Summary
Background
RhoGTPases have been implicated in the regulation of cancer metastasis. Invasive carcinoma cells form invadopodia, F-actin-rich matrix degrading protrusions that are thought to be important for tumor cell invasion and intravasation. Regulation of actin dynamics at invadopodial protrusions is crucial to drive invasion. This process requires the severing activity of cofilin to generate actin-free barbed ends. Previous work demonstrates that cofilin’s severing activity is tightly regulated through multiple mechanisms including regulation of cofilin serine phosphorylation by Rho GTPases. However, it is not known which Rho GTPase is involved in regulating cofilin’s phosphorylation status at invadopodia.
Results
We show here, for the first time, how RhoC activation is controlled at invadopodia and how this activation regulates cofilin phosphorylation to control cofilin’s generation of actin-free barbed ends. Live-cell imaging of fluorescent RhoC biosensor reveals that RhoC activity is spatially confined to areas surrounding invadopodia. This spatiotemporal restriction of RhoC activity is controlled by “spatially distinct regulatory elements” that confines RhoC activation within this compartment. p190RhoGEF localizes around invadopodia to activate RhoC, while p190RhoGAP localizes inside invadopodia to deactivate the GTPase within the structure. RhoC activation enhances cofilin phosphorylation outside invadopodia.
Conclusion
These results show how RhoC activity is spatially regulated at invadopodia by p190RhoGEF and p190RhoGAP. RhoC activation in areas surrounding invadopodia restricts cofilin activity to within the invadopodium core resulting in a focused invadopodial protrusion. This mechanism likely enhances tumor cell invasion during metastasis.
Keywords: Metastasis, Invadopodia, RhoC, p190RhoGEF, p190RhoGAP, Cofilin, Cofilin phosphorylation, tumor invasion
Introduction
The steps of invasion and intravasation during metastasis require tumor cells to degrade through dense basement membrane barriers. To accomplish this process, tumor cells are believed to form invadopodia, F-actin-based membrane protrusions with matrix metalloproteinase activity to degrade the extracellular matrix [1–3]. Invadopodia are enriched in proteins that regulate the actin cytoskeleton including cortactin, cofilin, Arp2/3, and N-WASp [1, 4]; as well as proteins that regulate matrix degradation, such as MT1-MMP [2, 5, 6]. Actin polymerization is essential for maturation of invadopodia [6].
In mammary carcinoma cells, the cofilin pathway is an essential generator of free barbed ends both at the leading edge [7] and at invadopodia [6]. Cofilin pathway activity is essential for tumor cell invasion, migration and metastasis [8, 9]. Cofilin is phosphorylated at serine 3 by LIM- and TES-family kinases, which inhibits its ability to bind F-actin, thereby inactivating it [10]. The Rho family of small GTPases has been shown to activate Rho-associated coiled-coil kinase (ROCK) leading to the phosphorylation and activation of LIM kinase (LIMK) [11].
During tumor cell migration, pathways that control cofilin activity must be spatially and temporally coordinated to create areas of high cofilin activity in specific subcellular compartments [12, 13]. However, the mechanism that controls the local activation of cofilin at invadopodia remains unclear.
Another major regulator of tumor cell migration and invasion are Rho GTPases [14]. Rho GTPases cycle between two states: 1) GDP-bound inactive state and a 2) GTP-bound active state that can bind and activate downstream effectors. These states are controlled by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). How RhoGTPase activation is spatially regulated by GEFs and GAPs is not well documented. Different GEFs and GAPs have been shown to influence the activities of many GTPases [15, 16]. Understanding how GTPase activities are controlled at specific subcellular compartments is crucial to elucidating how each GTPase is involved in different cellular processes.
The Rho subfamily of GTPases consists of 3 isoforms: RhoA, RhoB and RhoC [17]. In particular, RhoC plays a critical role during tumor metastasis [18] and has been identified as a biomarker used for invasive breast cancer in human patients [19]. RhoC expression has been positively correlated with increased invasion and motility in vitro and in vivo [20], and RhoC knockdowns have been effective in suppressing breast cancer proliferation and metastasis [21, 22]. Recently, expression profiling of human mammary tumors has shown that RhoC mRNA is highly upregulated in invasive tumor cells compared to average primary tumor cells of the same tumor (Patsialou, A., unpublished observations). Although this evidence points to RhoC as a master regulator of tumor cell invasion, the mechanism by which RhoC promotes metastasis is unknown.
The development of fluorescence resonance energy transfer (FRET)-based biosensors for Rho GTPases [23] has allowed the study of Rho GTPase activation in cells with high spatiotemporal resolution. Here, we have utilized a new biosensor for RhoC [24] to study RhoC spatiotemporal activation during invadopodium formation in mammary tumor cells. We describe how this activation is controlled by “spatially distinct regulatory elements” consisting of p190RhoGEF and p190RhoGAP, both contributing to spatially restrict the RhoC activity to areas surrounding invadopodia. We further demonstrate that activation of RhoC is important for focused cofilin activity and actin polymerization during invadopodial protrusion, contributing to efficient tumor cell invasion. Together, our data identify RhoC, tightly regulated by p190RhoGEF and p190RhoGAP, as a key GTPase in invadopodium formation, and provide new insights into how actin polymerization is controlled within the invadopodium.
