Abstract
Extinction of fear is important for treating stress-related conditions particularly post traumatic stress disorder (PTSD). Although traditional extinction presents the feared stimulus by itself, there is evidence from both clinical and basic research that repeatedly presenting the feared stimulus by itself does not prevent fear from returning. This renewal or relapse can be “thwarted” by unpaired extinction – presentations of the feared stimulus and the event producing the fear. However, no matter how effective standard unpaired extinction may be in the laboratory, repeated presentation of a traumatic event is untenable. To make an unpaired extinction procedure more clinically relevant, we classically conditioned the rabbit nictitating membrane response using electrical stimulation or air puff as the unconditioned stimulus and then during unpaired extinction reduced both the intensity of the unconditioned stimulus and the days of unpaired stimulus presentations. We found unpaired extinction reduced conditioned and exaggerated unconditioned responding (an animal analog of PTSD called conditioning-specific reflex modification) and could be accomplished with a weak unconditioned stimulus as long as extended presentations were used. Surprisingly, brief presentations of a weak unconditioned stimulus or extended presentations of a strong one made the exaggerated responses stronger. One implication is that brief treatment may not just be ineffectual; it may heighten the symptoms of PTSD. Another implication is that using strong stimuli may also heighten those symptoms.
Keywords: classical conditioning, fear, heart rate, nictitating membrane response, rabbit, relapse
Introduction
Extinction of fear has become increasingly important in treating stress-related conditions particularly post traumatic stress disorder (PTSD). Although traditional forms of extinction present the feared stimulus by itself, there is evidence from both clinical and basic research that repeated presentation of feared stimuli does not prevent fear from returning (Milad et al., 2006; Bouton et al., 2006; McNally, 2007; Hofmann, 2008; Chang et al., 2009). Moreover, PTSD is not just characterized by fear of stimuli associated with traumatic events but by exaggerated reactions to innately aversive/stressful stimuli (Pitman et al., 1993; Milad et al., 2006). Renewal or relapse of fear may be “thwarted” by unpaired presentations of both the feared stimulus and the event producing the fear (Rauhut et al., 2001; Thomas et al., 2005; Vervleit et al., 2010). It is also possible unpaired extinction may modulate exaggerated reflexive reactions (Schreurs, 2003; Burhans et al., 2008).
Unpaired extinction experiments conducted across different species show unpaired presentations of a conditioned stimulus (CS) and unconditioned stimulus (US) facilitate extinction of a conditioned response (CR). These experiments are drawn from human and rabbit eyelid conditioning (Spence, 1966; Leonard, 1975; Frey & Butler, 1977; Kehoe et al., 2004), pigeon autoshaping (Colwill, 2007), conditioned bar-press suppression in rats (Rauhut et al., 2001; Thomas et al., 2005), conditioned taste aversion (Mickley et al., 2009), and human discriminative fear conditioning (Vervleit et al., 2010). In the human study, Vervleit and coworkers found that compared to normal extinction, only unpaired extinction prevented renewal of fear responses in people trained to discriminate one of two pictures paired with shock (Vervleit et al., 2010).
In rabbit classical conditioning experiments designed to extinguish CRs, comparable extinction of CS responding occurs following CS-alone or unpaired CS/US presentations in the nictitating membrane response (NMR, Schreurs et al., 2000) and heart rate (HR) response (Burhans et al., 2009). However, unpaired presentations were able to extinguish conditioning-specific increases in the unconditioned response (UR) known as conditioning-specific reflex modification (CRM) better than CS-alone presentations (Schreurs et al., 2000). CRM is an increase in size and shape of URs that occurs when these responses are measured in the absence of the CS following classical conditioning of the rabbit NMR (Gruart & Yeo, 1995; Schreurs et al., 1995; Schreurs et al., 2000; Buck et al., 2001; Wikgren et al., 2002; Seager et al., 2003; Schreurs et al., 2006; Burhans et al., 2008) and HR (Schreurs et al., 2005; Burhans et al., 2008; Burhans et al., 2009).
The ability of unpaired presentations to diminish both CS responses and exaggerated US responses (i.e., CRM) suggests it may be relevant for treating symptoms of PTSD (Seager et al., 2003; Schreurs et al., 2006; Burhans et al., 2008). In theory, exaggerated responses following classical conditioning may be similar to exaggerated reactions to innately aversive/stressful stimuli considered a hallmark of PTSD (Shalev et al., 2000; Orr et al., 2002; Pole et al., 2009). However, no matter how effective unpaired extinction might be in the laboratory, it would be ethically unacceptable for treating PTSD. Repeated presentation of the traumatic event responsible for PTSD is untenable.
To determine if unpaired extinction could be made more clinically relevant, we classically conditioned the rabbit NMR and reduced US intensity and duration of unpaired extinction. We wanted to know if a weaker US or fewer days of extinction would be effective in extinguishing both CRs and CRM (Schreurs et al., 1995; Schreurs et al., 2000; Buck et al., 2001; Seager et al., 2003; Schreurs et al., 2005; Burhans et al., 2009). Given the heightened physiological indices found in patients with PTSD including HR (Pitman et al., 2006), we measured HR during testing as a physiological measure of rabbits’ emotional reactivity to the US.
Experiment 1
There is considerable evidence classical conditioning rabbit eyelid responses using periorbital electrical stimulation (ES) produces rapid acquisition that can be extinguished with either CS-alone or unpaired presentations (Frey & Butler, 1977). We have shown unpaired extinction will also greatly reduce the level of heightened US responding known as CRM (Schreurs et al., 2000). The aim of Experiment 1 was to determine if reductions in ES intensity and duration of unpaired extinction would affect CRs and CRM.
Methods
Subjects
Seventy two male New Zealand White rabbits (Oryctolagus cuniculus) supplied by Harlan weighed 2.0–2.2 kg. Animals were housed in individual cages, given free access to food and water, and maintained on a 12-hour light/dark cycle. Rabbits were maintained within guidelines issued by the National Institutes of Health, and the research was approved by the West Virginia University Animal Care and Use Committee.
Apparatus
The apparatus and NMR recording procedures have been detailed by Schreurs and Alkon (1990) who modeled apparatus after those described by Gormezano (Gormezano, 1966; Coleman & Gormezano, 1971). Each subject was restrained in a Plexiglas box and trained in a sound-attenuating, ventilated chamber (Coulbourn Instruments, Model E10-20). A stimulus panel containing a speaker and house light (1-W, 120-V, 60-lumen LED lamp) was mounted above the subject’s head and a noise level of 65 dB was provided by exhaust fan. Periorbital ES was delivered by programmable shocker (Coulbourn Instruments, Model E13-35) via stainless steel Autoclip wound clips positioned below and 1 posterior to the dorsal canthus of the right eye.
The apparatus, recording and analysis procedures for heart rate (HR) have been detailed previously (Schreurs et al., 2005). Wound clips were placed in shaved skin either side of the breast bone and on the right shoulder. The clips were coupled to individual custom amplifiers (10,000-fold) and ECG signals were routed to an analog-to-digital converter and stored for offline analysis.
Details of transducing NM movements have been reported previously (Gormezano & Gibbs, 1988; Schreurs & Alkon, 1990) A hook connected to an L-shaped lever was attached to a 6-0 nylon loop sutured into the NM. The other end of the lever was attached to a rotary potentiometer connected to a 12-bit analog-to-digital converter and computer. Individual analog-to-digital outputs were stored for subsequent analysis.
