Abstract
In this work we obtain the thermodynamic properties of mixed (1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine) PC and (1-stearoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (sodium salt)) PS monolayers. Measurements of compressibility (isotherms, bulk modulus, and excess area per molecule) and surface potential show that the properties of monolayers at the air-water interface depend on the concentration of ions (Na+ and K+) and the proportion of PS in the mixture. The dependence on PS arises because the molecule is originally bound to a Na+ counterion; by increasing the concentration of ions the entropy changes, creating a favorable system for the bound counterions of PS to join the bulk, leaving a negatively charged molecule. This change leads to an increase in electrostatic repulsions which is reflected by the increase in area per molecule versus surface pressure and a higher surface potential. The results lead to the conclusion that this mixture of phospholipids follows a non ideal behavior and can help to understand the thermodynamic behavior of membranes made of binary mixtures of a zwitterionic and an anionic phospholipid with a bound counterion.
Keywords: Phosphatidylserine, Phosphatidylcholine, monolayer, isotherms, counterions, non-ideality
1. Introduction
Cells, nuclei, and other organelles are compartmentalized by thin membranes which control the transport of molecules between the interior and exterior of the respective compartments [1]. Membranes play an active role in many biochemical processes, including signaling, cell fusion, and, adhesion [1]. To complete these functions, biological membranes must be dynamic, changing their composition and geometry in response to the environment. As a result, their properties vary depending on their components (phospholipids, proteins, and/or cholesterol) and local environment (ions, proteins, and other charged molecules) [2].
Phospholipids are the major components of membranes, and because of their amphiphilic nature they spontaneously self-assemble when hydrated to form the membrane layer [3]. The main phospholipids of membranes can be divided by charge into zwitterionic (phosphatidylcholine and phosphatidylethanolamine) and anionic (phosphatidylserine, phosphatidylinositol, phosphatidyic acid and phosphatidylglycerol) [2]. The mixing of these phospholipids determines the mechanical and electrical properties of the membranes and their concentrations vary between different types of cells [2; 3].
Because biological membranes are involved in many complex phenomena, simpler models, such as vesicles, supported bilayers, and monolayers, have been used to understand their mechanical and thermodynamical properties [3]. For example, model membranes created by mixtures of phospholipids have been shown to reproduce biologically-relevant processes such as ion binding, transport [4], protein adsorption, electrostatic interactions [5], the appearance of lipid domains, and fusion events [6].
Membranes composed of binary mixtures of zwitterionic and anionic phospholipids are of special interest due to their unique electrostatic and thermodynamic properties [2]. Previous reports have shown that the charge on the phospholipid headgroup is affected considerably due to ion binding [7]. The effects of ions are based on the phenomena of adsorption, described by the Langmuir equation [7–11], together with electrostatic interactions explained by the Grahame equation [10]. For an initially negative phosphatidylserine molecule, addition of monovalent ions at concentrations between 10−1 and 10−3 M promotes ion binding to nearly half of the molecules in the system, with no significant change on the fraction of bound molecules between these concentrations [12]. These theoretical results describe the behavior of initially negatively charged phospholipids and the binding of positive ions; however, in our case we will be analyzing the behavior of phospholipids containing a bound counterion.
Characterization of phospholipids with a phosphatidylserine headgroup and Na+ counterion has been done mainly in bilayers and vesicles. Using x-ray diffraction and NMR spectroscopy on dioleoylphosphatidylserine (DOPS) and dioleoylphosphatidylcholine (DOPC) bilayers it has been found that the area per molecule of DOPS (65.3 Å2) is smaller than that obtained for DOPC (72.5 Å2), although DOPS is considered to be anionic. These results are explained by the absence of electrostatic repulsion due to the existence of the counterion within the bilayer, which can induce an attractive force between headgroups (hydrogen bonding) [13]. Supporting this finding, molecular simulations of palmitoyl-oleoyl phosphatidylserine (POPS) bilayers [14] showed that an added Na+ counterion stays localized at the headgroup of POPS near the carboxyl group. Further, the counterion increases the probability of tightly packed zones where bonding occurs between two POPS molecules by means of the NH3+ group with PO4− and Na+ with the COO− group. This scenario may explain not only the reduction in electrostatic repulsion, but also the attractive interactions between phosphatidylserine molecules that have bound counterions. Additionally, molecular simulations of dipalmitoylphosphatidylserine (DPPS) bilayers with Na+ counterions [15; 16] have estimated that two-thirds of the Na+ ions can be found near the membrane. Furthermore, experimental results with charged phospholipids other than phosphatidylserine show a similar effect on the reduction of electrostatic interactions. For example, analysis on the interactions between egg lecithin bilayers containing the anionic phosphatidylglycerol and phosphatidylinositol found that the repulsive forces described by electrostatic theory are congruent only if the charged phospholipids are taken to be half of the total number of phospholipids present in the bilayer [17], meaning that not all the charged phospholipids are deprotonated. Experiments and simulations both suggest that the presence of headgroup-bound counterions is a strong factor which affects the properties of the membrane.
Tubular vesicles made by mixing different proportions of stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC) with other biomolecules can deform into coiled cylinders [18–24]. This structural change is produced by adding different concentrations of Ca2+ [19] or a polymer [20], and by mixing PC and 1-stearoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (PS) in different proportions [21; 22]. Coiling of vesicles containing PS is believed to occur due to an instability in the surface tension induced by headgroup interactions (e.g. electrostatic forces produce surface charge relaxation which gives rise to coiling or pearling of vesicles) [24]. Further analysis has shown that on the addition of monovalent ions, vesicle tube coiling or even vesicle tube formation is diminished, yielding mostly spherical vesicles [23; 24], while the addition of a divalent ion, Ca2+, mediates phase separation [19]. In this study, we are also interested in the behavior of a binary mixture composed of PC and PS. The PC and PS headgroups are the most abundant types in the plasma membrane [2] and since they possess the same hydrophobic segments, changes in the monolayer parameters (area per molecule, surface potential) are mainly caused by headgroup interactions (Figure 1).
Figure 1.
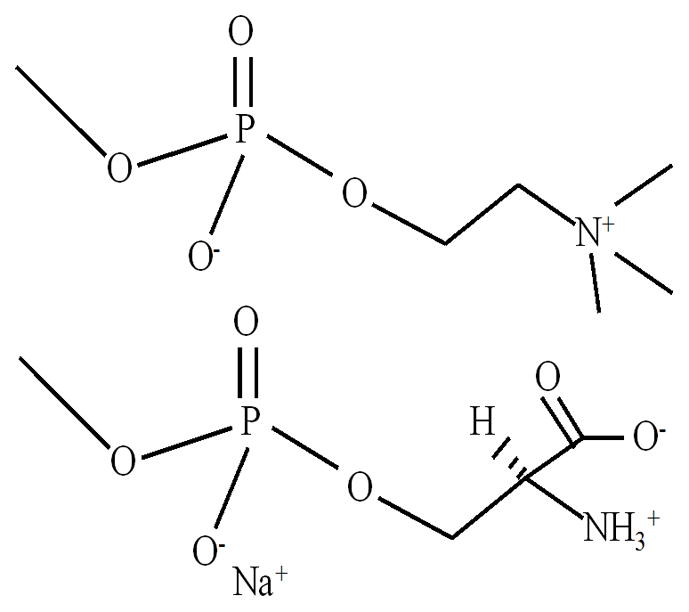
The figure shows the phosphatidylcholine (top) and phosphatidylserine (bottom) headgroup chemical structure and charges, including the Na+ counterion position.
