Abstract
Little is known about the effects of non-injection drug use (NIDU) on HIV antiretroviral treatment outcomes. We conducted a systematic literature search and identified 9 publications from prospective cohort studies investigating the relationship between NIDU and clinical HIV disease progression. Hazard ratios from studies estimating the effect of drug use on time to AIDS-related mortality ranged from 0.89 to 3.61 and only two of these were statistically significant. Hazard ratios from studies assessing time to an AIDS-defining event ranged from 1.19 to 2.51, with 8 of the 14 estimates falling between 1.55 to 1.65 regardless of drug use definition and measurement of use or frequency. It is suggested that NIDU may have a moderate effect of increasing the risk of progression to AIDS, but its impact on AIDS-related mortality is uncertain. NIDU may affect HIV antiretroviral treatment outcomes primarily through interaction with antiretroviral therapy and, to a lesser extent, through immune-modulation and deterioration of general health. The limitations about published studies are discussed and future perspectives on research on this topic are provided.
Keywords: Non-injection drug use, HIV, antiretroviral therapy, disease progression
1. Introduction
Illicit drug use is an important factor in the HIV/AIDS pandemic. The annual prevalence of illicit drug use is 4.8% worldwide, representing 208 million people between 15–64 years of age who have used an illicit drug at least once in the past 12 months (United Nations Office of Drugs and Crime, 2008); only 5–10% of those drug users are injecting drugs (Mathers, et al., 2008). Injection drug use (IDU), particularly sharing of contaminated injection tools, is an efficient means of HIV transmission, and an estimated 3 million (range 1–7 million) injectors may be living with HIV. Non-injection drug use (NIDU), defined as the non-medical, self-administration of any psychoactive substance by any route other than intravenous (e.g. via intranasal, inhalation, or ingestion), is also an important risk factors indirectly associated with HIV infection by various mechanisms: (1) the effects of drug use adversely affect judgment and decision-making, resulting in high-risk sexual behavior; (2) trading sex or participating in risky sexual practices in exchange for drugs; and (3) the immune-modulating effects of drugs have been shown to increase HIV viral load, decrease host defense to viral exposure, up-regulate HIV-specific cellular receptors, and increase the presence of lesions due to other bacterial or viral infections (Astemborski, Vlahov, Warren, Solomon, & Nelson, 1994; Brewer, Zhao, Metsch, Coltes, & Zenilman, 2007; Cabral, 2006; de Souza, Diaz, Sutmoller, & Bastos, 2002; Edlin, et al., 1994; Ramirez-Valles, Garcia, Campbell, Diaz, & Heckathorn, 2008).
Illicit drug use, and NIDU in particular, are common among HIV-infected individuals. A nationally representative probability sample in the United States of persons receiving HIV care found that 40% reported using an illicit drug other than marijuana during the preceding 12 months (Bing, et al., 2001). Another study found that 28% of HIV-infected men who have sex with men (MSM) reported illicit drug use in the past 30 days (Cofrancesco, et al., 2008). The Women’s Interagency HIV Study (WIHS) specifically evaluated the prevalence of NIDU among a prospective cohort in 6 U.S. sites and found 59% of HIV-positive women were actively using non-injection drugs (Kapadia, et al., 2005). Many other studies of HIV-infected individuals have found the prevalence of recent marijuana use to range from 10% to 63% and recent cocaine use to range from 4% to 47% (Cofrancesco, et al., 2008; Hessol, et al., 2007; Prestage, et al., 2007; Purcell, Moss, Remien, Woods, & Parsons, 2005; Sohler, et al., 2007; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003).
Since highly active combination antiretroviral therapy (cART) became available in the mid-1990s, the effect of illicit drug use on cART treatment outcomes has become a medical and public health concern and has been widely studied, primarily among IDUs. The Antiretroviral Therapy Cohort Collaboration (ART-CC) (Antiretroviral Therapy Cohort Collaboration, 2007; Egger, et al., 2002; May, et al., 2007) and others (Nicastri, et al., 2005; Perez-Hoyos, et al., 2006) have reported a positive relationship between IDU and more rapid disease progression. Some smaller studies, however, have reported no association (Eskild, et al., 1997; Pehrson, Lindback, Lidman, Gaines, & Giesecke, 1997; Wood, et al., 2008). Numerous methodological issues arise from these studies. First, the measure of IDU is often based on reported HIV transmission category. This measure likely represents a history of drug use rather than active drug use and, therefore, misclassification may occur. Furthermore, it does not distinguish duration and/or frequency of drug injection. Second, the IDUs are often compared to MSM, which necessarily restricts the results to men, discarding all information on IDUs who are women. Few studies have assessed the effect of active IDU, duration, or frequency with disease progression and they have reported inconsistent findings (Greub, et al., 2000; Pezzotti, et al., 1999).
Non-injection drug use likely plays an important role in HIV disease progression because it shares similar biologic, social, and behavioral consequences as IDU. Nevertheless, its role in HIV disease progression is an understudied medical and public health concern given that the majority of illicit drug users consume drugs via non-injection. The purpose of this review is to systematically report on studies of NIDU and its effect on HIV disease progression in the cART era.
2. Methods
2.1. Definition of exposure and outcome variables
For the purposes of this review, the exposure of interest is NIDU. Studies were considered to have measured NIDU if the study definition of drug use included at least one reference to a non-injection drug (such as marijuana), crack (a form of inhalational cocaine), or use of an illicit drug via non-injection routes. As such, some studies have a general definition of drug use that combines both injection and non-injection use. Alcohol abuse is often considered NIDU, but will not be considered here because a separate review by Azar et al. will be published soon (Azar, Springer, Meyer, & Altice, 2010).
The outcome of interest in this review is clinical HIV disease progression identified as an AIDS-defining illness or AIDS-related death. Studies that focused only on surrogate measures of disease progression, such as CD4 cell count decline or an increase in HIV viral load, were not considered. While these are precursors to AIDS, they may vary over time and are reversible with effective treatment. A clinical diagnosis of AIDS is not a reversible event (Centers for Disease Control and Prevention, 1992).