Results
RhoC is important for tumor cell invasion and invadopodial protrusion
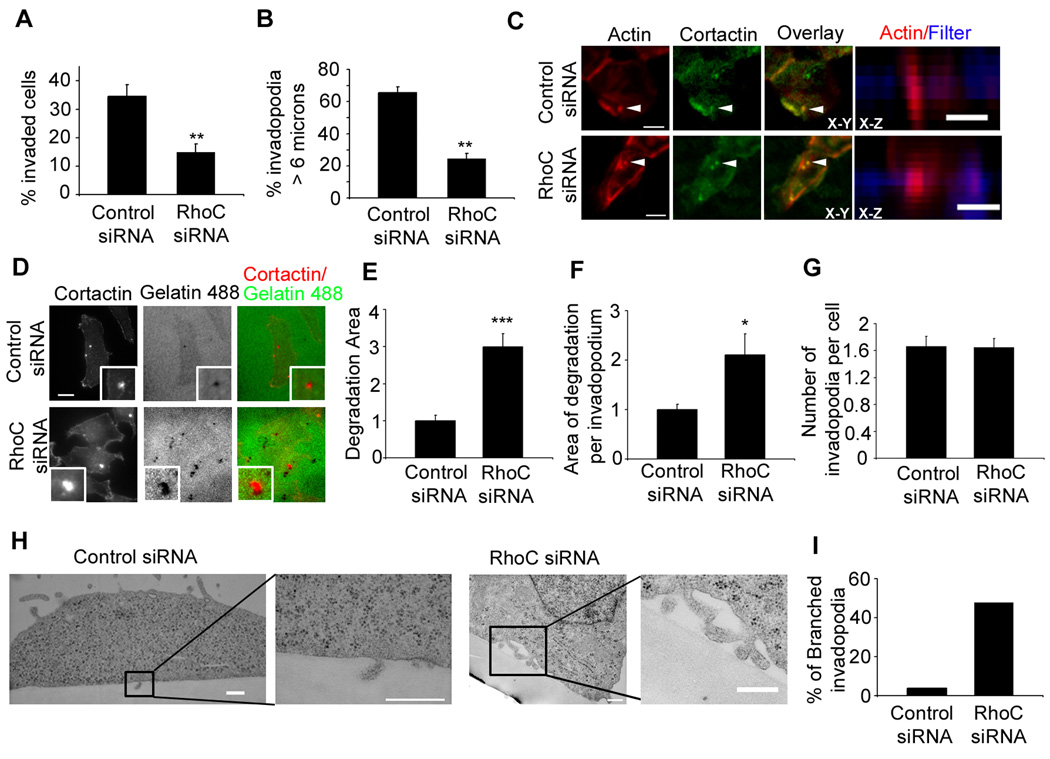
To investigate the role of RhoC in invasion, we performed an in vitro Matrigel-coated Transwell assay using MTLn3 cells, a highly metastatic rat mammary adenocarcinoma cell line. RhoC expression was knocked down by siRNA treatment with 95% efficiency without affecting RhoA, Rac and Cdc42 expression (Figure S1). We found that RhoC siRNA cells showed significantly decreased invasion (Figure 1a).
Figure 1. RhoC expression is required for invasion and invadopodial protrusion.
a) Transwell invasion assay in control or RhoC siRNA treated MTLn3 cells, n= 3 experiments. b) Quantification of protrusive depth by invadopodia. n = 100 invadopodia in each condition from 2 different experiments. c) Representative X-Y and X-Z images of cells plated on 1 µm pore Transwell filters. Scale bar = 5 µm. d) Matrix degradation of Alexa 488-gelatin matrix. Insets here and throughout the figures show higher magnifications of invadopodium. Scale bar = 10 µm. e) Quantification of the degradation area per field, f) the area of degradation per invadopodium and g) the number of invadopodia. p values are compared to control siRNA, and n>40 (number of fields) scored from 3 independent experiments. h) TEM sections of MTLn3 cells plated on thick matrix (FN/ gelatin). Scale bar = 500 nm (control siRNA), Scale bar= 400 nm (RhoC siRNA cells). i) Quantification of the percentage of “branched” invadopodia n=25 invadopodium per condition. ***, p < 0.001,* *p < 0.01.
Since invadopodia are involved in matrix degradation and invasion, we sought to determine if RhoC affects invasion by regulating invadopodia in MTLn3 cells, characterized for the formation of prominent invadopodia, rich in actin filaments, at the ventral cell membrane [1]. We quantified invadopodial protrusion depths by confocal microscopy in control and RhoC siRNA cells invading through the Matrigel-coated Transwell filters (1 µm pore size). Under these assay conditions, cells protrude through the 1 µm pores via invadopodia that were identified by the presence of cortactin and F-actin (Figure 1c and Figure S2a). RhoC-depleted cells clearly showed a significant decrease in invadopodial protrusion length compared to the control cells (Figure 1b and c and Figure S2a). Taken together, these results suggest that RhoC is critical for the formation of longer and more invasive invadopodia during tumor cell invasion.
RhoC spatially confines invadopodium formation and function
Since an important function of invadopodia is matrix degradation, we studied whether depletion of RhoC affects this process. RhoC siRNA MTLn3 cells were plated on Alexa 488-gelatin matrix and assayed for matrix degradation (Figure 1d). Surprisingly, in RhoC siRNA cells, invadopodia degraded more matrix area overall and per invadopodium compared to control cells (Figure 1e, f). This effect was not due to an increase in invadopodium formation since the number of invadopodia per cell was unaffected (Figure 1g). Similar results were observed when cells were plated on a fibronectin (FN)/gelatin matrix (Figure S2 c,d,e). Furthermore, the enhanced matrix degradation observed in RhoC siRNA cells was not due to increased MT1-MMP levels (Figure S2 f,g).
To determine whether the effect observed on matrix degradation after RhoC depletion was specific to RhoC as compared to other members of the family, we also investigated the role of RhoA, that has been shown to be important for invadopodial function [5, 25]. RhoA depletion by siRNA (Figure S3a) dramatically decreased matrix degradation by invadopodia as well as the number of invadopodia per cell, (Figure S3 c,d,e,f) in agreement with a previously described result [5].
To reconcile increased matrix degradation and decreased invasion after RhoC knockdown, we analyzed the ultrastructure of invadopodia in RhoC siRNA cells. Surprisingly, we found that invadopodia that formed in RhoC siRNA cells were unable to protrude deeply into the fibronectin (FN)/gelatin matrix while control cells form invadopodia that penetrate into the matrix (Figure 1h and Figure S2b). Furthermore, RhoC siRNA cells formed abnormal branched invadopodia, rather than an unbranched focal invadopodium (Figure 1 h,i and Figure S2b). Since changes in extracellular matrix rigidity has been shown to affect invadopodia activity [26] we plated RhoC siRNA cells on Matrigel, a softer matrix and obtained the same branched invadopodial phenotype (Figure S2b) suggesting that the abnormal morphology of RhoC siRNA invadopodia are independent of matrix rigidity.
These results clearly show that while invadopodia formed in RhoC depleted cells are functional in terms of the matrix degradation activity, the morphological defects observed by both light and electron microscopy analysis demonstrate an inability to protrude and focus matrix degradation in RhoC-depleted cells resulting in only superficial degradation of matrix. In contrast, control cells both penetrate and degrade matrix, resulting in increased cell invasion.
