Abstract
Background
Intravenous (IV) infusion of ice cold saline is an effective method to initiate induction of mild therapeutic hypothermia (MTH) following resuscitation from out-of-hospital cardiac arrest (OOHCA). Intraosseous (IO) infusion of cold saline may be an alternative method to induce MTH.
Objective
The goal of this study was to determine if IO infusion of cold saline is a comparable alternative to IV infusion for inducing MTH in a laboratory swine model of cardiac arrest.
Methods
Ten mixed breed swine were resuscitated from cardiac arrest and randomized post-resuscitation to infusion with ice cold saline using either IO (n=5) or IV (n=5) access. The study endpoints were either a goal esophageal temperature of 34°C or the elapse of a 30 minute time period, simulating a long prehospital transport.
Results
Four of five pigs in the IV infusion group achieved goal temperature within 30 minutes compared to 0/5 in the IO infusion group (p=0.048). The mean esophageal temperature change was significantly higher in the IV group when compared to the IO group (p<0.001). Post-arrest hemodynamic parameters were similar between the two groups.
Conclusions
IV infusion of ice cold saline is an efficacious method to achieve MTH in this swine model of cardiac arrest. Furthermore, IO infusion of cold saline is not sufficient to induce MTH in the time routinely available in the prehospital setting following OOHCA.
Keywords: hypothermia, cardiac arrest, intraosseous infusion, emergency medical services
Introduction
During out-of-hospital-cardiac arrest (OOHCA), cerebral oxygen delivery does not meet metabolic demands and global anoxic cerebral cellular injury commonly follows, exacerbated by a cascade of inflammatory mediators. Mild therapeutic hypothermia (MTH) decreases both cerebral and systemic metabolic rates of oxygen consumption and mediates metabolic pathways to help prevent oxygen debt and tissue injury.[1] Therapeutic hypothermia is associated with improvements in the rate of favorable neurologic outcomes following cardiac arrest and reduces the incidence of mortality due to this injury. [2, 3] Intravenous (IV) infusion of iced saline has been shown to rapidly decrease body temperature to achieve therapeutic hypothermic conditions (32–34°C).[4] Although the method is not sufficient to maintain hypothermic temperatures for the duration of the recommended 24 hour cooling period, the use of IV infusion of cold saline has been shown to be a safe and effective method to initiate hypothermia treatment in the prehospital arena.[5–8]
Obtaining IV access can sometimes be difficult in the OOHCA setting. Failure to achieve IV access on the first attempt occurs in 1 out of 4 patients with an average successful line placement time of 4 ½ minutes.[9, 10] Resuscitation guidelines suggest cooling should begin as soon as possible following return of spontaneous circulation (ROSC), although the optimal time window to achieve MTH remains unclear.[11]
One possible alternative to achieve MTH in the prehospital setting is intraosseous (IO) infusion, which is considered a rapid and effective route for the delivery of fluid and medications.[12] Establishing IO access in the proximal tibia has been achieved in the out-of-hospital setting in less than 1 minute with a high success rate on the first attempt.[13, 14] This technique is easily taught to EMS and pre-hospital personnel, is part of the National DOT curriculum for paramedics, and is associated with minimal complications.[13] The ease in which IO needles are placed may lead to valuable time saved in initiating therapeutic hypothermia en route to the hospital. It remains unclear whether IO infusion of saline is able to decrease body temperature in a manner similar to IV infusion.
The aim of this study was to evaluate the feasibility of inducing mild therapeutic hypothermia with IO infusion of cold saline as an alternative to IV infusion following cardiac arrest. The primary study outcome measurements were to compare the number of animals between the two groups that could achieve 34°C as measured by an esophageal temperature probe, and to compare the actual temperature change measured between the two groups within a 30 minute time window.
Methods
The animal protocol was approved by the institutional animal care and use committee of the University of Colorado Denver. Ten mixed breed swine weighing 35–50 kg were sedated using intramuscular ketamine (15 mg/kg) and acepromazine (0.2 mg/kg) and placed under general anesthesia using 2–3% isoflourane and 100% O2. The animals were endotracheally intubated and placed on a volume-controlled ventilator (Draeger Medical, Inc., Telford, PA, Model EV-A) at a tidal volume of 10 ml/kg and a respiratory rate of 10–14 breaths per minute. End-tidal CO2 was continuously monitored and minute ventilation was adjusted to maintain eucapnia. Isoflurane was adjusted to maintain an adequate plane of surgical anesthesia as assessed by blood pressure and pulse rate.
