Abstract
Background
Citicoline (cytidine-5’-diphosphate) is a mononucleotide composed of ribose, cytosine, pyrophosphate, and choline, and is involved in the biosynthesis of the structural phosopholipids of cell membranes. Treatment with citicoline, improves memory in patients with dementia, and reduces damage to the brain after traumatic brain injury or stroke. Recent research has been conducted to assess whether citicoline is an effective treatment for cocaine dependence. In cocaine-dependent individuals, withdrawal from cocaine is associated with disturbed sleep, which may contribute to the high rate of relapse to cocaine use. Therefore, it is important to know the impact of citicoline on the sleep/wake cycle in these individuals in order to rate its overall efficacy.
Method
In this double-blind, placebo-controlled trial, the effects of citicoline treatment on the sleep/wake cycles of cocaine dependent participants were assessed. The results of the current study are reported as part of a larger study, consisting of an eight-week treatment period to assess the efficacy of longer-term treatment with citicoline at decreasing cocaine consumption in cocaine-dependent polydrug using participants.
Results
In this non-abstinent, cocaine-dependent population, citicoline had no effect on any of the sleep parameters measured including sleep efficiency, sleep latency, total sleep time, number of waking episodes, time awake per episode, amount of time in bed spent moving, number of sleep episodes, time asleep per episode, and amount of time in bed spent immobile.
Conclusions
These data suggest that eight weeks of citicoline administration does not disturb sleep/wake cycles of cocaine-dependent individuals.
Keywords: citicoline, CDP-choline, sleep, cocaine
1. INTRODUCTION
Citicoline (cytidine-5’-diphosphate) is a mononucleotide composed of ribose, cytosine, pyrophosphate, and choline, and is involved in the biosynthesis of the structural phosopholipids of cell membranes (Kennedy and Weiss, 1956; Chida and Shimizu, 1973). Citicoline is absorbed with equal efficiency when administered via the intravenous or oral routes in both rodents (Galletti et al., 1991) and humans (Weiss, 1995). Once metabolites of citicoline cross the blood brain barrier, they enhance incorporation of phospholipids into neuronal membranes (Galletti et al., 1991; Secades and Frontera, 1995). Several benefits of treatment with citicoline make it a good candidate for the treatment of cocaine dependence.
Postmortem studies indicate that cocaine dependence causes a depletion of neuronal phospholipid metabolism in the putamen of cocaine dependent individuals (Ross et al., 2002); citicoline treatment improves neuronal membrane integrity. A recent phosphorus magnetic resonance spectroscopy study in healthy adults found that six weeks of oral citicoline dose-dependently increases high energy phosphates and membrane phospholipids (Silveri et al., 2008). Much of the research on the role of citicoline in membrane phospholipid turnover has come from studying the feasibility of using citicoline to augment neuronal repair after brain damage. In rats, citicoline treatment after experimental stroke increases motor function and neural plasticity (Hurtado et al., 2007), and after experimentally induced traumatic brain injury, citicoline treatment decreases both brain edema and the breakdown of the blood-brain barrier (Baskaya et al., 2000). In Europe, Asia, and more recently in the United States, citicoline has been used to treat human patients for ischemia as a result of traumatic brain injury (Spiers and Hochanadel, 1999; Baskaya et al., 2000) or stroke (Cho and Kim, 2009). After citicoline treatment, the volume of ischemic lesions are reduced, and structural integrity of the brain, levels of consciousness, neurological symptoms, motor deficits, hypertonia, psychomotor function, EEG activity, and general quality of life are all improved over placebo treatment (Calatayud Maldonado et al., 1991; Levin, 1991; Warach et al., 2000; Clark et al., 2001; Davalos et al., 2002). If citicoline treatment in cocaine dependent individuals is as effective at normalizing neuronal membrane phospholipids as it is in brain injury patients, citicoline may augment the ability of the brain to repair itself in these individuals during sustained cocaine abstinence.
A second effect of citicoline treatment which makes it a good candidate for treatment of cocaine dependence is improving cognitive performance in poorly functioning adults. Behavioral studies indicate that cocaine dependent individuals show deficits in performance on cognitive tasks (Tomasi et al., 2007). Citicoline protects against impairments in hippocampal-dependent long-term memory in middle-aged rats (Teather and Wurtman, 2003) and improves learning and memory task performance of older rats (Mosharrof et al., 1987; Drago et al., 1993). In humans, citicoline treatment improves both delayed and immediate logical memory in lower functioning older adults, although it has no effect on normally functioning older adults (Spiers et al., 1996). In Alzheimer’s patients, citicoline improves cognitive performance and cerebral blood flow velocities over placebo, and patients with more severe dementia show greater improvements (Alvarez et al., 1999). If citicoline treatment is as effective at normalizing cognitive function in cocaine dependent individuals as it is in lower functioning older adults, citicoline may augment the improvements in cognitive functioning in these individuals during sustained cocaine abstinence.
Research on the utility of citicoline as a treatment for cocaine dependence is limited. One study has assessed the effectiveness of citicoline treatment at reducing cocaine consumption within a psychiatric population. In 44 outpatients with a history of bipolar disorder or schizoaffective disorder of the bipolar type who were in early recovery from cocaine abuse or dependence, citicoline treatment decreased the probability of a cocaine-positive urine sample and improved scores on an auditory verbal learning task, without affecting mood (Brown et al., 2007). Renshaw et al. (1999) conducted a short-term (two week) citicoline treatment study in a population free of psychiatric disorders, and found that two-week treatment with citicoline did not affect psychomotor performance, EEG signals, cocaine cue reactivity, or subjective ratings of mood and craving. Participants did, however, report a decrease in feelings of “lack of control over use” from pre- to post-treatment periods, as well as lower “urge for cocaine” both before and after cocaine cue presentation. Unlike in inpatient rehabilitation centers, during outpatient trials, participants go about their daily lives where they are exposed to cues that can trigger cocaine craving and/or use; thus they may continue to use cocaine while they are taking citicoline. To assess safety issues associated with citicoline treatment in a population that may not be abstinent from cocaine, Lukas et al. (2001) conducted a study on the effects of citicoline with concomitant cocaine use and found that citicoline pre-treatment did not alter cocaine’s effect on heart rate, skin temperature, blood pressure, plasma levels of cocaine or its metabolites, or subjective ratings in cocaine-dependent individuals.