High levels of RhoC activity surround the cortactin core of invadopodia
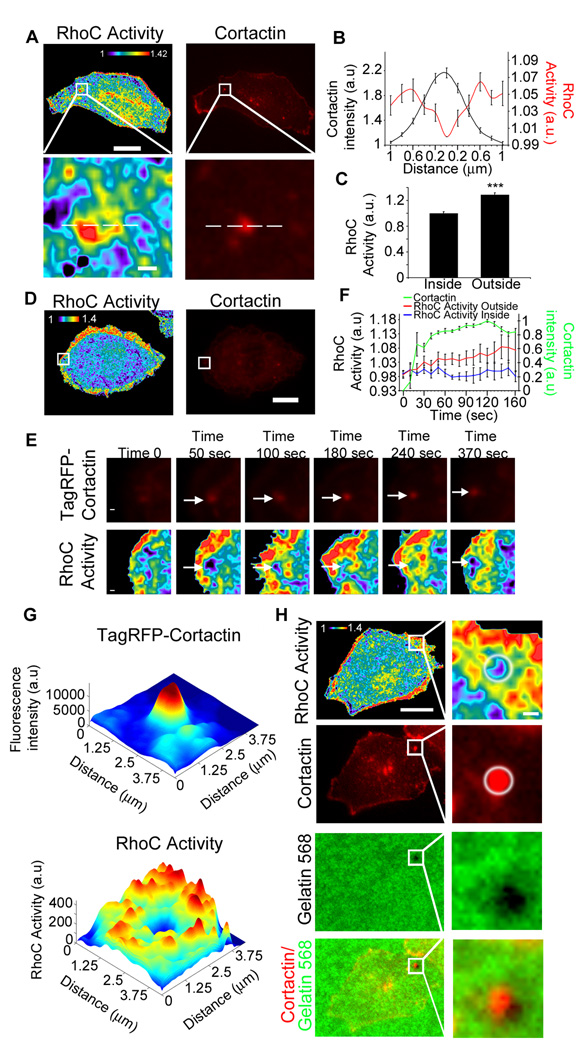
Spatiotemporal regulation of RhoGTPases activities is necessary to trigger signaling pathways that control various cellular processes including cell invasion. To determine the spatiotemporal localization of RhoC GTPase activity during invadopodium formation, we imaged cells expressing the fluorescence resonance energy transfer (FRET)-based biosensor for RhoC [24]. Expression of the RhoC biosensor in MTLn3 cells under a TET-off inducible system (Figure S4a) does not affect matrix degradation activity by invadopodia or the number of invadopodia formed (Figure S4 b,c,d).
Interestingly, we found that while RhoC activity is primarily excluded from the invadopodium core, it is elevated in the areas surrounding invadopodia (Figure 2 a,b). Analysis of RhoC activity levels inside the invadopodium core (identified by cortactin staining) to the average in a 3 µm wide circular band outside the cortactin core showed a 30% increase in RhoC activity (comparing the outside to the inside of an invadopodium) (Figure 2c). RhoC activity also surrounded invadopodia undergoing active matrix degradation (Figure 2h). This suggests that this spatiotemporal activation of RhoC may be necessary to regulate invadopodium formation and function.
Figure 2. RhoC activity surrounds cortactin in invadopodia.
a) Representative images of MTLn3 cells stably expressing RhoC biosensor plated on gelatin matrix and stained for cortactin to identify invadopodium core. b) Line scan measurements along invadopodia of RhoC activity normalized to lowest value inside and the cortactin intensity. n = 30 invadopodia from 3 different experiments c) Quantification of RhoC activity inside and outside of invadopodium. RhoC activity is averaged and normalized to the inside values. n > 20 invadopodia per group from 3 different experiments d) Representative image of MTLn3 cells stably expressing RhoC biosensor and TagRFP-Cortactin e) Representative images from various time points after EGF stimulation of MTLn3 cells expressing RhoC biosensor and TagRFP-Cortactin. Panels show magnified region represented by the white box in d. The pseudocolor scale here and throughout the figures shows ratio limits of (black) to (red) for RhoC activity. Arrows indicate the location of the invadopodium in the RhoC activity images. f) Quantification of RhoC activity outside and inside the cortactin core of the invadopodium, and TagRFP-Cortactin intensity during invadopodia formation. n = 7 invadopodia from 7 different cells. g) 3D plots of the maximum projection (12 time points) over time of RhoC activity and TagRFP-cortactin intensity from invadopodium in (e). h) Localization of RhoC activity around an invadopodium actively degrading the underlying Alexa 568-fluorescent matrix (Cortactin: Alexa 700). White circle indicates matrix-degrading invadopodium and associated RhoC activity localization. ***, p < 0.001. Scale bar = 10 µm; inserts scale bar = 1 µm.
Using MTLn3 cells co-expressing a RhoC biosensor together with TagRFP-cortactin to identify invadopodia, we visualized the RhoC activity patterns during the formation of invadopodia (Figure 2 d,e). EGF stimulation induces the formation of nascent invadopodium precursors that mature into invadopodia [6]. Surprisingly, during invadopodium assembly in response to EGF, transient activation of RhoC was observed surrounding, but not inside the invadopodium (Figure 2 d,e,f,g; Supplementary information Movie 1). Mutant versions of the RhoC biosensor (RhoC-PBD, RhoCF39A, RhoCQ63L and RhoCG14V, see Materials and Methods for detailed description) showed no measurable increase of RhoC activity surrounding the invadopodium over the background values confirming the specificity of the biosensor to report RhoC activity at invadopodia (Figure S4 e–i, Movie 3 and 4).
To determine whether this Rho GTPase activity pattern is specific to RhoC as compared to other members of the family, we investigated the spatiotemporal patterns of RhoA activation during invadopodium formation using the RhoA biosensor [27]. In contrast to the spatiotemporal localization of RhoC activity, RhoA activity showed no defined patterns of activity during invadopodium formation, essentially remaining as random fluctuations both inside and surrounding the invadopodial structures (Figure S3g–k, Movie 2).
