-1.0 mM. For example Krezel and Maret [14] report a total cellular zinc of 0.264mM in colon cancer HT-29 cells. There is also variation among the different cell types. For example, human prostate secretory epithelial cells contain about five times more zinc than most other mammalian cells such as liver (which we will discussing). There is also variation in the distribution and compartmentalization of zinc and zinc forms among different cell types. Notwithstanding these considerations, we, as others have, will attempt to extract some reasonable “common” relationships that might lead to the understanding of the physiological relationships that exist in the cells.
The free Zn2+ ion pool
The first and most important issue in our presentation is the cytoplasmic concentration of free Zn2+ ions. To highlight this issue we return to Vallee and Falchuk [8] that “In biological systems, very little, if any, zinc is free in solution.” In their excellent extensive reviews, Maret and colleagues [9, 22] provide a summary of several reports of estimates of the cellular free Zn2+ ion concentration is presented, in which values ranged from ~5 pM-1 nM. While there are issues relative to the precise forms or pools of zinc that are detected and represented in the various methods, there exists large agreement, if not consensus, that the component that we define as “free Zn2+ ion” is in the pM range, which for this presentation is the important consideration.
The cytosolic mobile reactive pool of Zn
The next important issue is the concentration of the mobile reactive pool of zinc that might exist in the cytosol. This requires some reasonable assumptions and expectations. Although the distribution varies for different cells, a reasonable estimate is that total cell Zn resides in the nucleus ~35%, and in the cytoplasm 65% [8,23]. Of the cytoplasmic Zn, an estimated ~50% resides between the cytoplasmic organelles and the cytosol. Most of the cytosolic zinc exists bound to immobile macromolecules and we will assume a value of~95%, and the balance exists as mobile Zn-ligands. These assumptions lead to an estimate of ~4 μM for the cytosolic pool of mobile reactive zinc. We could also consider the likelihood that nuclear and organellar matrix contains proportionately higher levels of tightly-bound Zn macromolecules than cytosol; which would favor an even higher estimate. This would make sense since the cytosol contains the mobile form of zinc that is distributed to the organelles where much of the zinc is tightly bound. Nevertheless, it is reasonable to expect that the cytosolic mobile reactive pool of zinc is near or in the μM range.
Perhaps a simpler and more direct calculation of the mobile reactive pool of cytosolic zinc can be derived from Maret's review [22]. We consider that Zn-MT along with the LMW Zn-Ligands comprise this cytosolic pool. Maret provides a value of 10% of total cellular zinc as being bound to MT in hepatocytes. Assuming the “representative” value of ~250 μM for the total zinc, then 25 μM Zn would be in the form of Zn-MT. If we assume that only one of the seven bound zincs is the physiological labile Zn (log Kf~8), ~3.5 μM would be available. An adjustment for some portion of total MT that might be sequestered in organelles will reduce this value somewhat. Even if we assign an unlikely high value of 50%, Zn-MT would constitute 1-2 μM Zn as cytosolic mobile reactive zinc. Adding this to the LMW Zn-Ligands provides an estimate of cytosolic pool being in the μM range. Our studies described below were focused on prostate cells which contain several-fold higher zinc concentration than most mammalian cells. Therefore, the estimated cytosolic pool of mobile reactive Zn would be ~20 μM, which is reflected in our studies presented below.
We further believe that this is a reasonable estimate because it is in the range of Km values for zinc transporters and for effects of zinc on some enzyme activities that we describe below. This is an important physiological relationship that we will emphasize. We think it to be highly unlikely that living systems evolved and exist under conditions in which the Km values for many transporters and for their effects on enzymes are 1000-fold, or more, greater than the existing concentration of their substrates in their natural environment.
5. Cellular zinc uptake transport processes
Free Zn2+ ions are impermeable by simple diffusion across the plasma membrane. Consequently, zinc uptake from ISF by cells requires some type of transport process. Any possible plasma membrane transport process must consider that the concentration of free Zn2+ ions in the ISF is in the low nM range. Despite these limitations, some reports purport to implicate free Zn2+ ions and ion channel processes or secondary active transport systems such as proton-ATPase transport mechanisms for the cellular uptake of zinc. However, the extremely low free Zn2+ ion concentration does not provide a likely pool that is sufficient for maintenance of the Zn requirements for most cells. Furthermore, the low concentration of free Zn2+ ions would be in competition with other divalent cations that exist in concentrations of over 1000-fold greater than the free Zn2+ concentration. In the relatively few reports with eukaryote cells that included kinetic Zn uptake studies, highly unphysiological excess free Zn2+ ions at μM-mM concentrations were employed. We agree with the conclusion of Magneson et al [17] that, “Because free Zn2+ is such a small fraction of the exchangeable Zn2+ in plasma, it seems doubtful that transport of Zn2+ across membranes involves free Zn2”. While not a likely major transport mechanism in most mammalian cells, we do not dismiss the possibility that free Zn2+ ions and ion channel transport might exist in highly specialized cells under unique localized conditions such as excitation in neuronal function.
Another possible process is the transport of zinc via a ligand transporter; notably co-transport of zinc via an amino acid transporter. It is likely that some zinc is delivered into some cells by this process. For example, luminal zinc absorption by intestinal mucosa cells occurs via co-transport with histidine and is affected by luminal amino acids [24]. However, for systemic and intracellular zinc uptake and maintenance, such a co-transport process is not a likely major mechanism. Circulating levels of zinc and cellular zinc uptake are not dependent upon regulation of amino acid transport.
It is now evident that the ZIP (Slc39) and ZnT (Slc30) family transporters constitute the major zinc transporters in mammalian cells. With some exception, the ZIP transporters are predominantly plasma membrane zinc uptake transporters; and the ZnT transporters are predominantly intracellular organelle zinc uptake transporters. Numerous excellent reviews of ZIP/ZnT transporters are available [2,5,25,26]. Consequently, the mechanism of zinc transport by the ZIP/ZnT transporters is a key to relating zinc trafficking and the zinc transporter process
6. The Zn exchange kinetics of plasma membrane zinc uptake transport from ISF
Despite the fact that the expression of many ZIP/ZnT members has been identified in mammalian cells, the functionality, kinetics, and transport mechanism of these transporters in specific cells are largely unknown. One of the important considerations is the relationship of the transporter Km value for zinc and the available concentration of zinc. ZIP1 and ZIP2 zinc uptake transporters exhibit Km values ~3-7 μM Zn [27,28]. Zinc uptake by several mammalian cells also reportedly exhibit Km values in the μM range [6]. It is highly unlikely that the pM-nM concentration range of free Zn2+ ion in ISF would constitute an effective substrate for a zinc transporter with a zinc Km in the μM range (i.e. >1000-fold differential). One should expect that the evolution of a physiologically relevant transporter is consistent with the conditions of its environment, including the concentration of its substrate. It is notable that Pattison and Cousins [19] studies of zinc uptake resulted in a transport Km >10 μM Zn., which caused them to conclude: “Because the amount of free Zn2+ available to hepatocytes is small, the Zn2+ uptake mechanism either a) has a high affinity for free Zn2+, true Kms and kds at least 100 times smaller than the measured values or b) the uptake is mediated via a zinc chelate”. Evolving evidence makes it apparent that the latter explanation is applicable.