One important aspect of using citicoline as a treatment for cocaine dependence that has not been assessed is citicoline’s effect on sleep. When cocaine-dependent individuals attempt to quit using cocaine, they experience a number of withdrawal symptoms, including disturbances to sleep. During cocaine use, latency to REM sleep is increased, and total REM sleep is decreased (Watson et al., 1992). Then with abstinence REM rebounds (Watson et al., 1992). During early (within one week of binge) abstinence, sleep efficiency, sleep latency, REM latency, and percent REM (Thompson et al., 1995; Johanson et al., 1999) are all improved. In fact, sleep efficiency is at its best during the first week of abstinence (Morgan et al., 2006). However as abstinence progresses, sleep architecture, as measured by polysomnography (Johanson et al., 1999; Morgan et al., 2008), as well as sleep quality and quantity as measured by actigraphy (Morgan et al., 2006) quickly deteriorate. In fact, total sleep time, time awake after sleep onset, number of arousals per hour of sleep, and number of awakenings of 1-minute or less are all at their worst at days 14–17 of abstinence (Morgan et al., 2006). This is in direct contrast to self reports of sleep quality and daytime sleepiness. During early abstinence, self-reported daytime sleepiness increases (Johanson et al., 1999), while during late abstinence when sleep architecture is at its worst, participants report better night time sleep quality (Morgan et al., 2006), and less daytime sleepiness (Johanson et al., 1999). In fact, self-assessed sleep is the best during days 14–17 (Morgan et al., 2006).
The deterioration in sleep is likely functionally important as it is accompanied by deficits on a variety of cognitive tasks including procedural learning tasks, digit vigilance tasks, and motor sequence tasks (Morgan et al., 2006). Following the pattern of quantitative sleep measures, and in direct contrast to self-reports of sleep quality, cognitive performance on these measures is best early in abstinence, and as abstinence continues, performance gets progressively worse (Morgan et al., 2006). In fact, it has been suggested that the cognitive deficits associated with cocaine withdrawal may not be a consequence of the withdrawal from the drug but rather due to disturbances to sleep architecture (Morgan and Malison, 2007; Morgan et al., 2008).
In addition to affecting cognitive performance, abstinence-induced disturbances to sleep may contribute to relapse (Pace-Schott et al., 2005). Several medications designed to treat cocaine dependence directly influence sleep quantity and quality. For example, modafinil improves slow-wave sleep, total sleep time, and sleep latency during the first three weeks of abstinence to levels seen in healthy controls (Morgan et al., 2010), and this is accompanied by a decrease in self-reported afternoon sleepiness. As a potential medical treatment for cocaine dependence, the effect of citicoline on sleep is an important aspect of its effectiveness at decreasing cocaine use. In addition, as sleep is so profoundly affected by abstinence from cocaine, any effect that treatment with citicoline may have on sleep is an important safety concern.
The results of the current study are reported as part of an outpatient trial, consisting of an eight-week treatment period to assess the efficacy of longer-term treatment with citicoline at decreasing cocaine consumption in cocaine dependent participants (Licata et al., 2011). In this randomized, double-blind, placebo controlled trial the effects of citicoline treatment on the sleep/wake cycles, and cognitive functioning of cocaine-dependent individuals were tested.
2. MATERIALS AND METHOD
2.1 Participants
The main study reported on 18 completers with partial data from other non-completers (Licata et al., 2011). The current study includes only those participants who completed the treatment phase, and for whom actigraphy data were available. Of a total of 43 original participants, 17 dropped out before the medication phase began, 8 dropped out during the medication phase, 4 had no actigraphy data, and 2 were removed because of non-compliance with sleep diaries. The remaining participants included 12 cocaine dependent African-American (n = 11) and “other” (n = 1) adult (average age = 36.33 ± 1.4 years old) males (n = 7) and females (n = 5) (see Table 1 for participant details). Eight participants were unemployed (placebo n = 4, citicoline n = 4). Of the four employed participants no participant indicated that they worked the night shift. However, for all participants, if days were shifted (ie participant slept from 7am–1pm), the defined “day” of assessment was changed accordingly. The beginning/end of the days did not necessarily start at 6am, but rather were tailored to the sleep patterns of the participant. The baseline phase lasted two weeks; the treatment phase (placebo n = 4, citicoline n = 8) lasted eight weeks; and the follow-up phase lasted two weeks. Participants were not abstinent from cocaine, alcohol, or (in several cases) marihuana during any study phase (See table 1 for drug use details by phase). Drug-use information was obtained from participant entries into the actigraphy device and onto daily diaries. Every participant produced cocaine positive urine tests during every phase.
Table 1.