p190RhoGAP decreases levels of RhoC activation in the invadopodium core
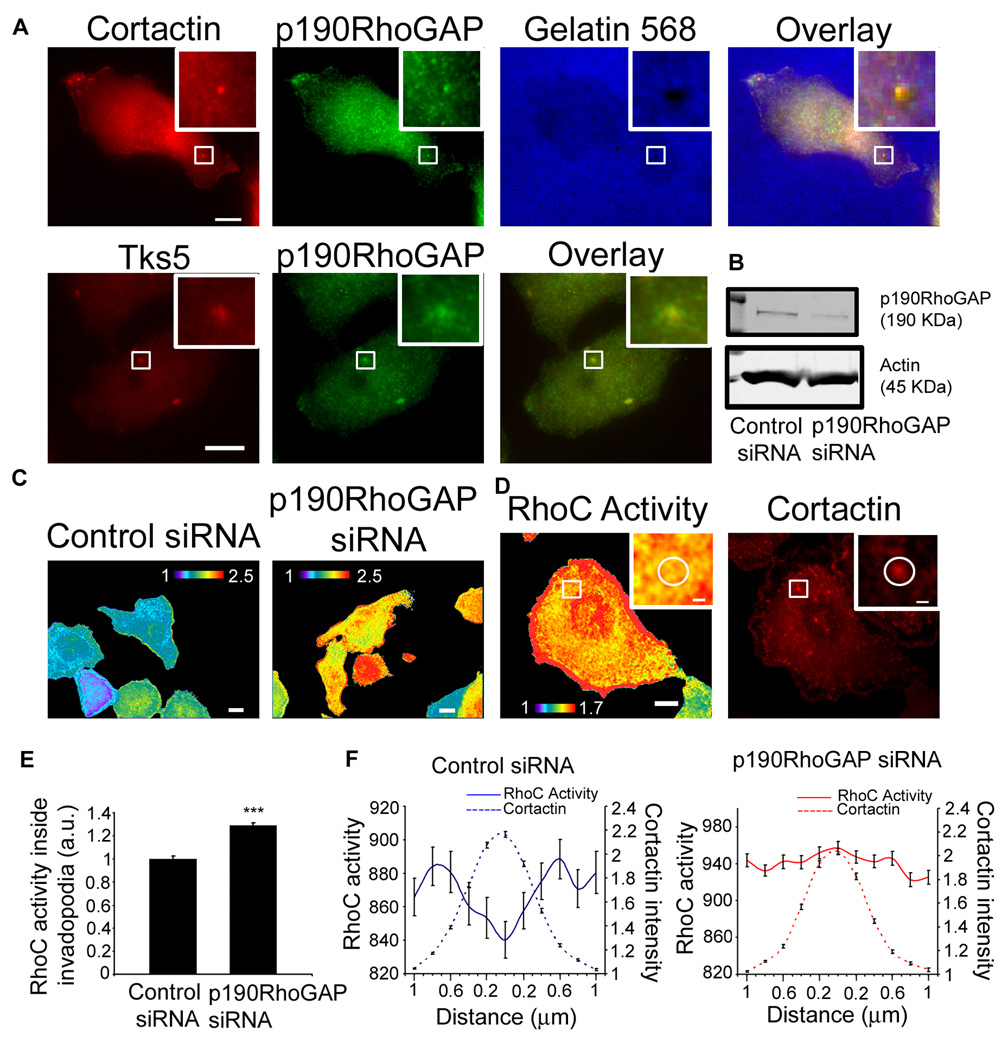
We sought to determine which factors regulate the spatiotemporal dynamics of RhoC activation at invadopodia. p190RhoGAP has been previously shown to localize to invadopodia [28] but its role in regulating GTPase activity at this actin compartment is unknown. In MTLn3 cells p190RhoGAP staining was clearly concentrated at invadopodia, identified by cortactin or Tks5 staining (Figure 3a). To investigate the contribution of p190RhoGAP to the regulation of RhoC activation, we knocked down p190RhoGAP by siRNA in MTLn3 cells expressing the RhoC biosensor (Figure 3b). RhoC activity levels were increased in p190RhoGAP siRNA cells (Figure 3c). Specifically in invadopodia, the spatial distribution of RhoC activity was lost and RhoC activity levels increased inside the cortactin invadopodium core in p190RhoGAP siRNA cells as compared to control siRNA cells (Figure 3 d,e,f). These data demonstrate that p190RhoGAP localization at the invadopodium core serves to keep RhoC inactivated within the core of the structure.
Figure 3. p190RhoGAP localization at invadopodia and regulation of RhoC activity.
a) Representative images of MTLn3 cells plated on a gelatin matrix and stained for cortactin, Tks5 and p190RhoGAP. b) Western blot of cell lysates from MTLn3 cells transfected with control siRNA or p190RhoGAP siRNA blotted for p190RhoGAP and β-actin. c) Representative images of RhoC activity in cells transfected with control or p190RhoGAP siRNA. d) Representative image of RhoC activity in p190RhoGAP siRNA cells and stained for cortactin. e) Quantification of RhoC activity inside invadopodium. n = 30 invadopodium per group from 3 independent experiments. f) Linescan measurements of RhoC activity and cortactin intensity along invadopodium. n = 30 invadopodium per group from 3 independent experiments. ***, p < 0.001. Scale bar = 10 µm; inserts scale bar = 1 µm.
p190RhoGEF activity is necessary to activate RhoC in areas surrounding invadopodia
RhoGTPases are held in balance by the opposing action of GEFs that activate them, and GAPs that inactivate them. Therefore, we hypothesized that a GEF could also play a role in this spatial regulation of RhoC activity, by activating it around the invadopodium core. p190RhoGEF is a ubiquitously expressed Rho-specific GEF that has been shown to interact with the 14-3-3 family of proteins [29]. This family of proteins binds pCofilinS3 preventing cofilin dephosphorylation [30]. Since both the 14-3-3 epsilon isoform and cofilin have been shown to localize to invadopodia [1, 31], we hypothesized that p190RhoGEF could regulate RhoC activation at invadopodia. We found that p190RhoGEF-GFP was enriched in areas around invadopodia (Figure 4a), as identified by cortactin or Tks5 staining, and completely excluded from the invadopodium core, in a manner similar to the localization of RhoC activity (Figure 4d). To study the contribution of p190RhoGEF activity to RhoC activation, we generated a catalytically-inactive mutant (p190RhoGEF-Y1003A mutant) [32] which is nucleotide exchange-deficient, thus acting in a dominant negative-like manner [33, 34]. Overexpression of p190RhoGEF-Y1003A-RFP in MTLn3 cells expressing the RhoC biosensor failed to activate RhoC (Figure 4b). At invadopodia, expression of p190RhoGEFY1003A-RFP show a significant decrease of RhoC activity levels outside invadopodia compared to control cells (Figure 4 c,e,f). These results show for the first time the involvement of p190RhoGEF in the regulation of the spatiotemporal activation dynamics of RhoC at invadopodia.
Figure 4. p190RhoGEF localization around invadopodia and regulation of RhoC activity.
a) Representative images of MTLn3 cells transfected with p190RhoGEF-GFP plated on a gelatin matrix and stained for cortactin, Tks5 and p190RhoGAP. b) and c) RhoC activity in cells transfected with p190RhoGEF-Y1003A-RFP. (Cortactin: Alexa 700) d) Quantification of p190RhoGEF intensity inside and outside the invadopodium. n = 20 invadopodium per group from 3 independent experiments, normalized to inside values. e) Quantification of RhoC activity outside the cortactin core of the invadopodium in control MTLn3 cells and MTLn3 cells transfected with p190RhoGEF-Y1003A-RFP, normalized to control MTLn3 cells value. n = 30 invadopodium per group from 3 independent experiments. f) Linescan measurements of RhoC activity and cortactin intensity along the invadopodium n = 30 invadopodium per group from 3 independent experiments. ***, p < 0.001. Scale bar = 10 µm; inserts scale bar = 1 µm.