Therefore, one must consider alternative pools of zinc in the ISF, which exist in μM concentrations that approximate the Km value of the physiologically relevant zinc uptake transporter. This is represented by the ISF Zn-Ligand pool that we described above. This same consideration applies to the cytosolic pools of zinc as described below. For this discussion, Table 1 provides a list of ligands that we employed in the following studies with their respective relative zinc-binding affinities and the resulting concentrations of free Zn2+ ions. The free Zn2+ concentrations were approximated using the web based Maxchelator programs (www.stanford.edu/~cpatton/maxc.html).
Table 1.
Estimates of the formation constants (log Kf) for zinc complexes
| Complex | 20μM ZnCl2 | ZnCit | ZnAsP | ZnHis | ZnCys | ZnEGTA | ZnEDTA |
| Log Kf | ~5 | ~6 | ~7 | ~10 | ~12 | ~16 | |
| Free Zn2+ ion at [Zn]/[Lig]=1/3 | 20μM | 4μM | 700nM | 110nM | 80pM | negligible | |
The selection of this group of ligands requires some explanation. Our studies described below originated from early experiments of effects of zinc on prostate cells, in which we employed μM ZnCl2 and ZnSO4 as the source of zinc. We came to the realization that these μM concentrations of free Zn2+ ions are unphysiological and do not exist in the cell's in situ environment. To reconcile this we repeated some studies with citrate and aspartate as zinc ligands. Both are present in high concentrations (~1.2mM) in prostate cells and were potentially important zinc ligands in these cells. Because of the observations relating to zinc effects with these ligands, we added histidine and cysteine to represent important amino acid ligands for other cells. Additionally, cysteine also provided a higher binding affinity, which was necessary for understanding the relationship of the ligands to the effects of zinc.
To address the issue of zinc uptake transport, studies [13,27] with prostate cells and ZIP1 provide the following critical information; and we refer the reader to those reports for details of the studies. The Zn uptake data presented in figure 1 show that free Zn2+ ions can be transported into the cells in a concentration-dependent mechanism consistent with Michaelis-Menton kinetics, which results in a Km~7 μM Zn. This is similar to the Km~3 μM Zn reported for ZIP2 in erythroleukemia cells [28]. The near-linear concentration-dependent range of free Zn2+ ions shown in figure 1A is ~1-10 μM; and this, as well as the Km~7 μM Zn, is ~1000-fold greater than the free Zn2+ ion concentration that exists in ISF. Therefore, free Zn2+ ion is not the likely physiological substrate for cellular Zn uptake transport. Instead, the results in figure 1B show that ZnCit and ZnCys are equally as effective as ZnCl2 for the uptake of Zn. Based on the formation constant log Kf values (table 1), at the equimolar concentrations of Zn, the free Zn2+ ion concentrations are estimated to be ~15 μM, 4 μM, 80 pM for ZnCl2, ZnCit, ZnCys, respectively. This is inconsistent with the free Zn2+ ion dose response curve in figure 1A, and demonstrates the Zn transport is dependent upon the total Zn concentration, and not dependent on the free Zn2+ ion concentration. This is also apparent from the effect of decreasing free Zn2+ ion concentration by increasing citrate concentrations (figure 1C). The addition of up to 300 μM citrate to 10 μM ZnCl2 has very little effect on zinc uptake; whereas the concentration of free Zn2+ ions decreased from 10 μM to ~1 μM. One would expect from Figure 1A that over this range of free Zn2+ ion, the Zn uptake rate would change by ~40-fold. Zn-EDTA (and EGTA shown in figure 6) provides no Zn for transport, which shows that Zn availability for transport is dependent upon the binding affinity of the Zn-Ligands. Figure 1D demonstrates that zinc uptake from ZnCit does not involve or require the co-transport of Zn and citrate into the cells
Figure 1.
The kinetic properties of Zn-Ligands in Zn-uptake transport by prostate PC-3 cells (modified from [11]). A. Zinc uptake as a function of ZnCl2 (i.e. free Zn2+ ion) concentration. The Km value was derived from Lineweaver-Burk application. B. Comparison of Zn uptake from free Zn2+ ion concentration vs., total Zn as Zn-Ligands. C. Effects of titration of ZnCl2 with increasing concentrations of citrate on the concentration of free Zn2+ ions and the Zn uptake transport. D. Comparison of Zn65 uptake and C14-citrate uptake from Zn-Citrate.
Figure 6.
Zinc uptake studies with liver mitochondria. A. Comparison of Zn uptake from Zn-Ligands vs. free Zn2+ions. B. Comparison of Zn accumulation by liver and prostate mitochondria. C. Comparison of Zn uptake by in-tact mitochondria and mitoplasts. D. Comparison of Zn accumulation from Zn-Metallothionein and Zn-Cysteine by liver and prostate mitochondria.
7. Interpretation: operation of a direct Zn-Ligand ----> Transporter exchange mechanism
These data allow for the following conclusions:
Although micromolar concentrations of free Zn2+ ions can be transported into the cells, the physiological millimicromolar or lower free Zn2+ concentration will not provide effective Zn transport for cellular uptake by transporters with Km values in the micromolar range.
Zinc uptake is dependent upon the total concentration of Zn that exists as Zn-Ligands with low to moderate binding affinities (log Kf~10 or lower); and is independent of the free Zn2+ ion concentration
As will be presented below, Zn uptake transport is energy-independent and occurs via a facilitative transport process rather than an active transport mechanism. This is consistent with the report of Gaither and Eide [28], which showed that ZIP2 zinc uptake by K562 erythroleukemia cells occurs by facilitated diffusion.
Such properties can best be accommodated by a mechanism that involves a direct exchange of Zn from donor Zn-Ligands to membrane Zn-transporter protein. Based on the formation constants of the donor Zn-Ligands (including studies described below), the Zn transporter protein exhibits an apparent formation constant of log Kf~10-11. Therefore we envision that the transport process for Zn-uptake transporters occurs as shown in figure 2A.
Figure 2.