2.2. Search strategy
We searched the PubMed database to identify peer-reviewed studies of NIDU and HIV disease progression published between January 1996 and December 2009. We used four categories of terms for literature search: study type, drug use, HIV/AIDS, and HIV disease progression. Specifically, the following combination of terms was used: (prospective OR longitudinal OR cohort) AND (“substance abuse” OR “drug abuse” OR “drug use” OR marijuana OR heroin OR amphetamine OR cocaine OR crack OR opioid OR opiate) AND (“HIV OR AIDS”) AND (“disease progression” OR “AIDS-defining illness” OR “AIDS-defining event” OR death).
2.3. Selection criteria and data extraction
All abstracts were independently reviewed by two of the authors (AMK and HQ) to determine which studies should be selected for more detailed review. When there was disagreement about whether to further review a study, the authors reviewed the abstract together and came to a consensus. Studies that met the following criteria were selected for inclusion in this review: non-injection drug use and disease progression as defined above, all or part of the follow-up occurring in 1996 or later, and published in English. References from included studies were reviewed to identify additional studies.
A secondary purpose of this review was to characterize the specific mechanisms of HIV disease progression, including immune-modulating effects of illicit drugs, utilization of and adherence to cART, and possible pharmacological interactions between antiretrovirals (ARVs) and illicit drugs. Published studies on these topics were not identified in a systematic fashion.
3. Results
3.1. Study selection and study characteristics
Abstracts from 315 publications were reviewed. Twenty-two papers were not in English and 5 were concluded prior to 1996. An additional 279 papers were excluded due to one or more of the following reasons: (1) study was conducted among IDUs only; (2) study did not use AIDS-defining even (ADE) or AIDS-related mortality as study endpoints (e.g., using CD+ cell count or HIV viral load); (3) study did not use drug use as the primary or confounding risk factor; and (4) study was not a prospective cohort design. Cross-sectional studies were not included because they could not ascertain temporal relationship of exposure (drug use) and outcome (ADE), and some used history data which could not determine the frequency and/or activity of drug use. A total of 9 publications met the inclusion criteria and were included in this review of the relationship of NIDU and HIV progression to ADE or AIDS-related mortality (Anastos, et al., 2005; Cohen, et al., 2002; Cook, et al., 2008; Cook, et al., 2004; Hershow, et al., 2005; Ickovics, et al., 2001; Kapadia, et al., 2005; Lucas, et al., 2006; Thorpe, et al., 2004).
All of the studies were performed in the United States, and most were conducted between 1994 and 2004, except two that began in 1989 (Hershow, et al., 2005; Thorpe, et al., 2004). Of note is that 8 of the studies were done among women only, with 5 of these coming from the WIHS (Anastos, et al., 2005; Cohen, et al., 2002; Cook, et al., 2008; Cook, et al., 2004; Kapadia, et al., 2005). Two publications used pregnant women study subjects and both were from the Women and Infant Transmission Study (WITS) (Hershow, et al., 2005; Thorpe, et al., 2004).
3.2. Measurements of exposure and outcome variables
The proportion of study participants who used drugs during the study period ranged from 25.5% (Cohen, et al., 2002) to 58.6% (Kapadia, et al., 2005). Five of the studies had the primary purpose of assessing drug use on disease progression (Cohen, et al., 2002; Cook, et al., 2008; Kapadia, et al., 2005; Lucas, et al., 2006; Thorpe, et al., 2004), while the remaining studies were not specifically performed to assess for the effect of drug use, but they did report an effect of drug use as a covariate. Three studies had a measure of drug use that included frequency rather than use or non-use of a particular drug or drugs (Cook, et al., 2008; Kapadia, et al., 2005; Lucas, et al., 2006).
Three studies assessed time to AIDS-related mortality only (Cohen, et al., 2002; Cook, et al., 2004; Ickovics, et al., 2001), 2 assessed time to AIDS-defining event only (Lucas, et al., 2006; Thorpe, et al., 2004), 3 performed separate analyses for both AIDS-related mortality and AIDS-defining event (Anastos, et al., 2005; Cook, et al., 2008; Kapadia, et al., 2005), and 1 study combined AIDS-defining event and all-cause mortality as the outcome under study (Hershow, et al., 2005).
3.3. Association of NIDU and HIV disease progression
Among 6 studies estimating the effect of drug use on time to AIDS-related mortality (Table 1), the point estimates of hazard ratios ranged from 0.89 to 3.61. Of the 11 hazard ratios, 5 were equal to or just below 1.0 while the remaining 6 were ≥1.27. Only two of these, however, were statistically significant (Anastos, et al., 2005; Cook, et al., 2008). The study by Cook et al. is a good example of how measurement of drug use can impact the results: compared with non-users, persistent drug users had nearly 4 times the risk of death (HR=3.6) while intermittent drug users had nearly the same risk (HR=0.9) (Cook, et al., 2008). The other positive study showed that current crack, cocaine, or heroine users (route unspecified) had 2.35 times the hazard of mortality than non-users (p<0.05) (Anastos, et al., 2005).
Table 1.