Vesicles are one of the most widely-used and efficient models to study cell membranes; however, they have some limitations when examining physico-chemical properties in more detail. For example, their area per molecule cannot be manipulated in a specific and controlled way [25]; further, the range of lipid composition cannot be varied without significantly changing the vesicle surface curvature [26]. We chose our experimental system to be monolayers formed at the air/water interface. Monolayers are a more appropriate system to study the thermodynamical properties of membranes because we are able to control the area per molecule, surface tension, and concentration of ions at the subphase. Measurements on the surface potential and compressibility of monolayers have been reported for various types of phospholipids and with subphases of different ionic strength [7–11; 27–30]. Although the effects of ions in the subphase have been widely analyzed, the fact that phospholipids can have a previously bound counterion has not yet been taken into account as a factor which affects the properties of monolayers. In this work we show that the non ideal behavior of PC:PS monolayers is dependent on the ionic strength of the subphase due to the bound counterion of the PS molecule. Furthermore, our observations correspond to a desorption mechanism in which the addition of ions promotes the release of bound counterions, leading to increased electrostatic repulsions between PS molecules. With this analysis we obtain an insight into the biophysical properties of PC:PS mixtures in a monolayer; these results lead to a more complete understanding of the thermodynamics of zwitterionic and anionic phospholipid mixtures when the anionic molecule has a bound counterion. The physical properties obtained, such as the variation of the surface pressure due to different concentrations of PS and ions, can help us understand the structural changes observed on PC:PS vesicles which are observed when varying the concentration of PS [21; 22].
2. Materials and Methods
2.1. Materials
The phospholipids used in this work are 1-stearoyl-oleoyl-sn-glycero-3-phosphocholine (PC) and 1-stearoyl-2-oleoyl-sn-glycero-3-phospho-L-serine [sodium salt] (PS) (Avanti Polar Lipids, Inc., Alabaster, AL). Pure PC, PS, and its mixtures were stocked in a chloroform solution (1 mg/ml). All the concentrations shown for mixtures are in molar percentage. PC and PS have an 18:0–18:1 hydrophobic tail and zwitterionic and anionic head groups, respectively.
Monovalent salt solutions, with concentrations of 10 mM, 50 mM and 100 mM of NaCl and KCl (Sigma-Aldrich chemical, St. Louis, MO) and divalent salt solutions of 1 mM and 5 mM CaCl2 (Sigma-Aldrich chemical, St. Louis, MO) were prepared with ultrapure water prior to the experiment.
2.2. Compression Isotherm
Using Microtrough X (Kibron, Inc., Helsinki, Finland), monolayers were prepared by carefully depositing a droplet of phospholipid at the air-water interface. Ultrapure water (18.2 MΩ) was deposited using 25 mm Syringe Filters with a pore size of 0.2 μm (Fisher Scientific, Co., Pittsburgh, PA). The subphase for monolayer experiments was deposited on a trough (59 mm × 208 mm) with two Teflon barriers and had a surface tension of 72.8 mN/m at room temperature (23±1°C). Mixtures were spread from a chloroform solution (1 mg/ml) using a 10 μl microsyringe (Hamilton, Co., Reno, NV). After deposition, a wait-time of 20 minutes was taken in order to let the chloroform evaporate. A compression isotherm was achieved by reducing the area per molecule available using the two symmetrical barriers with a constant velocity of 2 mm/min and under stable room temperature conditions (23±1°C). The surface pressure (mN/m) versus area per molecule (Å2/molecule) compression isotherm plot was obtained on a computer connected to the Microtrough X sensor through the FilmWare software. All experiments were repeated at least three times to ensure reproducibility with an error less than 0.5 mN/m.
2.3. Surface Potential
Measurements were done using the Microtrough X (Kibron, Inc., Helsinki, Finland) configuration for surface potential. This set up uses the vibrating plate capacitor technique which equilibrates the potential at the probe with the potential at the surface.
The surface potential was measured with respect to the subphase where the lipid was deposited which was zeroed for every experiment. The software calculation of the surface potential is as follows: ϕt = ϕ0 − ϕmon where ϕt is the measured surface potential, ϕ0is the reference potential and ϕmon is the potential of the monolayer. When zeroed, ϕ0 = 0, and the measured potential is: ϕt = −ϕmon.
After calibration, the phopsholipid was deposited in the same fashion as for compression isotherms. This procedure allowed for observation of the changes in the surface potential of the phospholipid monolayer with a resolution higher than 1 mV.
2.4. Bulk modulus and ideal area per molecule
Changes in the monolayer compressibility, as well as phase transitions, can be given by the isothermal compressibility modulus (C). The inverse of the isothermal compressibility modulus, or bulk modulus, is a measure of the resistance of the monolayer to compression, or, in other words, the amount of pressure needed to cause a change in the area per molecule. The bulk modulus is valid for mixed systems as well as for two phase multi-component systems [31] and is given by:
| (1) |
where A is the area per molecule at a certain pressure and the derivative is the change in surface pressure (π) over the change of area per molecule of the system at that point. The inverse of the isothermal compressibility modulus represents the slope of the isotherm and is used as a measure of phase transitions of the monolayer [27].
The area per molecule for an ideal mixed monolayer with two components can be expressed as the linear addition given by
| (2) |
where Xi is the mole fraction of pure component i and Ai is the area per molecule of the pure component i.
The excess area for a binary monolayer can be calculated from
| (3) |
wherein A12 is the experimental area per molecule. Thus, by calculating the ideal area per molecule we are able to estimate how much the monolayer is deviating from ideality; if different phases exist within the monolayer, or other cohesive forces are acting between the molecules, it will be reflected in a difference between the ideal area per molecule and the one experimentally obtained.