The animals were instrumented with a microprocessor-tipped pressure-transducing catheter (Millar Instruments, Houston, TX, Model SPC-450) placed into the descending aorta above the diaphragm via surgical cutdown of a femoral artery. A right atrial pressure-transducing catheter (Millar Instruments, Houston, TX, Model SPC-450) was placed either through a surgical cutdown of the external jugular vein or the femoral vein. Correct placement was confirmed by measurement and appropriate pressure waveform. A saggital sinus temperature probe (Physitemp Instruments, Inc., Clifton, New Jersey, Model IT-21) was placed through a burr hole into the saggital sinus, which was then sealed with bone wax. A second temperature probe (Physitemp Instruments, Inc. Clifton, NJ, Model ESO-1) was inserted approximately 30cm into the esophagus under direct visualization and continuous esophageal temperature readings were recorded. A vascular blood flow probe (Transonic Systems, Inc. Ithaca, NY, TS420 Perivascular Flowmeter/PS-4PSB 4mm flow probe) was placed on a carotid artery to measure carotid artery blood flow. All pressure-transducing catheters, temperature probes and the flowmeter data output were connected to a multi-channel analog to digital monitoring system (Powerlab 16SP, AD Instruments, Sydney, AUS) and data was converted and continuously saved to disc at 400 Hz (Chart 7, Powerlab, AD Instruments, Sydney, AUS).
Following instrumentation, cardiac arrest was induced using one of two methods: 1) a hypoxic pulseless electrical activity model (hypoxic PEA) or 2) a ventricular fibrillation (VF) model. The model used was dependent upon other ongoing experiments within the laboratory. For the hypoxic PEA model, a hypoxic gas mixture of 10% O2: 90% N2 was used to create a model of pseudo-PEA arrest, which was then continued until conversion to a full PEA arrest.[15] For the VF model, a bipolar pacing catheter was inserted via an internal jugular vein or femoral vein to the right atrium and VF was induced electrically. Animals in both models were resuscitated using standard ACLS protocols.
Upon return of spontaneous circulation (ROSC), the animals were monitored to ensure hemodynamic stability. During this time, the animals were randomized to receive either IV (n=5) or IO (n=5) infusion of cold saline. For the IV group, a peripheral 16 gauge catheter was placed in the cephalic vein. For the IO group, a 15 gauge IO needle (Illinois Bone Marrow Needle, Cardinal Health, Dublin, OH) was placed into the right or left proximal tibia. Aspiration of bone marrow and easy flush of saline were used to confirm correct placement of the IO needle. During the IO experiment, the leg of the animal was examined to assess proper functioning of the IO infusion set, including examination for significant extravasation of fluid into the soft tissue. The saline used for infusion was cooled overnight in a 4–6°C refrigerator and further cooled via immersion in an ice slurry prior to infusion. Each 1 L bag of saline was hung in a neoprene insulating sleeve (Neoprene pouch (1L), SIGG USA, Stamford, CT) to help maintain cold saline temperature during infusion.[16]
To maximize the infusion rate, Y-type blood tubing (Baxter Healthcare Corp, Deerfield Ill) and pressure bags inflated to 300mmHg were utilized. This equipment is readily available and easily applied in the prehospital setting. The volume of saline and the amount of time to achieve goal temperature (34°C) were recorded. The experiment was terminated after 30 minutes if the animal did not achieve the goal temperature. The 30 minute time period was selected to mimic the actual clinical time that EMS personnel may have to begin the cooling process during a prolonged transport. At the completion of the experiment, the animals were euthanized.
Descriptive statistics were used to compare data between the IV and IO groups. Group means for outcome data were compared using Student’s T-test with a Bonferroni correction for multiple comparisons as appropriate. Fischer’s exact test was used to compare categorical data with small cell numbers. A two-tailed p value of < 0.05 was considered significant. Data was analyzed using Systat statistical software (Systat v11 Software, Inc, Chicago, Ill).
