Abstract
The process of wound healing involves complex interactions between circulating immune cells and local epithelial and endothelial cells. Studies in murine models indicate that cells of the innate immune system activated via their Toll-like receptors (TLR) can accelerate wound healing. This work examines whether immunostimulatory CpG oligodeoxynucleotides (ODN) designed to trigger human immune cells via TLR9 can promote the healing of excisional skin biopsies in rhesus macaques. Results indicate that ‘K’ type CpG ODN significantly accelerate wound closure in non-human primates (p < 0.05). Contributing to this outcome was a CpG-dependent increase in both the production of basic fibroblast growth factor and in keratinocyte migration. Of interest, IL-1a and TGFa normally present at sites of skin injury facilitated these effects. Current findings support the conclusion that the local administration of CpG ODN may provide an effective strategy for accelerating wound healing in humans.
Keywords: CpG oligonucleotide, primate, TLR9, wound healing
Introduction
The skin is a physical barrier that serves many functions, including protecting the host from environmental pathogens. Wounding breaches this barrier and increases host susceptibility to infection, fluid loss, and other adverse consequences [1]. Methods that accelerate wound healing are thus of considerable therapeutic interest.
Wound repair is a multi-step process that culminates in the regeneration of normal dermal and epidermal tissue [2]. The first stage is marked by an inflammatory response characterized by the infiltration of neutrophils and macrophages [3]. This is followed by a proliferative phase marked by the accumulation and proliferation of fibroblasts and the subsequent formation of granulation tissue [2]. These events are influenced by cytokines, chemokines and growth factors (including basic fibroblast growth factor) secreted by inflammatory and epithelial cells at the wound margins [4-8].
Recent studies suggest that the innate immune system can contribute to wound healing by facilitating the elimination of invading microbes and promoting the production of factors that accelerate tissue repair. For example, local administration of the TLR4 ligands LPS and HMGB1 accelerate wound healing [9;10]. Conversely, healing is delayed in mice with a defect in the TLR adapter molecule MyD88 [11]. TLRs recognize common determinants expressed by a wide variety of infectious microorganisms. TLR9, for example, is triggered by the unmethylated CpG motifs present at high frequency in bacterial DNA [12;13]. Synthetic oligonucleotides (ODN) expressing CpG motifs mimic the ability of bacterial DNA to stimulate cells expressing TLR9 [14-19].
We recently examined the ability of CpG ODN to affect wound repair in mice, demonstrating that CpG ODN accelerated healing by >40% [20]. This effect was mediated via TLR9, as CpG ODN treatment did not accelerate wound repair in TLR9 KO mice (a strain unresponsive to CpG stimulation) [17].
It is perilous to extrapolate results from murine studies of CpG ODN to humans because i) the cell types expressing TLR9 differ between these species [21], ii) murine and human TLR9 differ by 24% at the AA level [17], iii) the CpG flanking regions optimally recognized by human TLR9 differs from those optimally recognized by murine TLR9 [22;23] and iv) humans but not mice respond to several structurally and functionally distinct classes of CpG ODN [23;24]. ‘D’ type ODN in particular (also referred to as ‘A’ type) stimulate human pDC to secrete IFNa but have little activity in mice [23;24]. ‘K’ type ODN (also referred to as ‘B’ type) stimulate both human and murine B cells to proliferate and secrete Ig while triggering pDC to differentiate and produce TNFa [23-25]. Fortunately, TLR9 expression, function, and sequence recognition has been highly conserved in primates, such that the response of rhesus macaques to CpG ODN is highly predictive of their activity in Man [26;27]. This work employs a rhesus macaque wound healing model to examine the therapeutic utility of ‘K’ and ‘D’ type ODN designed for human use.
Materials and Methods
Animals and cells
Healthy 4 - 6 Kg rhesus macaques (Macaca mulata) were obtained from the FDA colony in South Carolina. Specific pathogen free female BALB/c mice were housed in a barrier environment and studied at 8-12 wk of age. All experiments were approved by the appropriate Animal Care and Use Committee and were conducted in AAALAC accredited facilities. Animals were monitored daily by veterinarians.