RhoC regulates cofilin phosphorylation at serine 3 in a ROCK-dependent manner
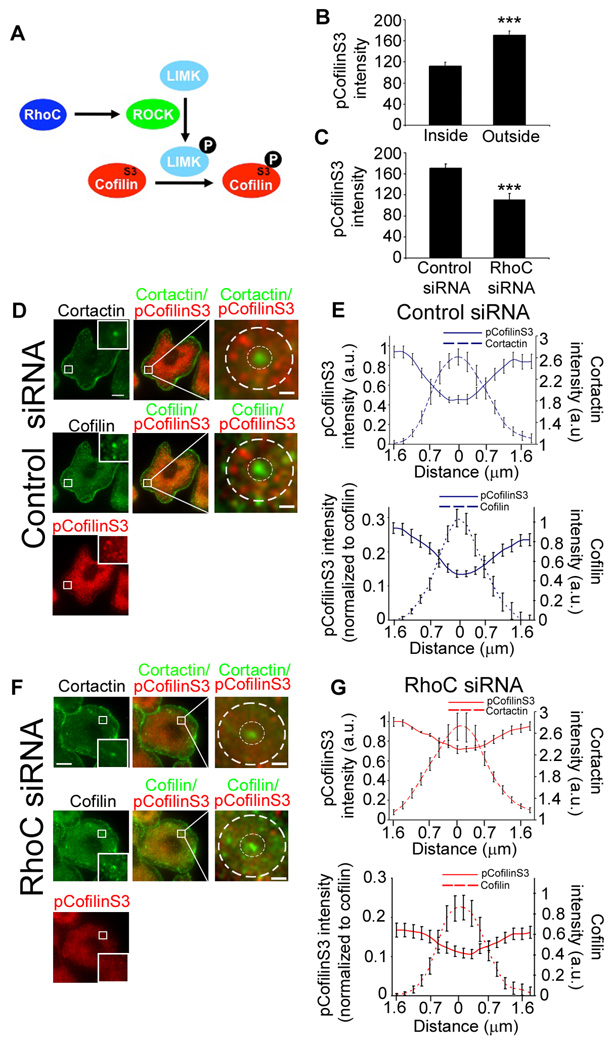
Actin dynamics play an important role during invadopodium formation and function. One of the best characterized effectors of Rho family GTPases that influence actin cytoskeleton dynamics is ROCK [17]. Activation of ROCK by RhoGTPases triggers cofilin phosphorylation through LIMK. Cofilin is essential for invadopodium maturation and is upregulated in invasive tumor cells [1, 35]. However, it is not yet known which Rho isoform regulates cofilin phosphorylation in tumor cells. Since precise regulatory control of the cofilin activity cycle is crucial in maintaining cell motility and invasive behavior [4], we examined how RhoC influences cofilin phosphorylation at invadopodia (Figure 5A).
Figure 5. RhoC controls the amount and spatial distribution of cofilin phosphorylation at invadopodia.
Distribution of cortactin, cofilin and pCofilinS3 at invadopodia in control and RhoC siRNA MTLn3 cells stimulated with 5 nM EGF for 1 min. a) Pathway for RhoC-dependent regulation of cofilin activity by phosphorylation at serine 3 (S3). b) Quantification of pCofilinS3 fluorescence intensity inside and outside the cortactin core of the invadopodium in control cells. n = 3 independent experiments with approximately 40 invadopodia per group. ***, p < 0.001 compared with inside values. c) Quantification of pCofilinS3 fluorescence intensity outside the cortactin core of the invadopodium in MTLn3 cells transfected with control or RhoC siRNA cells. p values are compared with control siRNA, n = 3 independent experiments with approximately 50 invadopodia per group. d and f) Representative images of control (d) and RhoC siRNA (f) cells stained for cortactin (Cy3), cofilin (Alexa 488) and pCofilinS3 (Cy5). Perimeter is indicated by outer dashed white circle; invadopodium core is indicated by inner dashed white circle. e and g) Measurements of fluorescence intensities of cofilin, cortactin and pCofilinS3 (normalized to the values at the outer most edge of the perimeter (upper panels) or normalized to cofilin, (lower panels)) from the perimeter to the center of invadopodia. n = 17–20 (number of invadopodia) from 3 independent experiments. Scale bar = 10 µm; inserts scale bar = 1 µm.
First, we investigated the effect of RhoC depletion on the spatial distribution of pCofilinS3 at invadopodia. To address this, we performed immunofluorescence studies in MTLn3 cells transfected with control or RhoC siRNA and co-stained with antibodies against cofilin and pCofilinS3 [12]. pCofilinS3 is concentrated outside the cortactin-containing invadopodium while cofilin is enriched inside (Figure 5 b,d,e,). RhoC siRNA cells showed a significant decrease in pCofilinS3 fluorescence intensity levels outside the invadopodium (Figure 5c). In control cells, pCofilinS3 is less concentrated inside the invadopodium (Figure 5e). In contrast, the spatial distribution of pCofilinS3 was more uniform across invadopodia in RhoC siRNA cells (Figure 5 f, g). These results suggest that the spatial distribution of pCofilinS3 in invadopodia is controlled by RhoC.
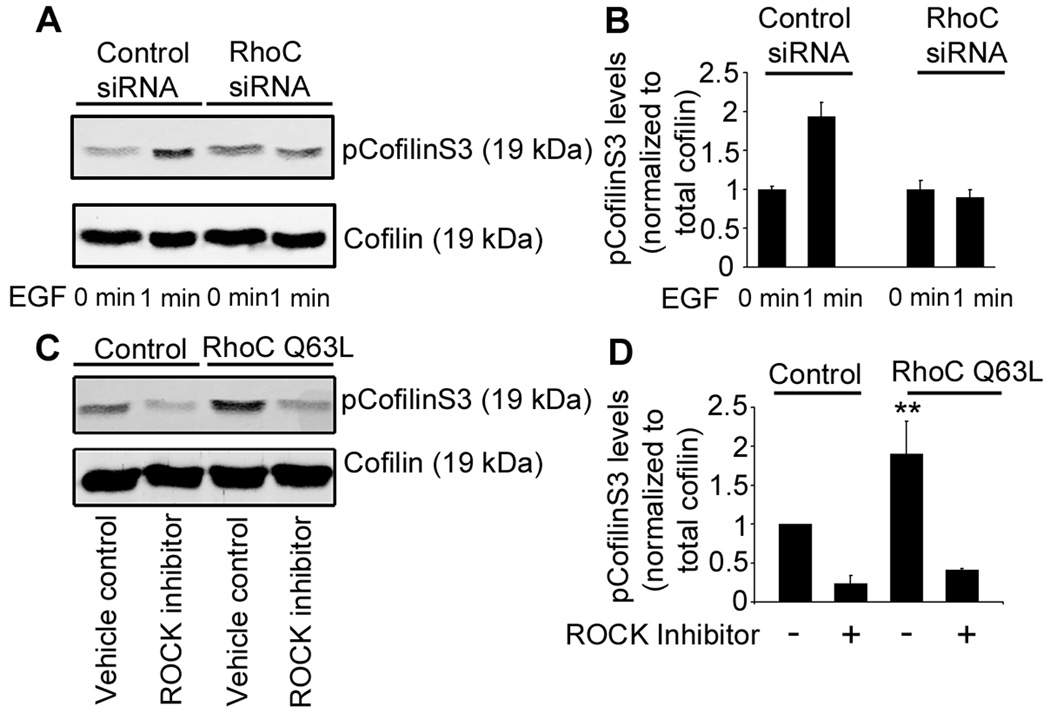
Upon EGF stimulation of MTLn3 cells, pCofilinS3 levels increase by approximately 2 fold [12]. In contrast to control cells, RhoC siRNA cells showed no significant increase in pCofilinS3 following EGF stimulation (Figure 6a and b). Interestingly, siRNA depletion of RhoA did not inhibit pCofilinS3 levels in response to EGF (Figure S3b) indicating that in MTLn3 cells, RhoC, but not RhoA, regulates the levels of pCofilinS3.
