Abstract
The goal of this study was to investigate the efflux of K+ from human corneal limbal epithelial cells (HCLE) exposed to ambient levels of UVB, which is known to cause apoptosis, and to examine the effect of K+ channel blockers on loss of potassium induced by UVB. HCLE cells were exposed to 100–200 mJ/cm2 UVB, followed by incubation in culture media with 5.5 – 100 mM K+, BDS-1, Ba2+ or ouabain. To measure intracellular cations, cells were washed in 280 mM sucrose and lysed in DI water. K+ and Na+ levels in lysates were measured by ion chromatography. HCLE cells showed maximal loss of [K+]i 10 minutes after exposure to UVB and 5.5 mM K+ media, with recovery of normal K+ levels after 90 minutes. Treatment with 1 µM BDS-1 following UVB exposure reduced the loss of [K+]i retained by HCLE cells. Exposure to 0.1–5 mM Ba2+ inhibited UVB-induced K+ loss in a time and dose dependent manner. These results confirm that blocking K+ channels in HCLE cells exposed to UVB prevents efflux of K+, confirming that UVB activates K+ channels in these cells. Electrophysiology data shows that K+ channels remain highly active at least 90 minutes after UVB exposure. HCLE cells exposed to UVB and incubated 0.01–1µM ouabain did not recover from UVB-induced K+ loss. These data suggest that the Na/K pump may act to restore [K+]i to control levels in HCLE cells following UVB exposure and that the pump is not damaged by exposure to UVB. Incubation of HCLE cells exposed to UVB in medium with 25–100mM K+ media prevented K+ efflux at extracellular concentrations as low as 25mM (the concentration in tear fluid), maintaining control levels of [K+]i. In all experiments inward fluxes and intracelluar Na+ levels mirrored K+ changes, albeit at the expected lower concentrations. The prevention of UVB-induced K+i loss by 25 mM K+o is consistent with the possible contribution of the relatively high K+ concentration in tears to protection of the corneal epithelium from ambient UVB.
Keywords: apoptosis, cornea, corneal epithelium, potassium ions, potassium channels, ultraviolet
1. Introduction
Apoptotic pathways in cells are highly complex and can be activated by a large number of signals and intracellular pathways. It has been reported using in vitro systems and various cell types that an early event in the initiation of apoptosis is activation of K+ channels with apparent loss of K+ from the cell. As reviewed below, this has been observed after exposure of cells to compounds such as staurosporin and dexamethasone, as well as exposure to ultraviolet (UV) radiation. This effect of UV is of particular interest with regards to the corneal epithelium since the cornea is continually exposed to ambient outdoor UVB and UVA, and because damage to the corneal epithelium by exposure to UV is due, in part, to apoptosis (Podskochy et al., 2000; Lu et al., 2003; Wang et al., 2003; Shimmura et al., 2004). The activation of K+ channels by UV in primary rabbit corneal epithelial cells, resulting in enhanced outward current and subsequent apoptosis, was demonstrated by Wang et al. and Lu et al. (2003). In their experiments cells were exposed to UVC to which corneas are not normally exposed since UVC is filtered by the atmosphere. We subsequently reported that UVB at doses relevant to ambient outdoor exposure also activates K+ channels and causes apoptosis of human corneal limbal epithelial (HCLE) cells in culture (Singleton et al., 2009) and that blocking the K+ channels after UVB exposure inhibits apoptosis (Ubels et al., 2010).
If initiation of apoptosis is due, at least in part, to loss of K+ from cells, then incubation of cells in elevated extracellular [K+] after exposure to conditions that cause apoptosis should inhibit apoptotic mechanisms. Bortner et al. (1997) and Hughes et al. (1997) reported that chemically induced apoptosis of lymphocytes can be prevented by incubation of the cells in isosmotic medium with 103 mM K+. This concentration is near intracellular levels, eliminating the concentration gradient and preventing loss of K+ from the cells. Yu et al. (1997) reported that 25 mM [K+]o, which is well above normal levels in culture medium (about 5 mM), reduced outward K+ current and inhibited neuronal cell apoptosis in vitro after serum deprivation or staurosporin exposure. They suggested that this was due to reduction of K+ loss from the cells in spite of channel activation. These observations are intriguing with respect to the corneal epithelium, which is continually bathed in tears that have a K+ concentration of about 25 mM, significantly higher than levels in extracellular fluid and other exocrine secretions (Atta et al., 1975; Botelho and Martinez, 1973; Rismondo et al., 1989). Previously, we proposed that the relatively high [K+] in tears reduces the outward gradient for K+ loss, inhibiting apoptotic pathways and preventing UVB damage to the corneal epithelium. In support of this hypothesis we reported that incubation of HCLE cells in isosmotic medium with 25–100 mM K+ for 6 hours after exposure of the cells to 100–200 mJ/cm2 UVB reduced DNA fragmentation and inhibited activation of caspase-3 and caspase-8 (Singleton et al., 2009).
The purposes of the present study were to further investigate effects of UVB on the magnitude and time course of intracellular K+ (K+i) loss from HCLE cells and to measure the effect of blocking K+ channels on UVB-induced loss of K+i. Most importantly, we show that incubation of the cells in medium with a [K+] as low as 25 mM after exposure to 150 mJ/cm2 UVB maintains [K+]i at levels consistent with inhibition of UVB-induced apoptosis. Since it has been suggested that Na+ fluxes resulting in increased [Na+]i are also involved in apoptosis (Bortner and Cidlowski, 2003, 2007; Bortner et al., 2008; Arrebola et al., 2005), UVB-induced changes in Na+ levels were also measured.
2. Materials and Methods
2.1. Cell Culture
Human corneal limbal epithelial cells (HCLE) were maintained as monolayer cultures in Keratinocyte-Serum Free Medium (KSFM, Invitrogen, Carlsbad, CA), as previously described (Gipson et al., 2003; Singleton et al., 2009).