3. Results and Discussion
3.1. Isotherms with ultrapure water subphase
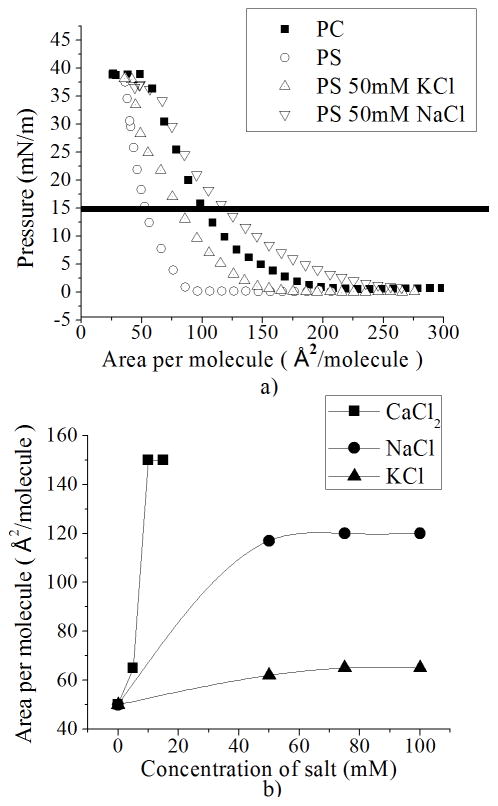
Monolayers were compressed at a constant velocity and temperature (see Experimental Section) to create plots of surface pressure versus area per molecule (isotherms). Isotherms of pure PC and PS (Figure 2) were found to be in agreement with previous reports [32–34]. Changing the area per molecule of the PS monolayer shows no significant variation in surface pressure from 200 to 75 Å2/molecule, whereas PC:PS mixtures and pure PC monolayers have already reached surface pressures above 15 mN/m. Above 15 mN/m monolayers increase in surface pressure until collapse without any significant difference. A negatively charged monolayer (PS) should have more or a similar area per molecule per surface pressure than a zwitterionic monolayer (PC), due to the electrostatic repulsion between PS headgroups. However, for the case of a bound counterion to PS it shows, in fact, more compression (less area per molecule) than the zwitterionic monolayer or even mixtures thereof (Figure 2), which can be due not only to the lack of electrostatic repulsion but also to possible hydrogen bonding [13; 14]. PS molecules are usually assumed to be in a monolayer bearing a negative charge [11] due to dissociation; however, in order to preserve stability, PS and other charged phospholipids are stored in an organic solvent with a bound counterion neutralizing the molecule. It has been observed in experiments and simulations [13–17; 35–37] that the counterion (Na+) does not always leave the monolayer.
Figure 2.
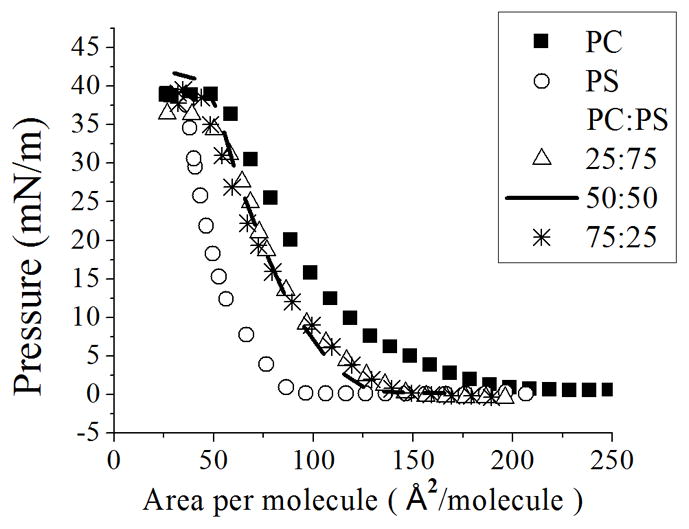
Compression isotherms of PC:PS mixtures. Surface pressure vs. area per molecule plots of PC:PS mixtures on ultrapure water. Standard deviation bars from three independent experiments are smaller than symbols
From the obtained results, we found that the PS molecule is bound to a Na+ counterion; this complex could behave as a molecule with dipole interactions or as a neutral molecule. If we consider the dipole-dipole interactions in the monolayer, this effect would induce the headgroups to orient themselves parallel to the interface, with the opposite charges of different dipoles facing each other, similar to a PC monolayer [2]. As a result, the monolayer would show more surface pressure upon compression, and the shift in the isotherm with respect to PC would be less significant, due to the fact that PS and PC have limiting areas per molecule in the same range (65.3 Å2 and 72.5 Å2) [13]. However, we found a greater increase in the compression of the monolayer, characteristic of a neutral molecule; thus, for the rest of the paper we will consider the PS-bound complex as neutral.
Thus, the bound counterion neutralizes the PS molecules, leaving no electrostatic repulsion between molecules. Therefore, the surface pressure values exist at areas per molecule much less for PS than for PC monolayers (Figure 2). Our results are consistent with the experimental findings for DOPC and DOPS, where DOPS shows less area per molecule in a bilayer due to a bound counterion [13].
Monolayers formed with 90:10, 95:5, 10:90 and 5:95 PC:PS mixtures have an isotherm similar to that of the corresponding major component (data not shown). However, for PC:PS mixtures of 75:25, 50:50 and 25:75; the isotherms are approximately the same as each other and lie in between those for pure PC and PS (Figure 2). This behavior could be caused by non-ideal mixing between PC and PS phospholipids probably due to the capability of the counterion to bind to PS. The non-ideal behavior of the mixtures is later discussed in more detail. In order to observe the electrostatic effects on monolayers containing PS, we next deposited the monolayers on a subphase containing different concentrations of ions.
3.2. Effect of Ions
To observe the electrostatic effects in PC:PS and pure PS monolayers, we created a physical environment in which the counterion binding would be affected. Different concentrations of ionic subphases were prepared from the monovalent salts NaCl and KCl, and also from the divalent salt CaCl2, and deposited in the Microtrough X system as described in the Experimental Section. The addition of ions to the subphase appeared to contribute to the release of ions from the monolayer into the bulk, increasing the negatively charged PS molecules (as will be illustrated in the next section); this is likely due to a more favorable energetic configuration which increases the entropy of mixing [12]. In order to support our theory that the shift of isotherms is due to increasing negatively charged PS molecules in the next section we observe the effect of the addition of ions into the subphase on the surface potential of the monolayer. During compression, monolayers formed with pure PC show the same isotherm on solutions with salt as on ultrapure water (Figure 3). Pure PS monolayers and PC:PS mixtures show an increase in the area per molecule per surface pressure (Figure 4a). This shift is dependent on the concentration and type of ion (Figure 4b). For the case of 50 mM NaCl, when the isotherm has high values of area per molecule (>200 Å2/molecule), we observe an increase in surface pressure, indicating that long range electrostatic interactions appear between PS molecules. For the case of KCl, an increase in surface pressure is shown at ~125 Å2/molecule (Figure 4a).
Figure 3.