Cohort studies on the association between non-injection drug use and risk of AIDS-related mortality
| Publication | Population | Sample size |
Study purpose | Definition of drug use | Definition of AIDS-related mortality |
Adjusted measure of effect § |
|---|---|---|---|---|---|---|
| Cohen, 2002* | Women | 2,059 | To examine the causes of AIDS and non-AIDS mortality | Non-injection cocaine or heroin use | Death due to AIDS-defining illness, or non-specific infection or organ failure with last CD4<200 | Drug use vs. no use: IRR=1.3 (0.9, 1.8) |
| Cook, 2004*,¶ | Women | 1,716 | To examine associations between depressive symptoms and AIDS-related mortality | Use of crack, cocaine or heroin at anytime during the study | Same as above | Drug use vs. no use: HR=0.9 (0.6, 1.4) |
| Cook, 2008* | Women | 1,686 | To examine the association between patterns of crack use and AIDS mortality | Repeated measures of frequency of crack use in the past 6 months over study period | Same as above | Intermittent vs. no use: HR=0.93, p ≥0.05 Persistent vs. no use: HR=3.61, p<0.001 |
| Anastos, 2005*,¶ | Women | 961 | To determine association between race and cART treatment effects | Current use of cocaine, heroin, or crack | Same as above | Current drug use vs. no use: HR=2.35, p<0.05 |
| Kapadia, 2005* | Women | 1,046 | To evaluate association of NIDU and HIV disease progression | Non-injection use of depressants (alcohol, marijuana, heroin), stimulants (crack, cocaine), or both (polydrug) | Same as above | Depressant vs. no use: HR=0.95 (0.66, 1.37) Stimulant vs. no use: HR=1.65 (0.85, 3.20) Polydrug vs. no use: HR=0.89 (0.59, 1.31) |
| Frequency of any non-injection drug use | Former vs. never use: HR=1.27 (0.82, 1.96) Inconsistent vs. never use: HR=0.99 (0.66, 1.50) Consistent vs. never use: HR=1.42 (0.87, 2.33) |
|||||
| Ickovics, 2001¶ | Women | 765 | To determine association between depressive symptoms and HIV-related mortality | Non-injection use of crack or cocaine, injection drug use, or both during the study period | HIV-related deaths were identified via review of medical records, no other details provided | Both non-injection crack/cocaine use and injection drug use were unassociated with HIV-related mortality in univariate analyses (p<0.05). |
Women’s Interagency HIV Study (WIHS);
Women and Infant Transmission Study (WITS);
Drug use was not primary exposure being assessed;
Variables included as covariates in the multivariable models:
Cohen 2002: age, alcohol, ART/cART, hepatitis B virus, hepatitis C virus, IDU (history), IDU (recent use), race, smoking, viral load.
Cook 2004: adherence, age, CD4 count, depression, education, employment, ART/cART use, income, marital status, mental health service utilization, race, residential status, study site, symptoms, viral load.
Cook 2008: age, CD4 count, education, income, race, study site, viral load, year of HIV diagnosis.
Anastos 2005: adherence, age, CD4 count, depression, ART/cART, HIV exposure, income, prior AIDS defining event, race, smoking, treatment naïve prior to cART initiation, viral load.
Kapadia 2005: age, CD4 count, emergency room visit in last 2 months, cART use, viral load.
In contrast to studies assessing time to AIDS-related mortality, there was more consistency among studies assessing time to an AIDS-defining event. Hazard ratios ranged from 1.19 to 2.51 with 8 of the 14 estimates falling between 1.55 to 1.65 regardless of drug use definition, measurement of use or frequency, or whether drug use was of primary or secondary interest. Eleven of 14 estimates were statistically significant (Table 2). Nearly all studies adjusted for age, race, cART use, CD4 count or percent, and viral load, but beyond this there was great heterogeneity in the covariates of the multivariable models.
Table 2.
Cohort studies on the association between non-injection drug use and risk of AIDS-defining event (ADE)
| Publication | Population | Sample size |
Study purpose | Definition of drug use | Definition of AIDS- defining event (1993 CDC case definition) |
Adjusted measure of effect§ |
|---|---|---|---|---|---|---|
| Cook, 2008* | Women | 1,686 | To examine the association between patterns of crack use and HIV disease progression | Repeated measures of frequency of crack use in the past 6 months over study period | New ADE excluding CD4 count <200 | Intermittent vs. no use: HR=1.57, p<0.001 Persistent vs. no use: HR=1.65, p<0.05 |
| Anastos, 2005*,¶ | Women | 961 | To determine association between race and cART treatment effects | Current use of cocaine, heroin, or crack | Same as above | Current drug use vs. no use: HR=1.49, p<0.05 |
| Kapadia, 2005* | Women | 701 | To evaluate association of NIDU and HIV disease progression | Non-injection use of depressants (alcohol, marijuana, heroin), stimulants (crack, cocaine), or both (polydrug) | Same as above | Depressant vs. no use: HR=1.19 (0.90, 1.58) Stimulant vs. no use: HR=2.04 (1.06, 3.94) Polydrug vs. no use: HR=1.65 (1.21, 2.25) |
| Frequency of any non-injection drug use | Former vs. never use: HR=1.56 (1.05, 2.32) Inconsistent vs. never use: HR=1.63 (1.15, 2.30) Consistent vs. never use: HR=2.51 (1.60, 3.96) |
|||||
| Lucas, 2006 | Men and women | 1,851 | To assess longitudinal association of drug use with HIV disease progression | Frequency of heroin or cocaine use (any route) | New/recurring ADE | Intermittent (abstinent period) vs. no use: HR=1.3 (0.9, 1.8) Intermittent. (active period) vs. no use: HR=1.6 (1.2, 2.3) Persistent vs. no use: HR=1.9 (1.2, 2.8) |
| Hershow, 2005**,¶ | Pregnant women | 652 | To examine association of HCV coinfection and HIV disease progression | Self-reported cocaine, crack, heroin, or other opiate (including methadone) use; or any IDU; or positive urine for drugs | First ADE or all-cause mortality | Drug use in past year: HR=1.62 (0.81, 3.24) |
| Thorpe, 2004** | Pregnant women | 1,148 | To examine association of drug use and HIV disease progression | Self-reported cocaine, crack, heroin, or other opiate (including methadone) use; or any IDU; or positive urine for drugs | First ADE excluding CD4 count <200 | Drug use vs. no use: HR=1.65 (1.00, 2.72) |
Women’s Interagency HIV Study (WIHS);
Women and Infant Transmission Study (WITS).
Drug use was not primary exposure being assessed
Variables included as covariates in the multivariable models:
Cook 2008: age, CD4 count, education, income, race, study site, viral load, year of HIV diagnosis.
Anastos 2005: adherence, age, CD4 count, depression, ART/cART, HIV exposure, income, prior AIDS defining event, race, smoking, treatment naïve prior to cART initiation, viral load.
Kapadia 2005: CD4 count, emergency room visit in last 2 months, cART use, viral load.
Lucas 2006: adherence, age, alcohol, CD4 count, gender, race, viral load.
Hershow 2005: age, CD4 percent, ART/cART, hepatitis C virus, viral load.