Participant characteristics including medication group, previous drug dependence, drug use during the baseline study phase, drug use during the medication phase, drug use during the follow up phase. Drug-use information was obtained from participant entries into the actigraphy device, daily diaries. Urine tests were performed for THC and cocaine. If any of the three measures (actigraphy device, daily diary, urine test) was positive, results were reported as positive. Sub = Subject Number
| Sub | Group | Sex | Drug Dependence | Baseline Stage Drug Use | Treatment Stage Drug Use | Follow-Up Stage Drug Use |
|---|---|---|---|---|---|---|
| 17 | Citicoline | Male | Cocaine | Cocaine Alcohol |
Cocaine Alcohol |
Cocaine Alcohol |
| 18 | Citicoline | Male | Cocaine | Cocaine Alcohol |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 26 | Citicoline | Male | Cocaine | Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 31 | Citicoline | Male | Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 34 | Citicoline | Female | Cocaine | Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 35 | Citicoline | Male | Cocaine | Cocaine Alcohol Marihuana |
Cocaine Alcohol |
Cocaine Alcohol |
| 38 | Citicoline | Male | Cocaine Alcohol |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 42 | Citicoline | Female | Cocaine | Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 15 | Placebo | Female | Cocaine | Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
Cocaine Alcohol Marihuana |
| 23 | Placebo | Male | Cocaine Alcohol Marihuana |
Cocaine Alcohol |
Cocaine Alcohol |
Cocaine Alcohol Marihuana |
| 33 | Placebo | Female | Cocaine | Cocaine Alcohol |
Cocaine Alcohol |
Cocaine Alcohol Marihuana |
| 43 | Placebo | Female | Cocaine Marihuana |
Cocaine Alcohol |
Cocaine Alcohol Marihuana |
Cocaine Alcohol |
2.2 Experimental Procedures
Participants were recruited from the community through advertisements in local and college newspapers as well as advertisements posted on bulletin boards in the Boston area. Responders who passed an initial phone screen visited the Behavioral Psychopharmacology Research Laboratory at McLean Hospital where they read and signed an Institutional Review Board approved informed consent form and passed a physical (EKG, complete blood panel including a test of liver function, drug urine screen (QuickTox Drug Screen Dipcard, Branan Medical Corporation, Irvine, CA), and pregnancy tests for females (Stanbio QuPID Procedure No. 1220, Studio Laboratory, Boerne, Texas)). They also were found to be free of any Axis I disorders (Structured Clinical Interview for DSM-IV disorders; First et al., 1997), with the exception of current cocaine, marihuana, or alcohol dependence.
Participants were fitted with a wrist actigraphy device (ActiWatch-Score, Phillips/Respironics, Murraysville, PA, USA), which is worn similarly to a wrist watch. For two weeks participants took part in a baseline no treatment period, followed by eight weeks of placebo or citicoline treatment (two 500 mg capsules a day for a total of 1,000 mg citicoline per day; Grupo-Ferrer International, Barcelona, Spain), then a two week follow-up period. Medication doses were taken twice a day, 12 hours apart. Medication compliance was determined by self-report on daily diaries. The compliance rate for participants in the placebo group was 86.7%, and the compliance rate for participants in the citicoline group was 90.3%.
Four cognitive tasks were used to assess cognitive function in these individuals. Tasks included the Trail Making B Test (a measure of processing speed and executive function), the Block Design task (a measure of spatial ability), the Digit Symbol Substitution Test (a measure of memory), and the Wisconsin Card Sorting task (a measure of executive function and set shifting). Each task was administered four times during the trial – once during the baseline phase, two during the treatment phase, and once at follow up.
2.3 Analysis
The ActiWatch-Score wrist actigraphy device contains an accelerometer that records the frequency and magnitude of motion. To calculate sleep variables, Acti-Ware Sleep software (Phillips/Respironics, Murraysville, PA, USA) uses an algorithm designed to take into account the magnitude of movement immediately surrounding the epoch in question. In this manner, the software can identify the different patterns of movement that constitute a waking episode versus a pattern of movement that constitutes a simple shift in body position during sleep (Mini Mitter Corporation Phillips/Respironics, 2009). Bedtime and rise time were entered from participants’ daily diaries. Acti-Ware software uses these times paired with accelerometer data collected via the ActiWatch device to calculate sleep parameters. Sleep parameters could not be calculated on days for which participants did not fill out sleep/wake diaries, on which participants did not sleep, and on which the ActiWatch malfunctioned.
Besides bedtime and rise times, participants’ daily diaries also assessed subjective ratings of sleep quality and quantity. Subjective ratings could not be analyzed for one participant as that person did fill out bedtime and rise times (allowing actigraphy measurements of sleep), but did not enter subjective sleep ratings into diaries.
Participants only removed ActiWatch devices to shower, so actigraphy data was collected almost 24 hours a day. The number of activity counts for each day was then averaged by the ActiWatch software. Daily activity data could not be calculated for days on which participants visited the lab to have the data from the ActiWatch downloaded into the computer, as there was not continuous activity data recorded for those days.
There were not an equal number of participants in the useable data for the citicoline and placebo phases (See Table 1). As such, there were not equal numbers of days in each phase for each participant. In order to account for the differing number of days so that no participant’s data would unequally affect calculated values, and to analyze data for each participant as a repeated data set (with study phase as the repeated measure), averages were first calculated for each participant for each dependent measure. This resulted in one value for each phase for each participant. Statistics were then calculated on these averaged data. All data are expressed as these phase averages ± standard error of the mean.
In order to assess the impact of citicoline on sleep parameters, within-subject repeated measures mixed model ANOVAs were run using SPSS (version #18, SPSS Inc., Chicago, IL) with the sleep/activity parameters and subjective ratings of sleep quality and quantity - sleep efficiency (calculated by dividing the time spent asleep by the time spent in bed), sleep latency (a measure of how long it took participants to fall asleep after “lights out”), total sleep time, number of waking episodes, time awake per episode, time spent moving, number of sleep episodes, time asleep per episode, time spent immobile, total daily activity score, subjective sleep quality, feeling rested, subjective number of waking episodes, and subjective number of hours slept - as the dependent variables. Medication type (citicoline or placebo) and study phase (baseline, medication, or follow-up) were fixed factor main effects. The Bonferroni method was used to correct for multiple comparisons.
In order to assess the impact of citicoline on cognitive function, within-subject repeated measures mixed model ANOVAs were run using SPSS (version #18, SPSS Inc., Chicago, IL) with cognitive tasks (Trail Making Task Part B, Digit Symbol Substitution Task, Block Design Task, and Wisconsin Card Sorting Task) as the dependent variables. Medication type (citicoline or placebo) and study phase (baseline, medication phase assessment 1, medication phase assessment 2, and follow-up) were fixed factor main effects. The Bonferroni method was used to correct for multiple comparisons.