Representation of the direct Zn ligand transport process as applied to: A. plasma membrane zinc uptake transporters (e.g. ZIP-family transporters); and B. intracellular organelle transporters (e.g., ZnT-family transporters). The representation shows Zn exchange directly between Zn-Ligands and Zn-transporter, in the absence of formation of free Zn2+ ions.
In support of such an exchange mechanism, Chao and Fu [29] concluded that, “The function of CDF (=ZnT) transporter may not be driven by Zn2+ thermodynamic equilibrium but, rather, operated in a kinetically controlled substitution fashion. This traffic of metal ions in the cell allows the delivery of metal ions through specific protein-protein interactions to overcome the kinetic binding barrier at an extremely low free metal-ion concentration.” In addition Cherezov et al [30] proposed a direct Zn-Chaperone→Zn-Transporter model for bacterial CzrB zinc transporter. Hathout et al [31] demonstrated that Zn can be transferred directly from metallothionein to zinc finger peptides. This is consistent with our demonstration that ZnMetallothionein is an effective Zn donor for transport into mitochondria via a direct exchange process with the transporter protein [11]. Also, Feng et al [32] demonstrated that ZnMetallothionein will transfer Zn to m-aconitase through a direct Zn exchange interaction. This is consistent with earlier reports of Costello et al [33,34] that zinc inhibits m-aconitase activity, and shows that Zn reactivity is dependent on direct Zn-Ligand exchange as we describe below. Feng et al and Krezel et al [32,35] suggested a direct Zn exchange process that eliminates the requirement for free Zn2+ ion, which is in agreement with our earlier reports and concept [10-13].
8. Mechanism of plasma membrane ZIP-family Zn uptake transport
The mechanism of Zn uptake transport by ZIP/ZnT transporters is poorly understood; as is the functional structure of the transporter proteins. Based on the relationships described above, the critical issue becomes the potential transporter structure that might be consistent with an intermolecular exchange mechanism from Zn-Ligand to ZIP transporter protein. Structurally, ZIP transporters across all species show a high degree of homology. Members of the ZIP family are generally predicted to contain 8 transmembrane domains (TM) with a large hydrophilic, histidine rich, intracellular loop between TM 3 and 4. The TMs at the large loops of Zip proteins are highly conserved and have been proposed to form the pore through which zinc passes [2,25,36] . ZnT family proteins generally contain 6 TMs with a large loop between TMs 4 and 5. ZnT members form homo and heterodimers which appear to be required for their function as zinc transporters [37-39]. The X-ray structure of Yiip the E. coli homologue of the mammalian ZnT family of transporters is a homodimer that is stabilized by zinc binding at the interface of the cytoplasmic domains [40]. The TMs of the Yiip homodimer form a void space through the membrane. This space contains extracellular and intracellular cavities that bind zinc and are likely involved in the transport of zinc across the membrane. While oligomerzation of ZnT members has been extensively studied, oligomeric interactions among Zip proteins have not been reported. However dimer structure likely exists for ZIP transporters and is likely requisite for zinc transport.
The large histidine rich loops are potential sites for zinc coordination during the transport process. The large loop between TM 3-4 in Zip1 contains two histidine residues and an aspartate residue that could participate in the formation of a zinc binding site. We showed that the histidine residues in the large intracellular loop of Zip1 (figure 3) are important for zinc binding and transport [13]. Mutation analysis showed that replacing either of the histidine residues (H158 or H160) in the loop with alanine decreased zinc accumulation by cells over expressing the mutated transporter [13] (Fig 4). These results suggest that these histidines are necessary for the normal function of the transporter. Consistent with the intermolecular exchange mechanism, the ligand bound form of zinc had no effect on zinc transport by wild type or mutated transporter, since zinc transport in the presence of ZnCl2, ZnCit and ZnCys was identical when the total zinc concentration was 15 μM. This was the case although the concentration of free Zn2+ ion was ~80 pM with Zn-Cysteine, ~3.8 μM with Zn-Citrate and ~15 μM with ZnCl2. The decrease in zinc transport by the mutated transporter also was not affected by the ligand form of zinc. Thus the conserved large cytoplasmic loop domain and the histidine residues in the loop are important in the transport process. Zinc binding sites have been described based on the three dimensional structure of known zinc binding proteins. Four types of zinc binding sites have been described [41]; structural, catalytic, cocatalytic and protein interface binding. The two histidines and the aspartate residues in the loop are not appropriately spaced, thus it is not likely that these residues form a zinc binding site. Other possibilities are that zinc binding by residues in the loop and histidine residues in the transmembrane domains function to coordinate zinc during the transport process. Indeed, mutation of the histidine residues in TM 4 and 5 alters zinc transport of Zip1 and Irt1 [13]. The issue of how extracellular ligands might exchange zinc with a binding site predicted to be in an intracellular loop must be addressed. Indirect evidence suggests that the loop may extend into the membrane as a reentrant loop similar to that described for several other transporters [42-44]. Pre-incubation with a Zip1 antibody directed against a peptide located at the beginning of the TM3-4 loop decreased zinc uptake compared to cells pre-incubated with antibody depleted serum (unpublished data). This suggests that the loop epitope is accessible from the outside of the cells.
Figure 3.
Transmembrane domains 3, 4 and 5 and the large loop of hZip1 are represented. The histidines (H) in the loop are conserved in ZIP family transporters. Mutation of histidines 158 and 160 significantly decreased zinc transport. The histidines in the loop and histidines 190 and 217 in transmembrane domains 4 and 5, respectively potentially form a zinc coordination-binding site that functions in the transport process.
Figure 4.
Comparison of Zn uptake from Zn-Ligands and from free Zn2+ ions in PC-3/ZIP1 cells vs. PC-3/mut ZIP1 cells. Zinc uptake is decreased in ZIP1-mutant cells compared to PC-3 ZIP1 wild-type cells. In both cases, Zn transport is dependent upon total Zn concentration and not on free Zn2+ ion conc. Mutations were the histidines in the TM3-4 loop as shown in figure 3.
9. The direct Zn-Ligand exchange kinetics of mitochondrial zinc uptake transport
Once within the cell, the cytosolic zinc is distributed and sequestered among the cytoplasmic organelles and nucleus. This involves organellar zinc uptake transport processes. Any such process must consider the fact that the cytosolic free Zn2+ ion concentration is negligible (i.e. pM range). Very little established information exists regarding the kinetics of organelle zinc transport in mammalian cells. The mitochondria constitute a major cytoplasmic compartment for zinc accumulation, and can serve as a model for addressing the issues of intracellular zinc transport mechanisms. Mitochondria also provide the advantage of being readily isolated as clean preparations and can readily be assayed for functional and kinetic determinations.