2.2. UVB exposure
Cells were grown to confluence in the four corner wells of six-well plates in KSFM with the standard 5.5 mM K+. The medium was changed to Hank’s Balanced Salt Solution (HBSS) without phenol red (Invitrogen), and the cells were exposed to 302 nm UVB at 100–200 mJ/cm2 using an Ultraviolet Products model UVM-57 lamp (UVP, Upland, CA), which delivers an equal dose of UVB to the cells in each well, as described by Singleton et al., (2009). UVB intensity was measured with a Solarmeter Model 6.2 (Solartech, Inc., Harrison Twp., MI). Control cells were subjected to the same medium changes as treated cells, without the exposure to UVB.
2.3. Measurement of Intracellular Cation Content by Ion Chromatography
After exposure to UVB, the HBSS was immediately aspirated and the cells were incubated in KSFM with varying K+ concentrations or pharmacologic agents for time periods ranging from 10–90 minutes, as explained in the Results section. The high K+ medium was prepared by mixing normal medium with custom-made KSFM containing 100 mM K+ and reduced Na+ to maintain osmolarity (Invitrogen).
The protocol used for measurement of intracellular cations was based on a method described by Friis et al. (2005), with major modifications. At the end of the incubation period the medium was aspirated, and the cells were washed twice with 2 ml of 280 mM sucrose in DI water to remove extracellular electrolytes. The cells were lysed by adding 500 µL of deionized water to each well and placing the plate in a −80°C freezer for five minutes, followed by rapid thawing in a 37°C incubator. The cell debris was scraped from the well using a cell scraper, and the lysate with the cell debris was transferred to a 1.5 mL microcentrifuge tube. After centrifugation at 10,000 × G for 3 minutes, 50 µL of supernatant was retained for use in the Bio-Rad protein assay (Bio-Rad, Hercules, CA). The remaining 350–400 µL of supernatant was transferred to an Amicon Ultra-0.5 Centrifugal Filter Device and centrifuged for 30 minutes at 14,000 × G to remove proteins greater than 3 kD, since larger proteins would interfere with the function of the ion chromatography column.
Cations in the lysates, all of which were of intracellular origin, were measured by ion chromatography using a Dionex DX500 ion chromatograph with a Dionex CS-12 cation column and ED50 electrochemical detector (Dionex, Sunnyvale, CA). The mobile phase was 11 mM H2SO4 in degassed, deionized water. K+ and Na+ concentrations in the samples were calculated based on a cation standard curve and PeakNet software integral to the ion chromatograph. Ion concentrations in the cell lysates were expressed as µg/mg protein.
2.4 Patch-clamp recording
HCLE cells were plated in a recording chamber at low density in bath solution containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES and 10 glucose at pH 7.4. Whole-cell voltage-clamp current recordings were made using patch pipettes (resistance 2–5 MOhms) filled with a recording solution containing (in mM) 145 K-methanesulfonate, 2.5 MgCl2, 2.5 CaCl2, 5 HEPES and 0.25 mg/ml amphotericin-B (Sigma, St. Louis, MO)at pH 7.3., as previous described (Singleton et al., 2009; Ubels et al., 2010). To determine the time course of UVB-induced K+ channel activation, from a holding potential of −80 mV command voltage steps were given at 10 mV, 250 msec intervals to +120 mV. After 3 control protocols cells were exposed to 80 mJ/cm2 UVB and the recording protocol was repeated immediately after exposure and at intervals of several minutes for as long a cell access could be maintained. Data were analyzed from cells in which access was maintained for 45 – 90 minutes. Although most ion chromatography experiments in this study used a 150 mJ/cm2 UVB dose, patch recordings were conducted using a dose of 80 mJ/cm2 because, as previous reported (Singleton et al. 2009), the higher dose causes unacceptably high access resistance, preventing long term recording.
2.5 Statistical analysis