Procedure
Rabbits were randomly assigned to nine groups (n’s=8) comprising a 3 × 3 factorial with days of unpaired extinction (Extinction Duration, 1, 3 or 6 days) and US intensity during unpaired extinction (Extinction Intensity, 0.25, 1.0 or 2.0 mA) as factors. Rabbits received adaptation, one 80-trial session of US testing (Pretest), six daily sessions of paired CS-US presentations (Acquisition), another 80-trial session of US testing (Post Test-1) and then one, three or six days of unpaired CS and US presentations with a US of 0.25-, 1.0-, or 2.0-mA (Unpaired Extinction), followed by a final 80-trial session of US testing (Post Test-2). On adaptation, rabbits were prepared for ES and NMR and HR recording and adapted to training chambers for the duration of subsequent sessions (80 min). On pre and post tests, subjects were prepared for HR recording and received 80 ES trials presented at an average interval of 60 s. Each trial involved presentation of 1 of 20 combinations of ES intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms). Four randomized sequences of the 20 stimulus combinations were presented each test day but the same intensity or duration could not occur on more than three consecutive trials.
Each of six paired conditioning sessions consisted of 80 presentations of a 400-ms, 1-kHz, 82-dB tone CS co-terminating with a 100-ms, 2.0-mA US (i.e., 300-ms inter-stimulus interval [ISI]). Paired presentations were delivered, on average, every 60 s (50–70 s range). One, three or six daily sessions of unpaired extinction consisted of 80 CS-alone and 80 US-alone presentations occurring in an explicitly unpaired manner delivered, on average, every 30 s (20–40 s range). CRs were defined as any NM extension exceeding 0.5 mm initiated after CS onset but before US onset. A UR was defined as any NM extension exceeding 0.5 mm initiated within 300 ms of US onset (ISI used to score CRs during pairings). Response amplitude was scored in millimeters as maximum NM extension. Response area was total area of the response (in arbitrary units) from stimulus onset until the end of trial. For URs during US testing, two additional measures were calculated to overcome statistical limitations of empty data cells produced by subthreshold US responses, particularly at lower intensities and durations. Magnitude of the response and magnitude of the response area included the amplitudes and areas of all NM movements above baseline (Gracia et al., 2003).
Details for detecting heartbeats and components of ECG signals have been described previously (Schreurs & Smith-Bell, 2005; Schreurs et al., 2005; Burhans et al., 2009). Heartbeats were detected in filtered ECG signals with a template-matching algorithm. Visual inspection of data corrected any false positives or negatives as a result of artifacts (e.g., movement). Data were expressed as a change in inter-beat interval (IBI) to the US from a pre-stimulus baseline. Individual components of the ECG – particularly the P wave, with amplitudes of 20 to 40 μV, were resolved with a peak detection algorithm (Origin Software, Origin Lab, MA). The distance between the P and Q components (PQ interval) has been used in previous animal ECG studies (Hayes et al., 1994; Nijsen et al., 1998; Schreurs et al., 2007; Burhans et al., 2009) and represents vagal-mediated parasympathetic contribution to HR (Nijsen et al., 1998). In addition to IBI and PQ measurements, topographies of changes in IBI during CRM testing examined timing of changes in heart beats.
Results
CR Acquisition
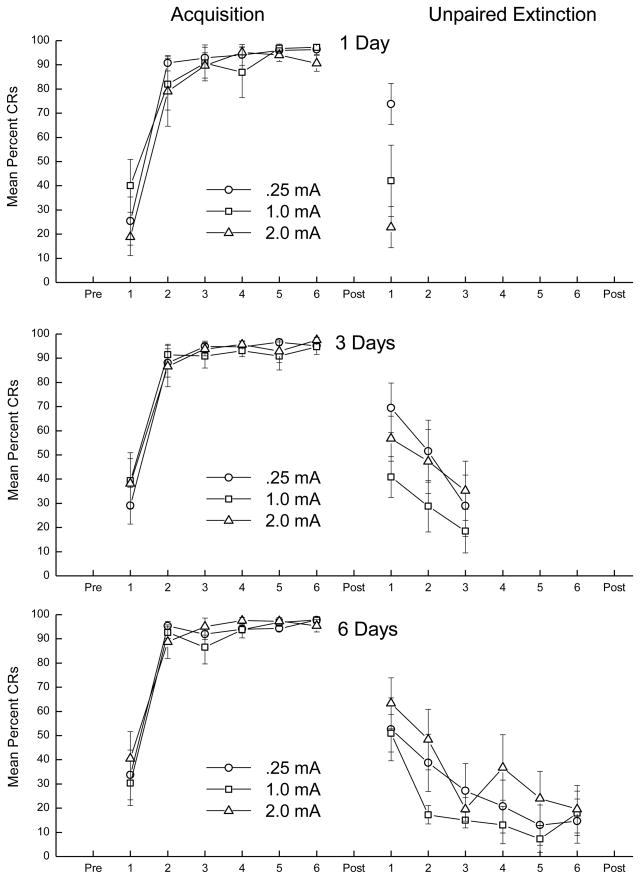
Panels of Figure 1 show CS responding during CS-US pairings (Acquisition) and unpaired presentations (Unpaired Extinction) for rabbits given one (top), three (middle), or six days (bottom) of extinction with a 0.25-mA (circle), 1.0-mA (square), or 2.0-mA (triangle) US. With the exception of one rabbit that did not meet a criterion of 80% CRs and whose data were eliminated, 71 rabbits acquired CRs in excess of 89% by the end of pairings. Analysis of variance (ANOVA) revealed a significant main effect of days of CR Acquisition (F(5, 310) = 273.22, p < .001) but no other effects (F’s < 1.1).
Figure 1.
The three panels show mean (± SEM) conditioned responses (CRs) to the tone conditioned stimulus (CS) during six days of CS-unconditioned stimulus (US) pairings (Acquisition) and during one (top), three (middle), or six (bottom) subsequent days of unpaired CS/US presentations (Unpaired Extinction) with either a 0.25-mA (circle), 1.0-mA (square), or 2.0-mA (triangle) US.
CR Extinction – CS Responding
The top middle panel of Figure 1 shows on the first day of extinction there was a higher level of CS responding (i.e., less extinction) in the 0.25-mA group than in the 1.0-mA and 2.0-mA groups. Because all nine groups received at least one day of unpaired extinction, an ANOVA was conducted on CS responding on the first day across all rabbits as a function of US intensity during extinction. There was a significant effect of Extinction Intensity (F(2, 62) = 3.99, p < .05) and post hoc comparisons confirmed the level of CS responding in 0.25-mA groups was higher than CS responding in 1.0-mA and 2.0-mA groups (p’s < .05).
Because six groups received at least three days of unpaired extinction (middle and bottom panels), an ANOVA was conducted on CS responding on the first three days for the 3-Day and 6-Day extinction groups as a function of Extinction Intensity. Analyses of CS responding revealed an effect of Days (F(2, 84) = 49.81, p < .001) but not other effects. Finally, analysis of CS responding across six days of extinction for 6-Day extinction groups also revealed an effect of Days (F(5, 105) = 23.35, p < .001) without any other effects.
CR Extinction – US Responding
Examination of CS responding as a function of US intensity during unpaired extinction revealed response levels to the 0.25-mA US averaged 21.9% (± 4.7%) whereas responding to 1.0- and 2.0-mA USs averaged 92.7% (± 3.5%) and 99.5% (± 0.2%), respectively. Analyses of percent URs across US intensity groups for one, three and six days of unpaired extinction yielded an effect of Extinction Intensity in each case (F’s > 86.08, p’s < .001) but no other effects. Post hoc comparisons confirmed the significant Extinction Intensity effects were due to lower levels of URs to the 0.25-mA US across all extinction groups (p’s < .001).
Together, acquisition and extinction data show CS responding increased to asymptotic levels of 90% CRs as a function of pairings and decreased during unpaired extinction as a function of US intensity during unpaired presentations. In addition, US responding during unpaired extinction was also a function of US intensity.
