Abstract
A computation docking study of the highly potent, non-nitrogen containing, acetylcholinesterase inhibitor (+)-Arisugacin A is presented. The model suggests that (+)-arisugacin A is a dual binding site covalent inhibitor of AChE. These findings are examined in the context of Alzheimer's disease-modifying therapeutic design. (+)-Arisugacin A's revealed mode of action is unique, and may serves as a basis for the development of AD therapeutics capable of treating the symptomatic aspects of AD, while being neuroprotective with long term efficacy.
Keywords: Arisugacin A, Acetylcholinesterase inhibitor, Alzheimer's disease, Dual binding site, Covalent inhibitor
Alzheimer's disease [AD]1 affects well over 24 million people worldwide.2–3 It is one of the most degenerative and devastating syndromes affecting mostly the geriatric population, causing loss of memory and cognitive abilities. The exact pathogenic mechanism of AD still remains unknown, however tremendous progress has been made in understanding the biochemical events involved. Through this understanding, targets and theories have emerged as to how to best combat this disease. The current leading theory in disease-modifying therapeutic research is the amyloid cascade hypothesis focusing on β-amyloid peptide (Aβ), a primary component of the neurotoxic senile plaques that are a major hallmark of AD.4 These efforts focus on inhibiting Aβ production, preventing Aβ aggregation, or altering Aβ metabolism and clearance.5 The long standing cholinergic deficiency hypothesis3,6 has served almost exclusively as the rational basis in the development of known drugs (Figure 1). This hypothesis links the loss of acetylcholine, a neurotransmitter responsible for memory and cognitive functions, to AD. To maintain concentrations of acetylcholine in AD patients, current treatment involves the use of reversible and competitive inhibitors of acetylcholinesterase [AChE], a classical serine protease that catalyzes the hydrolysis of acetylcholine.6 Treatments using cholinesterase [ChE] inhibitors have been deemed symptomatic, and found to result in statistically significant but clinically marginal improvement in measures of cognition.7 However there have been a number of reports stemming from clinical trials that differential efficacies of therapeutics are not solely accounted for by differences in ChE inhibitory activities, suggestive of secondary modes of action of these therapeutics.6b,8–10
Figure 1.
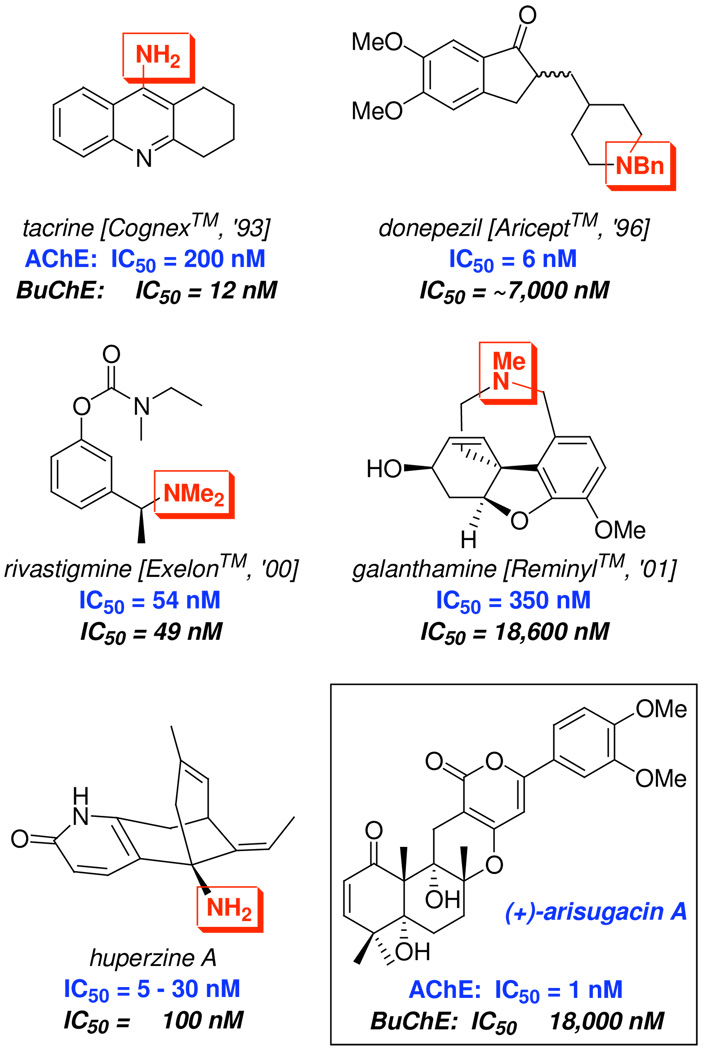
Five AChE inhibitors approved as therapeutic drugs for combating dementia, and (+)-arisugacin A.