Results
Descriptive characteristics comparing the IV and IO groups are shown in Table 1. Both groups had 3 animals that were resuscitated from a hypoxic-PEA arrest and 2 animals that were resuscitated from a VF arrest. Esophageal temperature recordings were available in all 5 animals in each group. Saggital sinus temperature data was available in 5 animals from the IO group and from 3 animals in the IV group due to probe malfunction. There were no differences in environmental temperature between the groups (mean IV: 20.1°C, mean IO: 20.2°C; p=NS). There were no changes in hemodynamic parameters including mean arterial pressure (MAP), pulse rate, and carotid artery flow between the groups at the time cold saline infusion was initiated and when the experiment was terminated. No vasoactive agents were required to achieve or maintain hemodynamic stability in any of the animals from either group.
Table 1.
Descriptive Characteristics
| IV (n=5) | IO (n=5) | |
|---|---|---|
| Weight (Kg) | 42.50 ± 4.75 | 37.82 ± 4.31 |
| Cardiac Arrest type (PEA/VF) | 3/2 | 3/2 |
| Total fluid infused (mL) | 3310 ± 1416 | 1320 ± 542 |
| Total saline per kg (cc/kg) | 76.3±27.5 | 34.6±13.4 |
| Mean Arterial Pressure (mmHg) | ||
| Initial | 138.8 ± 53.62 | 125.8 ± 57.50 |
| Final | 82.20 ± 14.74 | 61.80 ± 21.81 |
| Difference | 56.60 ± 40.76 | 64.00 ± 61.35 |
| Heart Rate (bpm) | ||
| Initial | 187.5 ± 38.13 | 160.4 ± 21.35 |
| Final | 122.3 ± 29.55 | 100.6 ± 23.44 |
| Difference | 52.20 ± 47.30 | 59.80 ± 25.50 |
| Carotid Flow (mL/min) | ||
| Initial | 2444.1 ± 606.78 | 1656.1 ± 804.07 |
| Final | 2262 ± 673.24 | 882.05 ± 308.75 |
| Difference | 145.70 ± 323.76 | 774.03 ± 688.98 |
Values are expressed as mean ± S.D.
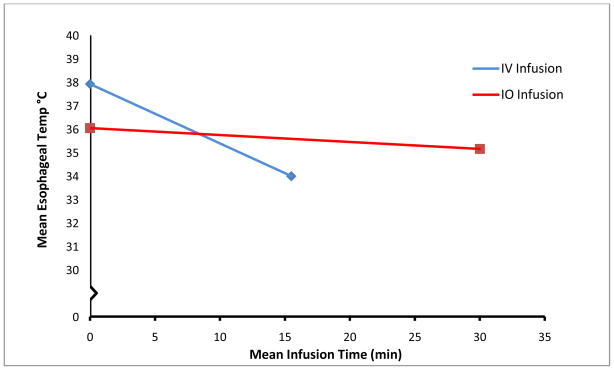
The main study results are shown in Table 2. The initial starting esophageal temperature was significantly greater in the IV group compared to the IO group (p=0.006). Despite this higher starting temperature, 4/5 animals achieved goal temperature in the IV group whereas 0/5 of the IO animals achieved goal temperature in the 30 minute time period (p=0.048). There was a difference in the esophageal temperature change between the two groups, with IV infusion esophageal temperature changes being significantly faster that IO infusion esophageal temperature changes (p<0.001). Time to achieve final esophageal temperature was significantly less in the IV group (p=0.047, Figure 1). The rate of infusion (ml/kg/min) between the methods was also significantly different (p=0.001).
Table 2.