CRM Acquisition
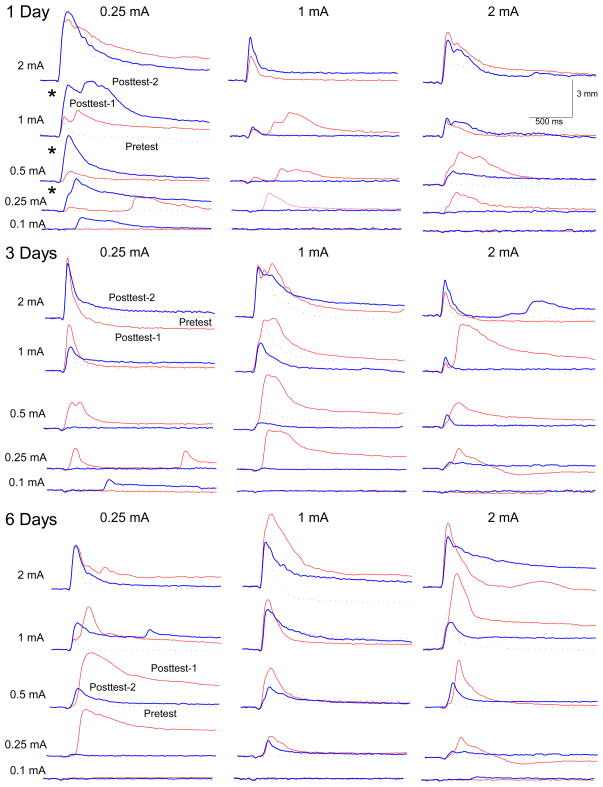
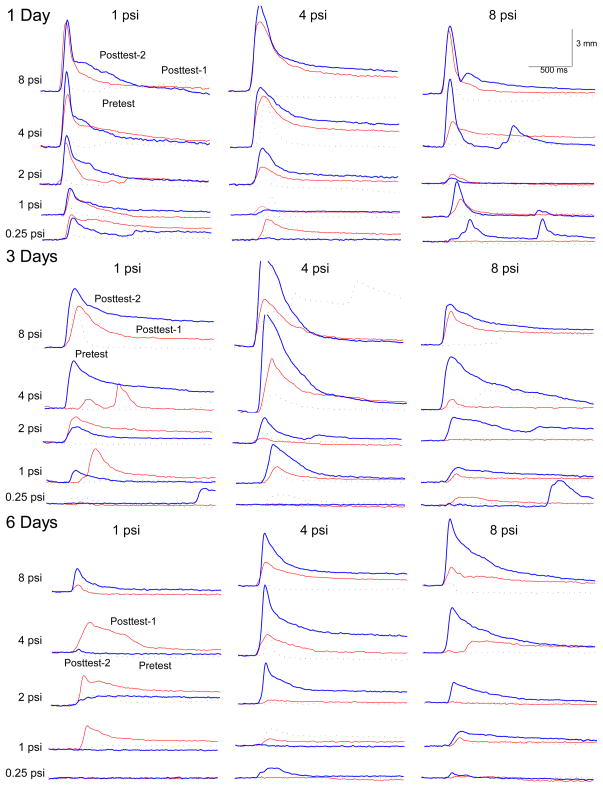
Panels in Figure 2 show representative UR response topographies for a rabbit in each of nine unpaired extinction groups as a function of the first 20-trial block of US test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before CS-US pairings (Pretest) and the post test before (Post Test-1) and after unpaired extinction (Post Test-2). Focusing on CRM acquisition (Pretest versus Post Test-1), panels show responding increased as a function of US test intensities and testing before or after CS-US pairings with more responses occurring at lower US testing intensities after pairings. These observations were confirmed by ANOVA of UR frequency which revealed significant effects of US testing intensities (F(4, 248) = 731.37, p < .001) and CRM Test (F(2, 124) = 5.31, p <.01), and an interaction of US testing intensities and CRM Test (F(8, 496) = 3.51, p <.001). Panels also show at intermediate US testing intensities of 0.25, 0.5 and 1.0 mA – where we typically see CRM – responses were larger on Post Test-1 than on Pretest indicating CRM occurred before unpaired extinction. This observation was confirmed by an effect of CRM Test on the magnitude of UR area (Pretest vs. Post Test-1; F(1, 62) = 7.40, p < .01).
Figure 2.
The nine panels show representative individual unconditioned response topographies for rabbits in each of the unpaired extinction groups as a function of the first block of unconditioned stimulus (US) test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented on Pretest (dashed black line) and the post test before (red line, Post Test-1) and after (blue line, Post Test-2) one, three or six days of unpaired extinction with a 0.25-mA, 1.0-mA or 2.0-mA US.
CRM Extinction
Although nearly all panels of Figure 2 show responding to different US intensities after unpaired extinction (Post Test-2) was at or below pre-extinction levels (Post Test-1) indicating CRM had been extinguished (Schreurs et al., 2000), the top left panel shows a representative rabbit subjected to brief unpaired extinction (1 Day) with a weak US (0.25 mA) had larger responses on Post Test-2 than on Post Test-1. In other words, CRM actually increased as a result of brief, weak unpaired extinction. This observation was confirmed by analysis of the magnitude of UR area which revealed the significant difference between Post Test-1 and Post Test-2 interacted with Extinction Intensity and Extinction Duration (F(4, 62) = 2.80, p < .05). A separate analysis of the magnitude of UR area for that group confirmed rabbits showed significantly larger responses on Post Test-2 than on Post Test-1 (F(1, 7) = 11.56, p < .05).
The CRM data show extended unpaired extinction even with a weak US is effective in reducing and even eliminating CRM. Importantly, the data show brief extinction with a weak US can actually increase the level of CRM.
Change in heart beat
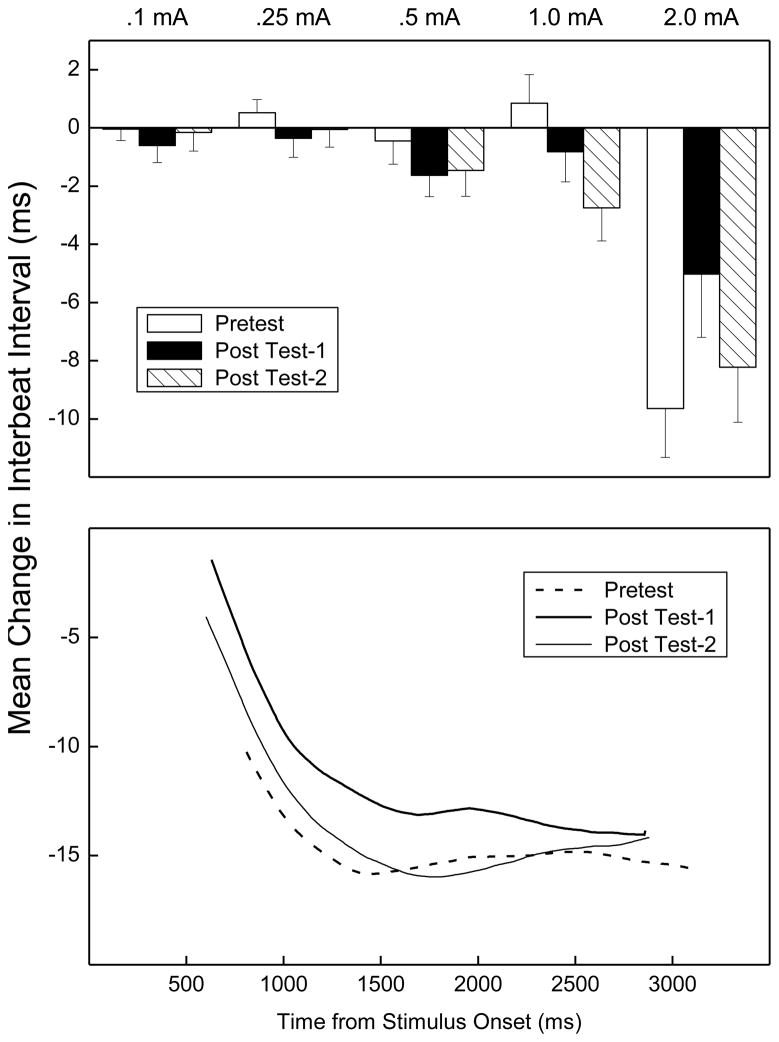
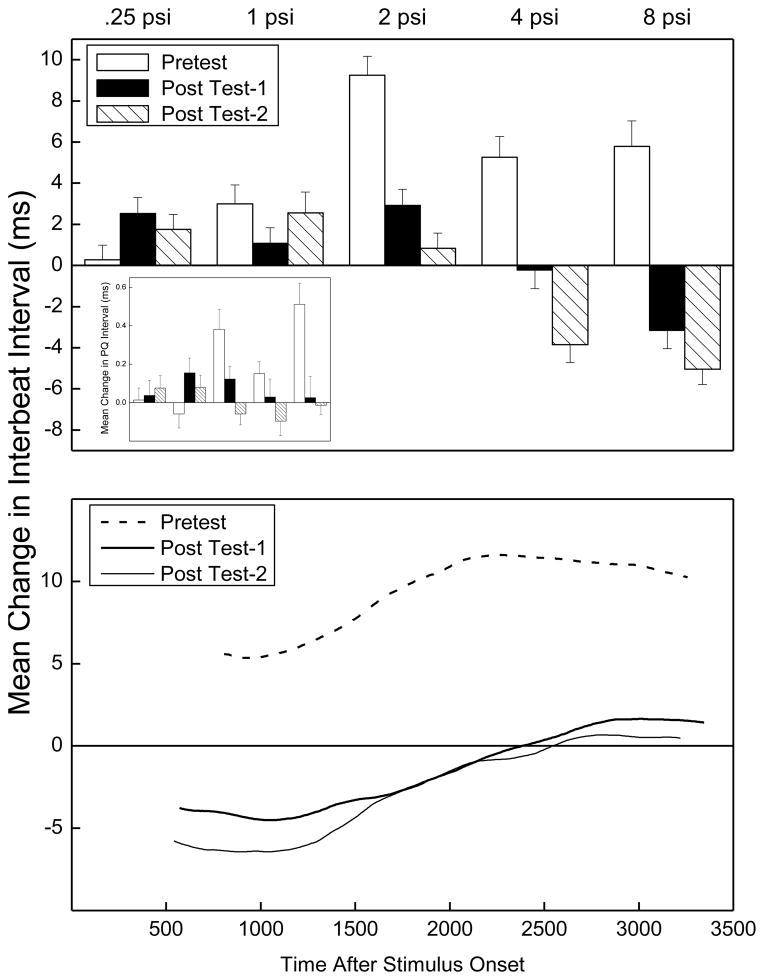
The top panel of Figure 3 shows overall mean change in IBI between a 2000-ms baseline before and a 2000-ms interval after US onset for all rabbits on Pretest, Post Test-1 and Post Test-2 for five US intensities collapsed across US duration. A negative change in IBI reflects an increase in heart rate. The panel shows there was little change in IBI except at 2.0 mA where there was a general decrease (HR acceleration) which changed as a function of when tested. Analysis of changes in IBI during CRM testing revealed significant effects of US intensities used during CRM Test (F(4, 248) = 20.00 p < .001), Extinction Intensity (F(2, 62) = 4.42, p < .05), and an interaction of the two (F(8, 248) = 4.15, p < .001). Importantly, there was a significant interaction of US testing intensities and CRM Test (Pretest vs. Post Test-1 vs. Post Test-2; F(8, 496) = 2.94, p < .01) suggesting the change in IBI varied as a function of acquisition and extinction of CRM. A separate analysis of changes in IBI at 2.00 mA – the intensity at which we see the largest changes in IBI – revealed the change in IBI was initially quite large during Pretest (strong HR acceleration) but decreased significantly (i.e., showed less HR acceleration) after pairings (Pretest vs. Post Test-1; (F1, 62) = 4.12, p < .05)). The change in IBI at 2.00 mA then tended to increase again (back to strong HR acceleration) after unpaired extinction (F(1, 62) = 3.82, p = .055), suggesting a return to an innate defensive/fear response to the US.