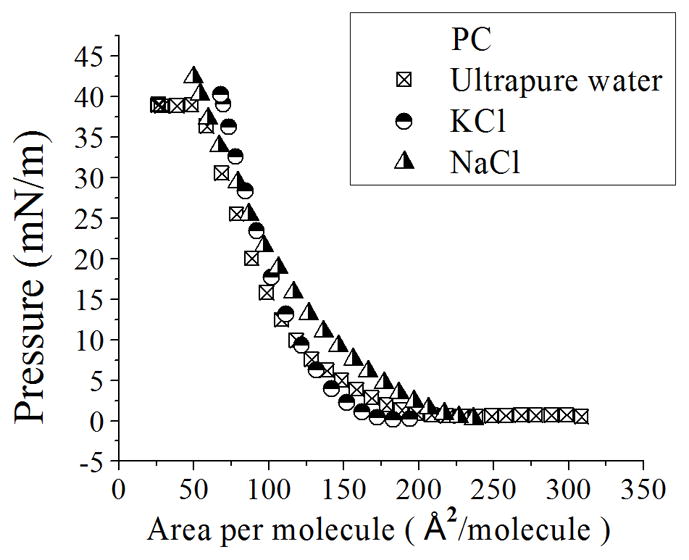
Compression isotherms of PC with different concentrations of ions. Comparison between isotherms of PC with ultrapure water, 50 mM KCl and NaCl at the subphase. Standard deviation bars from three independent experiments are smaller than symbols
Figure 4.
PS monolayer with different concentrations of ions. a) Comparison between isotherms of PS with 50 mM NaCl and KCl, b) Area per molecule at a constant pressure of 15 mN/m (as indicated by the horizontal line in a)) versus concentration of NaCl, KCl and CaCl2 at the subphase. Standard deviation bars from three independent experiments are smaller than symbols
Changes in the monolayer with respect to the type and concentration of ions are better illustrated in Figure 4b, where we see different values of area per molecule versus concentration of salt taken at a surface pressure of 15 mN/m (data taken from isotherms shown in Figure 4a). There is clearly a steady-state effect (such that the area per molecule maintains a constant value) at concentrations of salt above 50 mM. This is consistent with an adsorption-desorption mechanism of ions which affects the compressibility of monolayers [38] and the trend follows what would be expected for ions being dissociated from the monolayer. Further, the shift in the compression isotherm is more pronounced for divalent ions than for monovalent ions (Figure 4b), as discussed below.
Changes in the pH of the sample can induce electrostatic interactions in charged monolayers, beyond varying the concentration of ions in the subphase [11]. However, PC is known to preserve its zwitterionic characteristics over the entire pH range [39], and therefore effects due to protonation are negligible. Also, PS bears a negative charge at values of pH above 4.5 and protonates at a pH of 4.5 [39] and is found in a zwitterionic form. Furthermore, pH values of 4.5 are not present in our system, and thus changes in electrostatics are not due to pH.
The fact that the isotherm of pure PC is not shifted for concentrations of 50 mM NaCl and KCl indicates that there is no binding effect between these ions and PC. In contrast, for PS, the addition of monovalent ions leads to a shift in the isotherm to values similar to that of PC, which we believe is due to increased electrostatic repulsions (Figure 4a), with Na+ ions having a stronger effect than K+, which is reflected in the larger surface pressure versus area per molecule. We believe this is due to the stronger affinity of Na+ (0.6 M−1) to phosphatidylserine than K+ (0.15 M−1) [11]. Although the concentration of divalent ion Ca2+ needed to produce a shift is less than for monovalent ions, other changes may occur besides electrostatics for a divalent ion; for example, Ca2+ can alter the molecular structure of the PS headgroup [40–43]. Therefore, for this case a more detailed analysis that includes molecular alterations is required [11; 29–30] and is beyond the scope of this paper.
Changes in the concentration of ions bound to the monolayer and in the bulk are determined by the entropy of mixing. For a monolayer, it has been shown that the entropy of mixing depends on the concentration of salt and the binding affinity [12]. From our results with ultrapure water we have found that most of the PS molecules maintain their bound Na+ counterion. Thus, when ions are added to the subphase (Na+ or K+) they interact, but not bind to the PS-Na+ monolayer depending on its affinity. Those with higher affinity (Na+) will interact more with the PS-bound complex. This induces the solvation of the bound Na+ counterions, as it becomes more favorable for them to dissociate from PS and instead associate with water molecules, contributing to the entropy of mixing.
The entropy of mixing maintains a favorable energetic configuration in the system by controlling the number of bound and unbound molecules [44], reaching a stable configuration where the charge at the monolayer is similar to that at the subphase. Therefore, in this case, by adding ions to the subphase of a PS monolayer, we induce the PS-Na+ molecule to release the bound counterion.
Using Equation 1, we obtained the bulk modulus for PS monolayers. A PS monolayer on ultrapure water has a low bulk modulus for most values of area per molecule, resembling the behavior of an ideal gas (Figure 5). This holds true until it increases greatly at values of area per molecule less than 60 Å2/molecule, when molecules are being tightly packed, nearly at the point of collapsing the monolayer (Figure 1). The low values of bulk modulus for PS on ultrapure water indicate a lack of electrostatic repulsions between molecules (PS with bound counterion) as observed experimentally for DOPS [13].
Figure 5.
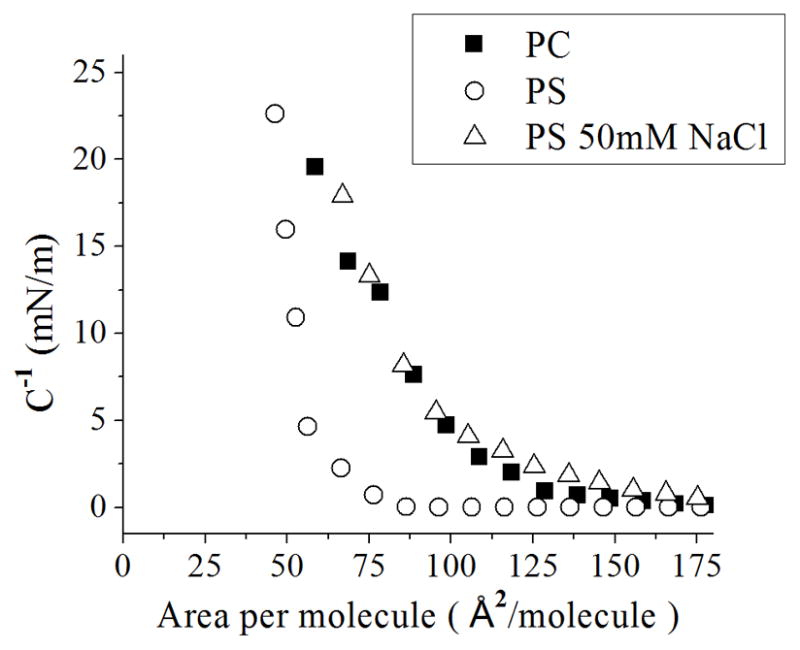
Bulk modulus (C−1) of pure PC and PS monolayers on ultrapure water, as well as PS monolayer on 50 mM NaCl, as a function of area per molecule.