Recent findings show the cholinergic and amyloid hypotheses are not independent of one another. Namely, acetylcholine activation of the muscarinic receptors leads indirectly to increases in the non-amyloidogenic β-secretase processing of Aβ precursor protein (APP),11 thus linking AChE inhibition with the diversion of APP away from Aβ formation. Another key connection has been the identification and mechanistic elucidation of the pro-aggregating action of AChE on Aβ.12 This secondary activity of AChE involves the enzyme's peripheral anionic site (PAS) located at the entrance of the catalytic gorge. AChE inhibitors able to bind to the PAS have been found to inhibit this pro-aggregating activity.12a These connections point to the disease-modifying potential of AChE inhibitors.
In light of these findings there has been a re-emerging interest in AChE inhibitor-based drug development, and their extension to molecules that serve to modulate both the cholinergic and amyloid targets. Many efforts have focused on designing dual binding site inhibitors that maintain or improve ACh-hydrolysis inhibition while adding or improving PAS binding, leading to improved inhibition of Aβ pro-aggregating activity of AChE.13,14 Dual binding site inhibitors of AChE have also been designed to concomitantly inhibit β-secretase (BACE-1), the enzyme involved in the rate-limiting step of APP processing into Aβ.15 Furthermore, the understanding that AChE colocalizes with AB has lead to the design of AChE inhibitors with the added ability to chelate or release metal ion chelators, ions also implicated in Aβ aggregation and neurotoxicity.16 The development of these multi-target-directed ligands holds great promise in development of AD therapeutics capable of targeting early stages of the neurotoxic cascade as well as mitigating the cholinergic deficit.
With the value of AChE modulation clearly established, we were inspired to continue pursuing the understanding of the biological activity of the natural product (+)-arisugacin A. (+)-Arisugacin A [box in Figure 1], isolated from Penicillium Sp. Fo-4259 by Õmura, is a highly potent inhibitor of AChE with an IC50 of 1 nM.17 The inhibitory activity of (+)-arisugacin A is also highly selective for AChE