Ion chromatography data were analyzed statistically by analysis of variance and the Student –Newman-Keuls test. All data are presented as mean ± standard deviation, and differences were judged to be statistically significant at p ≤ 0.05.
3. Results
3.1. Effect of UVB on intracellular K+ and Na+ levels of HCLE cells
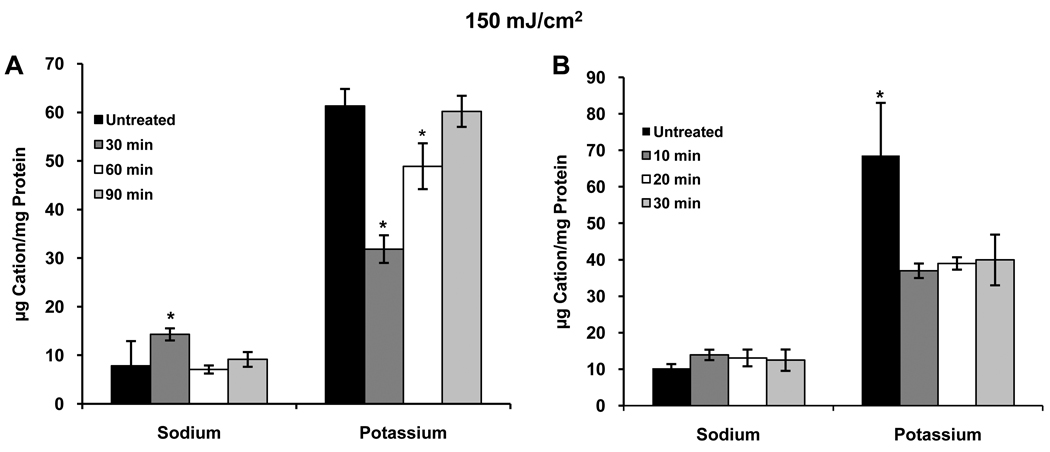
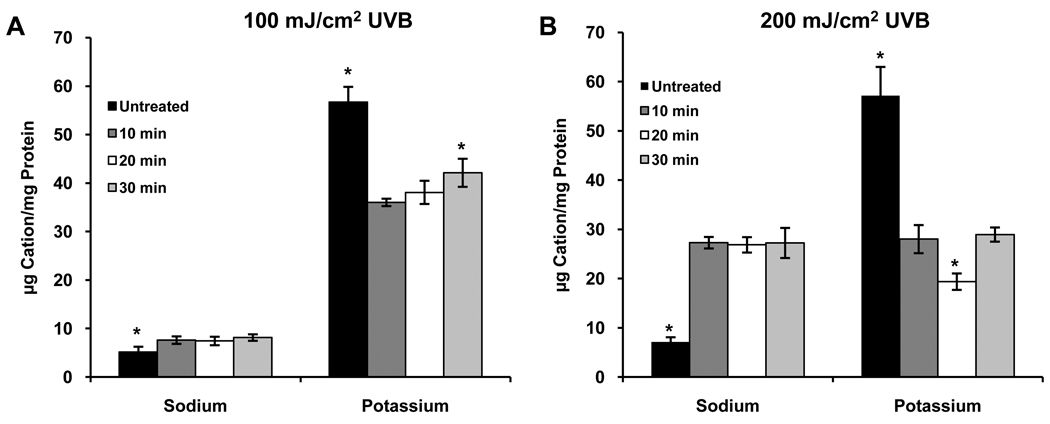
Initial experiments were conducted by exposing HCLE cells to 150 mJ/cm2 UVB and incubating the cells in KSFM for 2–6 hours, a time range chosen based on the response of apoptotic signaling pathways to UVB in our previous studies (Singleton et al., 2009; Ubels et al., 2010). No effect on [K+]i as compared to untreated controls was observed (data not shown). Since patch-clamp recordings had demonstrated a strong activation of K+ current within minutes following UVB treatment (Singleton et al., 2009), a subsequent study was conducted in which cells were incubated for 30 – 90 minutes after UVB exposure. At 30 minutes a marked reduction in [K+]i was observed, followed by a time dependent recovery to control levels 90 minutes after UVB treatment (Fig. 1A). Having confirmed a loss of intracellular K+ after UVB treatment, cells were exposed to 150 mJ/cm2 UVB and incubated for 10–30 minutes (10 minutes being the shortest practical incubation period) to determine how quickly [K+]i decreases. The loss of K+ from the cells was maximal by 10 minutes, dropping to about 54% of control, and remained low for 30 minutes (Fig. 1B). After this, as described above, [K+]i recovered to normal levels. Exposure to 100 or 200 mJ/cm2 UVB had similar effects on [K+]i with decreases to about 64% and 52% of control levels, respectively, 10 minutes after exposure to UVB (Figs. 2). The recovery of [K+]i was not consistently observed after exposure of cells to 200 mJ/cm2 UVB (data not shown). Based on these observations all subsequent experiments were conducted using a UVB dose of 150 mJ/cm2.
Figure 1.
(A) Exposure of HCLE cells to 150 mJ/cm2 UVB results in a decrease in [K+]i within 30 minutes, followed by recovery to normal levels by 90 minutes after exposure. Na+ moves into and out of the cells with the same time course. (B) The decrease in [K+]i and increase in [Na+]i in response to UVB are rapid, occurring within 10 minutes. *significantly different from all other values within groups (p ≤ 0.05, n=4) These, and all subsequent ion chomatography experiments were repeated 3 times with similar results.
Figure 2.
Effect of (A) 100 mJ/cm2 UBV or (B) 200 mJ/cm2 UBV on intracellular K+ and Na+. *significantly different from all other values within groups (p ≤ 0.05, n=4)
Since studies of lymphocytes exposed to UV or other apoptosis-inducing agents have suggested that Na+ levels in cells increase as K+ is lost, Na+ was also measured in all experiments. Control [Na+]i for all experiments conducted in this study was about 13% of control [K+]i, confirming that the cations measured by the assay were of intracellular origin. In all experiments (Figs. 1 and 2, and see below) Na+ entered cells in response to UVB. All movements and intercellular concentrations of Na+ mirrored the K+ movements and levels, albeit at the expected lower concentrations.
3.2 Effect of K+ channel blockers on [K+]i
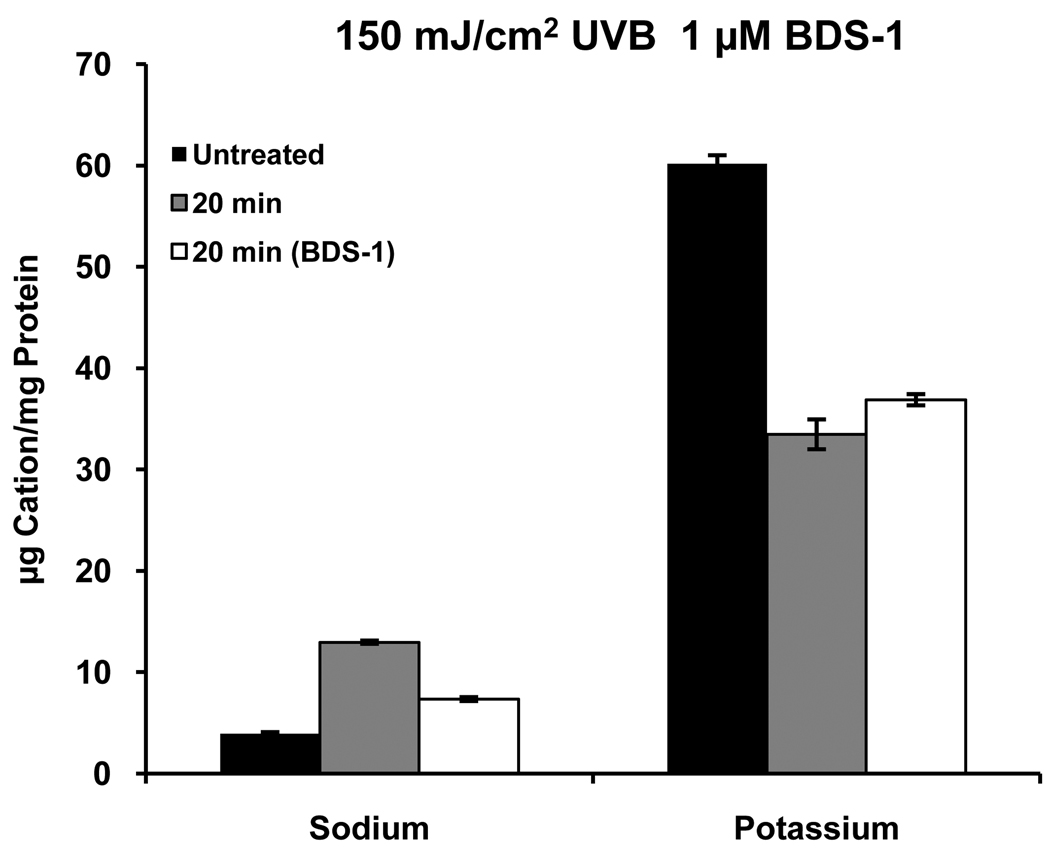
In our previous study of activation of K+ channels by UVB we showed by patch-clamp recording that the specific Kv3.4 channel (Wang et al., 2004) blocker, BDS-1, applied to single cells at 1 µM blocks about 50% of UVB-activated current in response to voltage steps. In the present study [K+]i was measured 20 minutes after exposure to UVB in the presence or absence of 1 µM BDS-1 (Alamone Labs, Jerusalem, Israel). BDS-1 caused a small, but significant decrease in the UVB-induced loss of K+ from the cells (Fig. 3); however, given the constraints of the experimental design, which would have required very large amounts of BDS-1, it was not feasible to do a complete dose-response and time course studies with BDS-1. Therefore, more extensive experiments were conducted using the broad-spectrum K+ channel blocker, Ba2+.