Thorpe 2004: age, CD4 percent, ART/cART, smoking, subsequent pregnancies
3.4. Mediators of drug use and disease progression
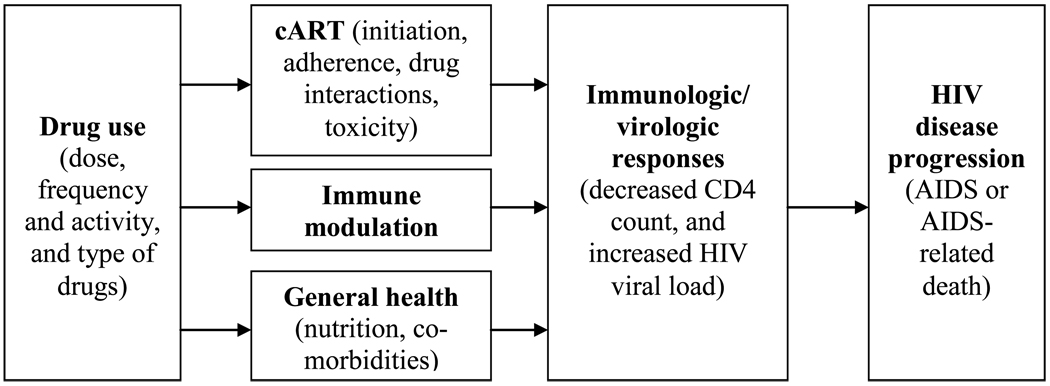
Drug use, either non-injection or injection, may affect HIV disease progression to AIDS or AIDS-related mortality through multiple mechanisms (Figure 1). Compared with HIV-infected people who do not use drugs, HIV-infected drug users may have poorer general health due to malnutrition and co-morbidities such as sexually transmitted infections, tuberculosis, and hepatitis (Girardi, et al., 2005; Hendricks, Erzen, Wanke, & Tang, 2010; Kuyper, et al., 2005; Lelutiu-Weinberger, et al., 2009; Mitchell & Latimer, 2009; Quach, et al., 2008). Poorer nutrition and co-morbidities have been associated with suboptimal immune reconstitution or HIV virologic failure (Hermans, et al., 2010; Modjarrad & Vermund, 2010; Quach, et al., 2008; Smit, et al., 2008).
Figure 1.
Conceptual diagram for mediators of the association between drug use and HIV disease progression
In vitro studies with animal models have shown that illicit drugs such as amphetamines, cocaine, marijuana, and opiates may affect HIV disease outcomes by altering immune function and increasing susceptibility to infection. This immunomodulatory effect is mainly receptor-mediated, either directly by interaction with specific receptors on immune cells or indirectly by reaction with similar receptors on cells of the nervous system (Friedman, Newton, & Klein, 2003). However, there is a paucity of controlled epidemiological studies that definitively correlate immunosuppressive effects with increased incidence of infections or immune disorders in humans, including disease progression to AIDS (Cabral, 2006; Friedman, Pross, & Klein, 2006).
However, the primary effect of drugs on HIV disease progression might be mediated via factors that may limit access and/or adherence to cART (Celentano & Lucas, 2007). In addition, the pharmacokinetic interactions between ARVs and drugs of abuse may also reduce the effectiveness of cART.
NIDU and access to cART
Drug use is associated with delayed initiation of cART (starting cART when meeting treatment guidelines) (Rodriguez-Arenas, et al., 2006; Tegger, et al., 2008), and deferred initiation of combination therapy is associated with a higher risk of mortality (Sterne, et al., 2009). While there are few studies assessing NIDU and cART initiation, several studies have investigated NIDU and cART utilization (currently being prescribed ART). A study assessing the relationship between current cART use and abuse of heroin, cocaine, sedative, amphetamine, and marijuana in the last 12 months found that the odds ratios for each drug ranged from 0.40 to 0.75 while controlling for CD4 count, AIDS, and other sociodemographic variables (Turner, et al., 2001). Among patients with CD4 cell counts ≤350 who warranted treatment per the current guidelines, NIDU (including heavy alcohol use) in the past year was associated with not having been on ARVs in the previous 6 months (OR 0.35; 95% CI: 0.21 – 0.57 (Sohler, et al., 2007). Another study also showed that active crack/cocaine users with an AIDS diagnosis were less likely to have been prescribed cART than non-users who also had an AIDS diagnosis (OR 0.40; 95% CI: 0.19 – 0.85) (Cofrancesco, et al., 2008).
Of the 9 studies we reviewed for effects of NIDU on HIV disease progression, only two reported descriptive data on cART use and both showed that a lower proportion of drug users were on cART, though they had lower CD4 cell counts at baseline compared with non-users (Cook, et al., 2008; Lucas, et al., 2006).
NIDU and adherence to cART
Illicit drug use is a consistently reported barrier to cART adherence (Hinkin, et al., 2007; Howard, et al., 2002; Mills, et al., 2006; Tucker, et al., 2003; Wilson, et al., 2002). Cocaine, marijuana, amphetamine, or sedative use in the prior month were associated with nonadherence with odds ratios ranging from 1.6 to 2.3 for each drug type (Tucker, et al., 2003). Another study reported that NIDU in the prior 6 months was associated with an overall adherence of <90% during the study period (OR 4.1; 95% CI: 1.8 – 9.3), with stimulant users having seven times the odds of non-adherence compared to those who didn’t use any drugs (Hinkin, et al., 2007). In the WIHS study, use of crack, cocaine or heroin was associated with a lower level of adherence to treatment regimens (<95%) (OR 2.27; 95% CI: 1.32 – 3.91) (Wilson, et al., 2002). Drug use is frequently co-morbid with depression, anxiety and severe mental illness (Chander, Himelhoch, & Moore, 2006; Pence, Miller, Gaynes, & Eron, 2007; Torrens, Gilchrist, & Domingo-Salvany, 2010), which are well-established barriers to adherence.