Figure 6. RhoC regulates cofilin phosphorylation at serine 3 through ROCK activation.
a) Western blot and quantification (b) of MTLn3 lysates from control or RhoC siRNA cells blotted for cofilin and pCofilinS3. Cells were starved for 3 h and stimulated with 5 nM EGF for 1 min. n = 3 independent experiments. c) Western blot and quantification (d) of MTLn3 lysates from cells transfected with RFP or RhoC-Q63L-RFP in the presence of vehicle control (DMSO) or the ROCK inhibitor (5 µM, H-1152) showing pCofilinS3 and cofilin levels. n = 3 independent experiments. p values are compared to RFP vehicle control **, p < 0.01.
MTLn3 cells depleted of cofilin exhibited an elongated morphology [36]. Since RhoC controls cofilin phosphorylation, we hypothesize that a constitutively-activated mutant of RhoC would mimic the cofilin knockdown phenotype. Indeed, cells that overexpressed the constitutively-activated mutant RhoC-Q63L-RFP showed an elongated phenotype compared to control cells (Figure S5a,b).
Based on these observations, we hypothesized that RhoC could regulate cofilin activity through its known effector, ROCK (Figure 5a). To test this hypothesis, we first measured the pCofilinS3 levels in cells overexpressing RhoC-Q63L. Cells that overexpressed RhoC-Q63L showed a greater than two-fold increase in pCofilinS3 (Figure 6c,d) compared to control cells transfected with RFP alone. This increase in pCofilinS3 is ROCK-dependent since incubation with the ROCK inhibitors H-1152 (5 µM) (Figure 6c,d) and Y-27632 (10 µM) (Figure S6a,b) abrogated the increase in cofilin phosphorylation.
To test the involvement of LIMK activity in pCofilinS3 regulation, we knocked down LIMK1 and LIMK2 and measured the change in pCofilinS3 levels. The double knockdown of LIMK1/LIMK2 dramatically reduced pCofilinS3 levels (Figure S6 e–j). Based on our results, we propose that ROCK can phosphorylate LIMK to inactivate cofilin and this pathway is controlled by RhoC.
RhoC regulates cofilin-dependent formation of free barbed ends at invadopodia
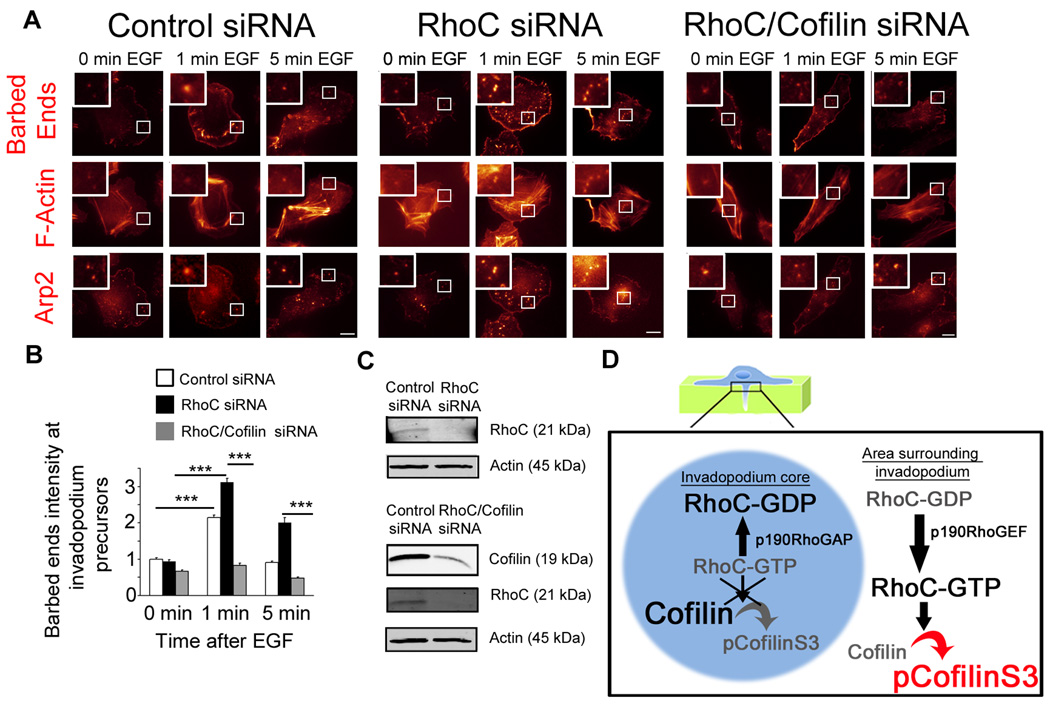
EGF stimulation of MTLn3 cells increases the number of cofilin-dependent actin barbed ends at invadopodium precursors [6]. We hypothesized that the regulation of pCofilinS3 by RhoC at invadopodia may affect the number of actin barbed ends at this structure. Indeed, RhoC siRNA cells showed an increase in the number of actin barbed ends upon EGF stimulation over control (Figure 7a and b), prolonging the actin barbed-end persistence even at 5 min following EGF stimulation, a time when actin barbed-end activity in control cells has been switched off (Figure 7B). Furthermore, cofilin is the primary isoform involved in barbed end generation at invadopodia, since siRNA depletion of ADF (Figure S7d) (the other cofilin isoform expressed in MTLn3 cells) did not affect barbed end formation at invadopodia (Figure S7e). Based on the RhoC-dependent change in pCofilinS3 levels, we hypothesized that the prolonged barbed-end response observed in RhoC siRNA cells was due to an inability to inactivate cofilin severing activity at invadopodia. Knockdown of cofilin (without affecting the levels of ADF (Figure S7a,b,c)) and RhoC (Figure 7c) resulted in a decrease in the relative number of barbed ends compared to RhoC knockdown only (Figure 7b). These results indicate that the barbed ends induced by RhoC knockdown are cofilin-dependent.