Earlier studies regarding zinc uptake by mitochondria were conducted with unphysiological concentrations of free Zn2+ ions. Brierley and Knight [45] reported that heart mitochondria accumulated zinc by energy-dependent transport, but employed 100-500 μM free Zn2+ ions. Saris and Niva [46] employed 1-30 μM Zn2+ and suggested that free Zn2+ ions enter liver mitochondria via the calcium uniporter; but no direct measurements of mitochondrial uptake of zinc were provided. Malaiyandi et al [47] reported that brain cortex mitochondrial Zn uptake occurred through the calcium uniporter, but they employed concentrations of free Zn2+ ions in the range of 1-80 μM. However, they also reported that some mitochondrial zinc uptake occurred through an unidentified “passive, uniporter-independent” mechanism. The major conclusion that is common to all these studies is that Zn uptake occurs via a Ca2+ ion channel mechanism. Since the cytosolic free Zn2+ ion concentration is in the pM range, and the Ca2+ concentration is ~1000-fold over Zn2+, it is unlikely that this provides a major mechanism for mitochondrial uptake and accumulation of Zn. Dineley et al [48] in the review of the literature and their own studies reached the conclusion, “we could find no evidence supporting mitochondrial import of Zn2+ through the uniporter, or indeed by any other mechanism. Furthermore, published data in support of such a mechanism(s) are scant and not wholly convincing.”
Despite the fact that mitochondria accumulate a major pool of cellular zinc and zinc exerts important mitochondrial functional and metabolic activities, reported studies of mitochondrial zinc transport are scant. Surprisingly, mitochondrial zinc uptake transporters have not been identified. Consequently this discussion of the mechanism of mitochondrial zinc uptake relies predominantly on the reported kinetic studies of zinc uptake transport by prostate and liver mitochondria reported by Guan et al [10] and Costello et al [11] to provide the basis for a different zinc uptake transport mechanism. The following data are extracted from those reports. Figure 5A shows that the mitochondrial uptake of zinc is essentially the same for ZnCl2, Zn-Cit, Zn-His, and Zn-Cys; despite the fact that the free Zn2+ ion concentration ranged from 20 μM down to ~80 pM. This reveals that zinc uptake is dependent upon the total zinc, not the free Zn2+ ion availability. No zinc uptake occurred with Zn-EGTA and Zn-EDTA; which reveals that tightly bound Zn-Ligands (log Kf~12 and greater) are not zinc donors for zinc transport; whereas Zn-Ligands of log Kf~10 (Zn-Cys) and less are effective Zn donors for transport. It also demonstrates the integrity of the mitochondrial preparations. Figure 5B shows that the Zn-uptake exhibits Michaelis-Menton transport kinetics that is essentially the same for ZnCl2 and Zn-Ligands. The calculated Km values are quite similar and are in the μM range of the expected mobile Zn pool that exists in cytosol. Notably, the uptake of zinc from 20 μM ZnCl2 remains constant in the presence of added citrate, although the concentration of free Zn2+ ions decreases over the range from 20 μM to <1 μM (Figure 5C). The uptake of zinc from Zn-Ligand does not involve the co-transport with citrate uptake (figure 5D). As shown in figure 5E, mitochondrial uptake of zinc is not attenuated by Ca++ or Mg++, which indicates that zinc uptake occurs via a specific transporter that is not associated with specific ion channel mechanisms. In addition to these kinetic properties, the mitochondrial uptake of zinc is unaffected by pH changes over the range of 6.0-8.0; is not dependent upon substrate oxidation; is unchanged in coupled vs. uncoupled condition; is the same in state 3 and state 4 respiration; and is unchanged by cyanide inhibition of respiration [10].
Figure 5.
Kinetic studies of zinc uptake transport by prostate mitochondria. A. Time-dependent uptake of Zn from Zn-Ligands as a function of total Zn vs. free Zn2+ ion. B. Concentration-dependent zinc uptake from Zn-Ligands vs. from free Zn2+ ion; and corresponding Km values. C. Effect of titration of ZnCl2 source of free Zn2+ ions by increasing concentrations of citrate on Zn uptake as a function of total Zn concentration vs. free Zn2+ ion concentration. D. Comparison of Zn65 uptake and C14-citrate uptake from Zn-Citrate. E. Effect of Ca2+, Mg2+, and Cd2+ on Zn transport.
Studies with liver mitochondria demonstrate M-M kinetics that is essentially the same as prostate mitochondria [10]. Figure 6A shows the similar rapid zinc accumulation from ZnCl2, Zn-Cit, and Zn-Cys, although the free Zn2+ is 20 μM, ~4 μM, and ~80 pM, respectively. Also, no Zn accumulation results from Zn-EDTA. Also, the total zinc accumulation based on mitochondrial protein is the same for liver and prostate. LMW compounds (up to ~5 kDa) can passively permeate the outer mitochondrial membrane, but the inner mitochondrial membrane is highly impermeable. Therefore, mitochondrial transporters are associated with the inner membrane. Figure 6C shows that mitoplasts exhibit zinc transport capability similar to whole mitochondria, which supports the association of the putative facilitative zinc uptake transporter with the inner membrane.
A major physiological mobile zinc ligand in cells is metallothionein (MT); so it is important to determine if Zn-MT is a Zn donor for mitochondrial uptake. Zn-MT permeates the outer mitochondrial membrane for entry into the mitochondrial intermembrane space [49] Figure 6E (modified from [11]) shows that Zn-MT is an effective zinc donor for both liver and prostate mitochondrial zinc uptake. The most labile Zn-cysteine site (Zn3-b cluster) has a log Kf~8 [50]; which is the donor Zn that gives the same results as Zn-Cys that has a log Kf~10. Also notable is the absence of zinc uptake from Zn-EDTA, which also demonstrates the integrity of the mitochondrial inner membrane in the mitoplast preparation.
It has been suggested [50] that localized mitochondrial oxidative metabolism and redox changes will cause Zn release from Zn-MT at the intermembrane space, which could provide localized free Zn2+ ions for transport into the mitochondrial matrix. Although this possibility exists, our zinc uptake studies employed non-respiring, oxidative inactive intact mitochondria and mitoplasts. Therefore the uptake of zinc from Zn-MT did not require metabolically-induced release of free Zn2+ ions for transport into the mitochondria.
The collective kinetic data demonstrate that:
mitochondrial uptake of zinc occurs via facilitative transport.
free Zn2+ ions are not required for Zn uptake transport
Zn-Ligands with formation constants log Kf~10 or lower and permeate the outer membrane serve as effective Zn donors.
the putative Zn uptake transporter has an apparent formation constant of log Kf~11.
the transport Km for Zn is in the range of cytosolic concentration of donor Zn-Ligands.