For all dependent variables, because of the unequal number of participants in the placebo and citicoline groups, we conducted a within-subjects, post hoc repeated measures ANOVA using SPSS on data only from participants in the citicoline group using the same parameters as above. Because much of the effect of cocaine abstinence on sleep is dependent on how long the person has been abstinent (see Introduction for details), the treatment phase was divided into early and late stages, and mixed model within-subject repeated measures ANOVAs were run using SPSS for each dependent variable. The Bonferroni method was used to correct for multiple comparisons.
3. RESULTS
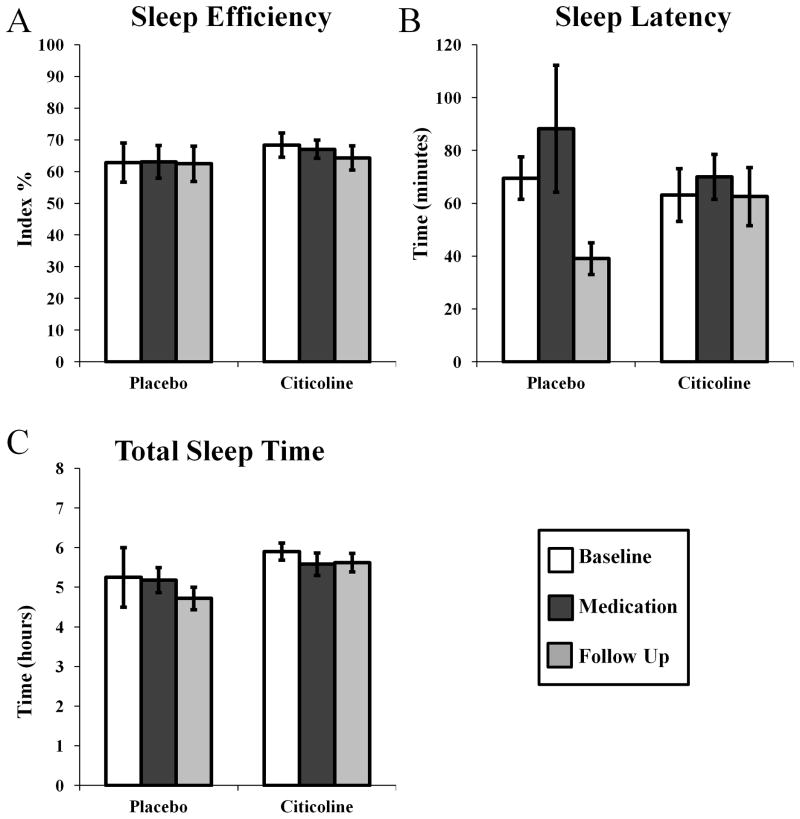
In these cocaine-dependent participants, who were not abstinent from cocaine during the study (see Table 1 for details of participant drug use), citicoline treatment did not affect standard parameters of sleep. Sleep efficiency (Fig 1A; phase by medication F(2,29) = 0.084, p = 0.920), sleep latency, (Fig 1B; phase by medication F(2,29) = 1.040, p = 0.961), and the total sleep time per night were not affected by citicoline treatment (Fig 1C; phase by medication F(2,29) = 0.237, p = 0.791). There was an effect of treatment group on total sleep time (Fig 1C; medication F(1,29) = 5.540, p < 0.05) as participants in the placebo group spent less time asleep overall than participants in the citicoline group (95% CI = 0.024–3.12 hours, difference = 1.56 hours).
Figure 1.
Effects of citicoline on sleep efficiency (A), sleep latency (B), and the total time asleep per night (C) assessed using wrist actigraphy.
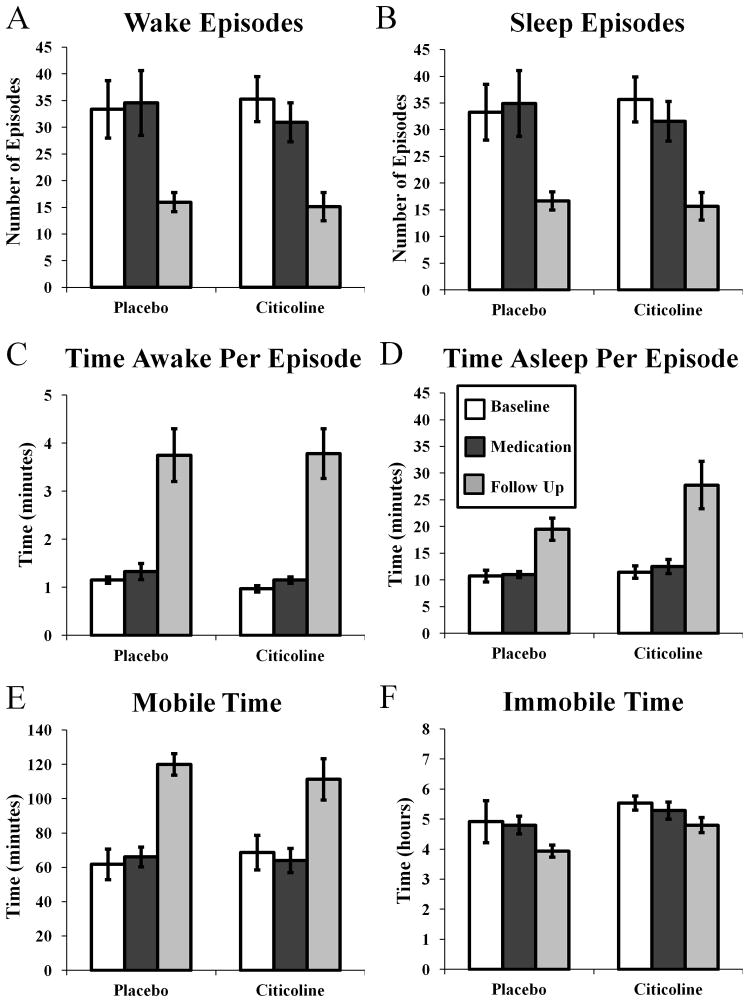
Citicoline treatment did not affect nighttime wakefulness. There was no effect of treatment on the number of waking episodes (Fig 2A; phase by medication F(2,29) = 0.206, p = 0.815), the time awake per episode (Fig 2C; phase by medication F(2,29) = 2.075, p = 0.114), or on the amount of time spent moving (Fig 2E; phase by medication F(2,29) = 0.754, p = 0.285). There was an effect of study phase on the number of waking episodes per night (Fig 2A; phase F(2,29) = 11.383, p < 0.0001), with pairwise comparisons indicating that participants in both groups experienced fewer waking episodes in the follow-up phase than in the baseline (p < 0.001, 95% CI = 7.718–29.804 episodes, difference = 18.761 episodes) and the treatment phases (p < 0.01, 95% CI = 6.146–28.232 episodes, difference = 17.189 episodes). Similarly, there was an effect of study phase on the amount of time in bed spent moving (phase Fig 2E; F(2,29) = 15.653, p < 0.0001), with pairwise comparisons indicating that participants in both groups experienced more minutes moving in the follow-up phase than during the baseline phase (p < 0.0001, 95% CI = 24.060–76.877 minutes, difference = 50.469 minutes) and the treatment phase (p < 0.0001, 95% CI = 24.233–77.049 minutes, difference = 50.641 minutes).