since > 18,000 nM is required to achieve the desired inhibition of a structurally related serine protease, butyrylcholinesterase [BuChE]. What is most intriguing about (+)-arisugacin A's AChE inhibition is that the molecule lacks a quaternizable nitrogen atom. Of the five inhibitors of AChE approved as therapeutic drugs for combating dementia [Figure 1]: Tacrine, donepezil, huperzine A, rivastigimine, and galanthamine all contain at least one quaternizable nitrogen atom.18 We recognized that this structural distinction of (+)-arisugacin A could lead to the potential discovery of a different mode of inhibitory action against AChE.
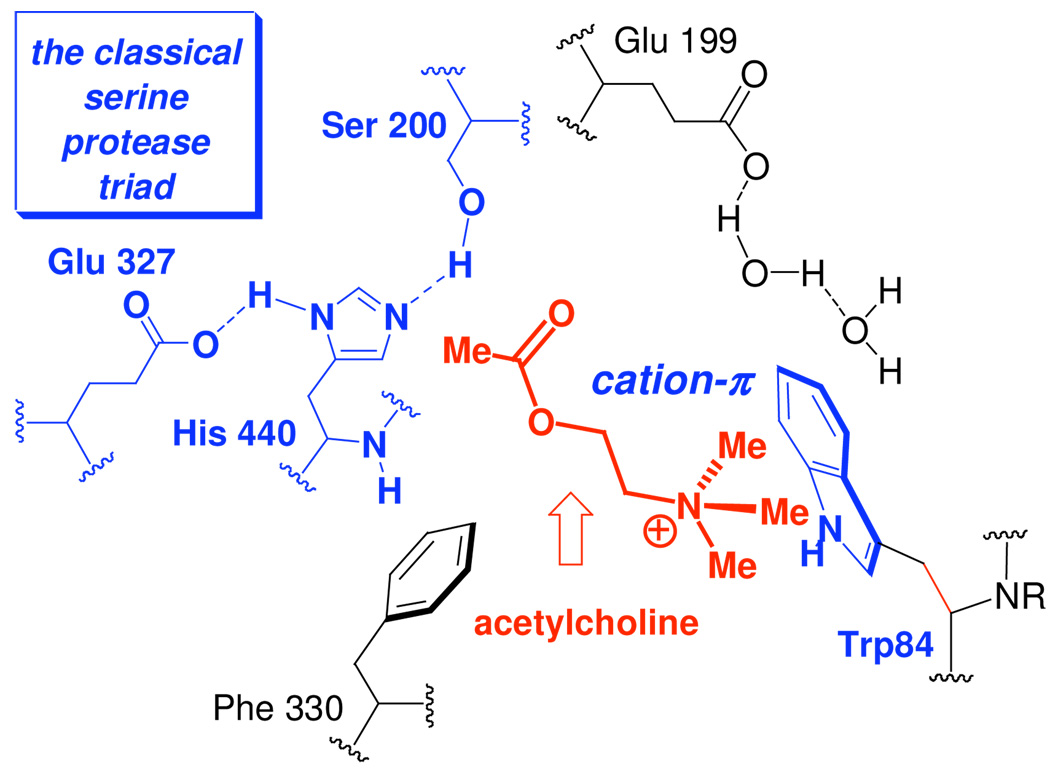
Effective AChE inhibitors should contain a nitrogen atom to mimic the quaternized nitrogen atom of acetylcholine in the binding to AChE [Figure 2]. At the active site of AChE, this quaternizable nitrogen atom interacts with the π-electrons of the indole ring of Trp84 electrostatically (residue numbering from the Torpedo Californica sequence will be used throughout).19 This cation-π interaction19 is the most significant factor for the binding of acetylcholine or other known inhibitors to AChE.
Figure 2.
The binding and active site of AChE.
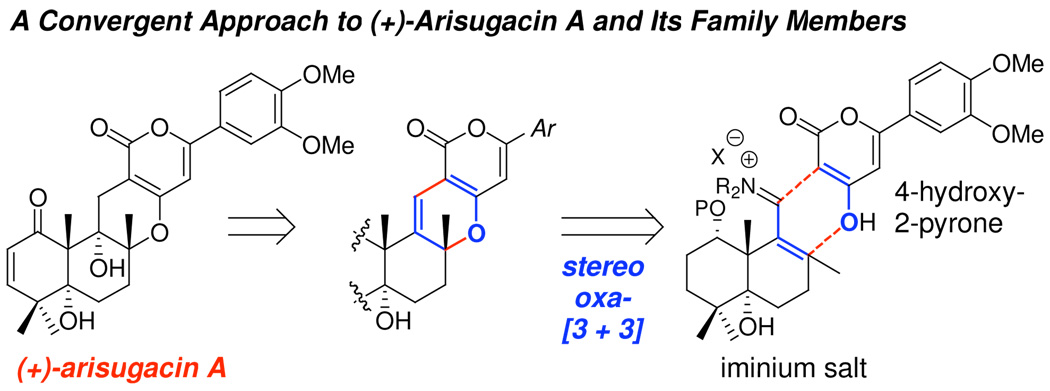
We made this unique structural observation and connection to the requisite cation-π interaction.20 This recognition is novel and has led to the initiation of a significant medicinal chemistry program in our group for therapeutic development of anti-Alzheimer's disease and related dementias with (+)-arisugacin A as a centerpiece. We have succeeded in our total synthesis21–23 of (+)-arisugacin A via oxa-[3 + 3]24 annulation [Figure 3]. Our convergent approach provides a facile and practical entry to (+)-arisugacin A and a diverse array of its structural analogs enabling key SAR studies to elucidate the mode of action as well as discover new leads. We have undertaken efforts in elucidation and comprehensive understanding of the mode of action through unprecedented computational docking. We communicate here our preliminary finding of computational evidence that (+)-arisugacin A is a dual binding site covalent inhibitor of AChE.
Figure 3.
Total synthesis of (+)-arisugacin A.