These are the same properties that characterize ZIP plasma membrane zinc uptake transporter. Unfortunately, the genetic or proteomic identity of the mitochondrial zinc uptake transporter has not been established. It is reasonable to expect that the transporter might be a ZnT family transporter; and to expect that the zinc uptake transport process identified for mitochondria and for plasma membrane will also apply to other organelles (figure 2A and B). Consequently, we propose that the direct Zn exchange from donor Zn-Ligand to Zn-transporter will universally apply to all ZnT and ZIP transporters.
10. The direct Zn-Ligand mechanism of zinc reactivity
The preceding discussion dealt with the mechanism of zinc trafficking and transport in a cellular environment in which the concentration of free Zn2+ ions is negligible. This same issue also applies to the involvement of zinc in cellular biological/biochemical reactions. Essentially all reported studies regarding effects of zinc on cellular activities (with perhaps some exception) have been conducted with free Zn2+ ions at various concentrations generally in the μM-mM range, i.e. conditions that do not exist in the cellular environment. Therefore, the issue remains regarding the mechanism by which cellular Zn activity occurs
To address this issue we will employ the results of studies regarding the effects of zinc on mitochondrial respiration and terminal oxidation. Several studies with mammalian cells have reported that zinc inhibits mitochondrial respiration and terminal oxidation [51-56]. While there was some consistency in that μM concentrations of Zn inhibited aspects of terminal oxidation, those studies employed μM concentrations of free Zn2+ ions; i.e. unphysiological conditions.
This issue is addressed by the following results of zinc inhibition of mitochondrial respiration and terminal oxidation taken from the report of Costello et al (12). Figure 7 shows that the Zn-Ligands were equally effective as ZnCl2 in inhibiting the succinate-stimulated respiration of both liver and prostate mitochondria. Thus the reactivity effect of zinc is dependent upon the total concentration of zinc as donor Zn-Ligands and not on the concentration of free Zn2+ ion. The absence of any effect by ZnEDTA confirms that the effectiveness of Zn is dependent upon the binding affinity of the Zn-Ligands. This effect occurs in coupled and uncoupled mitochondria [12]. The tracing in Figure 7C also shows the rapid inhibitory effects of added Zn to the succinate-stimulated respiring mitochondria. Upon the addition of the Zn-Ligands, rapid initiation of inhibition and the same level of inhibition of respiration occur at the levels obtained with the free Zn2+ ions. This shows that a rapid Zn exchange process with the electron transport component(s) is associated with the respiration effect of Zn. Figure 8 presents tracings of the effects of Zn-Ligands on electron transport activities of liver mitochondria. The results show that the inhibition of succinate oxidation of cytochrome c (complexes II, III) is dependent upon the total concentration of Zn in Zn-donor ligands; and not dependent upon the concentration of free Zn2+ ions. In contrast, cytochrome oxidase (complex IV) is unaffected by all forms of Zn. This further reveals that the Zn inhibition is enzyme-specific and that the ligands are not responsible for the Zn-Ligand effects. The same results occur with prostate and liver mitochondria [12]. Collectively, these data show that Zn-Ligands with log Kf~10 or lower and at representative physiological levels of Zn are effective donors of Zn for direct interaction with metabolic enzymes; and free Zn2+ ions are not required for this reactivity. The μM range for the inhibitory effects of Zn is similar to the reports of others described above, and now provides a corresponding physiological pool of Zn-Ligands to achieve those effects. Additional corroboration is provided by the observation described above that Zn is directly transferred from Zn-MT to purified m-aconitase [32]. It is also important to note that the Ki for zinc inhibition of purified m-aconitase as well as mitochondrial preparation m-aconitase is ~2-10 μM [33]; which is the range of the mobile reactive Zn-Ligand pool, not the free Zn2+ ion pool.
Figure 7.
Comparative effects of free Zn2+ ions and Zn-Ligands on the respiration of liver and prostate mitochondria. A and B. Tracings obtained following five minute incubation with Zn solution to the mitochondrial preparation. Numbers at tracings are relative respiration rates. ZnCl, ZnCit, ZnCys have similar rates. C. Tracing begun prior to succinate and Zn treatments to obtain immediate effects Zn on respiration.
Figure 8.
The effect of Zn from Zn-Ligands on terminal oxidation of liver mitochondria. A. Zn effects on succinate oxidation via Complexes II and III. B. Zn effects on cytochrome oxidase (Complex IV) activity.
11. What do these collective studies demonstrate?
The results for plasma membrane zinc uptake transport, mitochondrial zinc uptake transport, and Zn effects on respiration and terminal oxidation all exhibit the same zinc relationships: 1) mobile reactive Zn-Ligands (including Zn-MT where applicable) with log Kf~10 and lower are equally as effective as free Zn ions as Zn donors; and Zn-Ligands with log Kf ~11 and greater are not Zn-donors; 2) the kinetics for these activities are dependent upon the total concentration of Zn represented in the Zn-Ligand pool; and not on the concentration of free Zn2+ ions; 3) the equimolar effects of the Zn-Ligands are independent of the ligand form; 4) the concentration of zinc and the Km/Ki values associated with these activities are in the μM range; 5) a free Zn2+ ion concentration of ~1 nM or less that exists in ISF and in cytosol is unlikely to be the source of Zn for activities that exhibit cellular μM concentrations and Km values that are >1000-fold higher than the cellular environment of free Zn2+ ions. The explanation for these relationships is best fitted by the concept of a direct Zn exchange from the Zn-Ligand donor to the acceptor protein without any requirement for the presence or involvement of production of free Zn2+ ions. We hope that our presentation conveyed that this relationship has been suggested by others in the search to reconcile the differences between the extremely low concentrations of free Zn2+ ions in biological fluids and the >1000-fold greater concentration of zinc associated with many of its cellular/physiological activities. We believe that this concept and the supporting data for the trafficking, transport, and reactivity of zinc contribute to the understanding and resolution of this important cellular relationship.
11. The physiological zinc donor ligands in mammalian cells
Our studies described above focused on selected LMW organic acid ligands, which are natural and important metabolites in mammalian cells that bind zinc over a wide range of binding affinities. The LMW ligands along with MT comprise the mobile reactive pool of Zn-Ligands. In addition, the studies employed prostate cells because of their uniquely high zinc levels and their specialized function as zinc accumulating cells; as compared to hepatic cells which are more representative of zinc relationships that typify most mammalian cells. Despite these differing zinc related functional characteristics of the cell-type, the consistent relationship that emerges from the composite studies is that: all the Zn-Ligands with formation constants of log Kf~10 or lower are equally effective Zn donors and as effective as free Zn2+ ion at equimolar concentrations of total Zn. The effectiveness is independent of free Zn 2+ ion concentration. Conversely, Zn-Ligands that exhibit log Kf~11 or greater, are not Zn-donor ligands for cellular transport or reactivity.