Upon the addition of a monovalent salt (50 mM NaCl) the bulk modulus is approximately the same as for PC monolayers on ultrapure water (Figure 5). Furthermore, the increase in bulk modulus at the liquid phase of the monolayer is a reflection of repulsive forces between molecules of electrostatic origin (i.e. negatively-charged PS molecules). We expect that the negatively-charged PS molecules will induce a change in the surface potential of the monolayer, a measurement which should be proportional to the concentration of ions in the subphase, as observed in the thermodynamic analysis shown in Figure 4b and again in Figure 5. Therefore, the behavior of the surface potential will be studied in the next section.
3.3. Surface Potential of a pure PS monolayer
We measured the surface potential as a function of salt concentration as indicated in the Experimental Section. A positive surface potential indicates a negatively charged monolayer, or in the case of zwitterionic molecules, that the dipole has the negative charge close to the air-water interface [45]. When there is no salt in the system, there is no significant change in the surface potential with respect to the area per molecule, as shown in Figure 6 for areas per molecule between 150-100 Å2/molecule. When ions are added to the subphase, the surface potential is higher than in ultrapure water and increases as ions are added to the subphase (Figure 6). On concentrations of 10 mM NaCl, the change in surface potential between 150-125 Å2/molecule is approximately 12 mV and between 125-100 Å2/molecule is of 25 mV. For the case of 50 mM NaCl, the first change is slightly higher (20 mV) and the second change remains the same (25 mV). Our results (Figure 6) are consistent with theoretical results, where the surface potential increases as the concentration of ions increases for negatively-charged monolayers [45].
Figure 6.
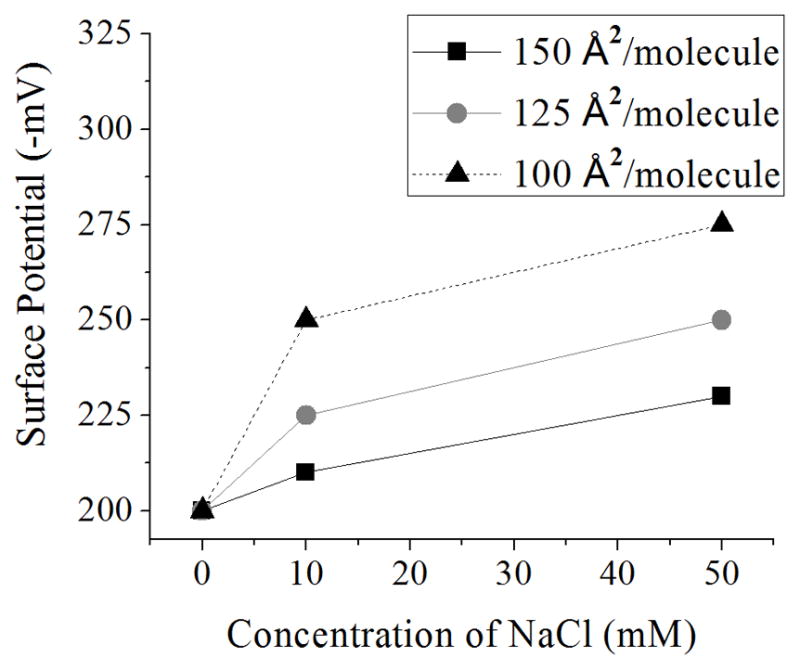
Surface potential of a PS monolayer with different concentrations of NaCl. Standard deviation bars from three independent experiments are smaller than symbols
The fact that the surface potential doesn’t change when we compress the monolayer (50 Å2/molecule) indicates the lack of electrostatic repulsions, a result that is consistent with the isotherm in ultrapure water (Figure 2). Furthermore, when molecules are farther apart (150-125 Å2/molecule) and the concentration of ions is increased, the change in the surface potential is higher; this indicates that electrostatic interactions increased. These results are similar to those observed for changes in isotherms (Figure 4b). Therefore, we conclude that the change in surface potential with increased salt concentration occurs because the bound counterion leaves the PS molecule. The monolayer becomes more negatively charged, leading to an increase in surface charge and therefore in surface potential. This change in surface charge can be related to the compressibility isotherms if we compare to previous experimental and theoretical results [46], where the surface charge is proportional to the surface pressure. In the next section, we use the data from isotherms and surface potential in pure systems to understand the behavior of mixed PC:PS monolayers.
3.4. Mixed PC:PS monolayers
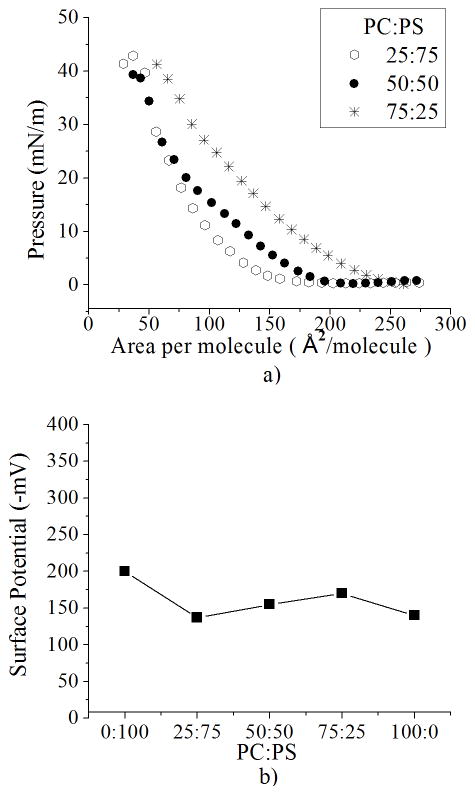
Previously we observed that isotherms of PC:PS mixtures (75:25, 50:50 and 25:75) on ultrapure water are similar; here, we show that differences in the isotherms become obvious when ions are added to the subphase (Figure 7a). We find that with 50 mM NaCl, each of the mixed PC:PS monolayers has a different isotherm. The isotherms change with respect to the concentration of PS, where 75:25 has a greater shift in area per molecule than 25:75. Further, isotherms with higher concentration of PS (50:50 and 25:75) have similar values of surface pressure at low areas per molecule (<75 Å2/molecule). These results might seem counterintuitive; however, since we have shown that the surface potential and isotherms are related (Figure 4b and 6), we evaluated the surface potential of these mixtures. The surface potential of PC:PS mixtures at large area per molecule (75 Å2/molecule) in 50 mM NaCl shows a slight change between mixtures, where the difference between each is around 10 mV. The mixture with more PS (25:75) has a surface potential closer to pure PC than the mixture with more PC (75:25) (Figure 7b). In the following analysis, we use these results as an indication of how many PS molecules are dissociated and present a negative charge (i.e. surface charge).
Figure 7.