Of the 9 studies we reviewed, only one provided descriptive information on adherence and showed that persistent and inconsistent users were less likely to report >=95% adherence at every study visit compared with non-users (7% and 16% vs. 29%; p<0.001) (Cook, et al., 2008). Another study reported that adherence did not differ between NIDU and non-users but did not provide the data (Kapadia, et al., 2005). Two studies adjusted for adherence in the analyses of disease progression, but did not provide descriptive data on adherence by drug use category (Anastos, et al., 2005; Lucas, et al., 2006).
NIDU and ARV drug interactions
Several commonly abused drugs, including cocaine, marijuana, ecstasy and amphetamines as well as opioid agonist medications such as methadone and buprenorphine, are metabolized by the cytochrome P450 (CYP) enzymes (Gruber & McCance-Katz, 2010; Lakhman, Ma, & Morse, 2009; Pasanen, et al., 1995; Wynn, Cozza, Zapor, Wortmann, & Armstrong, 2005), as are antiretrovirals (ARVs), particularly non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) (Kakuda, Scholler-Gyure, & Hoetelmans, 2010; Seden, Back, & Khoo, 2009; Slish, et al., 2007; Wynn, et al., 2004; Zapor, Cozza, Wynn, Wortmann, & Armstrong, 2004). In addition, both abused drugs and ARVs may induce and inhibit specific CYP450 enzymes (Antoniou & Tseng, 2002; Hariparsad, et al., 2004; Harrington, Woodward, Hooton, & Horn, 1999; Ma, et al., 2005; Zhou, Xue, Yu, Li, & Wang, 2007). The pharmacokinetic interactions can result in increased toxicity or reduced effectiveness (Altice, Kamarulzaman, Soriano, Schechter, & Friedland, 2010; Baker, et al., 2010; Clarke, et al., 2001; Iribarne, et al., 1998; Khalsa & Elkashef, 2010; McCance-Katz, Moody, et al., 2006; McCance-Katz, et al., 2007; McCance-Katz, Rainey, et al., 2006; Wynn, et al., 2005). For example, amphetamines are metabolized by CYP2D6, and amphetamine abusers are likely to have an increased serum concentration level and increased risk of toxicity when using CYP inhibitors, e.g., ritonavir, nelfinavir or efavirenz (Lin, et al., 1997; Lin, et al., 1995). NNRTIs and several PIs may induce methadone metabolism and decrease methadone plasma concentrations in methadone maintenance patients, and cause withdrawal symptoms (Gruber & McCance-Katz, 2010). Therefore, patients should have a higher dose to avoid withdrawal symptoms when they start AVRs and have a lower dose to avoid toxicity when they stop ARVs. On the other hand, methadone can reduce absorption of ARV (e.g., stavudine) in the gastrointestinal (GI) tract,(Rainey, et al., 2000) which may result in failure of ARV treatment and development of HIV drug resistance; methadone can also inhibit glocuronidation of zidovudine (McCance-Katz, Rainey, Jatlow, & Friedland, 1998), which may lead to toxicity.
4. Discussion
4.1. Summary of findings
This is the first review to specifically address the effect of NIDU on clinical HIV disease progression in the new cART era. Among the studies included in this review, there is considerable agreement about the effect of NIDU on increased progression to AIDS, regardless of whether NIDU duration or frequency was measured. The effects seem to be moderate, as 8 of the 14 hazard ratios in the 6 papers were between 1.55 and 1.65. It should be noted that 7 papers came from either the WIHS or the WITS parent study; therefore, some agreement is to be expected. However, the reviewed studies had inconsistent results for the association of NIDU and AIDS-related mortality, and the hazard ratios were both above and below the null value of 1.0. One possible explanation is that there is truly no significant effect that can be detected with the available sample sizes in the reviewed studies. An alternative explanation is due to inaccurate measurement of death, as death was ascertained using multiple data sources instead of solely being based on clinical evaluation.
It should be noted that the collective findings from this review may have limited generalizability given that 8 of the 9 studies were conducted exclusively among women in the WIHS and WITS studies, with only one study in both men and women (Lucas, et al., 2006). More studies on this topic are highly recommended considering high prevalence of NIDU among HIV-infected patients.
4.2. Methodological concerns and challenges
A number of methodological concerns and challenges remain both in measurement and analysis in the studies assessing drug use and HIV disease progression. The type of drugs abused, route of administration, dose and frequency, and polydrug use may have different effects on HIV disease progression. The challenge of collecting information on all aspects of drug use is daunting, as a drug user may use multiple drugs, change dose, frequency and administration route of drugs over time, and even stop and resume using drugs in different periods of his lifetime. The vague definitions of IDU or NIDU, active drug use versus history of drug use, and heroin use opposed to cocaine use in data analysis are likely to lead to misclassification of drug use groups.
There is a similar challenge for measurement of outcome variables, particularly death. It is often difficult to differentiate AIDS-related causes from non-AIDS-related causes particularly for drug users who also have high risks of non-AIDS mortality. In addition, death is often assessed based on secondary databases, e.g., National Death Index, and the misclassification of AIDS-related and non-AIDS-related mortality is likely and may lead to biased analysis results.
Adherence to ARV regimens is a crucial determinant of treatment outcome that is also affected by drug use. Measurement of treatment adherence is challenging, and there is currently no readily available measure used in routine clinical practice. Patient self-report is easy to implement but is subjected to bias and poor accuracy; microelectronic monitoring systems (MEMS) are accurate but expensive; therapeutic monitoring of plasma drug concentrations is infeasible for routine use at every clinic visit; use of pharmacy data on drug pick-up is more reliable than self-reporting but may not elucidate actual patterns of suboptimal adherence or short-term changes in adherence. Even if data on treatment adherence are properly collected, it remains a question of over-adjustment in the data analysis of NIDU and HIV disease progression. Poor adherence might be one mechanism by which NIDU impacts HIV disease progression. Meanwhile, there may be other biologic mechanisms by which NIDU hastens disease progression. Differentiating the biologic effects of drug use from the behavioral effects of adherence is critical in data analysis. Some studies have addressed this issue by adjusting for adherence during the study, but it is generally not advised to adjust for variables that lie on the causal pathway from exposure (NIDU) to outcome (AIDS) (Rothman, Greenland, & Lash, 2008) unless specific assumptions are made or complex analytic methods are used (Cole & Hernan, 2002; Kaufman, Maclehose, & Kaufman, 2004; Rothman, et al., 2008).