The important issue is, “what are the physiologically relevant cytosolic zinc-donor ligands that exist in mammalian cells?” For the following discussion we will consider the physiological importance of each of the two “sub-pools”: 1) the LMW organic acid pool; and 2) the metallothionein pool (MTs).
A. The LMW pool of organic acids
The organic acids in our studies included citrate, aspartate, histidine, and cysteine. However, the potential LMW Zn-donor ligands will include many other organic compounds; and will be different for different cell types. To exemplify this, normal prostate epithelial cells have very high citrate and aspartate levels (~1.2 mM) and cytosolic mobile reactive zinc levels that are several-fold higher than found in most other cells such as hepatocytes. At these concentrations, Zn-Citrate and Zn-Aspartate likely constitute a relevant pool of Zn-donor ligands in prostate cells, which would not exist in hepatic cells. Unfortunately, the variability among different cell types of the individual organic acids and their concentrations have resulted in a largely “dismissive” view of their relevance as Zn-donor ligands. This view arises when one considers each zinc-binding organic acid as a separate and individual Zn-Ligand, rather than as a component of a larger pool of Zn-donor ligands. The latter is the more appropriate consideration since the equimolar effectiveness as Zn-donors of each organic acid is similar as long as it meets the log Kf requirements as we have described. Therefore the sum of the concentrations of the Zn-binding organic acids constitutes and approximates the total concentration of the LMW Zn-Ligand pool. This also becomes the important consideration in estimating the free Zn2+ ion concentration that will likely exist in the milieu of the total Zn concentration of the mobile reactive organic acid pool of Zn donors; in the absence of any considerations for MT. Thus, the LMW organic acid component of Zn-Ligands should be considered as a relevant contributor to “the control of cellular zinc distribution, translocation, and availability”, as suggested for the primary function of metallothionein [50].
However, it is important to recognize that the cellular concentration of the organic acids is not regulated in response to the status of cellular zinc. Therefore, the LMW Zn-Ligand pool is not the physiological/homeostatic component that is regulated in response to cellular Zn changes.
B. The Metallothionein (MT) component
In contrast to the LMW Zn-Ligand pool, MTs constitute the major component of the mobile reactive Zn-Ligand pool that is regulated in response to changes in cellular zinc concentration in mammalian cells. For example, increased cellular zinc levels increases the gene expression and levels of MTs in prostate cells [57,58]. Therefore, MT has evolved with chemical and physiological/biochemical properties that make it ideally suited for this function.
Mammalian MTs are small (6 –7 kDa) non-enzymatic proteins, which contain 20 cysteine sites and bind up to seven Zn atoms [9, 59-61]. The molecular size of MT is especially important for the delivery of Zn to organellar zinc reactive sites. This is exemplified by the mitochondria in which Zn transport activity and electron transport components occurs at the inner mitochondrial membrane juxtaposed with the intermembrane space. That MTs permeate the outer mitochondrial membrane has been established [e.g. 50]; so that Zn-MT is delivered to the intermembrane space and the inner membrane for reactivity and for transport into the mitochondrial matrix.
MT is comprised of two domains that form thermodynamically stable and kinetically labile complexes with zinc ions [59,62]. Cysteine residues are exposed at the surface of the protein, not buried within; which allows them to participate in rapid direct transfer of zinc in site-specific binding complexes formed between MT and small molecules or other proteins [9]. The Zn-Ligand exchange mechanism presumes initial formation of a protein/protein complex to facilitate formation of a series of intermediates with the eventual transfer Zn from the donor protein to the acceptor protein as represented in figure 9. In this process, no formation of free Zn2+ ions is involved in the transfer process. Hathout et al [31] showed that Zn exchange from donor Zn-MT to a zinc-recipient peptide, separated by dialysis membrane to allow free Zn2+ ion diffusion, zinc transfer was not detectable after three hours. However, electrospray mass spectrometry showed that transfer of zinc ions occurred in less than 2 min when the apo-peptide was mixed directly with Zn7-MT. Thus, the transfer of zinc is a slow process if the zinc must be dissociated from the donor Zn-Ligand and the free Zn2+ ion then binds to the acceptor protein. As all of our studies have shown, the effects of donor Zn-Ligands are rapid effects, and this applies to LMW Zn-Ligands as well as Zn-MT.
Figure 9.
Representation of the direct Zn intermolecular exchange process. The representation eliminates the involvement or requirement for the production of free Zn ions for the Zn transfer mechanism. Although this representation has been applied to protein—protein interaction, we propose that it also applies to LWM Zn-Ligand--protein exchange of Zn.
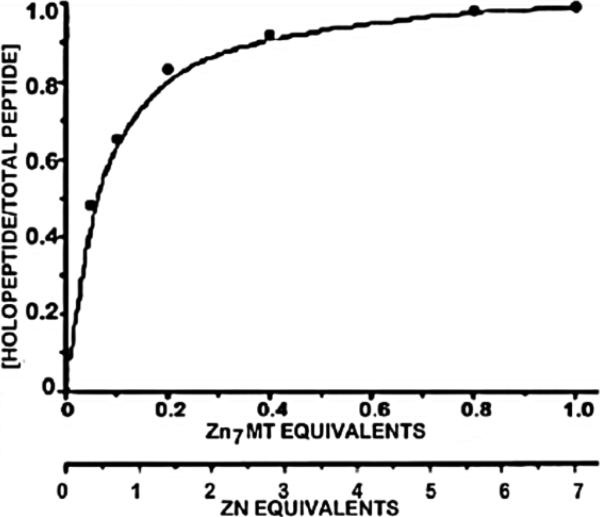
Multiple forms of MT exist in cells and each of these proteins exhibits its own log Kf values; and each of the zinc binding sites within MT also has a unique binding affinity [42]. This provides a range of binding or formation constants that falls midway in the range of formation constants known for zinc-binding proteins. However, the most labile Zn site (i.e. the Zn3-b cluster) exhibits a formation constant of log Kf~8, which is the important physiological relevant Zn donor for direct intermolecular exchange. Figure 10 shows the stoichiometry of metal ion transfer from MT to apo-CP peptide [31]. The results show that complete reconstitution of the CCHH zinc finger is achieved with one molar equivalent of Zn-MT, which indicates that zinc from only one of the seven sites is donated. A similar observation was reported by Jiang et al [63]. This is also apparent for the mitochondrial transport of zinc from Zn-MT as we showed in figure 6.
Figure 10.