Figure 2.
Effects of citicoline on the number of waking episodes (A), the number of sleep episodes (B), time awake per episode (C), time asleep per episode (D), mobile time (E), and the immobile time (F) assessed using wrist actigraphy.
Citicoline treatment did not affect measures of sleep quantity. There was no effect of treatment on the number of sleep episodes (Fig 2B; phase by medication F(2,29) = 0.220, p = 0.804), on the time asleep per sleep episode (Fig 2D; phase by medication F(2,29) = 0.610, p = 0.550), or on the amount of time in bed spent immobile (Fig 2F: phase by medication F(2,29) = 0.174, p = 0.841). There was an effect of study phase on the number of sleep episodes per night (Fig 2B; phase F(2,29) = 10.939, p < 0.0001), with pairwise comparisons indicating that participants in both groups experienced fewer sleep episodes in the follow-up phase than in the baseline (p = 0.001, 95% CI = 7.232–29.401 episodes, difference = 18.317 episodes) and the treatment phases (p = 0.001, 95% CI = 6.012–28.181 episodes, difference = 17.096 episodes). Similarly, there was an effect of study phase on the amount of time spent immobile (Fig 2F; phase F(2,29) = 3.459, p < 0.05), however pairwise comparisons were not significant. In addition, there was an effect of treatment group on the amount of time spent immobile (Fig 2F; medication F(2,29) = 5.726, p < 0.05), with pairwise comparisons indicating that participants in the citicoline group spent more time immobile overall than participants in the placebo group (95% CI = 5.805–74.063 minutes, difference = 39.933 minutes).
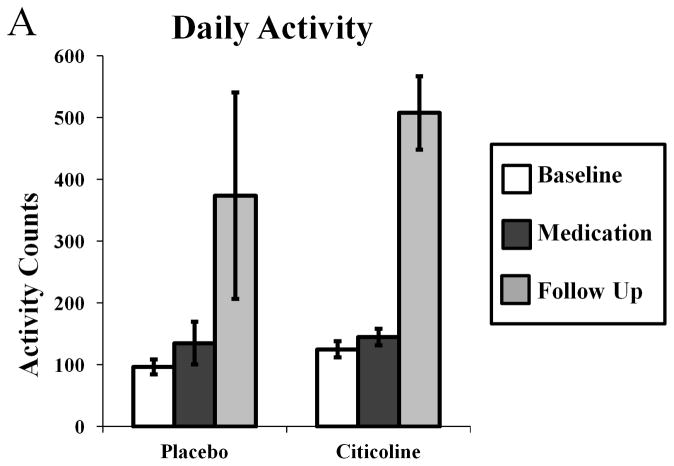
Because data from several of the sleep parameters were highly variable, and during several nights participants did not sleep at all, total daily activity was assessed. As with sleep parameters, there was no effect of citicoline treatment on daily activity (Fig 3A; phase by medication F(2,29) = 0.634, p = 0.538). There was an effect of study phase on total daily activity (Fig 3A; F(2,29) = 18.805, p < 0.001), with pairwise comparisons indicating participants in both groups moved more during the follow up phase than during both the baseline phase (p < 0.001, 95% CI = 178.961–481.187 activity counts, difference = 330.074 activity counts) and the medication phase (p < 0.001, 95% CI = 149.800–452.026 activity counts, difference = 300.913 activity counts).
Figure 3.
Effects of citicoline on average total daily activity (A) assessed using wrist actigraphy.