Figure 3.
The top panel depicts overall mean (±SEM) change in the inter-beat interval (IBI) between a 2000-ms baseline interval before and 2000-ms interval after unconditioned stimulus (US) onset during US testing on Pretest, Post Test-1 and Post Test-2 for five US intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) collapsed across US duration. A reduction in IBI (from baseline) represents heart rate acceleration. The bottom panel shows the average topography of changes in IBI to the 2-mA US for a period of 3000 ms following US onset from a 2000-ms baseline period before the US.
Changes in HR are part of an animal’s acute defense reaction to stressful stimulation (Raskin et al., 1969; Richter et al., 1990; Duan et al., 1997; Schadt & Hasser, 1998). This impression is reinforced by the bottom panel of Figure 3 which shows average topography of changes in IBI for 3000 ms following US onset from a 2000-ms baseline before the UR to the 2-mA US. The panel shows although there are initial differences in IBI change immediately after US onset reflected by the data in the top panel, change in the interval between beats tended to converge for all tests as the observation interval increased. Finally, there were no discernable differences in heart beat PQ intervals during CRM testing.
Discussion
A main purpose of Experiment 1 was to determine the parameters of unpaired presentations that would lead to both CR and CRM extinction. The data revealed extinction of both CRs and CRM was a function of the US intensity used during unpaired presentations and the duration of those presentations. Both CRs and CRM seemed to extinguish best with prolonged stimulus presentations. Of note and of potential clinical relevance was the finding extinction of CRM occurred even if a weak US was used during unpaired extinction. This weak stimulus was effective in extinguishing CMR despite the fact it produced relatively low response levels (less than 25%) and did not produce any change in HR, suggesting it was not stressful. Surprisingly, rather than simply producing poor or negligible CRM extinction, a brief period of unpaired presentations with the weak US increased the size of US responding suggesting CRM actually increased. This cannot be explained by brief exposure to the CS that can increase responding known as paradoxical enhancement (Riccio et al., 2006) because rabbits received an extended number of CS (as well as US) presentations even during 1 day of unpaired extinction.
These results have implications for the use of unpaired extinction to extinguish fear and the exaggerated responses accompanying fear and therefore, as a potential treatment for PTSD. The most important implication is a CS and weak US can be used to extinguish fear and exaggerated responding if it is presented repeatedly over several days. One might envisage repeated exposure to a virtual reality environment (Rothbaum et al., 2001; Cukor et al., 2009; Gerardi et al., 2010) in which loud noises are accompanied by low-frequency percussive pulses to simulate the impact of explosions. However, if a weak US is only presented briefly – as in the case of a single virtual reality session with limited presentations of simulated events – it could actually cause a relapse and heighten PTSD symptoms.
Experiment 2
The results of Experiment 1 indicated CRM as well as CRs acquired using ES can be extinguished using unpaired presentations of the CS and the ES. Electrodermal stimulation has been shown to elicit acute responses such as HR increases measured as part of the animal’s defense reaction (Raskin et al., 1969; Richter et al., 1990; Duan et al., 1997; Schadt & Hasser, 1998). On the other hand, unless particularly strong, air puff (AP) is considered less stressful and evidence is mixed whether it will support fear conditioning (McEchron et al., 1991; Powell et al., 1993). We have found it difficult to obtain reliable CRM using AP unless we use a pressure twice that normally used during eyeblink conditioning (Buck et al., 2001).
The purpose of Experiment 2 was to determine whether CRs and CRM acquired using a strong AP could be extinguished using unpaired presentations of the CS and the AP. The reasons for conducting this experiment included a need to determine: (1) the generality of unpaired extinction effects; and (2) whether a US different from ES and potentially less stressful but still capable of eliciting an NMR and establishing CRM could be used to extinguish CRM.