Titration of 100 μM apo-CP (CCHH) with different concentrations of rabbit Zn7MT-2. pH 7.4. Results show that only one Zn site donates Zn from Zn7MT to the acceptor protein. (Taken from [31] with permission from Elsevier Science)
Thus the direct Zn exchange process is dependent upon the formation constant of Zn donors including Zn-MT; which is exactly what the cellular mitochondrial zinc uptake and trafficking studies above has revealed, as well as the cellular zinc uptake transport and Zn reactivity. For discussion purposes, we described the LMW Zn-donor pool separately from Zn-MT as components of the mobile reactive Zn-Ligand pool. This should not imply that these components are not interactive. It is highly plausible that direct exchange could occur between the LMW Zn-Ligands and Zn-MT; especially in the direction of transfer of Zn from LMW Zn-Ligands to MT. For example, the log Kf values for citrate, aspartate, histidine are lower than that of MT. Therefore, significant flexibility exists within cells without sacrificing the effectiveness of the Zn-Ligand form.
12. The impact of the direct Zn-Ligand exchange mechanism on biomedical research and clinical application of zinc relationships
It is fair to state that many and possibly most biomedical studies have employed zinc conditions that do not reflect the physiological/biochemical relationships that exist in the in situ cellular environment. Especially relevant are the unphysiological concentrations of zinc and the forms of zinc that have been commonly employed. The zinc relationships described in this paper should be given careful consideration in the planning and conduct of biomedical and clinical studies, and in the interpretation and translational application of the results obtained. The overwhelming studies of extracellular zinc effects on cell function and transport have been conducted with medium supplemented with μM-mM concentrations of free Zn2+ ions, and in the absence of Zn ligands. This is not reflective of the physiological conditions of the cell's environment in situ; whether one is dealing with representation of the ISF environment, the cerebrospinal fluid environment, or other specialized fluid environments. The same applies to studies regarding the intracellular trafficking, transport, and reactivity of zinc. Most such studies also employ medium containing μM free Zn2+ ions, although the physiological condition involves pM levels of free Zn2+ ions. So, one must inquire as to the translational value and physiological/pathological/clinical relevance and application of such studies.
We do not imply that studies that employ free Zn2+ ion as the source of Zn are necessarily inappropriate. This largely depends upon the intent of the study. Free Zn2+ ion at concentrations that reflect the physiological concentration range of the mobile reactive pool (for example, ~1-20 μM Zn for the extracellular medium; ~1-50 μM for the cytosolic medium) can be an acceptable condition. As this paper shows, at such concentrations of total mobile reactive Zn, free Zn2+ ions provide the same effects as the Zn-Ligands. However, it is likely to be inappropriate to employ μM concentrations of free Zn2+ ion in a system devoid of Zn ligands in studies that deal with the mechanism or process that requires ionic zinc such as: ion channel transport; modulator effects on excitability and synaptic transmission; or other studies of reactivity of the ionic form of zinc.
13. Conclusion
Figure 11 is a conceptualization of the direct Zn-Ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells; as represented by the cellular uptake of zinc and its cytosolic trafficking and uptake by mitochondria. In the absence of a physiologically-relevant pool of free Zn2+ ion in extracellular or intracellular fluid, the donor Zn-Ligand direct exchange mechanism provides a unifying physiological concept that integrates cellular Zn trafficking, Zn transport, and Zn reactivity. We recognize that further studies are required to establish this concept as a general principal. It is important to acknowledge that we do not dismiss the possibility and likelihood that localized generation of free Zn2+ ions exists and is important in specialized conditions; but this does not represent the pool and process for zinc involved in trafficking of Zn for transport and reactivity.
Figure 11.
Illustration of the transport and trafficking of Zn from blood plasma to mitochondria by the direct Zn-Ligand exchange mechanism. LMW donor Zn-Ligands and Zn-MT directly transfer Zn to the Zn uptake transporter proteins transporters and to other Zn acceptor zinc ligands. No formation of free Zn2+ ions is involved.
Perhaps this presentation will have identified zinc relationships that others will find important for consideration in the planning of studies and the translational interpretation of results. We fully anticipate that alternative and opposing views will be expressed; and, if so, this will be a healthy scientific discourse from which all will benefit.
Acknowledgement
This review and the cited studies of LCC and RBF have been supported by NIH grants RO1CA79903 and R01DK42839.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eide DJ. Eur. J. Physiol. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 2.Gaither AL, Eide DJ. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- 3.Franklin RB, Milon B, Feng P P, Costello LC. Front. Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichten LA, Cousins RJ. Ann. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 5.Liuzzi JP, Cousins RJ. Ann. Rev. Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 6.Reyes JG. Am. J. Physiol. 1996;270:C401–C410. doi: 10.1152/ajpcell.1996.270.2.C401. [DOI] [PubMed] [Google Scholar]
- 7.Vallee BL. Physiol. Rev. 1959;39:443–490. doi: 10.1152/physrev.1959.39.3.443. [DOI] [PubMed] [Google Scholar]
- 8.Vallee BL, Falchuk KH. Physiol. Rev. 1993;73:79–117. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Maret W, Li Y. Chem. Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 10.Guan Z, Kukoyi B, Feng P, Kennedy MC, Franklin RB, Costello LC. J. Inorg. Biochem. 2003;97:199–206. doi: 10.1016/s0162-0134(03)00291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello LC, Guan Z, Franklin RB, Feng P. J. Inorg. Biochem. 2004;98:664–666. doi: 10.1016/j.jinorgbio.2004.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello LC, Guan Z, Kukoyi B, Feng P, Franklin RB. Mitochond. 2004;4:331–338. doi: 10.1016/j.mito.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milon B, Wu Q, Zou J, Costello LC, Franklin RB. Biochim. Biophys. Acta. 2006;1758:1696–1701. doi: 10.1016/j.bbamem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Krezel A, Maret W. J Biol Inorg Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 15.Pilch SM, Senti FR. J Nutr. 1985;115(1985):1393–1397. doi: 10.1093/jn/115.11.1393. [DOI] [PubMed] [Google Scholar]