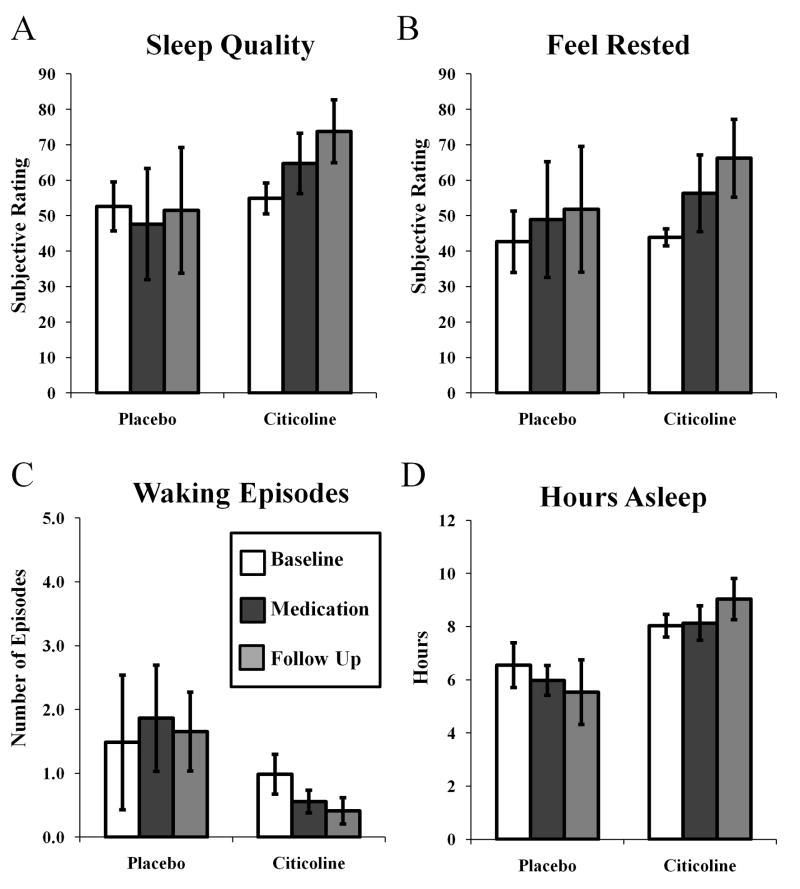
Along with bedtimes and rise times, daily diaries also recorded several subjective ratings of sleep quality and quantity. As with sleep parameters measured with actigraphy, citicoline treatment did not affect subjective measures of sleep. There was no effect of citicoline treatment on subjective ratings of sleep quality (Fig 4A; phase by medication F(2,24) = 0.082, p = 0.922), subjective ratings of feeling rested (Fig 4B; phase by medication F (2,24) = 0.0006, p = 0.994), subjective number of waking episodes (Fig 4C; phase by medication F (2,23) = 0.236, p = 0.792), or subjective number of hours slept (Fig 4D; phase by medication F (2,22) = 1.719, p = 0.202). There was an effect of treatment group on subjective ratings of sleep quality (Fig 4A; F(1,24) = 4.416, p < 0.05) with participants in the citicoline group overall rating sleep quality better than participants in the placebo group (p < 0.05, 95% CI = 0.330–36.576, difference = 18.453).
Figure 4.
Effects of citicoline on subjective ratings of sleep quality (A), feeling rested (B), number of waking episodes (C), and hours asleep (D).
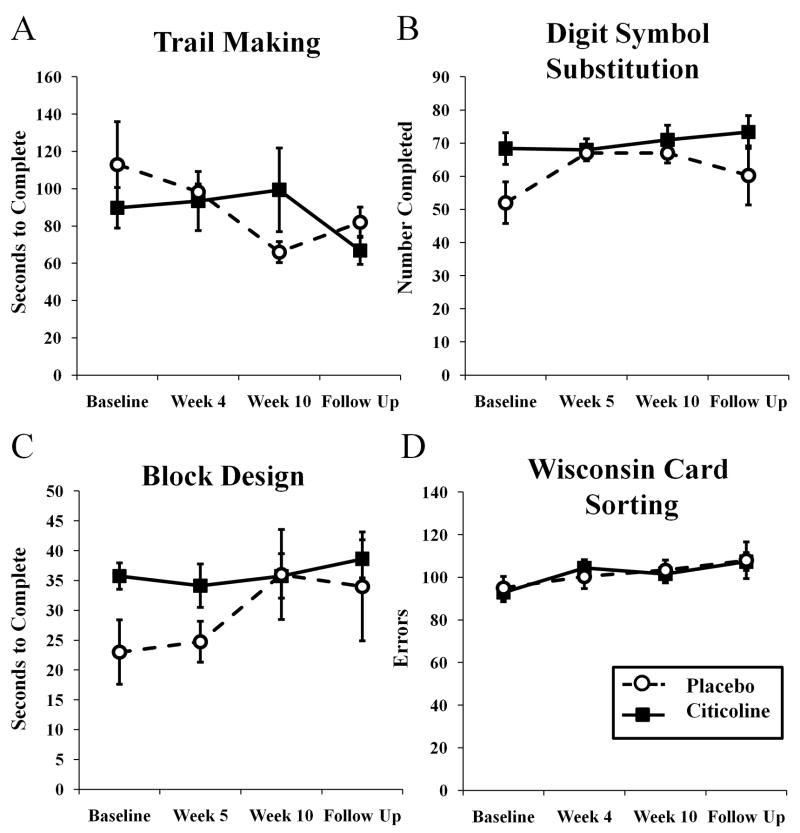
Finally, citicoline treatment had no effect on cognitive function as assessed in this study. There was no effect of treatment on performance on the Trail Making Test part B (Fig 5A; F(3,29) = 0.954, p = 0.424), on the Digit Symbol Substitution Test (Fig 5B; F (3,38) = 0.833, p = 0.484), the Block Design Task (Fig 5C; F (3,39) = 0.701, p = 0.557), or the Wisconsin Card Sorting Task (Fig 5D; F(3,38) = 0.124, p = 0.945). There was an effect of treatment group on performance on the Digit Symbol Substitution Task (Fig 5B, F(1,38) = 4.645, p < 0.05),with participants in the citicoline group overall completing more pairs in two minutes than participants in the placebo group (p < 0.05, 95% CI = 0.523–16.727 pairs, difference = 8.625) .
Figure 5.
Effects of citicoline on performance on the trail making task (A), the digit symbol substitution task (B), the block design task (C), and the Wisconsin card sorting task (D).