PC:PS mixtures. a) Isotherms of PC:PS mixtures and b) Surface potential of PC:PS mixtures (200 Å2/molecule) on 50 mM NaCl. Standard deviation bars from three independent experiments are smaller than symbols
Since PC remains unaffected to different concentrations of salt, changes in the surface pressure on PC:PS mixtures must be related to the dissociation of the counterion from PS in the mixture. Therefore, to understand the mixtures we must consider first the area per molecule of PC molecules; this is similar to that of negatively charged PS. Thus, its isotherm is close to that of pure PC. Then we take into account the effect of PS molecules with or without a bound counterion (Figure 8). This shifts the isotherm to the right or left, depending on how many negatively-charged PS molecules the mixture has compared to the total. This second effect is better understood by looking at the surface potential. The similarity between surface potential measurements indicates that the number of negatively-charged PS molecules between mixtures is comparable. The mixture 25:75 is closer to pure PC, indicating that it has few negatively-charged PS molecules and thus more PS with a bound counterion. Further, 75:25 has a potential closer to pure PS (Figure 6) which reflects that the amount of PS molecules in this mixture is mostly negatively-charged. Thus, as we increase the number of PS molecules in the mixture, the number of bound PS molecules increase as well.
Figure 8.
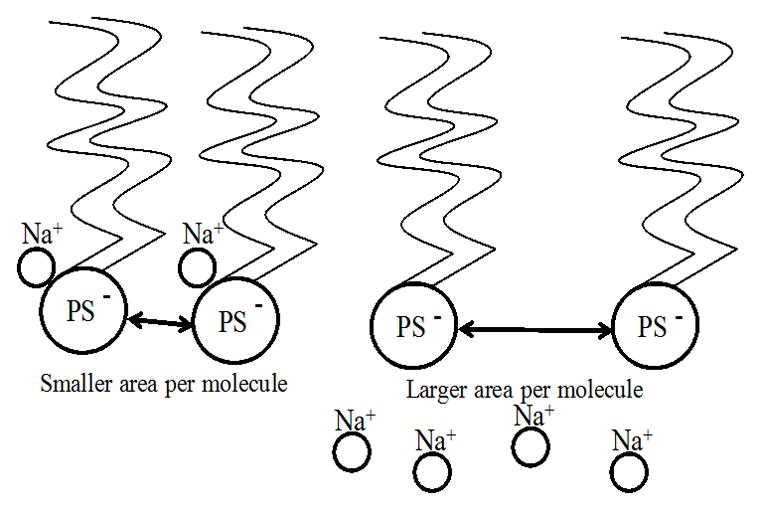
Phosphatidylserine molecule and Na+ counterion. Phosphatidylserine molecules with a bound counterion have less area per molecule (left) than those where the counterion has joined the subphase (right). This is due to electrostatic repulsion between negatively charged phosphatidylserine headgroups.
As we have shown, the isotherm of the mixture with more PC (75:25) is similar to pure PC but shifted to the right due to the electrostatic repulsions caused by the PS molecules present. As the concentration of PS is increased, the amount of bound PS with less area per molecule also increases, and therefore the isotherm of the mixture 25:75 is shifted to the left (Figure 7a). Further, the isotherm of 50:50 shows better the existence of both states of PS: (1) electrostatic repulsions due to unbound PS at areas per molecule between ~200-100 Å2/molecule which are shown by the increase in surface pressure, and (2) bound PS molecules indicated by the isotherm shifting left to the same values as 25:75 at areas per molecule <100 Å2/molecule. The observed behavior suggests non-ideality of the mixed monolayers, and thus in the next section we analyze the deviation from ideality by calculating the excess area per molecule and bulk modulus.
3.5. Deviation from ideality
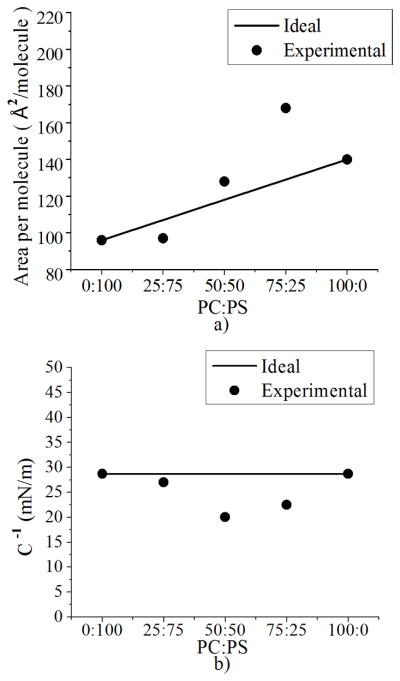
The deviation from ideality was calculated from the isotherms (Figure 4 and 5a) using equations 1 and 2 (see Experimental Section). If a monolayer mixture is ideal, the area per molecule at a certain pressure should be the one calculated with equation 2; when the area per molecule of the mixture is away from the ideal line it is an indication of non-ideality [47; 48]. We find that for monolayers containing less PS molecules (75:25), the excess area per molecule is positive (above the ideal line), indicating more expanded states [47; 48]. Meanwhile, for mixtures containing more PS molecules (25:75) the excess area per molecule is negative, indicating the existence of compressed states (Figure 9a). Further, the bulk modulus was calculated for each mixture using the isotherms and compared with the one obtained by the linear addition of pure PS and PC bulk moduli (ideal), similar to our analysis for the excess area per molecule (Figure 9b). When salt is added to the subphase, pure PS and PC have the same bulk modulus. For the case of PC this is due to the effective area of the headgroup and for the case of PS this is due to electrostatic repulsions, a result which is expected, based on the similar isotherms between both monolayers (Figure 4a). However, the mixtures deviate from ideality, indicating the presence of different states in the monolayer.
Figure 9.
Deviation from ideality. a) Ideal area per molecule and experimental area per molecule (calculated at a surface pressure of 25 mN/m) as a function of concentration b) Experimental and theoretical bulk modulus of PC:PS monolayers, calculated at 100 Å2/molecule.
These calculations together signify a non-ideal behavior of the monolayer. The excess area per molecule indicates how much area the monolayer exceeds beyond its ideal behavior, and we observe that the mixture of 75:25 has a large positive excess area per molecule which suggests that electrostatic repulsions are present between molecules. For the mixture with more PS (25:75) the negative excess area per molecule occurs if PS molecules in the monolayer have a bound counterion and thus more compressed states. These results are consistent with the ones obtained with the surface potential analysis (Figure 7b) where we showed that 75:25 PC:PS has more electrostatic repulsions than 25:75. Our analysis of bulk modulus supports the fact that parts in the monolayer exist in a different physical state than the rest of the monolayer. Indeed, the force it takes to compress the monolayer is different than the case when all the molecules are the same (ideal line). Thus, because of the non-ideal mixing they have less or more area per molecule and a different resistance to compression. While our results suggest that PS molecules in mixed PC:PS monolayers exist in different states (Figure 8 - bound to a counterion for the case of ultrapure water, left; unbound when salt is added to the subphase, right), further analysis of the mixing behavior is needed to better understand how these molecules are arranged and how this arrangement affects the monolayer properties.