There are examples of different rates and levels of CR extinction when AP and periorbital ES have been used as a US (Powell et al., 1996; Oswald et al., 2006). Issues of emotional arousal were proposed to explain that an ES US supported higher levels of acquisition and faster extinction than AP (Oswald et al., 2006). In the current study, we used an 8-psi AP to ensure there were robust levels of CR acquisition during pairings and an increased likelihood of strong levels of CRM during testing (Buck et al., 2001).
Methods
Subjects
Seventy two male New Zealand White rabbits (Oryctolagus cuniculus) supplied by Harlan weighed 2.0–2.2 kg.
Except where noted, apparatus, recording, and experimental procedures were the same as those used in Experiment 1.
Apparatus
A programmable air delivery system (Model ER-3000, Tescom Corp., Elk River, MN) presented air puff through a tube (1 mm internal diameter) positioned 5 mm from the cornea. AP was calibrated at the tube tip using a digital manometer (Smart Manometer 350, Meriam Instruments, Cleveland, OH).
Procedure
Rabbits were randomly assigned to nine groups (n’s=8) comprising a 3 × 3 factorial with days of unpaired extinction (Extinction Duration, 1, 3 or 6 days) and US intensity during extinction (Extinction Intensity,1, 4 or 8 psi) as factors. Rabbits received adaptation, a 75-trial session of AP US pretesting (Pretest), six sessions of paired CS-US presentations (Acquisition), a 75-trial session of AP US post-testing (Post test-1) to assess CRM, then one, three or six days of unpaired presentations with an AP US of 1, 4 or 8 psi (Unpaired Extinction), followed by a final 75-trial session of AP US post-testing (Post Test-2).
Due to high CR levels during unpaired extinction (see Results) extinction was continued after Post Test-2 in subsets of six rabbits per group until they received a total of eight days of extinction (i.e., seven, five or two additional sessions for the 1-, 3- and 6-Day groups). Unlike the ES, AP has a hiss that increases in volume as the AP intensity increases (Flaten & Blumenthal, 1998), and at 8 psi, we measured a 2-3 dB increase in sound level. To determine if the hiss of the 8-psi AP used during pairings might have played a role in high levels of responding during unpaired extinction, the additional extinction sessions consisted of unpaired presentations but with the AP tubing removed from near the rabbit’s eye and placed out of view at the front of the chamber. This phase of unpaired extinction was designated “CS Extinction”. On AP pretest and post-test days, subjects were prepared for HR measurements and received 75 trials of AP presented at an average ITI of 60 s. Each trial involved 1 of 15 possible combinations of stimulus intensity (0.5, 1.0, 2.0, 4.0, 8.0 psi) and duration (25, 50, or 100 ms). Five randomized sequences of 15 stimulus combinations were presented but the same intensity or duration could not occur on more than three consecutive trials.
Each of six conditioning sessions consisted of 80 presentations of a 400-ms, 1-kHz, 82-dB tone CS that coterminated with a 100-ms, 8-psi AP US (i.e., 300-ms ISI). One, three or six daily sessions of unpaired extinction consisted of 80 CS-alone and 80 US-alone presentations (1, 4 or 8 psi) in an explicitly unpaired manner delivered, on average, every 30 s. CS extinction was identical to unpaired extinction except the AP tubing was removed from near the rabbit’s eye as noted above.
Results
CR Acquisition
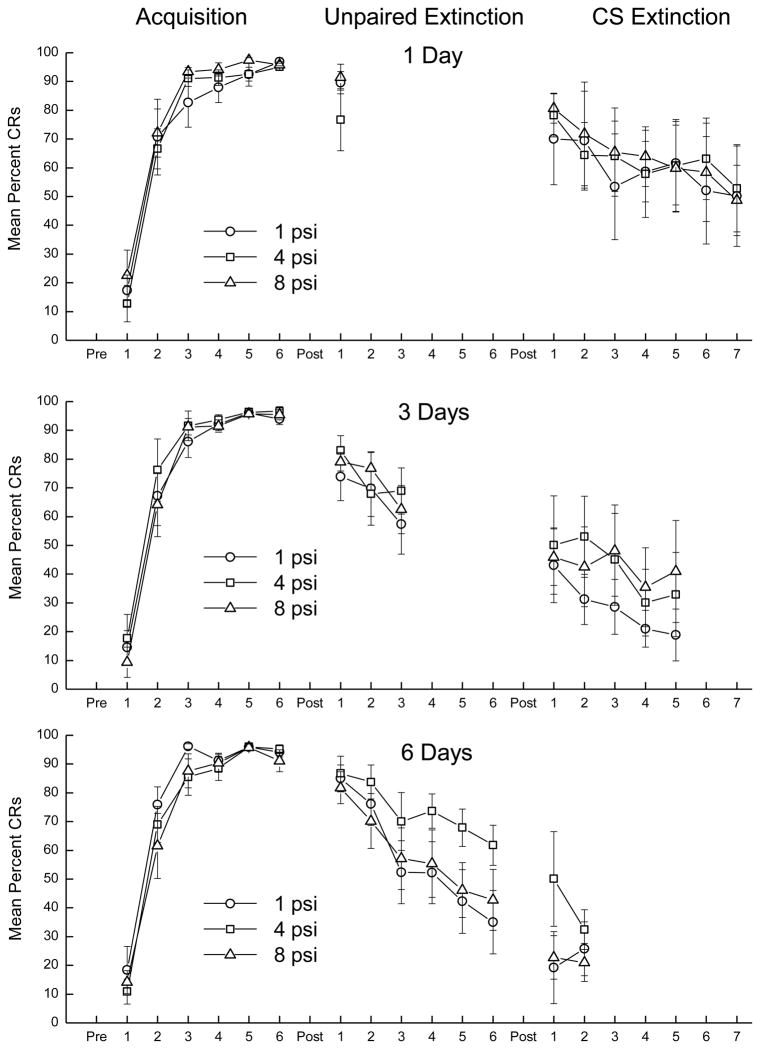
Panels in Figure 4 show CS responding during Acquisition, Unpaired Extinction for rabbits given one (top), three (middle), or six days (bottom) of extinction with either a 1-psi (circle), 4-psi (square), or 8-psi (triangle) US, and CS Extinction for seven days in three groups given one day of unpaired extinction (top), five days for groups given three days of unpaired extinction (middle) and two days for groups given six days of unpaired extinction (bottom). With the exception of two rabbits that did not meet a criterion of 80% CRs whose data were eliminated, rabbits in all groups acquired CRs in excess of 92% by the end of pairings. Analysis of variance revealed there was a significant effect of days of CR Acquisition (F(5, 305) = 528.66, p < .001) but no other effects (F’s < 1.0).
Figure 4.
The three panels show conditioned responses (CRs) to the tone conditioned stimulus (CS) during six days of CS-unconditioned stimulus (US) pairings (Acquisition), during one (top), three (middle), or six (bottom) subsequent days of unpaired CS/US presentations (Unpaired Extinction) with either a 1-psi (circle), 4-psi (square), or 8-psi (triangle) US, and then a further seven (top), five (middle), or two (bottom) days of unpaired CS/“US” presentations with the 1-, 4, or 8-psi US air puff tubing moved to the front of the chamber and away from the eye (CS Extinction).
CR Extinction – CS Responding
The panels of Figure 4 show very high levels of CS responding at the end of acquisition that decreased slightly on the first day of unpaired extinction and then continued to decrease rather modestly in those groups that received further unpaired extinction. An analysis comparing responding on the last day of CR acquisition (94.9% CRs) and the first day of unpaired extinction (83.0% CRs) revealed a significant main effect (F(1, 61) = 41.09, p < .001) indicating the decrease in responding during the first day of unpaired presentations from terminal acquisition levels was significant. There were no other significant effects. An ANOVA conducted on CS responding for 3-Day and 6-Day extinction groups as a function of Extinction Intensity revealed an effect of Days (F(2, 82) = 23.05, p < .001), indicating all groups showed comparable levels of extinction, but no other effects. Finally, analysis of CS responding for 6-Day extinction groups also revealed an effect of Days (F(5, 105) = 20.73, p < .001) without any other effects.