Figure 3.
The Kv3.4 channel blocker, BDS-1, has a small, but significant, inhibitory effect on UVB-induced loss of K+ from HCLE cells, which is reflected by Na+ fluxes. All values differ from one another within groups (p ≤ 0.05, n=4).
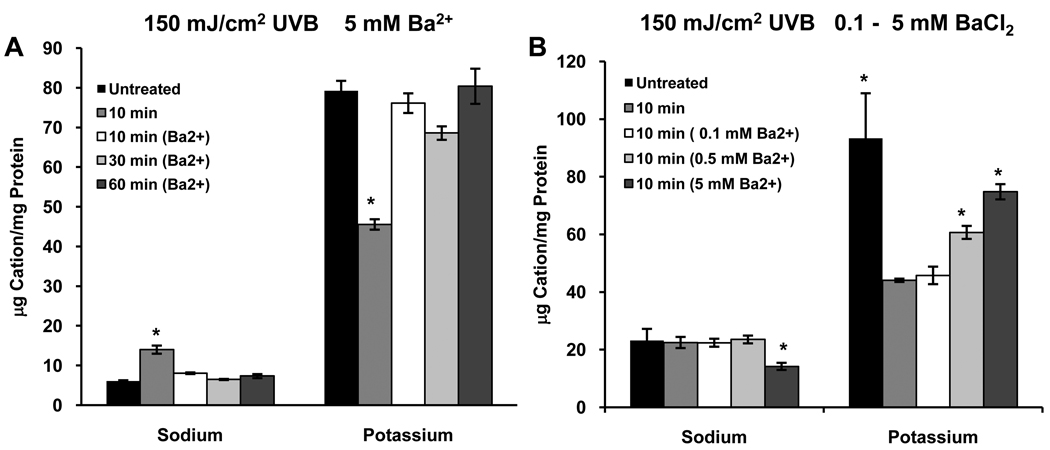
Cells were exposed to 150 mJ/cm2 UVB and incubated in KSFM for 10 minutes or in KSFM with 5 mM BaCl2 (Sigma, St. Louis, MO) for 10–60 minutes. Exposure to UVB resulted in the expected decrease in [K+]i after 10 minutes (about 55%); however, 5 mM Ba2+ blocked the loss of K+ from cells that were exposed to UVB and incubated with for 10, 30 or 60 minutes (Fig. 4A). When cells were treated similarly and incubated with 0.1 – 5 mM Ba2+ for 10 minutes, UVB-induced loss of K+ from the cells was inhibited in a dose-dependent manner (Fig. 4B).
Figure 4.
(A) Ba2+ blocks the UVB-induced loss of K+ from HCLE cells. [K+]i does not differ from control in the presence of Ba2+ at 10–60 minutes after UVB exposure. Blocking K+ flux also prevents the increase in [Na+]i. (B) Ba2+ has a dose-dependent effect on the UVB-induced change in [K+]i 10 minutes after exposure to UVB. *significantly different from all other values within groups (p ≤ 0.05, n=4)
3.4 Time course of K+ currents after UVB exposure
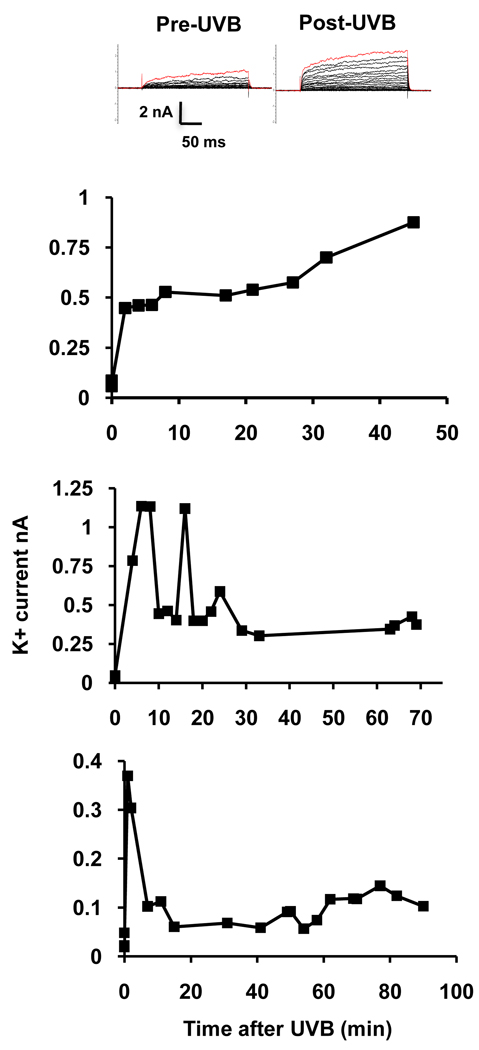
To determine whether the recovery of normal [K+]i by 90 minutes after the initial UVB-induced loss of K+ was due to inactivation of K+ channels, patch clamp data were analyzed by plotting the whole-cell outward K+ current at a command voltage of +60 mV during a period of 45–90 minutes after exposure to UVB. Data was collected from cells in which access was maintained for at least 45 minutes and up to 90 minutes. Data are presented from three representative cells from which recordings were made for 45, 70 or 90 minutes. Most cells exhibited high amplitude K+ currents during the first 10–15 minutes after UVB treatment. This is consistent with the rapid loss of intracellular K+. The current then decreased by about 20 minutes but was maintained at levels well above pre-UVB levels for the duration of the experiment (Fig. 5). The maintenance of high currents was observed in all 13 cells from which recordings were made. The decrease in current at 20 minutes did not occur in 5 of these cells.