Adult human dermal fibroblasts were purchased from Lifeline Cell Technology (Walkersville, MD). Cells were maintained in Fiblolife basal medium supplemented with Fibrolife serum-free life factors (Lifeline Cell Technology, Walkersville, MD). For studies of bFGF production, cells were maintained in medium without rh FGF basic. The human HaCaT keratinocyte cell line was purchased from the German Cancer Research Center (Heidelberg, Germany). Cells were maintained in DMEM supplemented with 10% FCS, 100 U/ml penicillin, 100 ug/ml streptomycin, 25 mM HEPES, 1 mM sodium pyruvate, 0.1 mM non essential amino acids.
Oligonucleotides and reagents
An equimolar mixture of three ‘K’ type phosphorothioate ODN (TCGTTCGTTCTC, ATCGACTCTCGAGCGTTCTC and TCGAGCGTTCTC), and three ‘D’ type ODN (GGtgcatcgatgcaggGGGG, GGtgcaccggtgcaggGGGG and GGtgcatcgatgcaggggGG (upper case nucleotides were phosphorothioate and lower case were phosphodiester backbone), were used in all primate studies. These ODN mixtures were previously shown to be highly stimulatory in both rhesus macaques and human PBMC [27]. Phosphorothiate CpG ODN 1555 (GCTAGACGTTAGCGT) was used in murine studies. All ODNs were synthesized at the CBER Core Facility and were free of endotoxin and protein contamination. Growth factor reduced basement membrane extract (BME) without phenol red was purchased from Trevigen (Gaithersburg, MD) and used as a carrier of these ODN in animal studies.
Macaque in vivo wound repair model
A modification of the wound repair model of Devalaraja et al was used to analyze the effects of CpG ODN in vivo [28]. Animals were anesthetized by intramuscular injection of ketamine (10-12 mg/kg) plus xylazine (2 mg/kg) on the day of surgery [28]. The skin on the back was cleaned, shaved, and sterilized with betadine followed by 2% chlorhexidine in 70% isopropyl alcohol. 6 mm full thickness excisional punch biopsies (Acu Punch, Fort Lauderdale, FL) were taken from the right and left paravertebral region of each animal [29]. Individual Bx sites were coated with BME + 50 ug of ‘K’ or ‘D’ ODN (an amount selected on the basis of preliminary dose-ranging studies) on days 0, 2 and 4. Each site was covered with an Opsite FlexGrid dressing (Smith&Nephew, London, UK) secured in place with adhesive tape and then covered with a cotton bandage and Vetrap (3M, St. Paul, MN). The animals were placed in a jacket (Lomir Biomedical, Inc., Quebec, Canada) that did not restrict mobility but prevented them from scratching the biopsy site. Animals were treated with buprenorphine (0.02 mg/kg) twice daily to prevent pain. Biopsy sites were digitally photographed and Image J software (Ver 1.37) used to calculate changes in wound area over time. Repair of the wound area was calculated as [1 - (wound area) / (original wound area)] ) 100%. No systemic or local adverse reactions to treatment were observed.
Murine in vivo wound repair model
Mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) plus xylazine (10 mg/kg). As previously described, skin on the back was cleaned, shaved, and sterilized with a betadine solution followed by 70% ethanol [20]. A 6 mm full thickness (including the Pannilulus carnosus) excisional punch biopsies (Acu Punch, Fort Lauderdale, FL) were taken from the right and left upper paravertebral region of each animal [29]. Individual Bx sites were coated with BME mixed + 50 ug of ODN and the bx sites covered with non-adhesive sterile gauze. Mice were wrapped with a form fitting bandage to further protect the bx sites. Changes in wound size were monitored as described for rhesus macaques.
Quantitative RT-PCR
Tissue from the biopsy site was excised, homogenized in Trizol, and extracted with chloroform. Total RNA was isolated from the aqueous phase and passed through an RNeasy column as per manufacturer's suggestion. Five ug of total RNA was reverse-transcribed using the Quantitect Reverse Transcription Kit (Qiagen). Gene expression levels were analyzed using the StepOnePlus RT-PCR system (Applied Biosystems, Foster City, CA). All reagents and probes used in this system were purchased from Applied Biosystems.
In vitro bFGF assay
Human adult dermal fibroblasts were seeded at 105 cells/well in 96 well microtiter plates. After overnight incubation (to allow the formation of an adherent monolayer) cells were stimulated with human rIL-1∀ (R&D systems, Minneapolis, MN) and/or 1 uM of ODN for 48 h. FGF basic levels in the culture supernatants were measured using a commercial ELISA kit (RayBiotech Inc., Norcross, GA) as recommended by the manufacturer.