The main conclusions of this paper are the following: (1) While PS is a negatively charged molecule, it can be found bound to a counterion, rendering an effectively neutral molecule. (2) The mixing of PC and PS is non-ideal and varies according to the PS concentration. If we translate these findings to a bilayer, we would expect that some of the PS molecules in the membrane would be bound to a counterion, changing the bilayer structural and electrostatic properties. Our experimental data is supported by those found in numerical simulations for PS containing bilayers, where the majority of the Na+ counterions are found in the bilayer and not at the bulk [15]. The change in counterion binding due to the concentration of salt produces a change in the surface potential in monolayers. This feature could play an important role in biological membranes and their interactions with other molecules such as proteins [20] and DNA [49, 50].
The conclusions are even more striking when we use the mixtures of PC and PS to help us understand the behavior of a more complex membrane. There, due to the bound counter ion, a certain number of PS molecules will occupy less area per molecule compared to a different group of negatively charged PS molecules. This effect induces the non-ideal mixing of PC and PS, which can induce the formation of lipid domains in a bilayer. Thus, according to our results, bilayers with a higher concentration of PS molecules will have a greater tendency to form domains with less area per molecule and surface potential. This would affect whole bilayer properties such as mechanical tension and shape, as observed for mixed PC:PS vesicles [21,22].
4. Conclusions
In this work mixed monolayers made of the zwitterionic lipid PC and the anionic lipid PS were formed at the air-water interface. We found that when deposited on ultrapure water, the behavior of PS within the monolayer is similar to that of a neutral molecule due to the bound Na+ counterion. When ions are added into the subphase, the electrostatic interactions between PS molecules increase; this causes an increase both in area per molecule versus surface pressure and in the surface potential. Further, the excess area per molecule and bulk modulus together indicate a non-ideal behavior of mixed PC:PS monolayers, which is attributed to the existence of PS molecules with bound and unbound counterions. PS-Na+ molecules have a smaller effective area per molecule due to the lack of electrostatic repulsions. Unbound PS molecules increase the surface charge of the monolayer and thus also the measured surface potential. The fraction of molecules in each state depends on the concentration of PS in the mixture and of the ions in the subphase.
Previously, it has been shown that varying the amount of PS and salt in the system changes the geometrical properties of PC:PS vesicles. Our results further explain this behavior, by showing that the surface pressure and charge of the membrane change as a function of PS and salt concentration; these modifications likely lead to previously-observed geometrical alterations such as vesicle coiling. This analysis can be extrapolated to other phospholipid systems where the salt in the media changes, such as cell membranes, supported bilayers, and self-assembled monolayers. Further exploration on this system is needed to demonstrate how the mixing occurs; including how the different PS and PC molecules arrange within the monolayer, and whether the existence of PS in two different states induces domain formation.
Acknowledgments
This work was supported by a fellowship from CONACYT-Mexico to CL, a NINDS Award Number F31NS068028 to KMS, and NSF Awards CMMI-0643783 to HAE and DMR-0847558 to HB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts B. Molecular biology of the cell. Garland Science; 2007. [Google Scholar]
- 2.Cevc G. Phospholipids handbook. CRC Press; 1993. [Google Scholar]
- 3.Chan YHM, Boxer SG. Model Membrane Systems and their applications. Curr Opin Chem Bio. 2007;11:581–587. doi: 10.1016/j.cbpa.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattai J, Hauser H, Demel RA, Shipley GG. Interactions of metal ions with phosphatidylserine bilayer membranes: effect of hydrocarbon chain unsaturation. Biochem. 1989;28:2322–2330. doi: 10.1021/bi00431a051. [DOI] [PubMed] [Google Scholar]
- 5.Langner M, Kubica K. The electrostatics of lipid surfaces. Chem Phys Lipids. 1999;101:3–35. doi: 10.1016/s0009-3084(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 6.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 7.Helm CA, Laxhuber L, Losche M, Möhwald H. Electrostatic interactions in phospholipid membranes I: Influence of monovalent ions. Colloid Polym Sci. 1986;264:46–55. [Google Scholar]
- 8.Domènech Ò, Torrent-Burgués J, Merino S, Sanz F, Montero MT, Hernández-Borrell J. Surface Thermodynamics study of monolayers formed with heteroacid phospholipids of biological interest. Colloids Surf B. 2005;41:233–238. doi: 10.1016/j.colsurfb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 10.Grahame DC. The electrical double layer and the theory of electrocapillarity. Chem Rev. 1947;41:441–501. doi: 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin S, Mulrine N, Gresalfi T, Vaio G, McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981;77:445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travesset A, Vangaveti S. Electrostatic interactions at the Stern layer: Physics or Chemistry? J Chem Phys. 2009;131:185102. doi: 10.1063/1.3257735. [DOI] [PubMed] [Google Scholar]
- 13.Petrache HL, Tristram-Nagle S, Gawrisch K, Harries D, Parsegian VA, Nagle JF. Structure and fluctuations of charged phosphatidylserine bilayers in the absence of salt. Biophys J. 2004;86:1574–1586. doi: 10.1016/S0006-3495(04)74225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukhopadhyay P, Monticelli L, Tieleman DP. Molecular dynamics simulations of a palmitoyl-oleoyl phosphatidylserine bilayer with Na+ counterions and NaCl. Biophys J. 2004;86:1601–1609. doi: 10.1016/S0006-3495(04)74227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandit SA, Berkowitz ML. Molecular dynamics simulation of dipalmitoylphosphatidylserine bilayer with Na+ counterions. Biophys J. 2002;82:1818–1827. doi: 10.1016/S0006-3495(02)75532-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandit SA, Bostick D, Berkowitz ML. Mixed bilayer containing dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylserine lipid complexation, ion binding and electrostatics. Biophys J. 2003;85:3120–3131. doi: 10.1016/S0006-3495(03)74730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowley AC, Fuller NL, Rand RP, Parsegian VA. Measurement of repulsion forces between charged phospholipid bilayers. Biochem. 1978;17:3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- 18.Santangelo CD, Pincus P. Coiling instabilities of multilamellar tubes. Phys Rev E. 2002;66:061501. doi: 10.1103/PhysRevE.66.061501. [DOI] [PubMed] [Google Scholar]