Main Results
| IV (n=5) | IO(n=5) | p-value | |
|---|---|---|---|
| Animals that Achieved 34°C | 4 | 0 | 0.048 |
| Time to 34°C (min) | 15.49 ± 8.94 | 30 ± 0.007 | 0.047 |
| Infusion Rate (mL/kg/min) | 5.491 ± 1.55 | 1.153 ± 0.447 | 0.001 |
| Esophageal Temp (°C) | |||
| Initial | 37.92 ± 0.709 | 36.04 ± 0.391 | 0.006 |
| Final | 33.98 ± 0.148 | 35.14 ± 0.483 | 0.004 |
| Difference | 3.94 ± 0.666 | 0.900 ± 0.534 | <0.001 |
| Saggital Sinus Temp (°C)* | |||
| Initial | 37.93 ± 0.451 | 35.94 ± 0.896 | NS |
| Final | 33.9 ± 0.265 | 34.54 ± 1.37 | NS |
| Difference | 4.033 ± 0.462 | 1.40 ± 0.919 | 0.028 |
Values are expressed as mean ± SD
Saggital Sinus temperatures were only available for 3/5 animals in IV group due to probe malfunction
Figure 1.
Change in mean esophageal temperature over time by infusion route
Saggital sinus temperatures were not significantly different at the start and completion of the experiment between the two groups. However, the difference in mean saggital sinus temperature changes between the IV and IO groups, reflecting brain temperatures, was significantly different (p=0.028), with the mean temperature change being lower in the IV group.
Discussion
In this study, induction of MTH was more rapidly achieved using IV infusion of ice cold saline when compared to IO infusion, despite a higher initial starting temperature in the IV group. The target temperature was not achieved within the 30 minute time period for any of the animals using the IO infusion method. The rate of infusion (mL/kg/min) for IV infusion was higher than IO infusion, in agreement with prior IV vs. IO infusion studies unrelated to hypothermia.[17] The increased rate of IV infusion allowed for a larger volume of cold saline to be infused over a shorter period of time, which is the likely reason the IV group was able to reach the goal temperature in the allotted time. The large volume of cold saline given to the IV group is more than is typically recommended (30cc/kg) for field induction of MTH. Despite the larger volumes, we found no significant differences in hemodynamic parameters including MAP, heart rate, and carotid artery flow between the time the experiment was initiated and the time of completion. Additionally, we observed no change in continuous ETCO2 measurements throughout the experiment to suggest that the large volume of infused saline was detrimental to the respiratory status of the animals. The difference in volume infused between the groups serves to highlight the difference in efficacy of the two methods, and is not intended to advocate for a larger infusion volume for MTH induction.
The differences the current study found between IV and IO infusion is in contrast to the final conclusions reported by Mader, et.al., who stated that IO infusion of iced saline was as efficacious in initiation of MTH as IV infusion.[18] In their study, both IV and IO groups were able to achieve a similar rate of esophageal temperature decrease by maintaining a constant infusion rate of cold saline (ml/kg/min) using fluid resuscitation pumps for both groups. In the current study, the rate of infusion (ml/kg/min) was clearly different between the two groups and was limited by time. Comparing the two studies, then, would suggest that the rate of infusion of the cold saline may be a key determinant for the induction of MTH by either method. This idea is supported in Mader’s study as well, where they noted an extremely weak correlation between the rate of infusion and the core temperature decrease. However, the use of a fluid resuscitation pump to set a specific infusion rate is not typical for prehospital personnel in our region. A pressure bag is more likely to be available on the ambulances, making the model in the current study more clinically relevant for EMS implementation.
Previous studies have determined that IV infusion of ice cold saline is effective in rapidly initiating therapeutic hypothermia in the pre-hospital setting.[5, 6] Our results indicate that the higher rate of infusion achieved with IV access is associated with a more rapid induction of hypothermic conditions. This study demonstrates that IV infusion may be the preferred method to initate the cooling process within the limited time period available in the prehospital setting, and use of this method may actually help the patient to achieve goal temperature prior to ED arrival. If there is difficulty gaining IV access in a patient during resuscitation from OOHCA, IO access should be initiated for delivery of ACLS medications and to begin the induction of MTH, in keeping with the theoretical notion that the earliest possible induction of MTH is warranted, but with the expectation that goal temperature may not be achieved prior to hospital arrival.
Conclusions
Intravenous infusion of ice cold saline may be the preferred method for induction of therapeutic hypothermia when compared to intraosseous infusion in this swine model of cardiac arrest. When attempting to mimic a true clinical scenario by utilizing a time period that is typical of the prehospital setting, and by using a standard pressure bag for infusing the iced saline, IV access had a higher rate of infusion of cold saline than IO access. Intraosseous infusion of cold saline was not sufficient to induce mild therapeutic hypothermia within a 30 minute time period using these methods.