In vitro scratch assay
HaCaT cells were seeded at 105 cells/well in 24 well Linbro plates. After the cells formed an adherent monolayer, a linear scratch was created using a sterile P 200 pipette tip as previously described [9;30;31]. The monolayer was washed twice to remove cellular debris and incubated in DMEM containing 2% FCS plus 5 ng/ml of TGF∀ and/or 1 uM of ODN. “Healing” was quantified by analysis of photomicrographs taken with an IX50 inverted microscope (Olympus, Tokyo, Japan). Changes in migration area over time were calculated using NIH Image J software. Results were derived from at least 4 independent experiments.
Statistical analysis
Statistical analysis was performed using SigmaStat, version 3.11 (Systat Software, Inc., Chicago, IL). Differences in the rate of healing of Bx sites were assessed using one way repeated measures analysis of variance (ANOVA). Area under the curve was calculated for overall changes in wound area using serial measurement. All values are expressed as means ∀ SE unless otherwise noted.
Results
Effect of CpG ODN on wound healing in rhesus macaques
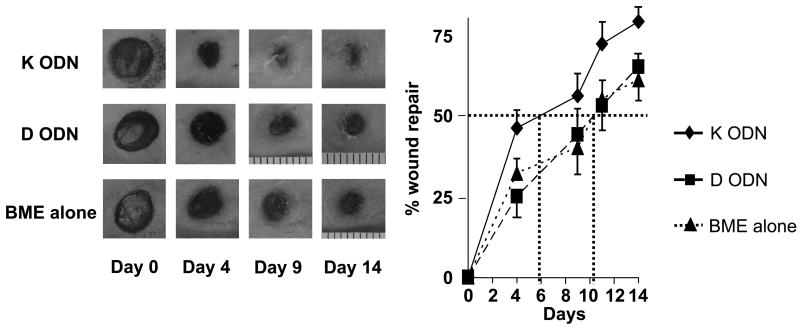
We previously demonstrated that CpG ODN could be formulated in liquid basement membrane extract (BME) and that the combination gelled when applied to wounded skin to create a protective layer from which ODN was released in bioactive form over 2 – 3 days [20]. To evaluate whether CpG ODN designed for human could accelerate wound healing in primates, multiple 6 mm full-thickness excisional skin biopsies were taken from the dorsal lumber area of individual rhesus macaques. Different biopsies from the same animal were treated on days 0, 2 and 4 with ‘K’ or ‘D’ type CpG ODN formulated in BME. Sequential photographs provided a permanent record of the behavior of each biopsy site on each animal (Fig 1).
Figure 1.
Effect of CpG ODN on wound healing in rhesus macaques
Multiple independent 6 mm excisional biopsies, spaced 4 cm apart, were taken from the dorsum of rhesus macaques. BME combined with 50 ug of ‘K’ or ‘D’ ODN (or BME alone as negative control) was administered to each Bx site (N = 6/treatment) on days 0, 2 and 4. Wound size recorded by digital photography. The best fit first-order regression curve was calculated for each group, and the time needed to achieve a 50% reduction in the original area determined (dotted lines).
*, p < .05 for the integrated area under the curve of BME + ‘K’ ODN vs BME alone or BME + ‘D’ ODN by two way repeated measures ANOVA.
Those sites treated with ‘K’ ODN healed significantly more rapidly than those treated with ‘D’ ODN or BME alone (p < 0.05; two way repeated measures ANOVA). A 50% reduction in wound area was achieved after 5.9 ∀ 0.7 days at sites treated with ‘K’ ODN vs 10.3 ∀ 1.9 days for sites treated with ‘D’ ODN or BME alone (p < 0.05 for both comparisons, Fig 1). These findings indicate that ‘K’ (but not ‘D’) type CpG ODN significantly accelerate wound healing, confirming and extending previous results obtained using ODN optimized for use in mice [20].