Figure 5.
Time course of UVB-induced whole-cell K+ currents from HCLE cells. The top panel shows the recording protocol in 10 mV, 250 ms steps from a holding potential of −80 mV to +120 mV before and immediately following UVB exposure. The graphs show UVB-induced increases in K+ currents recorded at intervals from 3 representative cells at a command voltage of +60 mV over periods of 45 – 90 minutes following UVB exposure. The 0 time currents were recorded before UVB treatment. In two cells UVB caused high amplitude current during the first 10–15 minutes after followed by a reduction in current which is maintained at levels well above untreated values.
3.5 Effect of ouabain on recovery of [K+]i after UVB
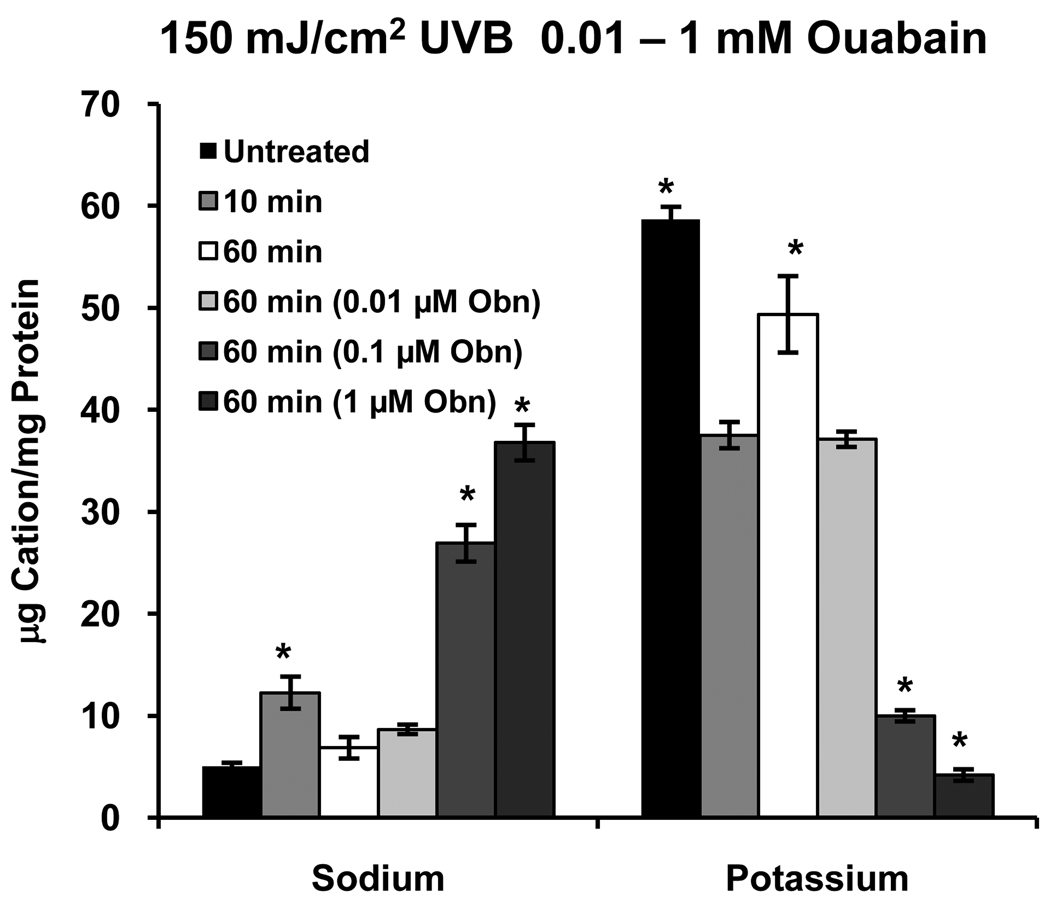
To determine whether the Na/K pump is responsible for the recovery of [K+]i that occurs between 30 – 90 minutes after UVB exposure (Fig. 1), cells were exposed to 150 mJ/cm2 UVB and incubated for 10 or 60 minutes. At 10 minutes the expected decrease in [K+]i occurred, followed by recovery to control levels by 60 minutes (Fig. 6). When UVB-treated cells were incubated with 0.01 µM ouabain for 60 minutes the recovery of [K+]i did not occur, and as expected if the pump were inhibited, a further, dose-dependent decrease in [K+]i occurred at 60 minutes in the presence of 0.1 and 1 µM ouabain. Intracellular Na+ levels also reflected inhibition of the pump. It should also be noted that ouabain caused the expected decrease in [K+]i and increase in [Na+]i in cells that were not exposed to UVB (data not shown).
Figure 6.