- 16.Faure H, Favier A, Tripier M, Arnaud J. Biol. Trace Elem. Res. 1990;24:25–37. doi: 10.1007/BF02789138. [DOI] [PubMed] [Google Scholar]
- 17.Magneson GR, Puvathingal JM, Ray WJ. J. Biol. Chem. 1987;262:11140–11148. [PubMed] [Google Scholar]
- 18.Guyton AC, Granger HJ, Taylor AE. Physiol. Rev. 1971;51:527–563. doi: 10.1152/physrev.1971.51.3.527. [DOI] [PubMed] [Google Scholar]
- 19.Pattison SE, Cousins RJ. Am. J. Physiol. 250(1986):E677–E685. doi: 10.1152/ajpendo.1986.250.6.E677. [DOI] [PubMed] [Google Scholar]
- 20.Gálvez M, Moreno JA, Elósegui LM, Escanero JF. Biol. Trace Elem. Res. 2001;84:45–56. doi: 10.1385/BTER:84:1-3:045. [DOI] [PubMed] [Google Scholar]
- 21.De Kok J, Van Der Schoot C, Veldhuizen M, Wolterbeek HT. Biol. Trace Elem. Res. 1993;38:13–26. doi: 10.1007/BF02783978. [DOI] [PubMed] [Google Scholar]
- 22.Maret W. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 23.Dhar NK, Goel TC, Dube PC, Chowdhury AR. Exper. Mol. Pathol. 1973;19:139–142. doi: 10.1016/0014-4800(73)90073-7. [DOI] [PubMed] [Google Scholar]
- 24.Conrad EM, Ahearn G,A. J. Exp. Biol. 2005;208:287–296. doi: 10.1242/jeb.01401. [DOI] [PubMed] [Google Scholar]
- 25.Cousins RJ, Liuzzi JP, Lichten LA. J. Biol. Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 26.Eide DJ. Biochim. Biophys. Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P P, Costello LC. J. Inorgan. Biochem. 2003;96:435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaither AL, Eide DJ. J. Biol. Chem. 2001;276:22258–22264. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- 29.Chao Y, Fu D. J. Biol. Chem. 2004;279:17173–17180. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]
- 30.Cherezov V, Hofer N, Szebenyi DM, Kolaj O, Wal JG, Gillilan R, Srinivasan V V, Jaroniec CP, Caffrey M. Structure. 2008;16:1378–1388. doi: 10.1016/j.str.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hathout Y, Fabris D, Fenselau C. Internat. J. Mass Spectrom. 2001;204:1–6. [Google Scholar]
- 32.Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, Kang Y J YJ. Biochemi. Biophys. Res. Comm. 2005;332:853–858. doi: 10.1016/j.bbrc.2005.04.170. [DOI] [PubMed] [Google Scholar]
- 33.Costello LC, Liu T, Franklin RB, Kennedy MC. J. Biol. Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 34.Costello LC, Franklin RB, Liu Y, Kennedy MC. J. Inorg. Biochem. 2000;78:161–165. doi: 10.1016/s0162-0134(99)00225-1. [DOI] [PubMed] [Google Scholar]
- 35.Krezel A, Hao Q, Maret W. Arch. Biochem. Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Rogers EE, Eide DJ, Guerinot ML. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis CD, MacDiarmid CW, Eide DJ. J. Biol. Chem. 2005;280:28811–28818. doi: 10.1074/jbc.M505500200. [DOI] [PubMed] [Google Scholar]
- 38.Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T. J. Biol. Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, Yamaguchi-Iwai Y, Kambe T. J. Biol. Chem. 2006;281:17743–17750. doi: 10.1074/jbc.M602470200. [DOI] [PubMed] [Google Scholar]
- 40.Lu M. Structure of the zinc transporter Yiip. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 41.Auld DS. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- 42.Chen N, Li B, Murphy TRH, Raymond LA. Mol. Pharmacol. 2004;65:157–164. doi: 10.1124/mol.65.1.157. [DOI] [PubMed] [Google Scholar]
- 43.Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. J. Biol. Chem. 2004;279:31228–31236. doi: 10.1074/jbc.M402428200. [DOI] [PubMed] [Google Scholar]
- 44.Grunewald M, Menaker D, Kanner BI. J. Biol. Chem. 2002;277:26074–26080. doi: 10.1074/jbc.M202248200. [DOI] [PubMed] [Google Scholar]
- 45.Brierley GP, Knight VA. Biochemistry. 1967;6:3892–3901. doi: 10.1021/bi00864a035. [DOI] [PubMed] [Google Scholar]
- 46.Saris NE, Niva K. FEBS Lett. 1994;356:195–198. doi: 10.1016/0014-5793(94)01256-3. [DOI] [PubMed] [Google Scholar]
- 47.Malaiyandi LM, Vergun O, Dineley KE, Reynolds IJ, Neurochem J. 2005;93:1242–1250. doi: 10.1111/j.1471-4159.2005.03116.x. [DOI] [PubMed] [Google Scholar]
- 48.Dineley KE, Votyakova TV, Reynolds IJ, Neurochem J. 85(2003):563–570. doi: 10.1046/j.1471-4159.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- 49.Krezel A, Maret W. J. Biol. Inorg. Chem. 2008;13:401–409. doi: 10.1007/s00775-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 50.Ye B, Maret W, Vallee BL. Proc. Natl. Acad. Sci. U S A. 98(2001):2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter FE, Ford L. J Biol Chem. 216(1955):357–369. [PubMed] [Google Scholar]
- 52.Skulachev VP, Chistyakov VV, Jasaitis AA, Smirnova EG. Biochem. Biophys. Res. Commun. 26(1067):1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- 53.Nicholls P, Malviya AN. Biochemistry. 1968;7:305–310. doi: 10.1021/bi00841a038. [DOI] [PubMed] [Google Scholar]
- 54.Kleiner D, von Jagow G. FEBS Lett. 1972;20:229–232. doi: 10.1016/0014-5793(72)80802-0. [DOI] [PubMed] [Google Scholar]
- 55.Lorusso M, Cocco T, Sardanelli AM, Minuto M, Bonomi F, Papa S. Eur. J. Biochem. 1997;197:555–561. doi: 10.1111/j.1432-1033.1991.tb15944.x. [DOI] [PubMed] [Google Scholar]
- 56.Link TA, von Jagow G. J. Biol. Chem. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- 57.Hatsumi M, Suzuki K, Marsui H, Koike H, Ito k., Yamanaki H. Canc. Lett. 2003;200:187–195. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- 58.Lin SF, Maeda d., Franklin RB, Feng P. Nutr. Biochem. 2008;29:1000–1012. doi: 10.1016/j.jnutbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ngu TT, Stillman MJ. Dalton Trans. 2009;28:5425–5433. doi: 10.1039/b902008j. [DOI] [PubMed] [Google Scholar]
- 60.Otvos JD, Petering DH, Shaw CF. Comm. Inorg. Chem. 1989;9:1–35. [Google Scholar]
- 61.Romero-Isart N, Jensen LT, Zerbe O, Winge DR, Vasak M. J. Biol. Chem. 2002;277:37023–37028. doi: 10.1074/jbc.M205730200. [DOI] [PubMed] [Google Scholar]
- 62.Winge DR, Miklossy KA. J. Biol. Chem. 1982;257:3471–3476. [PubMed] [Google Scholar]
- 63.Jiang LJ, Maret W, Vallee BL. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]