Because of the unequal number of participants in the placebo and citicoline groups, a within-subjects, post hoc analysis on data only from participants in the citicoline group was conducted with similar results to analysis containing all participants. There was no effect of citicoline treatment on standard parameters of sleep, measures of sleep quality, or measures of quantity obtained from actigraphy data. There was no effect of citicoline treatment on subjective measures of sleep or cognitive function as assessed in this study. For a subset of variables (number of waking episodes (Fig 2A; F(1,6) = 60.154, p < 0.001), the time spent awake per episode (Fig 2C; F(1,6) = 13.842, p < 0.05), the amount of time spent mobile (Fig 2E; F (1,6) = 12.556, p < 0.05), the number of sleep episodes (Fig 2B; F(1,6) = 63.158, p < 0.001), the amount of time spent immobile (Fig 2F; F(1,6) = 32.839, p < 0.01), total daily activity (Fig 3A; F(1,7) = 48.761, p < 0.001), subjective ratings of number of waking episodes (Fig 4C; F(1,4) = 11.274, p < 0.05), subjective ratings of the time spent asleep (Fig 4D; F(1,4) = 8.615, p < 0.05), and the Wisconsin Card Sorting Task (Fig 5D; F(1,7) = 7.515, p < 0.05), there was an effect of phase. However, when pairwise comparisons were run, each variable showed no significant differences between any of the phases (subjective ratings of number of waking episodes, subjective ratings of time spent asleep, and the Wisconsin Card Sorting Task) or only the follow-up phase was significantly different with no change between baseline and citicoline treatment periods (number of waking episodes p = 0.445; time awake per episode p = 0.445, time spent mobile p = 1.00, number of sleep episodes p = 0.551, time spent immobile p = 0.958, total daily activity p = 0.533).
Because much of the effect of cocaine abstinence on sleep is dependent on the length of abstinence, data from the citicoline treatment phase was separated into early and late periods. Analyses on the sleep/wake data separated in this way yielded similar results to all previous analyses. There was no difference between baseline and early or late treatment phases for any variable. For a subset of variables (number of waking episodes F(3,27) = 5.459, p = 0.005, time awake per episode F(3,27) = 33.326, p = 0.000, time spent mobile F(3,27) = 7.181, p = 0.001, number of sleep episodes F(3,27) = 5.306, p = 0.005, and time asleep per episode F(3,27) = 6.963, p =0.001) there was an effect of phase. However, as with previous analyses when pairwise comparisons were run, only the follow-up phase was significantly different (p = 1.00 for all other pairwise comparisons).
4. DISCUSSION
Taken together, our data demonstrate that in cocaine-dependent individuals who are not abstinent from cocaine, citicoline treatment has no effect on any of the measured parameters of sleep quality or quantity. Sleep onset latency, sleep efficiency, and waking after sleep onset were all similar during placebo and citicoline treatment periods. Citicoline did not increase nighttime wakefulness or movement, measured by the number of waking episodes per night, the time awake per episode, and the number of moving minutes per night. Finally, treatment with citicoline did not affect sleep quantity as measured by the number of sleep episodes, the time asleep per episode, and the amount of time spent immobile. Similarly, citicoline did not affect participants’ activity throughout the day or subjective ratings of sleep quality and quantity. Our original hypothesis was that cocaine abstinence would occur, but since there was no abstinence, it is reasonable that there was no change in sleep across the treatment period, either when separated into segments or collapsed across the entire treatment period.
There was an effect of study phase on the number of waking episodes, the time spent moving, the number of sleep episodes, the time spent immobile, and on total daily activity scores. It is unclear why these sleep variables were different during the follow-up phase, however this change occurred in both the citicoline and placebo treatment groups and so was not likely due to citicoline treatment.
Although the sleep data were obtained via objective means (wrist actigraphy), participants did provide self-reports of their bed times and wake times. It is possible that the large variability in this study was due to participant errors on the daily diaries. However, total daily activity, which does not rely on reports from participants, was also quite variable. Further, when sleep quality data collected in this study are compared to sleep quality data in other studies conducted in this lab, it is clear that sleep is highly disturbed (lower sleep efficiency, longer sleep latency, less total sleep time) in these cocaine-dependent participants, regardless of treatment group. So it is unlikely that the variability in the data is due to participant errors in filling out the daily diaries. It is more likely that in this population of cocaine-dependent participants, sleep and activity are both disrupted and variable. A second limitation is that nighttime polysomnography was not recorded, and sleep architecture was not assessed. So, it is not known if citicoline treatment affects the timing or length of sleep stages. As any effect that citicoline treatment may have on sleep is an important safety concern, it would be interesting to assess details of sleep architecture during citicoline treatment. However, requiring participants to spend several nights sleeping at the laboratory would have affected the drug using and behavioral patterns of the participants. Thus, assessment of sleep using the wrist actigraphy device permitted us to continually assess sleep in participants’ home environments for the entire twelve weeks of the study.
This study shows that citicoline treatment in non-abstinent cocaine-dependent individuals does not affect sleep. Because participants did not maintain abstinence during any phase of the study (See Table 1 for details), these data indicate that within a population of current cocaine (and other drug) users, citicoline does not further perturb or normalize sleep parameters. Additional studies will be required to assess its effects, if any, on sleep within a cocaine dependent population during cocaine abstinence.
Acknowledgments
This research was supported by NIDA grants DA011098, T32 DA15036, and K05DA00343. We thank Ronna Shostak, Carol Buchanan, and Barbara Beake for administrative support in conducting these studies.