Effect of CpG ODN on fibroblast production of bFGF
Murine studies indicated that CpG ODN treatment induced the production of vascular endothelial growth factor (VEGF) by cells at the wound site. VEGF accelerates blood vessel formation and thus promotes wound healing [20]. Consistent with that finding, VEGF production was significantly increased when human PBMC treated with CpG ODN (p. <.05, supplementary Fig 1). However, this effect was mediated by both ‘K’ and ‘D’ ODN. Thus, while increased levels of VEGF might contribute to the repair of wounds treated with CpG DNA, production of VEGF could not explain the difference in activity between ‘K’ vs ‘D’ ODNs.
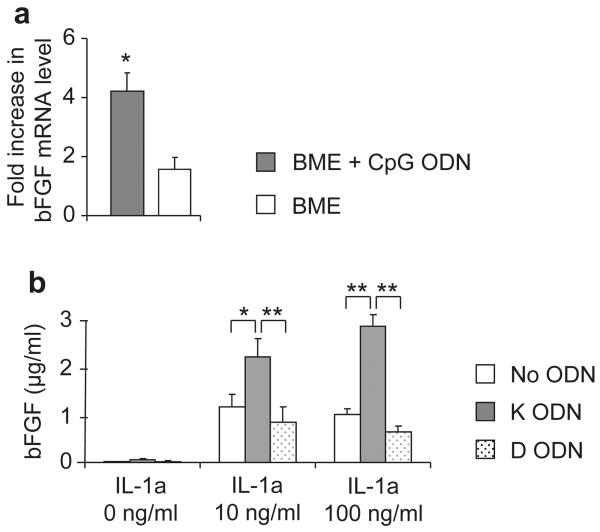
Fibroblasts contribute to wound repair, as they facilitate the formation of granulation tissue and differentiate into myofibroblasts that provide the contractile force needed for wound closure. This cell type is also a source of basic fibroblast growth factor (bFGF) [2], which is known to promote fibroblast proliferation and migration during wound healing [5]. Animal Care and Use Guidelines prohibit multiple surgical interventions in primates, preventing us from monitoring bFGF production at wound sites in rhesus macaques. The mechanism by which ‘K’ type ODN accelerated wound repair was therefore investigated in vivo in mice. mRNA was prepared from biopsy sites of mice treated with BME + ‘K’ ODN (mice do not respond to ‘D’ ODN). bFGF mRNA levels were significantly increased in wounds treated with ‘K’ ODN vs BME alone during the acute phase of wound healing (Fig 2a, p < 0.05).
Figure 2.
Effect of CpG ODN on bFGF production.
A) Six mm excisional biopsies were taken from the right and left dorsum of individual BALB/c mice. One site (selected at random) was treated with BME alone while the other was treated with BME formulated with 50 :g of ‘K’ ODN. Total RNA was extracted from the Bx site at 24 hr. Quantitative RT-PCR was performed to detect bFGF expression. Results represent the mean ∀ SE of at least 4 independent samples/treatment group. B) The concentration of bFGF in supernatants of cultured human skin fibroblasts was measured by ELISA. Results represent the mean ∀ SD of 3 independent experiments performed in triplicate.
*; p <.05, **; p <.01.
Further evidence that CpG ODN could boost bFGF production was obtained during in vitro studies of human skin fibroblasts. Whereas CpG ODN alone had no effect on bFGF production, human fibroblasts treated with ‘K’ ODN plus IL-1∀ significantly increased bFGF secretion (Fig 2b). ‘D’ ODN (alone or in combination with IL-1∀) had no effect on bFGF levels. IL-1∀ is normally present at high concentrations at sites of tissue injury during the acute phase of wound repair, and PCR analysis of tissue obtained from murine biopsy sites (N = 7) showed that IL-1∀ mRNA levels were elevated 12.2 + 3.2 fold in wounded vs normal skin (p <.01). These findings suggest that the accelerated wound repair mediated by ‘K’ ODN involved increased production of bFGF, supported by IL-1∀ present at the wound site.