Inhibition of Na+/K+ATPase with 0.01 mM ouabain (Obn) inhibits the recovery of [K+]i 60 minutes after UVB exposure. The 10 and 60 minute values represent the effect of UVB without ouabain. At higher doses (0.1 and 1 mM) ouabain causes further loss of K+i in addition to that caused by UVB. Inhibition of the pump also has the expected effect on [Na+]i. *significantly different from all other values within groups. (p≤ 0.05, n=4)
3.6 Effect of elevated [K+]o on the UVB-induced decrease in [K+]i
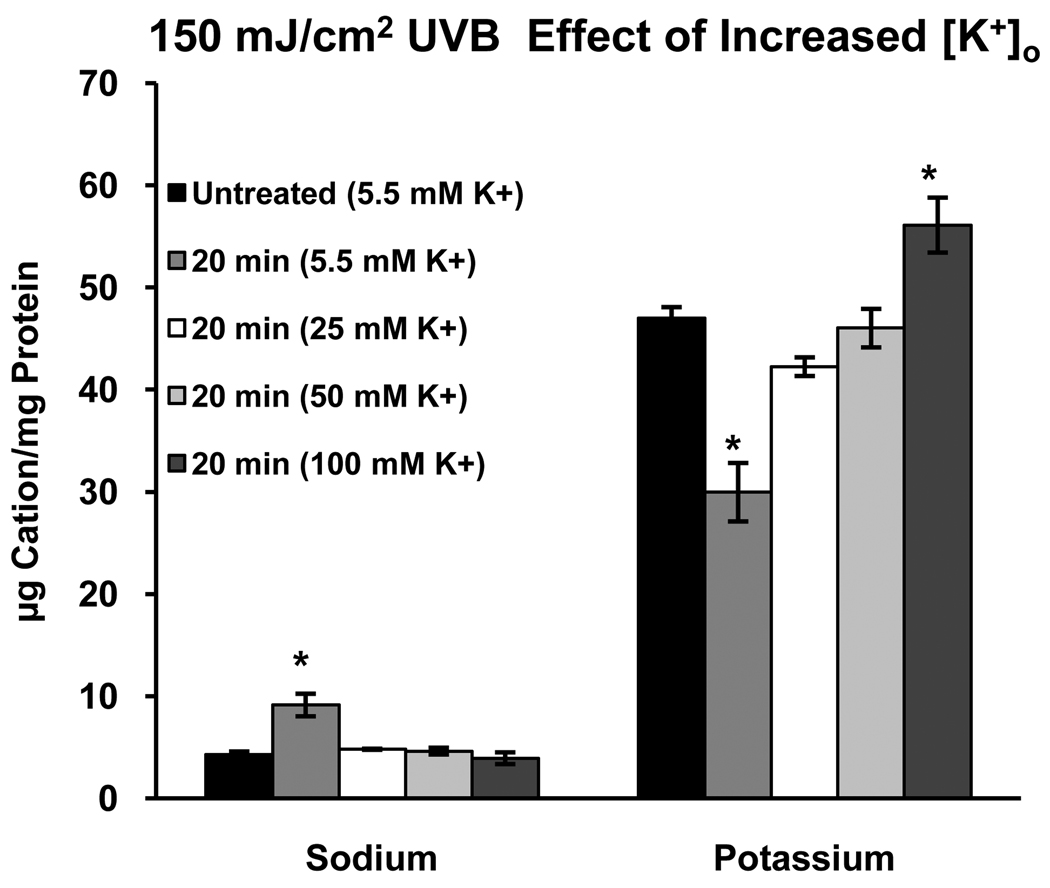
Having demonstrated the dynamics of the response of HCLE cells to UVB, we turn to the test of the primary hypothesis of the study, that elevated [K+]o will prevent UVB induced loss of K+ from the cells. Cells were exposed to 150 mJ/cm2 UVB and incubated for 20 minutes (the approximate midpoint of the period of decreased [K+]i) in KSFM with 5.5, 25, 50 or 100 mM K+. The expected UVB-induced decrease in [K+]i was observed in the presence of 5.5 mM K+. Incubation of the cells in 25 mM K+ prevented this loss of K+, as did higher levels of K+o (Fig. 7).
Figure 7.
Incubation of HCLE cells in elevated concentrations of extracellular K+ after exposure to UVB prevents loss of intracellular K+. Note that 25 mM K+, the concentration in tears, is effective in inhibiting UVB-induced K+ loss. Inhibiting loss of K+ also prevents Na+ influx. *significantly different from all other values within groups. (p≤ 0.05, n=4)
4. Discussion
The data on effects of UVB on [K+]i of HCLE cells show that doses relevant to ambient outdoor exposure cause a rapid loss of K+ from the cell in the presence of 5 mM K+o. The effect of K+ channel blockers on this loss of K+ is in agreement with our previous reports that UVB in this dose range activates K+ currents that are blocked by channel blockers (Singleton et al., 2009; Ubels et al., 2010). The rapid recovery of [K+]i that occurred by 60–90 minutes after UVB exposure was unexpected, and as discussed below, is in contrast to other reports on K+ levels in cells after treatment with inducers of apoptosis. Since normal levels of [K+]i were maintained in HCLE cells for as long as 4 hours after UVB treatment, the time at which caspase activation and DNA fragmentation approach a maximum (Singleton et al., 2009), it appears that the initial, rather brief drop in [K+]i is adequate to initiate apoptosis, which then continues in spite of the quick return to normal intracellular K+ levels. Finally, this study shows that increased [K+]o at levels as low as 25 mM, the concentration in tears, prevents UVB induced K+ loss. This is in agreement with our previous observation and the report by Yu et al. (1997) that 25 mM [K+]o can inhibit apoptosis of HCLE cells and a neuronal cell line, respectively.
Several previous studies have demonstrated the loss of intracellular K+ that occurs in response to apoptotic stimuli, including staurosporin, thapsigargin, glucocorticoids, Fas ligand and UV (Arrebola et al., 2005, 2006; Bortner et al., 1997, 2008; Dallaporta et al., 1998; Hughes et al., 1997; Vu et al., 2001). Most of these studies have been conducted using lymphocytic cell lines, and ion concentration changes were detected using cation sensitive dyes. In these studies K+ levels were generally measured at 4–6 hours after the beginning of exposure to the apoptotic stimulus with the loss of K+i reported to be a relatively late event, correlated with a strong activation of apoptotic mechanisms. In marked contrast to these reports, we detected no change in [K+]i at 4 hours in HCLE cells, as compared to controls. The present study is unique with respect to the studies cited above in that we measured cation levels at much earlier time points, demonstrating a very rapid loss of K+, which is in agreement with the rapid activation of K+ channels by UV (Wang et al., 2003; Singleton et al., 2009). We also noted a recovery of [K+]i, after exposure to 100 or 150 mJ/cm2 UVB, that has not been previously reported. Taken together, the early UVB induced loss of K+ from HCLE cells and previously reported apoptosis, in spite of the K+ recovery reported here, suggest that a sustained decrease in [K+]i is not required for apoptosis. The data indicate that there may be important differences in the apoptotic response of HCLE cells and cells of lymphocytic origin.