Acknowledgments
Funding: This project was supported by NHLBI NIH-SBIR grant #2R44HL080826-03A1
Footnotes
Presented as an abstract at American Heart Association/Resuscitation Sciences Symposium, November 13, 2010, Chicago, Ill.
Conflict of Interest: The authors report no conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Todd M. Larabee, Department of Emergency Medicine, University of Colorado Denver School of Medicine, 12401 East 17th Avenue B215, Denver, CO 80045.
Jenny A. Campbell, Department of Emergency Medicine, University of Colorado Denver School of Medicine, 12401 East 17th Avenue B215, Denver, CO 80045.
Fred A. Severyn, Department of Emergency Medicine, University of Colorado Denver School of Medicine, 12401 East 17th Avenue B215, Denver, CO 80045.
Charles M. Little, Department of Emergency Medicine, University of Colorado Denver School of Medicine, 12401 East 17th Avenue B215, Denver, CO 80045.
References
- 1.Gaieski DF, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Bruel C, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care. 2008;12(1):R31. doi: 10.1186/cc6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard S, et al. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56(1):9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 6.Virkkunen I, Yli-Hankala A, Silfvast T. Induction of therapeutic hypothermia after cardiac arrest in prehospital patients using ice-cold Ringer’s solution: a pilot study. Resuscitation. 2004;62(3):299–302. doi: 10.1016/j.resuscitation.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim F, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007;115(24):3064–70. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- 8.Hammer L, et al. Immediate prehospital hypothermia protocol in comatose survivors of out-of-hospital cardiac arrest. Am J Emerg Med. 2009;27(5):570–3. doi: 10.1016/j.ajem.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Minville V, et al. Prehospital intravenous line placement assessment in the French emergency system: a prospective study. Eur J Anaesthesiol. 2006;23(7):594–7. doi: 10.1017/S0265021506000202. [DOI] [PubMed] [Google Scholar]
- 10.Lapostolle F, et al. Prospective evaluation of peripheral venous access difficulty in emergency care. Intensive Care Med. 2007;33(8):1452–7. doi: 10.1007/s00134-007-0634-y. [DOI] [PubMed] [Google Scholar]
- 11.Nolan JP, et al. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67(Suppl 1):S39–86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Fowler R, et al. The role of intraosseous vascular access in the out-of-hospital environment (resource document to NAEMSP position statement) Prehosp Emerg Care. 2007;11(1):63–6. doi: 10.1080/10903120601021036. [DOI] [PubMed] [Google Scholar]
- 13.Seigler RS, Tecklenburg FW, Shealy R. Prehospital intraosseous infusion by emergency medical services personnel: a prospective study. Pediatrics. 1989;84(1):173–7. [PubMed] [Google Scholar]
- 14.Fuchs S, LaCovey D, Paris P. A prehospital model of intraosseous infusion. Ann Emerg Med. 1991;20(4):371–4. doi: 10.1016/s0196-0644(05)81657-9. [DOI] [PubMed] [Google Scholar]
- 15.Larabee TM, et al. A swine model of pseudo-pulseless electrical activity induced by partial asphyxiation. Resuscitation. 2008;78(2):196–9. doi: 10.1016/j.resuscitation.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Mader TJ. The effect of ambient temperature on cold saline during simulated infusion to induce therapeutic hypothermia. Resuscitation. 2009;80(7):766–8. doi: 10.1016/j.resuscitation.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Warren DW, et al. Comparison of fluid infusion rates among peripheral intravenous and humerus, femur, malleolus, and tibial intraosseous sites in normovolemic and hypovolemic piglets. Ann Emerg Med. 1993;22(2):183–186. doi: 10.1016/s0196-0644(05)80199-4. [DOI] [PubMed] [Google Scholar]
- 18.Mader TJ, et al. The feasibility of inducing mild therapeutic hypothermia after cardiac resuscitation using iced saline infusion via an intraosseous needle. Resuscitation. 81(1):82–6. doi: 10.1016/j.resuscitation.2009.10.003. [DOI] [PubMed] [Google Scholar]