- 19.Ke-Chun L, Weis RM, McConnell HM. Induction of helical liposomes by Ca2+ mediated intermembrane binding. Nature. 1982;296:164–165. doi: 10.1038/296164a0. [DOI] [PubMed] [Google Scholar]
- 20.Frette V, Tsafrir L, Guedeau-Boudeville MA, Jullien L, Kandel D, Stavans J. Coiling of cylindrical membrane stacks with anchored proteins. Phys Rev Lett. 1999;72:2465–2468. [Google Scholar]
- 21.Paredes-Quijada G, Aranda-Espinoza H, Maldonado A. Shapes of mixed phospholipid vesicles. J Biol Phys. 2006;32:177–181. doi: 10.1007/s10867-006-9007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paredes-Quijada G, Aranda-Espinoza H, Maldonado A. Shapes and Coiling of Mixed Phospholipid Vesicles. Lipids. 2009;44:283–289. doi: 10.1007/s11745-009-3280-z. [DOI] [PubMed] [Google Scholar]
- 23.Mishima K, Fukuda K, Suzuki K. Double helix formation of phosphatidylcholine myelin figures. Biochim Biophys Acta, Biomembr. 1992;1108:115–118. doi: 10.1016/0005-2736(92)90121-2. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen T, Gopal A, Lee KYC, Witten A. Surface charge relaxation and the pearling instability of charged surfactant tubes. Phys Rev Lett. 2005;72:051930. doi: 10.1103/PhysRevE.72.051930. [DOI] [PubMed] [Google Scholar]
- 25.Brockman H. Lipid monolayers: why use a half membrane to characterize protein-membrane interactions? Curr Opin Struct Biol. 1999;9:438–443. doi: 10.1016/S0959-440X(99)80061-X. [DOI] [PubMed] [Google Scholar]
- 26.Micol V, Sanchez-Piñera P, Villalaín J, de Godos A, Gómez-Fernández JC. The cancer chemopreventive agent resveratrol is incorporated into model membranes and inhibits protein kinase C [alpha] activity. Biophys J. 1999;76:916–927. doi: 10.1006/abbi.1999.1507. [DOI] [PubMed] [Google Scholar]
- 27.Davies JT, Rideal EK. Interfacial Phenomena. Academic Press; New York: 1963. [Google Scholar]
- 28.Samsonov AV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–1500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen JA, Cohen M. Adsorption of monovalent and divalent ions by phospholipid membranes. The monomer-dimer problem. Biophys J. 1981;36:623–651. doi: 10.1016/S0006-3495(81)84756-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smoluchowski MV. Engineering biological structures of prescribed shape using self-assembled multicellular systems, Z. Phys Chem. 1917;92:129. [Google Scholar]
- 31.Firoozabadi A. Thermodynamics of hydrocarbon reservoirs. McGraw-Hill Professional; 1999. [Google Scholar]
- 32.Blois A, Holmsen H, Martino G, Corti A, Mertz-Boutigue MH, Helle KB. Interactions of chromogranin A-derived vasostatins and monolyers of phosphatidylserine, phosphatidylcholine and phosphatidylethanolamine. Regul Pept. 2006;134:30–37. doi: 10.1016/j.regpep.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Canale C, Torrasa S, Rispoli P, Relini A, Rolandi R, Bucciantini M, Stefani M, Gliozzi A. Natively folded HypF-N and its early amyolid aggregates interact with phospholipid monolayers and destabiliyze supported phospholipid bilayers. Biophys J. 2006;91:4575–4588. doi: 10.1529/biophysj.106.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dynarowicz-Latka P, Hac-Wydro K. Interactions between phosphatidylcholines and cholesterol in monolayers at the air/water interface. Colloids Surf B. 2004;37:21–25. doi: 10.1016/j.colsurfb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Loosley-Millman ME, Rand RP, Parsegian VA. Effect of monovalent ion binding and screening on measured electrostatic forces between charged phospholipid bilayers. Biophys J. 1982;40:221–232. doi: 10.1016/S0006-3495(82)84477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demé B, Dubois M, Gulik-Krzywicki T, Zemb T. Giant collective fluctuations of charged membranes at the lamellar to vesicles unbinding transition. 1. Characterization of a new lipid morphology by SANS, SAXS and Electron microscopy. Langmuir. 2002;18:997–1004. [Google Scholar]
- 37.Demé B, Dubois M, Zemb T. Giant collective fluctuations of charged membranes at the lamellar to vesicles unbinding transition. 2 Equation of state in the absence of salt. Langmuir. 2002;18:1005–1013. [Google Scholar]
- 38.Birdi KS, Gevod VS. Melittin and ionic surfactant interactions in monomolecular films. Colloid Polym Sci. 1987;265:257–261. [Google Scholar]
- 39.Hanahan DJ. A Guide to Phospholipid Chemistry. Oxford University Press; Oxford: 1997. [Google Scholar]
- 40.Marone PA, Elsmore SK, Sandulow J, Mannino RJ. Structural Determinants of Divalent Cation-Induced Phosphatidylserine Cochelate Crystallization. Microsc Microanal. 2005;11:1244–1245. [Google Scholar]
- 41.Hui SW, Boni LT, Stewart TP, Isac T. Identification of phosphatidylserine and phosphatidylcholine in calcium-induced phase separated domains. Biochem. 1983;22:3511–3516. [Google Scholar]
- 42.Silvius JR, Gagne J. Calcium-induced fusion and lateral phase separation in phosphatidylcholine-phosphatidylserine vesicles. Correlation by calorimetric and fusion measurements. Biochem. 1984;23:3241–3247. [Google Scholar]
- 43.Roux M, Bloom M. Calcium binding by phosphatidylserine headgroups. Deuterium NMR study. Biophys J. 1991;60:38–44. doi: 10.1016/S0006-3495(91)82028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth CM, Sader JE, Lenhoff AM. Electrostatic Contribution to the Energy and Entropy of Protein Adsorption. J Colloid Interface Sci. 1998;203:218–221. [Google Scholar]
- 45.Davies JT. The distribution of ions under a charged monolayer, and a surface equation of state for charged films. Proc R Soc London Ser A. 1951;208:224–247. [Google Scholar]
- 46.Levental I, Janmey PA, Cebers A. Electrostatic contribution to the surface pressure of charged monolayers containing polyphosphoinositides. Biophys J. 2008;95:1199–1205. doi: 10.1529/biophysj.107.126615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaines GL., Jr . Insoluble Monolayers at Liquid-Gas Interfaces. Wiley-Interscience; New York: 1996. [Google Scholar]
- 48.Birdi KS. Lipid and Biopolymer Monolayers at the Liquid Interface. Plenum; New York: 1989. [Google Scholar]
- 49.Schiessel H, Aranda-Espinoza H. Electrostatically induced undulations of lamellar DNA-lipid complexes. Eur Phys J E. 2001;5:499–506. [Google Scholar]
- 50.Trihn MU, Ralston J, Fornasiero D. Characterization and stability of lipid-DNA complexes. Colloid Surface B. 2008;67:85–91. doi: 10.1016/j.colsurfb.2008.08.002. [DOI] [PubMed] [Google Scholar]