Figure 7. RhoC regulates cofilin-dependent actin barbed ends.
Actin barbed end assay from control, RhoC or RhoC/cofilin siRNA-treated MTLn3 cells plated on gelatin matrix. a) Representative images of barbed ends, F-actin and Arp2 staining at invadopodia. Scale bar = 10 µm. b) Quantification of barbed end intensity at invadopodia normalized to control siRNA at 0 min. p values are compared to control siRNA or to RhoC siRNA, n = 3 independent experiments with approximately 120 invadopodia per group. ***, p < 0.001. c) Western blot of lysates from MTLn3 control, RhoC or RhoC/cofilin siRNA cells blotted for cofilin, RhoC and β-actin. d) Model for the spatial regulation of cofilin activity by RhoC at invadopodia.
Similar to RhoC depletion, ROCK inhibition or knock down of LIMK1 and 2 increases the number of barbed ends at invadopodia (Figures S6C, S6D and S6K) suggesting that regulation of cofilin phosphorylation by RhoC activation of ROCK/LIMK pathway is important in regulating the generation of cofilin-dependent barbed ends at invadopodia.
Discussion
Based on our results, we propose a new model to explain the spatial restriction of cofilin activity in invadopodia (Figure 7d). In this model, the spatiotemporal dynamics of activated RhoC forms an activity barrier surrounding the invadopodium. This RhoC activation leads to cofilin phosphorylation thereby restricting the cofilin activity to the F-actin-rich core of invadopodia. This focuses cofilin’s severing activity to invadopodium core to create free barbed ends for actin polymerization and filament turnover required for the generation of protrusive force. Cells which cannot focus their cofilin activity would show defects in actin polymerization within the invadopodium core, leading to abnormal branched invadopodia capable of only superficial degradation of matrix but unable of penetrating the matrix to greater depths. The localization of RhoC activity surrounding the invadopodium core is achieved by activation of p190RhoGEF outside invadopodia and p190RhoGAP inside in invadopodia. This balance of GEF and GAP localization results in restricting RhoC activity to areas surrounding the invadopodium and its exclusion from the core of the invadopodium.
It has been shown previously that cofilin severing activity is essential for the generation of barbed ends in invadopodia [4, 6]. Here, we demonstrate that breast tumor cells spatially restrict RhoC activity to increase cofilin phosphorylation and cofilin inactivation in areas surrounding invadopodia. This novel finding could provide a mechanism for how tumor cells focus long protrusions with efficient matrix degradation during invasion by spatially focusing cofilin activity within invadopodia.
Regulation of RhoC activity at invadopodia
In response to chemotactic signals, GTPases can be activated in multiple compartments simultaneously. The spatiotemporal regulation of these activities is essential for conveying specificity to these signals. There is mounting evidence that the spatiotemporal control of these signals is achieved by specific GEFs/GAPs at specific locations within the cell. Little is known about GEFs and GAPs at invadopodia and only two GEFs have been shown to be important for invadopodial function: Fgd1, a Cdc42-specific GEF [37] and fabrin, a Rac/Cdc42 GEF [38]. As for GAPs, only p190RhoGAP has been localized to invadopodia and been shown to be important for the matrix degradation activity by invadopodia [28].
In this study, we provide evidence that the confinement of RhoC activation to areas surrounding the invadopodia is achieved by “spatially distinct regulatory elements”. These “spatially distinct regulatory elements” consists of p190RhoGEF, localized outside invadopodia, and p190RhoGAP localized within the core of the structure. Furthermore, we hypothesize that the localization of the pre-activated pool of p190RhoGEF to areas surrounding the invadopodium core directly activates RhoC upon RhoC delivery to this location during invadopodium formation. Activation of p190RhoGAP inside and p190RhoGEF outside the invadopodium will constrict the RhoC activation to within a specific area surrounding the core.
Understanding which GEFs and GAPs are upregulated or downregulated in invasive tumor cells and how these alterations affect GTPase activation in specific subcellular compartments will yield the clues on how spatiotemporal GTPase signaling contributes to the invasiveness of tumor cells.
RhoC and RhoA have different roles in invadopodium function
Although RhoC and RhoA are both members of the Rho family of GTPases, they seem to play distinct roles during invadopodium function. As previously described, RhoA regulates matrix degradation through a mechanism that involves delivery of MT1-MMP to the invadopodia [5]. In support of this observation we found that depletion of RhoA in MTLn3 cells blocks matrix degradation.
ROCK has also been shown to have a higher affinity for RhoC than for RhoA or RhoB [17], and RhoC appears to have a stronger ability to activate ROCK in epithelial cells[39]. These studies and our results suggest that regulation of ROCK/LIMK pathway by RhoC is the predominant pathway controlling the activity of cofilin by phosphorylation.
Regulation of cofilin activity at invadopodia by RhoC
In mammary tumors, the expression of genes involved in the cofilin pathway show alterations in the invasive tumor cell population implicating an increase in the output of the cofilin pathway as an important determinant of tumor cell invasion and metastasis [40, 41]. Regulation of cofilin activity is critical for chemotaxis, invasion and metastasis of mammary tumor cells [8, 9, 35]. ADF, a cofilin isoform, does not appear to have a significant contribution to protrusion at the leading edge [7, 36] and actin dynamics at invadopodia in MTLn3 cells (Figure S7e). Cofilin activity is predominant in these processes, since ADF cannot compensate for the loss of cofilin activity [6, 36]. As an extension to our model, we speculate that RhoC may regulate membrane protrusions at the leading edge via the spatial confinement of cofilin activity to enhance the local excitation global inhibition (LEGI) response [13, 34] in a manner analogous to what we have described here for invadopodia.
This work demonstrates that RhoC enhances pCofilinS3 levels outside invadopodia. pCofilinS3 has been shown to activate phospholipase D1 and generate phosphatidic acid at the plasma membrane that can activate DOCK family of proteins [42]. This suggests that pCofilinS3 could contribute to the activation of p190RhoGEF resulting in a feed-forward activation cycle of RhoC.
The pathway described here (Figure 5a) predicts that inhibition of any of the components of the RhoC/ROCK/LIMK pathway will unbalance the cofilin activity cycle at invadopodia, affecting tumor cell invasion. Indeed, ROCK inhibition decreases invasion and motility in vivo of invasive tumor cells [43] and LIMK inhibition in tumor cells correlates with reduced invasion of human breast cancer MDA-MB-231 cells by affecting the formation of invadopodia [9].