Effect of CpG ODN on re-epithelialization and keratinocyte migration
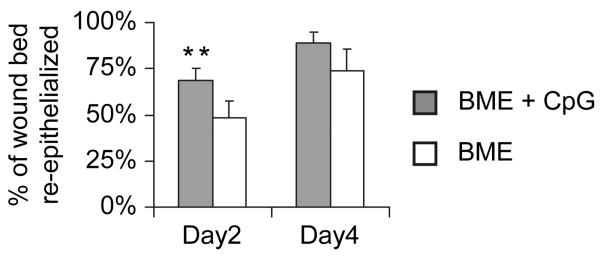
Histologic evaluation of Bx sites in mice confirmed earlier results that CpG ODN Rx significantly accelerated re-epithelialization (Fig 3, p < 0.01). A key determinant of the rate of re-epithelialization is how effectively keratinocytes migrate into the wound [32]. To explore the effect of CpG ODN on primate keratinocyte migration, an in vitro wound repair model utilizing the human keratinocyte HaCaT cell line was employed. PCR studies confirmed that HaCaT cells, like primary murine and human keratinocytes [33], express TLR9 (data not shown).
Figure 3.
Effect of CpG ODN on wound re-epithelialization.
a) Six mm excisional biopsies were taken from BALB/c mice as described in Fig 2. On each animal, one site was treated with BME alone while the other was treated with BME formulated with 50 :g of CpG ODN. Mean + SE of the total re-epithelialized area was measured on days 2 and 4. Results reflect the mean + SE of 6 independent samples/group.
**, p < .01 for % re-epithelialized area of the entire wound bed of CpG + BME vs BME alone.
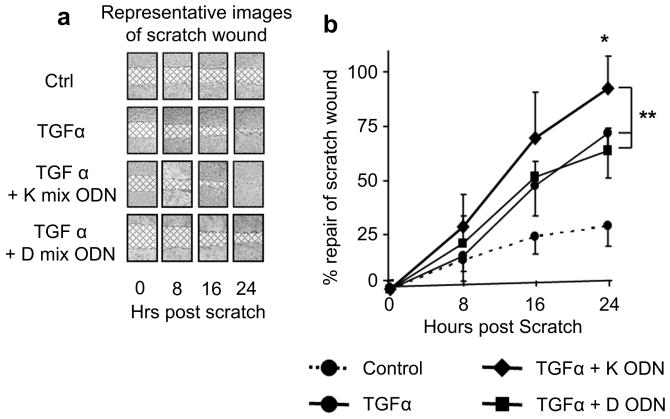
In a pattern reminiscent of it's effect on bFGF production, ‘K’ ODN alone had little effect on HaCaT cells (data not shown) but significantly accelerated cell migration when administered in combination with TGF∀ (which is present at high concentration at sites of skin injury [34]) (Fig 4). This effect was attributable to increased keratinocyte migration, as there was no difference in cell proliferation in cultures treated with TGF∀ + CpG ODN. ‘D’ ODN had no effect on human keratinocyte migration, indicating that ‘K’ ODN accelerate wound re-epithelialization in part by stimulating keratinocyte migration.
Figure 4.
Effect of ‘K’ CpG ODN on keratinocyte migration.
a) A linear “scratch wound” was made on a confluent monolayer of HaCaT cells. Digital photographs of representative wounds are shown, with the wound area cross hatched. b) The rate of repair of these scratch wounds was determined for each treatment over time. Results represent the mean ∀ SD of 4 independent studies.
**, p < .01 for the overall speed of wound healing of TGF∀ + ‘K’ ODN vs TGF∀ alone or TGF + ‘D’ ODN (Two way repeated mesures ANOVA)
Discussion
This work is the first to show that ‘K’ type CpG ODN optimized for human use significantly accelerate wound healing in primates. The ability of TLR ligands to impact wound repair was initially established in studies of the TLR4 agonists LPS and HMGB1. The former promoted the migration of human bronchial cells in vitro [9] while the latter accelerated wound repair in diabetic mice in vivo [10]. Similarly, MyD88 KO mice show delayed healing (MyD88 is a TLR adapter molecule through which both TLR4 and TLR9 signal) [35]. Pursuing those observations, we recently demonstrated that CpG ODN significantly accelerated wound healing in mice [20]. That activity was TLR9 dependent, since CpG treatment had no effect on TLR9 KO mice [20].