The recovery of [K+]i after UVB-induced efflux is intriguing because it suggests that corneal epithelial cells may have an intrinsic mechanism for protection from UV. In our previous study 150 mJ/cm2 UVB caused apoptosis of only about 10% of the cell population 6 hr after exposure and elevated [K+]o protected this subset of cells from apoptosis (Singleton et al., 2009). Intracellular K+ levels did not recover consistently in cells exposed to 200 mJ/cm2 UVB. This is probably due to a greater degree of damage to the cells, since this higher dose of UVB causes apoptosis of 40% of the cell population and 25 mM K+o does not protect these cells from apoptosis (Singleton et al., 2009).
It may be that the recovery of normal [K+]i after the initial 100–150 mJ/cm2 UVB-induced loss prevents apoptosis in the majority of the cell population. If so, the mechanism of recovery must be considered. At least two possibilities exist which are not mutually exclusive. The K+ channels might deactivate so that K+ efflux is reduced, and/or the Na/K pump replaces lost K+i. The patch clamp data show that after an initial UVB-induced increase in activity, consistent with loss of large amounts of K+ from the cell, the high open probability of the channels continues for up to 90 minutes, and perhaps longer, so that outward K+ current remains elevated. This leaves Na/K pump activity as a reasonable explanation for most of the K+i recovery.
Previous studies, however, indicate that Na/K ATPase is inhibited during apoptosis of lymphocytes caused by chemical treatments and UV (Mann et al., 2001; Panayiotidis et al., 2006, 2010). Inhibition of the Na/K pump by ouabain also can in itself cause apoptosis, as demonstrated in Jurkat cells by Nobel et al. (2000). Cejkova and coworkers, who have done extensive studies of damage to corneas by high doses of UV (Cejkova et al., 2004; Cejka et al., 2007), reported that histochemical staining of Na/K ATPase in corneal epithelium is reduced by exposure to a dose of UVB that also caused structural damage to the cornea and was apparently much higher than the doses used in the present study.(Cejkova and Lojda, 1994). Loss of pump function will, of course, cause loss of K+ from the cell, and since decreased [K+]i causes apoptosis, loss via this mechanism would be expected to promote apoptosis. In the present study, however, it is shown that Na/K ATPase apparently was not inhibited by 150 mJ/cm2 UVB, which is much lower than the repeated doses of 1 J/cm2 used by the Cejkova group (Cejkova and Lojda, 1994; Cejka et al., 2007). Rather, activity of the pump appears to be essential for the recovery of intracellular K+ levels that occurred after the initial UVB induced decrease, since ouabain prevented recovery. This appears to be a protective mechanism in corneal epithelial cells that may explain the relatively low number of cells that become apoptotic after exposure to ambient levels of UVB. The fact that inhibition of the pump by ouabain prevented recovery of K+i in HCLE, which would then be expected to result in apoptosis, supports the proposal from previous studies that inhibition of the pump is involved in apoptosis.
Although the primary focus of our studies on the effect of UVB on HCLE cells has been on K+, it was noted that [Na+]i also increased after UVB exposure and that the movements of Na+ mirrored the movements of K+, albeit at the expected lower concentrations. Previous studies have also noted an increase in [Na+]i during early stages of apoptosis, and it has been suggested that these Na+ fluxes are required for initiation of apoptosis (Arrebola et al., 2005, 2006; Bortner and Cidlowski, 2003; 2007; Bortner et al., 2008). The mechanism of the influx of Na+ in response to UV has not been studied in detail. It has been reported that UVA modifies activity of Na+ channels of cardiomyocytes in culture. Wang and Wang (2002) showed that UVA reduces the magnitude of the fast activating component and slows inactivation of Na+ currents when cardiac Na+ channels are expressed in HEK 293t cells, but that this effect only occurs while the light is on. In contrast, La et al. (2006) reported an increase in a slowly inactivating inward Na+ current in a cardiac cell line 20–30 minutes after UVA irradiation. These studies do not, however, provide an explanation for our findings on effects of UVB on HCLE cells because we observed the increase in [Na+]i only 10 minutes after UVB exposure, simultaneous with K+ efflux. We also showed that blocking K+ channels or raising [K+]o prevents the UVB induced increase in [Na+]i. Based on these observations we suggest that the movement of Na+ into the cell is simply via leak channels in response to the loss of positive charge as K+ moves out of the cell. As with K+, recovery of [Na+]i after UVB is accomplished by the pump since ouabain inhibited recovery.
In conclusion, we have shown that in HCLE cells UVB-induced loss of K+ from cells is a very early event. The protection of the cells from this effect of UV by elevated [K+]o at concentrations as low as 25 mM is consistent with our overall hypothesis that high [K+] in tears contributes to protection of the corneal epithelium from ambient UVB. The novel observation that HCLE cells quickly recover normal [K+]i after UVB exposure suggests that the cells themselves also have an intrinsic mechanism via the Na/K pump for restoration of normal cation levels that inhibits activation of apoptotic signaling pathways.