In summary, our results show that the spatiotemporally restricted regulation of RhoC activity at invadopodia is necessary to balance the activation status of cofilin, and ultimately control tumor cell invasion. We hypothesize that perturbation of the upstream regulators of RhoC activity at invadopodia, such as p190RhoGAP and p190RhoGEF, will also affect invasion of tumor cells. This study identifies new players in the regulation of the cofilin regulatory cycle that can potentially provide therapeutic drug targets to block tumor metastasis.
Experimental procedures
Details of the invadopodium degradation assay, invasion assay, invadopodium protrusion assay, barbed end assay, inmunofluorescence, electron microscopy, fluorescence imaging and imaging analysis, as well as all the reagents used in this study can be found in the Supplemental Experimental Procedures.
Cell culture and DNA transfection
For all experiments, MTLn3 cells, derived from 13762NF rat mammary adenocarcinoma, were cultured in α-MEM supplemented with 5% FBS plus antibiotics and starved and stimulated with EGF as described previously [7]. For transient expression experiments, MTLn3 cells were transfected with Lipofectamine (Invitrogen, Carlsbad, CA) 24 hr before each experiment. MTLn3 cell lines stably expressing RhoC, RhoC-PBD, RhoC-F39A or RhoA biosensor [24, 27] were cultured in the presence of doxycyclin (1 µg/ml). Doxycyclin was removed 72 h prior to the experiment in order to induce the biosensor expression which is under the control of the tet-OFF system (Clontech).
Biosensor imaging
Activation levels of RhoA or RhoC were measured in living cells by monitoring the ratio of Citrine-YFP FRET over the donor ECFP intensities for the RhoA biosensor [27], and the ratio of Venus FRET over the donor Cerulean intensities for the RhoC biosensor.
Details of the biosensor imaging analysis can be found in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank the Condeelis, Cox, and Segall laboratories for helpful discussions; the Analytical Imaging Facility of the Gruss Lipper Biophotonics Center at Albert Einstein College of Medicine for technical help. We thank Geoffrey Perumal for his technical help with TEM. We thank Dr. Vladislav Verkhusha (Einstein) for providing pm-TagRFP-T-C1 and Dr. David D. Schlaepfer for providing p190RhoGEF-GFP. This work was funded by GM064346 (J.J.B-C.), CA150344 (J.J.B-C., M.O., R.E., J.C.), CA100324 (X.C.), GM093121 (L.H., J.J.B-C.) and “Sinsheimer Foundation Young Investigator Award” (LH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
J.J.B.C., designed and performed the experiments that constitute the main body of this work; M.O., X.C., R.E., L.H. designed and performed additional experiments; J.C., L.H. designed experiments and provided expertise and intellectual input, assisted in interpreting the data and coordinated the project; J.J.B.C. wrote the manuscript and J.C., L.H., R.E., M.O. edited the manuscript.
References
- 1.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 3.Gimona M, Buccione R. Adhesions that mediate invasion. Int J Biochem Cell Biol. 2006;38:1875–1892. doi: 10.1016/j.biocel.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108:1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, Goswami S, Bresnick AR, Condeelis JS. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol. 2006;173:395–404. doi: 10.1083/jcb.200510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, Dawson J, Machesky L, Anderson KI, Sahai EA, Olson MF. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol. 2010;191:169–185. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 12.Song X, Chen X, Yamaguchi H, Mouneimne G, Condeelis JS, Eddy RJ. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J Cell Sci. 2006;119:2871–2881. doi: 10.1242/jcs.03017. [DOI] [PubMed] [Google Scholar]
- 13.Mouneimne G, DesMarais V, Sidani M, Scemes E, Wang W, Song X, Eddy R, Condeelis J. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr Biol. 2006;16:2193–2205. doi: 10.1016/j.cub.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Price LS, Collard JG. Regulation of the cytoskeleton by Rho-family GTPases: implications for tumour cell invasion. Semin Cancer Biol. 2001;11:167–173. doi: 10.1006/scbi.2000.0367. [DOI] [PubMed] [Google Scholar]
- 15.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 16.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, Mak TW. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleer CG, Griffith KA, Sabel MS, Gallagher G, van Golen KL, Wu ZF, Merajver SD. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat. 2005;93:101–110. doi: 10.1007/s10549-005-4170-6. [DOI] [PubMed] [Google Scholar]
- 20.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 21.Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694–8701. doi: 10.1158/0008-5472.CAN-04-2247. [DOI] [PubMed] [Google Scholar]
- 22.Pille JY, Denoyelle C, Varet J, Bertrand JR, Soria J, Opolon P, Lu H, Pritchard LL, Vannier JP, Malvy C, Soria C, Li H. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267–274. doi: 10.1016/j.ymthe.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Hodgson L, Pertz O, Hahn KM. Design and optimization of genetically encoded fluorescent biosensors: GTPase biosensors. Methods Cell Biol. 2008;85:63–81. doi: 10.1016/S0091-679X(08)85004-2. [DOI] [PubMed] [Google Scholar]
- 24.Zawistowski J, Sabouri-Ghomi M, Hodgson L, Danuser G, Hahn K. Differential activation of RhoA and RhoC in migrating cells. Submitted. 2010 doi: 10.1371/journal.pone.0079877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–2800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 26.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 28.Nakahara H, Mueller SC, Nomizu M, Yamada Y, Yeh Y, Chen WT. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 29.Zhai J, Lin H, Shamim M, Schlaepfer WW, Canete-Soler R. Identification of a novel interaction of 14-3-3 with p190RhoGEF. J Biol Chem. 2001;276:41318–41324. doi: 10.1074/jbc.M107709200. [DOI] [PubMed] [Google Scholar]
- 30.Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol. 2002;12:1704–1710. doi: 10.1016/s0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- 31.Attanasio F, Caldieri G, Giacchetti G, van Horssen R, Wieringa B, Buccione R. Novel invadopodia components revealed by differential proteomic analysis. Novel invadopodia components revealed by differential proteomic analysis. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Horck FP, Ahmadian MR, Haeusler LC, Moolenaar WH, Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- 34.Lee JR, Ha YJ, Kim HJ. Cutting edge: induced expression of a RhoA-specific guanine nucleotide exchange factor, p190RhoGEF, following CD40 stimulation and WEHI 231 B cell activation. J Immunol. 2003;170:19–23. doi: 10.4049/jimmunol.170.1.19. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidani M, Wessels D, Mouneimne G, Ghosh M, Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, Eddy R, Soll D, Condeelis J. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol. 2007;179:777–791. doi: 10.1083/jcb.200707009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, Polishchuk R, Castronovo V, Buccione R. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009;69:747–752. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]
- 38.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 39.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE, Condeelis JS. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 42.Bamburg JR, Bernstein BW. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep. 2010;2:62. doi: 10.3410/B2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.