Unfortunately, mice and humans differ in their recognition of and response to CpG DNA. Mice fail to recognize ‘D’ type ODN, and respond poorly to ‘K’ ODN optimized for human use. In contrast, rhesus macaques mirror the human response to these structurally distinct classes of CpG ODN [23;24]. Using ODNs optimized to stimulate human PBMC, the healing of full-thickness biopsies in macaques treated with ‘K’ vs ‘D’ ODN was monitored. Results showed that 50% wound closure was achieved nearly twice as rapidly when biopsies on the same animal were treated with ‘K’ ODN vs ‘D’ ODN or BME alone (Fig 1). Previous studies in mice suggested that this acceleration in wound closure was attributable to multiple factors, including CpG-induced VEGF production [20]. While ‘K’ ODN stimulated a significant increase in the production of VEGF, so to did ‘D’ ODN, indicating that the distinctive ability of ‘K’ ODN to accelerate wound repair must have some additional basis.
Our studies of macaques and mice indicate that CpG treatment is most effective when administered immediately after wounding (Fig 1 and ref 20). Focusing on that early period, we found that CpG ODN boosted the expression of bFGF at wound sites in mice within 24 hr (Fig 2). This finding was of interest because bFGF contributes to wound healing by inducing fibroblast proliferation [36;37]. Yet when human skin fibroblasts were cultured with ODN alone, no bFGF production was observed. Pursuing this matter further, ODN was co-administered with IL-1∀. IL-1∀ was selected for study because i) it is known to stimulate growth factor production by fibroblasts [38;39] and ii) it is secreted at high levels by keratinocytes during the early inflammatory stage of wound healing when the effect of “K” type CpG was maximal [40]. Consistent with the latter finding, bFGF mRNA levels were increased 4-fold at biopsy sites. As seen in Fig. 2b, the combination of IL-1∀ plus ‘K’ (but not ‘D’) ODN induced significant bFGF production by human skin fibroblasts. This set of observations led us to postulate that CpG ODN may not accelerate wound repair autonomously but rather in combination with other factors (such as IL-1∀) present at the injury site.
This interpretation was buttressed by studies examining the effect of CpG ODN on HaCaT human keratinocytes. HaCaT cells express TLR9 and form a monolayer when cultured in vitro. When “wounded” by scratching, changes in keratinocyte migration are readily visualized. Results showed that CpG ODN alone had no effect in this model, yet when co-administered with TGF∀, ‘K’ (but not ‘D’) ODN significantly accelerated keratinocyte migration. Of note, TGF∀ up-regulates TLR9 expression by human keratinocytes [41] and is present at high levels at sites of skin injury [34]. This set of findings is consistent with the observation that the rate of wound re-epithelialization was significantly accelerated by CpG ODN in vivo (Fig 3).
CpG ODN are easy to manufacture, inexpensive, and stable. They have an excellent safety profile [42-45], and no adverse reactions were observed when these ODN were administered cutaneously to rhesus macaques or mice. Current results confirm and extend findings in mice by showing that CpG ODN designed for human use accelerate wound healing in primates. This was not accomplished simply by activating cells that express TLR9, but rather involved synergy with additional factors normally present at wound sites (such as TGF∀ and IL-1∀). Given the complexity of the wound healing process, additional factors and activities may also play primary or supportive roles in CpG-accelerated repair. As previous studies established that the response of rhesus macaques to CpG ODN closely mimics that of Man, current findings support the initiation of human clinical studies to assess the therapeutic utility of ‘K’ ODN on wound healing.
Conclusions
This work establishes that the topical administration of “K” type CpG ODN designed for clinical use significantly accelerates wound healing in rhesus macaques (a species that accurately models the response of humans). This effect is mediated by CpG-dependent activation of TLR9 expressing cells but is critically dependent on other factors present at the wound site.
Supplementary Material
Human PBMC were cultured in vitro with 1 uM of ‘K’ or ‘D’ ODN for 48 hr. The concentration of VEGF in culture supernatants was measured by ELISA. Results represent the mean + SE of 5 independently studied PBMC samples.