Footnotes
Clinical Trial Identification Number: NCT00158249
ETR is currently in the Department of Psychology, Ohio State University, Columbus, Ohio, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alvarez XA, Mouzo R, Pichel V, Perez P, Laredo M, Fernandez-Novoa L, Corzo L, Zas R, Alcaraz M, Secades JJ, Lozano R, Cacabelos R. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer's disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find Exp Clin Pharmacol. 1999;21:633–644. [PubMed] [Google Scholar]
- Baskaya MK, Dogan A, Rao AM, Dempsey RJ. Neuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injury. J Neurosurg. 2000;92:448–452. doi: 10.3171/jns.2000.92.3.0448. [DOI] [PubMed] [Google Scholar]
- Brown ES, Gorman AR, Hynan LS. A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. J Clin Psychopharmacol. 2007;27:498–502. doi: 10.1097/JCP.0b013e31814db4c4. [DOI] [PubMed] [Google Scholar]
- Calatayud Maldonado V, Calatayud Perez JB, Aso Escario J. Effects of CDP-choline on the recovery of patients with head injury. J Neurol Sci. 1991;103(Suppl):S15–18. doi: 10.1016/0022-510x(91)90003-p. [DOI] [PubMed] [Google Scholar]
- Chida N, Shimizu Y. Biosynthesis of myelin lipids of cultured nervous tissues--incorporation of choline and CDP-choline into myelin phospholipids. Tohoku J Exp Med. 1973;111:41–49. doi: 10.1620/tjem.111.41. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31:171–176. doi: 10.1358/mf.2009.31.3.1364241. [DOI] [PubMed] [Google Scholar]
- Clark WM, Wechsler LR, Sabounjian LA, Schwiderski UE. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–1602. doi: 10.1212/wnl.57.9.1595. [DOI] [PubMed] [Google Scholar]
- Davalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, Lopez S, Cobo E, Warach S, Sherman D, Clark WM, Lozano R. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–2857. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- Drago F, Mauceri F, Nardo L, Valerio C, Genazzani AA, Grassi M. Effects of cytidine-diphosphocholine on acetylcholine-mediated behaviors in the rat. Brain Res Bull. 1993;31:485–489. doi: 10.1016/0361-9230(93)90113-p. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) New York, NY: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Galletti P, De Rosa M, Cotticelli MG, Morana A, Vaccaro R, Zappia V. Biochemical rationale for the use of CDPcholine in traumatic brain injury: pharmacokinetics of the orally administered drug. J Neurol Sci. 1991;103(Suppl):S19–25. doi: 10.1016/0022-510x(91)90004-q. [DOI] [PubMed] [Google Scholar]
- Hurtado O, Cardenas A, Pradillo JM, Morales JR, Ortego F, Sobrino T, Castillo J, Moro MA, Lizasoain I. A chronic treatment with CDP-choline improves functional recovery and increases neuronal plasticity after experimental stroke. Neurobiol Dis. 2007;26:105–111. doi: 10.1016/j.nbd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Roehrs T, Schuh K, Warbasse L. The effects of cocaine on mood and sleep in cocaine-dependent males. Exp Clin Psychopharmacol. 1999;7:338–346. doi: 10.1037//1064-1297.7.4.338. [DOI] [PubMed] [Google Scholar]
- Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(Suppl):S39–42. doi: 10.1016/0022-510x(91)90007-t. [DOI] [PubMed] [Google Scholar]
- Licata SC, Penetar DM, Ravichandran C, Rodolico J, Palmer C, Berko J, Geaghan T, Looby A, Peters E, Ryan E, Renshaw PF, Lukas SE. Effects of daily treatment with citicoline: A double-blind, placebo-controlled study in cocaine-dependent volunteers. Journal of Addiction Medicine. 2011;5:57–64. doi: 10.1097/ADM.0b013e3181d80c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Kouri EM, Rhee C, Madrid A, Renshaw PF. Effects of short-term citicoline treatment on acute cocaine intoxication and cardiovascular effects. Psychopharmacology (Berl) 2001;157:163–167. doi: 10.1007/s002130100824. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Cocaine and sleep: early abstinence. ScientificWorldJournal. 2007;7:223–230. doi: 10.1100/tsw.2007.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2010;167:331–340. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep architecture, cocaine and visual learning. Addiction. 2008;103:1344–1352. doi: 10.1111/j.1360-0443.2008.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharrof AH, Petkov VD, Petkov VV. Effects of meclofenoxate and citicholine on learning and memory in aged rats. Acta Physiol Pharmacol Bulg. 1987;13:17–24. [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Phillips/Respironics. Actiware and Actiware CT Software and Hardware Manual. Murrysville, PA USA: Respironics, Inc; 2009. [Google Scholar]
- Renshaw PF, Daniels S, Lundahl LH, Rogers V, Lukas SE. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology (Berl) 1999;142:132–138. doi: 10.1007/s002130050871. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Peretti FJ, Adams V, Schmunk GA, Kalasinsky KS, Ang L, Mamalias N, Turenne SD, Kish SJ. Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug Alcohol Depend. 2002;67:73–79. doi: 10.1016/s0376-8716(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Secades JJ, Frontera G. CDP-choline: pharmacological and clinical review. Methods Find Exp Clin Pharmacol. 1995;17(Suppl B):1–54. [PubMed] [Google Scholar]
- Silveri MM, Dikan J, Ross AJ, Jensen JE, Kamiya T, Kawada Y, Renshaw PF, Yurgelun-Todd DA. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. 2008;21:1066–1075. doi: 10.1002/nbm.1281. [DOI] [PubMed] [Google Scholar]
- Spiers PA, Hochanadel G. Citicoline for traumatic brain injury: report of two cases, including my own. J Int Neuropsychol Soc. 1999;5:260–264. doi: 10.1017/s1355617799533092. [DOI] [PubMed] [Google Scholar]
- Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Arch Neurol. 1996;53:441–448. doi: 10.1001/archneur.1996.00550050071026. [DOI] [PubMed] [Google Scholar]
- Teather LA, Wurtman RJ. Dietary cytidine (5')-diphosphocholine supplementation protects against development of memory deficits in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:711–717. doi: 10.1016/S0278-5846(03)00086-1. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38:831–836. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- Watson R, Bakos L, Compton P, Gawin F. Cocaine use and withdrawal: the effect on sleep and mood. Am J Drug Alcohol Abuse. 1992;18:21–28. doi: 10.3109/00952999209001608. [DOI] [PubMed] [Google Scholar]
- Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56:637–660. doi: 10.1016/0024-3205(94)00427-t. [DOI] [PubMed] [Google Scholar]