Another methodological issue is the time of entry to study cohort. The ideal time is at HIV infection,(Perez-Hoyos, et al., 2006) or prior to the availability of cART (Dorrucci, Pezzotti, Phillips, Alliegro, & Rezza, 1997; Pezzotti, et al., 1996). If the time of infection is not considered in studies assessing HIV disease progression, study findings may be biased toward a positive association with disease progression, as drug users are more likely to enter care later than non-users and have later HIV disease stages at study entry. Though baseline immunologic and virologic data can be adjusted in data analysis, residual confounding of the impact of disease stage is still possible considering the length of the HIV latent period. An alternative time of entry to study is at initiation of cART. In this case, drug users may still have worse baseline immunologic and virologic status than non-users, but because both groups meet the criteria for initiating cART, the findings may be less biased.
4.3. Future perspectives
As few independent studies have been conducted to evaluate NIDU and HIV disease progression and had inconsistent findings, particularly on the effect on AIDS-related mortality, more studies are needed with sound research methodologies, namely prospective cohort study design, diversity of study population (e.g., both men and women), proper time of entry to study, and accurate assessments of drug use, disease progression outcomes, and ARV treatment adherence. It is suggested that injection and non-injection drug users may have different disease outcomes;(Collaboration, 2007; Cook, et al., 2008; Egger, et al., 2002; Krol, et al., 1999; May, et al., 2007; Nicastri, et al., 2005; Perez-Hoyos, et al., 2006) however, none of the 9 studies we reviewed compared the effect of route of drug use. Ideally, this question should be addressed in one study that includes both injection and non-injection drug users, as this study design may avoid confounding by study design and population. We did a retrospective study assessing both effects of IDU and NIDU, and found that HIV-infected IDUs had faster disease progression than NIDU patients [Qian et al, unpublished data]. Explanations for the difference are that IDUs might have a longer history of drug use and use a high dose of drugs than NIDUs.
The effect and mechanism of illicit drugs on ARV treatment outcomes remains unclear. While in vitro information has proved helpful, this is a topic that has received little attention in public health and epidemiologic AIDS literature and the clinical implications remain largely unknown. Further work is needed to identify the pharmacokinetics of various drugs on ARVs, as well as the clinical effects these drugs have on plasma concentrations, separate from the effects of adherence.
In conclusion, we propose a hypothesized mechanisms diagram which highlights the complex immunological, pharmacological, and behavioral effects that drug use has on HIV disease progression (Figure 1). Basic science, epidemiological and clinical research is needed to fully understand the link between NIDU and HIV outcomes. Knowledge of the complex mechanisms will help improve clinical service and outcomes for this increasingly common problem in HIV/AIDS treatment and care.
Acknowledgment
This study was sponsored by Vanderbilt Center for AIDS Research (CFAR) Grant (3P30AI054999).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastos K, Schneider MF, Gange SJ, Minkoff H, Greenblatt RM, Feldman J, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39(5):537–544. [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46(5):607–615. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother. 2002;36(10):1598–1613. doi: 10.1345/aph.1A447. [DOI] [PubMed] [Google Scholar]
- Astemborski J, Vlahov D, Warren D, Solomon L, Nelson KE. The trading of sex for drugs or money and HIV seropositivity among female intravenous drug users. Am J Public Health. 1994;84(3):382–387. doi: 10.2105/ajph.84.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend (in print) 2010 doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Rainey PM, Moody DE, Morse GD, Ma Q, McCance-Katz EF. Interactions between Buprenorphine and Antiretrovirals: Nucleos(t)ide Reverse Transcriptase Inhibitors (NRTI) Didanosine, Lamivudine, and Tenofovir. Am J Addict. 2010;19(1):17–29. doi: 10.1111/j.1521-0391.2009.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Brewer TH, Zhao W, Metsch LR, Coltes A, Zenilman J. High-risk behaviors in women who use crack: knowledge of HIV serostatus and risk behavior. Ann Epidemiol. 2007;17(7):533–539. doi: 10.1016/j.annepidem.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1(3):280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Lucas G. Optimizing treatment outcomes in HIV-infected patients with substance abuse issues. Clin Infect Dis. 2007;45 Suppl 4:S318–S323. doi: 10.1086/522557. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. MMWR Morb Mortal Wkly Rep. 1992;41(RR-17) [PubMed] [Google Scholar]
- Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Mulcahy FM, Tjia J, Reynolds HE, Gibbons SE, Barry MG, et al. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br J Clin Pharmacol. 2001;51(3):213–217. doi: 10.1046/j.1365-2125.2001.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofrancesco J, Jr, Scherzer R, Tien PC, Gibert CL, Southwell H, Sidney S, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22(3):357–365. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113(2):91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- Collaboration ATC. Importance of baseline prognostic factors with increasing time since initiation of highly active antiretroviral therapy: collaborative analysis of cohorts of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46(5):607–615. doi: 10.1097/QAI.0b013e31815b7dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza CT, Diaz T, Sutmoller F, Bastos FI. The association of socioeconomic status and use of crack/cocaine with unprotected anal sex in a cohort of men who have sex with men in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2002;29(1):95–100. doi: 10.1097/00126334-200201010-00013. [DOI] [PubMed] [Google Scholar]
- Dorrucci M, Pezzotti P, Phillips AN, Alliegro MB, Rezza G. Antiretroviral treatment and progression to AIDS in HIV seroconverters from different risk groups. HIV Italian Seroconversion Study. AIDS. 1997;11(4):461–467. doi: 10.1097/00002030-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Irwin KL, Faruque S, McCoy CB, Word C, Serrano Y, et al. Intersecting epidemics--crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med. 1994;331(21):1422–1427. doi: 10.1056/NEJM199411243312106. [DOI] [PubMed] [Google Scholar]