Acknowledgements
This work was supported by NIH grant R01 EY018100, NSF grant DBI-0321049, NSF grant DBI-0520840 and the Den Ouden Fellowship for summer undergraduate research. We thank Dr. Ilene K. Gipson, Schepens Eye Research Institute, Harvard Medical School for providing the HCLE cells. Scott Prentice and Daniel Evans provided technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrebola F, Fernández-Segura E, Campos A, Crespo PV, Skepper JN, Warley A. Changes in intracellular electrolyte concentrations during apoptosis induced by UV irradiation of human myeloblastic cells. Am. J. Physiol. Cell Physiol. 2006;290:C638–C649. doi: 10.1152/ajpcell.00364.2005. [DOI] [PubMed] [Google Scholar]
- Arrebola F, Zabiti S, Cañizares FJ, Cubero MA, Crespo PV, Fernández-Segura E. Changes in intracellular sodium, chlorine, and potassium concentrations in staurosporine-induced apoptosis. J Cell Physiol. 2005;204:500–507. doi: 10.1002/jcp.20306. [DOI] [PubMed] [Google Scholar]
- Atta AG, Maringoni RL, Borsio Netto JB, Silveira Filho B. Potassium and sodium levels in the initial saliva of the dog’s submandibular and parotid glands. Rev. Bras. Pesqui. Med. Bio. 1975;3:37–43. [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J. Biol. Chem. 2003;278:39176–39184. doi: 10.1074/jbc.M303516200. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch. Biochem. Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner CD, Hughes FM, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J. Biol. Chem. 1997;272:32436–32542. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Sifre MI, Cidlowski JA. Cationic gradient reversal and cytoskeleton-independent volume regulatory pathways define an early stage of apoptosis. 2008 doi: 10.1074/jbc.M707809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho SY, Martinez EV. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am. J. Physiol. 1973;225:606–609. doi: 10.1152/ajplegacy.1973.225.3.606. [DOI] [PubMed] [Google Scholar]
- Cejka C, Pláteník J, Guryca V, Sirc J, Michálek J, Brůunová B, Cejková J. Light absorption properties of the rabbit cornea repeatedly irradiated with UVB rays. Photochem. Photobiol. 2007;83:652–657. doi: 10.1111/j.1751-1097.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Cejková J, Lojda Z. The damaging effect of UV rays (with the wavelength shorter than 320 nm) on the rabbit anterior eye segment. I. Early changes and their prevention by catalase-aprotinin application. Acta Histochem. 1994;96:281–286. doi: 10.1016/S0065-1281(11)80036-X. [DOI] [PubMed] [Google Scholar]
- Cejková J, Stípek S, Crkovská J, Ardan T, Pláteník J, Cejka C, Midelfart A. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol. Res. 2004;53:1–10. [PubMed] [Google Scholar]
- Dallaporta B, Hirsch T, Susin SA, Zamzami N, Larochette N, Brenner C, Marzo I, Kroemer G. Potassium leakage during the apoptotic degradation phase. J. Immunol. 1998;160:5605–5615. [PubMed] [Google Scholar]
- Friis MB, Friborg CR, Schneider L, Nielsen MB, Lambert IH, Christensen ST, Hoffmann EK. Cell shrinkage as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol. 2005;567:427–443. doi: 10.1113/jphysiol.2005.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol .Vis. Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. J. Biol. Chem. 283, 7219–7229. [DOI] [PubMed] [Google Scholar]
- La C, You Y, Zhabyeyev P, Pelzer DJ, McDonald TF. Ultraviolet photoalteration of late Na+ current in guinea-pig ventricular myocytes. J. Membr. Biol. 2006;210:43–50. doi: 10.1007/s00232-005-0844-6. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang L, Shell B. UV-induced signaling pathways associated with corneal epithelial cell apoptosis. Invest. Ophthalmol. Vis. Sci. 2003;44:5102–5109. doi: 10.1167/iovs.03-0591. [DOI] [PubMed] [Google Scholar]
- Mann CL, Bortner CD, Jewell CM, Cidlowski JA. Glucocorticoid-induced plasma membrane depolarization during thymocyte apoptosis: association with cell shrinkage and degradation of the Na+/K+-adenosine triphosphatase. Endocrinology. 2001;142:5059–5068. doi: 10.1210/endo.142.12.8516. [DOI] [PubMed] [Google Scholar]
- Nobel CS, Aronson JK, van den Dobbelsteen DJ, Slater AF. Inhibition of Na+/K+-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis. 2000;5:153–163. doi: 10.1023/a:1009684713784. [DOI] [PubMed] [Google Scholar]
- Panayiotidis MI, Bortner CD, Cidlowski JA. On the mechanism of ionic regulation of apoptosis: would the Na+/K+-ATPase please stand up? Acta. Physiol. (Oxf) 2006;187:205–215. doi: 10.1111/j.1748-1716.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- Panayiotidis MI, Franco R, Bortner CD, Cidlowski JA. Ouabain-induced perturbations in intracellular ionic homeostasis regulate death receptor-mediated apoptosis. Apoptosis. 2010;15:834–949. doi: 10.1007/s10495-010-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podskochy A, Gan L, Fagerholm P. Apoptosis in UV-exposed rabbit corneas. Cornea. 2000;19:99–103. doi: 10.1097/00003226-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989;15:222–229. [PubMed] [Google Scholar]
- Shimmura S, Tadano K, Tsubota K. UV dose-dependent caspase activation in a corneal epithelial cell line. Curr. Eye Res. 2004;28:85–92. doi: 10.1076/ceyr.28.2.85.26237. [DOI] [PubMed] [Google Scholar]
- Singleton KR, Will DS, Schotanus MP, Haarsma LD, Koetje LR, Bardolph SL, Ubels JL. Elevated extracellular potassium inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp. Eye Res. 2009;89:140–151. doi: 10.1016/j.exer.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Schotanus MP, Bardolph SL, Haarsma LD, Koetje LR, Louters JR. Inhibition of UV-B induced apoptosis in corneal epithelial cells by potassium channel modulators. Exp. Eye Res. 2010;90:216–222. doi: 10.1016/j.exer.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu CC, Bortner CD, Cidlowski JA. Differential involvement of initiator caspases in apoptotic volume decrease and potassium efflux during Fas- and UV-induced cell death. J. Biol. Chem. 2001;276:37602–37611. doi: 10.1074/jbc.M104810200. [DOI] [PubMed] [Google Scholar]
- Wang L, Li T, Lu L. UV-induced corneal epithelial cell death by activation of potassium channels. Invest. Ophthalmol. Vis Sci. 2003;44:5095–5101. doi: 10.1167/iovs.03-0590. [DOI] [PubMed] [Google Scholar]
- Wang L, Fyffe RE, Lu L. Identification of a Kv3.4 channel in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1796–1803. doi: 10.1167/iovs.03-1056. [DOI] [PubMed] [Google Scholar]
- Wang GK, Wang SY. Modifications of human cardiac sodium channel gating by UVA light. J. Membr. Biol. 2002;189:153–165. doi: 10.1007/s00232-002-1010-z. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]