*; p <.05.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
Abbreviations
- BME
basement membrane extract
- ODN
phosphorothioate oligodeoxynucleotide
- LPS
lipopolysaccharide
- bFGF
basic fibroblast growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madison KC. Barrier function of the skin: “la raison d'etre” of the epidermis. J Invest Dermatol. 2003;121:231–41. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury. 2006;37:S5–12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 6.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 7.Frantz S, Vincent KA, Feron O, Kelly RA. Innate immunity and angiogenesis. Circ Res. 2005;96:15–26. doi: 10.1161/01.RES.0000153188.68898.ac. [DOI] [PubMed] [Google Scholar]
- 8.Broadley KN, Aquino AM, Woodward SC, Buckley-Sturrock A, Sato Y, Rifkin DB, et al. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989;61:571–5. [PubMed] [Google Scholar]
- 9.Koff JL, Shao MX, Kim S, Ueki IF, Nadel JA. Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J Immunol. 2006;177:8693–700. doi: 10.4049/jimmunol.177.12.8693. [DOI] [PubMed] [Google Scholar]
- 10.Straino S, Di CA, Mangoni A, De MR, Guerra L, Maurelli R, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128:1545–53. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 11.Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–88. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razin A, Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- 13.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci. 1994;91:3799–803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieg AM, Yi A, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–8. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 15.Klinman DM, Yi A, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc Natl Acad Sci USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner H. Bacterial CpG-DNA activates immune cells to signal “infectious danger”. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 17.Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita F, Leifer CA, Gursel I, Ishii K, Takeshita S, Gursel M, et al. Cutting Edge: role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–8. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 19.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–58. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Yamamoto M, Shimosato T, Klinman DM. Accelerated wound healing mediated by activation of Toll-like receptor 9. Wound Repair Regen. 2010;18:586–93. doi: 10.1111/j.1524-475X.2010.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, et al. Human TLR9 confers responsiveness to bacterial DNA via species -specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001;11:333–40. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- 23.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J Immunol. 2001;166:2372–7. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 24.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFNa/b in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–41. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 26.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002;168:1659–63. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 27.Verthelyi D, Klinman DM. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin Immunol. 2003;109:64–71. doi: 10.1016/s1521-6616(03)00202-x. [DOI] [PubMed] [Google Scholar]
- 28.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–44. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol. 2008;180:569–79. doi: 10.4049/jimmunol.180.1.569. [DOI] [PubMed] [Google Scholar]
- 30.White SR, Tse R, Marroquin BA. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:301–10. doi: 10.1165/rcmb.2004-0118OC. [DOI] [PubMed] [Google Scholar]
- 31.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van d V. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol. 2007;36:138–46. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 33.Miller LS. Toll-like receptors in skin. Adv Dermatol. 2008;24:71–87. doi: 10.1016/j.yadr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James LC, Moore AM, Wheeler LA, Murphy GM, Dowd PM, Greaves MW. Transforming growth factor alpha: in vivo release by normal human skin following UV irradiation and abrasion. Skin Pharmacol. 1991;4:61–4. doi: 10.1159/000210925. [DOI] [PubMed] [Google Scholar]
- 35.Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–88. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. ndocr Relat Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 37.Clark RAF. The molecular and cellular biology of wound repair. 2nd. New York: Plenum Press; 1996. [Google Scholar]
- 38.Werner S, Smola H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001;11:143–6. doi: 10.1016/s0962-8924(01)01955-9. [DOI] [PubMed] [Google Scholar]
- 39.Sato N, Fujii A. Effects of interleukin-1alpha on the production and release of basic fibroblast growth factor in cultured nifedipine-reactive gingival fibroblasts. J Oral Sci. 2008;50:83–90. doi: 10.2334/josnusd.50.83. [DOI] [PubMed] [Google Scholar]
- 40.Lee P, Lee DJ, Chan C, Chen SW, Ch'en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–23. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller LS, Sorensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174:6137–43. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- 42.Horner AA, Raz E. Immunostimulatory sequence oligodeoxynucleotide-based vaccination and immunomodulation: two unique but complementary strategies for the treatment of allergic diseases. J Allergy Clin Immunol. 2002;110:706–12. doi: 10.1067/mai.2002.129122. [DOI] [PubMed] [Google Scholar]
- 43.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–7. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 44.Klinman DM, Ishii KJ, Gursel M, Gursel I, Takeshita S, Takeshita F. Immunotherapeutic applications of CpG-containing oligodeoxynucleotides. Drug News Perspect. 2000;13:289–96. [PubMed] [Google Scholar]
- 45.Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2003;2:305–15. doi: 10.1586/14760584.2.2.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human PBMC were cultured in vitro with 1 uM of ‘K’ or ‘D’ ODN for 48 hr. The concentration of VEGF in culture supernatants was measured by ELISA. Results represent the mean + SE of 5 independently studied PBMC samples.
*; p <.05.