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- Eskild A, Magnus P, Brekke T, Bruun JN, Heger B, Froland SS, et al. The impact of exposure group on the progression rate to acquired immunodeficiency syndrome. A comparison between intravenous drug users, homosexual men and heterosexually infected subjects. Scand J Infect Dis. 1997;29(2):103–109. doi: 10.3109/00365549709035868. [DOI] [PubMed] [Google Scholar]
- Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16(2):209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47(3):330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41(12):1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- Gruber VA, McCance-Katz EF. Methadone, buprenorphine, and street drug interactions with antiretroviral medications. Curr HIV/AIDS Rep. 2010;7(3):152–160. doi: 10.1007/s11904-010-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44(11):1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-threatening interactions between HIV-1 protease inhibitors and the illicit drugs MDMA and gamma-hydroxybutyrate. Arch Intern Med. 1999;159(18):2221–2224. doi: 10.1001/archinte.159.18.2221. [DOI] [PubMed] [Google Scholar]
- Hendricks KM, Erzen HD, Wanke CA, Tang AM. Nutrition issues in the HIV-infected injection drug user: findings from the nutrition for healthy living cohort. J Am Coll Nutr. 2010;29(2):136–143. doi: 10.1080/07315724.2010.10719827. [DOI] [PubMed] [Google Scholar]
- Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershow RC, O'Driscoll PT, Handelsman E, Pitt J, Hillyer G, Serchuck L, et al. Hepatitis C virus coinfection and HIV load, CD4+ cell percentage, and clinical progression to AIDS or death among HIV-infected women: Women and Infants Transmission Study. Clin Infect Dis. 2005;40(6):859–867. doi: 10.1086/428121. [DOI] [PubMed] [Google Scholar]
- Hessol NA, Kalinowski A, Benning L, Mullen J, Young M, Palella F, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study. Clin Infect Dis. 2007;44(2):287–294. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AA, Arnsten JH, Lo Y, Vlahov D, Rich JD, Schuman P, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16(16):2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Iribarne C, Berthou F, Carlhant D, Dreano Y, Picart D, Lohezic F, et al. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metab Dispos. 1998;26(3):257–260. [PubMed] [Google Scholar]
- Kakuda TN, Scholler-Gyure M, Hoetelmans RM. Clinical perspective on antiretroviral drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor etravirine. Antivir Ther. 2010;15(6):817–829. doi: 10.3851/IMP1652. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Cook JA, Cohen MH, Sohler N, Kovacs A, Greenblatt RM, et al. The relationship between non-injection drug use behaviors on progression to AIDS and death in a cohort of HIV seropositive women in the era of highly active antiretroviral therapy use. Addiction. 2005;100(7):990–1002. doi: 10.1111/j.1360-0443.2005.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1(1):4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa JH, Elkashef A. Drug interactions between antiretroviral medications and medications used in the treatment of drug addiction: research needs. Am J Addict. 2010;19(1):96–100. doi: 10.1111/j.1521-0391.2009.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A, Flynn C, Vlahov D, Miedema F, Coutinho RA, van Ameijden EJ. New evidence to reconcile in vitro and epidemiologic data on the possible role of heroin on CD4+ decline among HIV-infected injecting drug users. Drug Alcohol Depend. 1999;54(2):145–154. doi: 10.1016/s0376-8716(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Kuyper LM, Collins CL, Kerr T, Hogg RS, Li K, Tyndall MW, et al. The prevalence and incidence of sexually transmitted infections in a prospective cohort of injection drug users in Vancouver, British Columbia. Can J Infect Dis Med Microbiol. 2005;16(4):225–229. doi: 10.1155/2005/617326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhman SS, Ma Q, Morse GD. Pharmacogenomics of CYP3A: considerations for HIV treatment. Pharmacogenomics. 2009;10(8):1323–1339. doi: 10.2217/pgs.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelutiu-Weinberger C, Pouget ER, Des Jarlais DD, Cooper HL, Scheinmann R, Stern R, et al. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Soc Sci Med. 2009;68(3):579–590. doi: 10.1016/j.socscimed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, et al. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997;25(9):1059–1064. [PubMed] [Google Scholar]
- Lin LY, Kumagai Y, Hiratsuka A, Narimatsu S, Suzuki T, Funae Y, et al. Cytochrome P4502D isozymes catalyze the 4-hydroxylation of methamphetamine enantiomers. Drug Metab Dispos. 1995;23(6):610–614. [PubMed] [Google Scholar]
- Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- Ma Q, Okusanya OO, Smith PF, Dicenzo R, Slish JC, Catanzaro LM, et al. Pharmacokinetic drug interactions with non-nucleoside reverse transcriptase inhibitors. Expert Opin Drug Metab Toxicol. 2005;1(3):473–485. doi: 10.1517/17425255.1.3.473. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21(9):1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, Friedland G, Pade P, Baker J, et al. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43 Suppl 4:S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse GD, Ma Q, DiFrancesco R, Friedland G, et al. Interaction between buprenorphine and atazanavir or atazanavir/ritonavir. Drug Alcohol Depend. 2007;91(2–3):269–278. doi: 10.1016/j.drugalcdep.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Jatlow P, Friedland G. Methadone effects on zidovudine disposition (AIDS Clinical Trials Group 262) J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(5):435–443. doi: 10.1097/00042560-199808150-00004. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey PM, Smith P, Morse GD, Friedland G, Boyarsky B, et al. Drug interactions between opioids and antiretroviral medications: interaction between methadone, LAAM, and delavirdine. Am J Addict. 2006;15(1):23–34. doi: 10.1080/10550490500419029. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MM, Latimer WW. Unprotected casual sex and perceived risk of contracting HIV among drug users in Baltimore, Maryland: evaluating the influence of non-injection versus injection drug user status. AIDS Care. 2009;21(2):221–230. doi: 10.1080/09540120801982897. [DOI] [PubMed] [Google Scholar]
- Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E, Angeletti C, Palmisano L, Sarmati L, Chiesi A, Geraci A, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS. 2005;19(6):577–583. doi: 10.1097/01.aids.0000163934.22273.06. [DOI] [PubMed] [Google Scholar]
- Pasanen M, Pellinen P, Stenback F, Juvonen RO, Raunio H, Pelkonen O. The role of CYP enzymes in cocaine-induced liver damage. Arch Toxicol. 1995;69(5):287–290. doi: 10.1007/s002040050172. [DOI] [PubMed] [Google Scholar]
- Pehrson P, Lindback S, Lidman C, Gaines H, Giesecke J. Longer survival after HIV infection for injecting drug users than for homosexual men: implications for immunology. AIDS. 1997;11(8):1007–1012. doi: 10.1097/00002030-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- Perez-Hoyos S, Ferreros I, del Amo J, Muga R, del Romero J, de Olalla PG, et al. Survival and progression to AIDS in a seroconverter cohort in the post-highly active antiretroviral therapy era: effectiveness goes on. AIDS. 2006;20(2):289–291. doi: 10.1097/01.aids.0000202651.41397.db. [DOI] [PubMed] [Google Scholar]
- Pezzotti P, Galai N, Vlahov D, Rezza G, Lyles CM, Astemborski J. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS Link to Intravenous Experiences. Italian Seroconversion Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(3):275–282. doi: 10.1097/00042560-199903010-00010. [DOI] [PubMed] [Google Scholar]
- Pezzotti P, Phillips AN, Dorrucci M, Lepri AC, Galai N, Vlahov D, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1199 people with known dates of seroconversion. HIV Italian Seroconversion Study Group. BMJ. 1996;313(7057):583–586. doi: 10.1136/bmj.313.7057.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestage G, Fogarty AS, Rawstorne P, Grierson J, Zablotska I, Grulich A, et al. Use of illicit drugs among gay men living with HIV in Sydney. AIDS. 2007;21 Suppl 1:S49–S55. doi: 10.1097/01.aids.0000255085.77470.bd. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Moss S, Remien RH, Woods WJ, Parsons JT. Illicit substance use, sexual risk, and HIV-positive gay and bisexual men: differences by serostatus of casual partners. AIDS. 2005;19 Suppl 1:S37–S47. doi: 10.1097/01.aids.0000167350.00503.db. [DOI] [PubMed] [Google Scholar]
- Quach LA, Wanke CA, Schmid CH, Gorbach SL, Mwamburi DM, Mayer KH, et al. Drug use and other risk factors related to lower body mass index among HIV-infected individuals. Drug Alcohol Depend. 2008;95(1–2):30–36. doi: 10.1016/j.drugalcdep.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey PM, Friedland G, McCance-Katz EF, Andrews L, Mitchell SM, Charles C, et al. Interaction of methadone with didanosine and stavudine. J Acquir Immune Defic Syndr. 2000;24(3):241–248. doi: 10.1097/00126334-200007010-00007. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valles J, Garcia D, Campbell RT, Diaz RM, Heckathorn DD. HIV infection, sexual risk behavior, and substance use among Latino gay and bisexual men and transgender persons. Am J Public Health. 2008;98(6):1036–1042. doi: 10.2105/AJPH.2006.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Arenas MA, Jarrin I, del Amo J, Iribarren JA, Moreno S, Viciana P, et al. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Res Hum Retroviruses. 2006;22(8):715–723. doi: 10.1089/aid.2006.22.715. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Validity in Epidemiologic Studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Third edition ed. Philadelphia: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- Seden K, Back D, Khoo S. Antiretroviral drug interactions: often unrecognized, frequently unavoidable, sometimes unmanageable. J Antimicrob Chemother. 2009;64(1):5–8. doi: 10.1093/jac/dkp152. [DOI] [PubMed] [Google Scholar]
- Slish J, Ma Q, Zingman BS, Reichman RC, Fischl MA, Gripshover B, et al. Assessing the impact of substance use and hepatitis coinfection on atazanavir and lopinavir trough concentrations in HIV-infected patients during therapeutic drug monitoring. Ther Drug Monit. 2007;29(5):560–565. doi: 10.1097/FTD.0b013e31806db8ae. [DOI] [PubMed] [Google Scholar]
- Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus: a 20-year prospective study. J Acquir Immune Defic Syndr. 2008;47(2):221–225. doi: 10.1097/QAI.0b013e31815d2f59. [DOI] [PubMed] [Google Scholar]
- Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni ML, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care STDS. 2007;21 Suppl 1:S68–S76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008;22(3):233–243. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- Thorpe LE, Frederick M, Pitt J, Cheng I, Watts DH, Buschur S, et al. Effect of hard-drug use on CD4 cell percentage, HIV RNA level, and progression to AIDS-defining class C events among HIV-infected women. J Acquir Immune Defic Syndr. 2004;37(3):1423–1430. doi: 10.1097/01.qai.0000127354.78706.5d. [DOI] [PubMed] [Google Scholar]
- Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: Substance-induced versus independent disorders. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, et al. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16(9):625–633. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office of Drugs and Crime. 2008 World Report. Vienna: United Nations; 2008. [Google Scholar]
- Wilson TE, Barron Y, Cohen M, Richardson J, Greenblatt R, Sacks HS, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- Wynn GH, Cozza KL, Zapor MJ, Wortmann GW, Armstrong SC. Medpsych drug-drug interactions update. Antiretrovirals, part III: antiretrovirals and drugs of abuse. Psychosomatics. 2005;46(1):79–87. doi: 10.1176/appi.psy.46.1.79. [DOI] [PubMed] [Google Scholar]
- Wynn GH, Zapor MJ, Smith BH, Wortmann G, Oesterheld JR, Armstrong SC, et al. Antiretrovirals, part 1: overview, history, and focus on protease inhibitors. Psychosomatics. 2004;45(3):262–270. doi: 10.1176/appi.psy.45.3.262. [DOI] [PubMed] [Google Scholar]
- Zapor MJ, Cozza KL, Wynn GH, Wortmann GW, Armstrong SC. Antiretrovirals, Part II: focus on non-protease inhibitor antiretrovirals (NRTIs, NNRTIs, and fusion inhibitors) Psychosomatics. 2004;45(6):524–535. doi: 10.1176/appi.psy.45.6.524. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Xue CC, Yu XQ, Li C, Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007;29(6):687–710. doi: 10.1097/FTD.0b013e31815c16f5. [DOI] [PubMed] [Google Scholar]