Levels of CS responding during CS Extinction continued to decrease from levels observed during Unpaired Extinction with clear differences between groups as a result of duration of initial unpaired extinction but there were no consistent differences among groups as a function of US intensities used during unpaired extinction. Analysis of CS responding during the first two days of CS Extinction for all nine groups revealed an effect of Extinction Duration (F(2, 44) = 14.38, p < .001) reflecting the different terminal levels of unpaired extinction. Similarly, analysis of CS responding during five days of CS Extinction for six groups that previously received one and three days of unpaired extinction revealed an effect of Extinction Duration (F(1, 29) = 9.44, p < .01) and Days of CS Extinction (F(4, 116) = 7.37, p < .001) suggesting CS responding continued to decrease across CS Extinction and was accelerated for groups that previously received more unpaired extinction. Analysis of CS responding during seven days of CS Extinction for three groups that previously received only one day of unpaired extinction revealed an effect of Days of CS Extinction (F(6, 84) = 3.86, p < .01) confirming continued decreases in responding characteristic of CR extinction. Finally, analysis of terminal levels of extinction across all groups yielded an effect of Days of CS Extinction (F(2, 44) = 3.67, p < .05) which post hoc comparisons confirmed to be due to less extinction for three groups that previously received only one day of unpaired extinction than those that received three or six days of unpaired extinction ( p < .05). Consequently, the further decrease in responding observed during CS extinction was a result of continued extinction and not of the switch from unpaired to CS extinction per se.
CR Extinction – US Responding
Examination of US response levels as a function of US intensity used during unpaired extinction revealed levels of responding to the 1-psi AP averaged 38% URs (± 9.04%) whereas responding to the 4- and 8-psi APs averaged 89.0% (± 4.24%) and 97.3% (± 2.38%), respectively. ANOVAs of percent URs across three US intensity groups for one, three and six days of unpaired extinction yielded an effect of Extinction Intensity in each case (F’s > 62.46, p’s < .001) but no other effects. Post hoc comparisons confirmed the significant US intensity effects were due to lower levels of responding to the 1-psi US than to the 4- or 8-psi US across extinction groups (p’s < .001).
Examination of responding to the sound of AP after the tubing had been removed from near the eye indicated rabbits continued to elicit low levels of responding even when AP no longer reached the cornea (mean = 7.69% ± 3.37% responses). In fact, some rabbits responded to the sound of AP at quite high levels. Three rabbits in particular that received only one day of unpaired extinction before continuing CS Extinction responded to the AP sound in excess of 20% “URs” with one rabbit reaching 74%. Interestingly, in each case, CS responding did not drop below 77% CRs over the course of six additional days of CS extinction. In other words, these rabbits did not extinguish their CRs or responses to the “US”. Not surprisingly, the level of responding to the sound of AP during this phase of unpaired extinction was a function of the different AP intensities used during extinction with overall response levels increasing from 5.7% to 8.5% to 13.8%, as AP pressure increased from 1 to 4 to 8 psi (Flaten & Blumenthal, 1998). The CR acquisition and extinction data show CS responding increased to asymptotic levels in excess of 90% CRs as a function of pairings and decreased modestly during unpaired extinction as a function of days of unpaired presentations but not as a function of US intensity used during unpaired extinction. Continued unpaired presentations with the US removed from near the eye produced additional CR extinction. Responding to the US during unpaired extinction as well as to the sound of the US during CS Extinction when AP was no longer near the eye was a function of US intensity.
CRM Acquisition
Panels in Figure 5 show representative UR response topographies for a rabbit in each of the nine unpaired extinction groups as a function of the first block of US test intensities (0.5, 1, 2, 4 and 8 psi) presented on Pretest and post test before (Post Test-1) and after unpaired extinction (Post Test-2). Focusing on CRM acquisition (Pretest versus Post-1), the panels show responses increased as a function of US test intensities and testing before (Pretest) or after pairings (Post Test-1) with more responses occurring at lower US testing intensities after pairings. These observations were confirmed by an ANOVA of UR frequency which revealed effects of US testing intensities (F(4, 244) = 111.13, p < .001) and CRM Test (F(2, 122) = 22.37, p <.001), and interaction of US testing intensities and CRM Test (F(8, 488) = 15.85, p <.001).
Figure 5.
The nine panels show representative individual unconditioned response topographies for rabbits in each of the unpaired extinction groups as a function of the first block of unconditioned stimulus (US) test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented on Pretest (dashed black line) and the post test before (red line, Post Test-1) and after (blue line, Post Test-2) one, three or six days of unpaired extinction with a 1-, 4- or 8-psi US.
CRM Extinction
Panels in Figure 5 also show that although there was good evidence of CRM with responses being larger on Post Test-1 than on Pretest, with the exception of the weakest US intensity (1 psi) when presented for six days, there is little evidence of extinction of CRM with a number of rabbits showing larger responses on Post Test-2 than on Post Test-1. This observation was confirmed for both magnitude of UR amplitude and magnitude of UR area by an effect of CRM Test (F’s(2, 118) = 7.47, p < .01 and 11.11, p < .001, respectively) and an interaction of CRM Test with US testing intensities (F’s(8, 472) = 11.73, p < .001 and 11.25, p < .001, respectively) and Extinction Intensity (F’s(8, 472) = 2.13, p < .05 and 2.29, p < .01, respectively). The latter effect was most clearly reflected by rabbits that received six days of unpaired extinction where there was significant extinction of CRM for the 1-psi group but enhanced CRM for the 4- and 8-psi groups.
The AP data show that although there is strong CR acquisition with an 8-psi US and CRM could be readily obtained, extended unpaired extinction was ineffective in extinguishing CRs or reducing CRM with CRM actually becoming larger in cases where stronger US intensities (4 and 8 psi) were used during unpaired extinction. However, a reduction in CRM did occur with extended unpaired extinction with a low intensity US (1 psi).
Change in heart beat
The top panel of Figure 6 shows mean change in IBI between a 2000-ms baseline before and a 2000-ms interval after US onset during US testing for all 70 rabbits on Pretest, Post Test-1 and Post Test-2 for five US intensities collapsed across US duration. The inset shows mean change in PQ interval for the IBI data. The panel and inset show there were substantial changes in IBI and PQ interval across US intensities particularly at higher intensities with an increase in IBI change (HR deceleration) and increase in PQ interval during Pretest and a decrease in IBI change and PQ interval following pairings and unpaired extinction (HR acceleration). The impression of significant changes in IBI is reinforced in the bottom panel which shows average topography of changes in IBI to the 8-psi AP for a period of 3000 ms following US onset from a 2000-ms baseline. The panel shows differences in IBI change immediately after US onset reflected by data in the top panel were maintained for each test as the observation interval increased.
Figure 6.
The top panel illustrates the overall mean (±SEM) change in the inter-beat interval (IBI) between a 2000-ms baseline interval before and 2000-ms interval after unconditioned stimulus (US) onset during US testing on Pretest, Post Test-1 and Post Test-2 for five US intensities ( 0.5, 1, 2, 4, and 8 psi) collapsed across US duration. A reduction in IBI (from baseline) represents heart rate acceleration. The inset shows changes in the PQ interval for the IBI data depicted in the top panel. The bottom panel shows the average topography of changes in IBI to the 8-psi US for a period of 3000 ms following US onset from a 2000-ms baseline period before the US.
Analysis of changes in IBI from baseline during CRM testing revealed effects of CRM Test (F(2, 122) = 55.12, p < .001), US intensities used during CRM Test (F(4, 244) = 14.10 p < .001), and an interaction of the two (F(8, 488) = 13.28, p < .001). Post hoc comparisons of the CRM Test effect showed mean change in IBI was significantly higher on Pretest than on either post test (p’s< .001) with the two post tests not differing. Analysis of changes in PQ interval during CRM testing revealed a significant main effect of CRM Test (F(2, 122) = 9.65, p < .001) and an interaction of CRM Test and the US intensities used during CRM Test (F(8, 488) = 5.16, p < .001). Post hoc comparisons of the CRM Test main effect showed the mean change in PQ interval was significantly higher on Pretest than on either post test (p’s< .05) with the two post tests not differing.
One interpretation of the IBI data is that HR initially decreased on pretest to the higher US testing intensities as part of the animal’s orienting response to a novel stimulus (Graham & Clifton, 1966; Raskin, 1972). When change in IBI was examined across blocks of trials, it became clear HR deceleration habituated across additional US testing and accelerated slightly (decrease in mean change in IBI) by the last block of Pretest trials (−2.56 ms ± 0.76 ms). The acceleration in HR towards the end of Pretest became stronger after CS-US pairings (Post Test-1) and unpaired extinction (Post Test-2) as shown in Figure 6 suggesting AP had lost its novelty and become stressful.
Discussion
Like Experiment 1, Experiment 2 shows extended unpaired extinction with a weak US can reduce the level of CR and extinguish CRM. Unlike Experiment 1, Experiment 2 showed CRs were only modestly extinguished and use of a strong US during unpaired extinction actually increased CRM rather than extinguishing it. Given the similar response topography between CRs and CRM and the suggestion CRM is a generalized CR (Schreurs, 2003; Burhans et al., 2008), it is possible failure to extinguish CRs even after six days of unpaired extinction meant CRM might also fail to extinguish. What was surprising was CRM actually increased after unpaired extinction with strong AP intensities.
To investigate the discrepancies in CR extinction between Experiment 1 and Experiment 2, we continued the unpaired extinction procedure with AP moved away from the eye. We observed rabbits continued to elicit low levels of responding to the sound of AP. Interestingly, many of the response topographies to the sound of AP were of the long-latency, slow-rise, and low-amplitude type usually elicited by the tone. Nevertheless, other topographies were the typical short-latency, rapid-rise, and large-amplitude responses elicited by APs.
From a clinical perspective, the results of Experiment 2, like those of Experiment 1, suggest a weak US can be used to extinguish exaggerated responses if presented repeatedly over several days. The US used here was sufficiently weak to elicit less than 40% responding and even then, did not cause the HR acceleration indicative of a stress response. However, if a strong US were used during extinction and responding to the feared stimulus was not extinguished, exaggerated reactions might have actually become worse.
General Discussion
The principal finding of these experiments was unpaired extinction can be used to extinguish CRs as well as CRM and this can be accomplished with a weak US as long as extended presentations are used. Rather surprisingly, depending on the nature of the US, brief presentations of a weak US or extended presentations of a strong US can actually make CRM stronger after unpaired extinction.
The Diagnostic and Statistical Manual (DSM-IV) indicates PTSD is characterized by psychological distress and/or physiological reactivity to "internal or external cues that symbolize or resemble an aspect of the traumatic event". This characterization indicates clearly there is a conditioning component to PTSD (Dobbs & Wilson, 1960; Pitman, 1988; Grahn et al., 2000; Orr et al., 2000). One of the most robust findings in PTSD research is stress-induced heightening of physiological responses particularly HR (Brunijnzeel et al., 2001; Orr et al., 2002; Elsesser et al., 2004; Pole et al., 2009). Some of the symptoms of PTSD are thought to be CRs to stimuli associated with traumatic events whereas others are exaggerated physiological responses to stress (Pitman, 1988). On the basis of CRM data, we suggest PTSD-like symptoms may also include unconditioned responses to weaker forms of the original traumatic event (Seager et al., 2003; Schreurs, 2003; Burhans et al., 2008). For example, a veteran may “hit the deck” when a car backfires not only because the backfire is a cue associated with explosions but because the backfire is an explosion, albeit a smaller one than those experienced during combat.
Despite some progress in the diagnosis and treatment of PTSD in the general population, evidence suggests treating veterans has been less than successful (Dierperink et al., 2005; English et al., 2006) and PTSD among veterans results in an increased risk of death (Boscarino, 2005). There are unprecedented numbers of military personnel returning from Iraq and Afghanistan and dramatic numbers are seeking mental health services (Hoge et al., 2006). Given the increasing number and duration of tours of duty occurring in these military conflicts, the incidence of PTSD may increase dramatically and every effort must be made to improve our understanding and treatment of the disorder.
From a clinical perspective, the present results suggest treating PTSD by extended exposure to the cues occurring in a traumatic environment should be augmented by exposure to weakened versions of the traumatic event. As noted above, a virtual reality environment could be created for combat veterans where loud noises are accompanied by low-frequency percussive pulses that would simulate the impact of explosions. For example, Geradri and colleagues have recently developed a Virtual Iraq environment involving various city- and landscapes through which a Humvee is driven (Gerardi et al., 2010). The Humvee scenes include not only sounds of the street but vibrations beneath the driver’s seat that coincide with movement of the vehicle and explosions. It does not seem too far a stretch to increase the intensity of these vibrations and the volume of explosions so they begin to approximate weakened versions of the unconditioned stimuli experienced in the real Iraq. Components of this scenario might also be employed for those who have suffered trauma after an auto wreck (Beck et al., 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Beck JG, Palyo SA, Winer EH, Schwagler BE, Ang EJ. Virtual reality exposure therapy for PTSD symptoms after a road accident: an uncontrolled case series. Behavior Therapy. 2007;38:39–48. doi: 10.1016/j.beth.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and mortality among U.S. army veterans 30 years after military service. Annals of Epidemiology. 2005;16:248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brunijnzeel AW, Stam R, Croiset G, Wiegant VM. Long-term sensitization of cardiovascular stress responses after a single stressful experience. Physiology & Behavior. 2001;73:81–86. doi: 10.1016/s0031-9384(01)00435-8. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behavioral Neuroscience. 2001;115:1039–1047. [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: behavioral rules, neural substrates, and potential applications to post-traumatic stress disorder. Behavioral Neuroscience. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behavioural Brain Research. 2009;206:127–134. doi: 10.1016/j.bbr.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S. Fear extinction in rodents. Current Protocols in Neuroscience. 2009;47:8.23.1–8.23.17. doi: 10.1002/0471142301.ns0823s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SR, Gormezano I. Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. Journal of Comparative and Physiological Psychology. 1971;77:447–455. doi: 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- Colwill RM. Effects of US identity on elimination and recovery of autoshaped responding with explicitly unpaired and degraded contingency extinction procedures. Behavioural Processes. 2007;74:1–12. doi: 10.1016/j.beproc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clinical Psychology Review. 2009;29:715–726. doi: 10.1016/j.cpr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Dierperink M, Erbes C, Leskela J, Kaloupek D, Farrer MK, Fisher L, Wolf E. Comparison of treatment for post-traumatic stress disorder among three Department of Veterans Affairs medical centers. Military Medicine. 2005;170:305–308. doi: 10.7205/milmed.170.4.305. [DOI] [PubMed] [Google Scholar]
- Dobbs D, Wilson WP. Observations on persistence war neurosis. Diseases of the Nervous System. 1960;21:686–691. [PubMed] [Google Scholar]
- Duan Y-F, Winters RW, McCabe PM, Green EJ, Huang Y, Schneiderman N. Cardiorespiratory components of defense reaction elicited from paraventricular nucleus. Physiology & Behavior. 1997;61:325–330. doi: 10.1016/s0031-9384(96)00410-6. [DOI] [PubMed] [Google Scholar]
- Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-related pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. Journal of Abnormal Psychology. 2004;113:289–301. doi: 10.1037/0021-843X.113.2.289. [DOI] [PubMed] [Google Scholar]
- English BA, Jewell M, Jewell G, Ambrose S, Davis LL. Treatment of chronic posttraumatic stress disorder in combat veterans with citalopram. Journal of Clinical Psychopharmacology. 2006;26:84–88. doi: 10.1097/01.jcp.0000195043.39853.bc. [DOI] [PubMed] [Google Scholar]
- Flaten MA, Blumenthal TD. A parametric study of the separate contributions of the tactile and acoustic components of airpuffs to the blink reflex. Biological Psychology. 1998;48:227–234. doi: 10.1016/s0301-0511(98)00018-0. [DOI] [PubMed] [Google Scholar]
- Frey PW, Butler CS. Extinction after aversive conditioning: an associative or nonassociative process? Learning and Motivation. 1977;8:1–17. [Google Scholar]
- Gerardi M, Cukor J, Difede J, Rizzo A, Rothbaum BO. Virtual reality exposure therapy for post-traumatic stress disorder and other anxiety disorders. Current Psychiatry Reports. 2010;12:298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- Gormezano I, Gibbs CM. Transduction of the rabbit’s nictitating membrane response. Behavior Research Methods, Instruments, & Computers. 1988;20:18–21. [Google Scholar]
- Gracia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Watkins LR, Maier SF. Impaired escape performance and enhanced conditioned fear in rats following exposure to an uncontrolled stressor are mediated by glutamate and nitric oxide in the dorsal raphe nucleus. Behavioural Brain Research. 2000;112:33–41. doi: 10.1016/s0166-4328(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Experimental Brain Research. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Hayes E, Pugsley MK, Penz WP, Adaikan G, Walker MJA. Relationship between QaT and RR intervals in rats, guinea pigs, rabbits, and primates. Journal of Pharmacological and Toxicological Methods. 1994;32:201–207. doi: 10.1016/1056-8719(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear conditioning and extinction in animals and humans: implications for exposure therapy for anxiety disorders. Clinical Psychology Review. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Auchterloine JL, Milliken CS. Mental health problems, use of mental health services, and attrition from miltary service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023–1032. doi: 10.1001/jama.295.9.1023. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Weidemann G, Dartnall S. Apparatus exposure produces profound declines in conditioned nictitating-membrane responses to discrete conditioned stimuli by the rabbit. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:259–270. doi: 10.1037/0097-7403.30.4.259. [DOI] [PubMed] [Google Scholar]
- Leonard DW. Partial reinforcement effects in classical conditioning in rabbits and human beings. Journal of Comparative and Physiological Psychology. 1975;88:596–608. doi: 10.1037/h0076419. [DOI] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Air puff versus shock unconditioned stimuli in rabbit heart rate conditioning. Physiology & Behavior. 1991;51:195–199. doi: 10.1016/0031-9384(92)90223-o. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Mickley GA, DiSorbo A, Wilson GN, Huffman J, Bacik S, Hoxha Z, Biada JM, Kim Y-H. Explicit dissociation of a conditioned stimulus and unconditioned stimulus during extinction training reduces both time to asymptotic extinction and spontaneous recovery of a conditioned taste aversion. Learning and Motivation. 2009;40:209–220. doi: 10.1016/j.lmot.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychiatry. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nijsen MJMA, Croiset G, Diamant M, Stam R, Delsing D, de Wied D, Wiegant VM. Conditioned fear-induced tachycardia in the rat; vagal involvement. European Journal of Pharmacology. 1998;350:211–222. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic tress disorder. Psychiatric Clinics of North America. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Knuckley B, Mahan K, Sanders C, Powell DA. Prefrontal control of trace versus delay eyeblink conditioning: role of the unconditioned stimulus in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2006;120:1033–1042. doi: 10.1037/0735-7044.120.5.1033. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, conditioning, and network theory. Psychiatric Annals. 1988;18:182–189. [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Annals New York Academy of Sciences. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Shalev AY. Once bitten, twice shy: beyond the conditioning model of PTSD. Biological Psychiatry. 1993;33:145–146. doi: 10.1016/0006-3223(93)90132-w. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Gibbs CM, Maxwell B, Levine-Bryce D. On the generality of the conditioned bradycardia in rabbits: assessment of CS and US modality. Animal Learning & Behavior. 1993;21:303–313. [Google Scholar]
- Powell DA, Maxwell B, Penney J. Neuronal activity in the medial prefrontal cortex during Pavlovian eyeblink and nictitating membrane conditioning. Journal of Neuroscience. 1996;16:6296–6306. doi: 10.1523/JNEUROSCI.16-19-06296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin DC. Orienting and defensive reflexes and conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 269–289. [Google Scholar]
- Raskin DC, Kotses H, Bever J. Autonomic indicators of orienting and defensive reflexes. Journal of Experimental Psychology. 1969;80:423–433. doi: 10.1037/h0027491. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Thomas BL, Ayres JJB. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: implications for treating human phobias. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:99–114. [PubMed] [Google Scholar]
- Riccio DC, Millin PM, Bogart AR. Reconsolidation: a brief history, a retrieval view, and some recent issues. Learning & Memory. 2006;13:536–544. doi: 10.1101/lm.290706. [DOI] [PubMed] [Google Scholar]
- Richter A, Schumann NP, Zweiner U. Characteristics of heart rate fluctuations and respiratory movements during orienting, passive avoidance and flight-fight behavior in rabbits. International Journal of Psychophysiology. 1990;10:75–83. doi: 10.1016/0167-8760(90)90048-i. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Hodges LF, Ready D, Graap K, Alarcon RD. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. Journal of Clinical Psychiatry. 2001;62:617–622. doi: 10.4088/jcp.v62n0808. [DOI] [PubMed] [Google Scholar]
- Schadt JC, Hasser EM. Hemodynamic effects of acute stressors in the conscious rabbit. American Journal of Physiology Regulatory Integrative Comparative Physiology. 1998;274:R814–R821. doi: 10.1152/ajpregu.1998.274.3.R814. [DOI] [PubMed] [Google Scholar]
- Schreurs BG. Classical conditioning and modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behavioral and Cognitive Neuroscience Reviews. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Alkon DL. US-US conditioning of the rabbit’s nictitating membrane response: emergence of a conditioned response without alpha conditioning. Psychobiology. 1990;18:312–320. [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behavioral Neuroscience. 2005;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gonzales-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learning & Behavior. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behavioral Neuroscience. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda SI, Buck DL. Conditioning the unconditioned response: modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA. Heart rate changes during conditioning-specific reflex modification of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response. Neurobiol Learn Mem. 2005;84:148–158. doi: 10.1016/j.nlm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Darwish DS, Wang D, Burhans L, Gonzales-Joekes J, Deci S, Stankovic G, Sparks DL. Cholesterol enhances classical conditioning of the rabbit heart rate response. Behavioural Brain Research. 2007;181:52–63. doi: 10.1016/j.bbr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learning & Behavior. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. American Journal of Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Spence KW. Extinction of the human eyelid CR as a function of presence or absence of the UCS during extinction. Journal of Experimental Psychology. 1966;71:642–648. doi: 10.1037/h0023108. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Longo CL, Ayres JJB. Thwarting the renewal (relapse) of conditioned fear with the explicitly unpaired procedure: Possible interpretations and implications for treating human fears and phobias. Learning and Motivation. 2005;36:374–407. [Google Scholar]
- Vervleit B, Vansteenwegen D, Hermans D. Unpaired shocks during extinction weaken the contextual renewal of a conditioned discrimination. Learning and Motivation. 2010;41:22–31. [Google Scholar]
- Wikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus) Behavioral Neuroscience. 2002;116:1052–1058. doi: 10.1037//0735-7044.116.6.1052. [DOI] [PubMed] [Google Scholar]