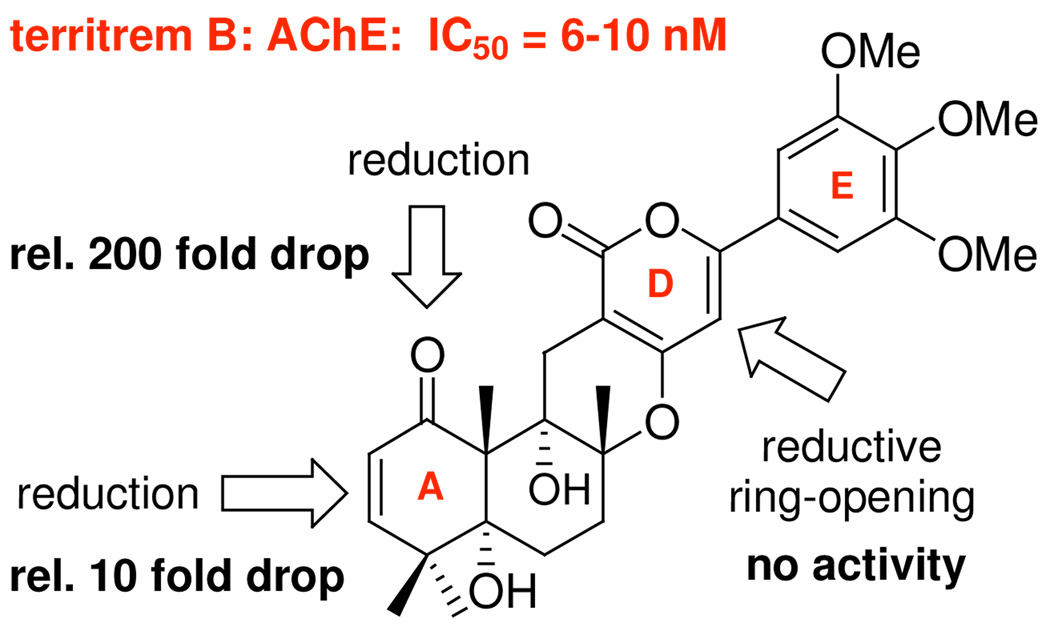
A Proposed Mode of Action. Although there are only limited structural activity relationship [SAR] studies,25,26 the structural significance of the αpyrone D-ring of territrem B [a structural relative of (+)-arisugacin A] in anti-cholinesterase activity has been reported by Peng25 [Figure 4]. While reduction of the enone motif in the A-ring produced some loss of activity, reductive ring-opening of the αpyrone D-ring led to a complete loss of activity.
Figure 4.
Established SARs for arisugacin-related territrem B.
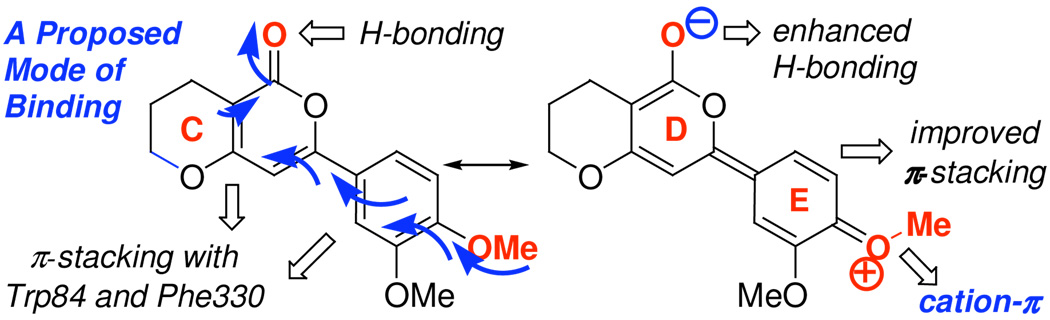
Based on these limited SAR data, while it appears that the A-ring could also be significant, we propose that the CDE portion of (+)-arisugacin A [Figure 5] is responsible for its binding to AChE. That is, the flat CDE-ring can potentially π–stack with Trp84 and Phe330 analogous to inhibitors such as tacrine, and the carbonyl oxygen of the αpyrone [D-ring] is capable of H-bonding with surrounding amino acids at the active site. In addition, the para-methoxy group on the E-ring can push electron density toward the α–pyrone carbonyl in the D-ring, thereby enhancing the H-bonding ability of the αpyrone carbonyl. The electron-deficiency of the E-ring can also improve its π–stacking ability and could elicit potential cation–π interactions.19 Hence, the D-ring along with the E-ring substituents could be critical for the binding of (+)-arisugacin A to AChE.
Figure 5.
Potential for π-stacking by the CDE portion of (+)-arisugacin A.
Docking of (+)-Arisugacin A. This study was powered by AutoDock v4.2 and the more recent version in AutoDock Vina,27 made available by The Scripps Research Institute under a General Public License. To ensure the accuracy of our docking experiments, we first compared several known crystal structures of ligand-bound AChE with their respective minimized docking structures using AutoDock. These successful trial runs involved Protein Data Bank’s data28 [www.pdb.org] obtained from co-crystallizations of AChE with known ligands donepezil, galanthamine, huperzine A, huperzine B, tacrine, and huprine X. Through these efforts it was identified that accurate prediction of receptor-ligand complexes were best obtained by allowing flexibility in residues Phe330, Trp279, and Asp72, holding the remainder of the enzyme rigid [see supplementary material].
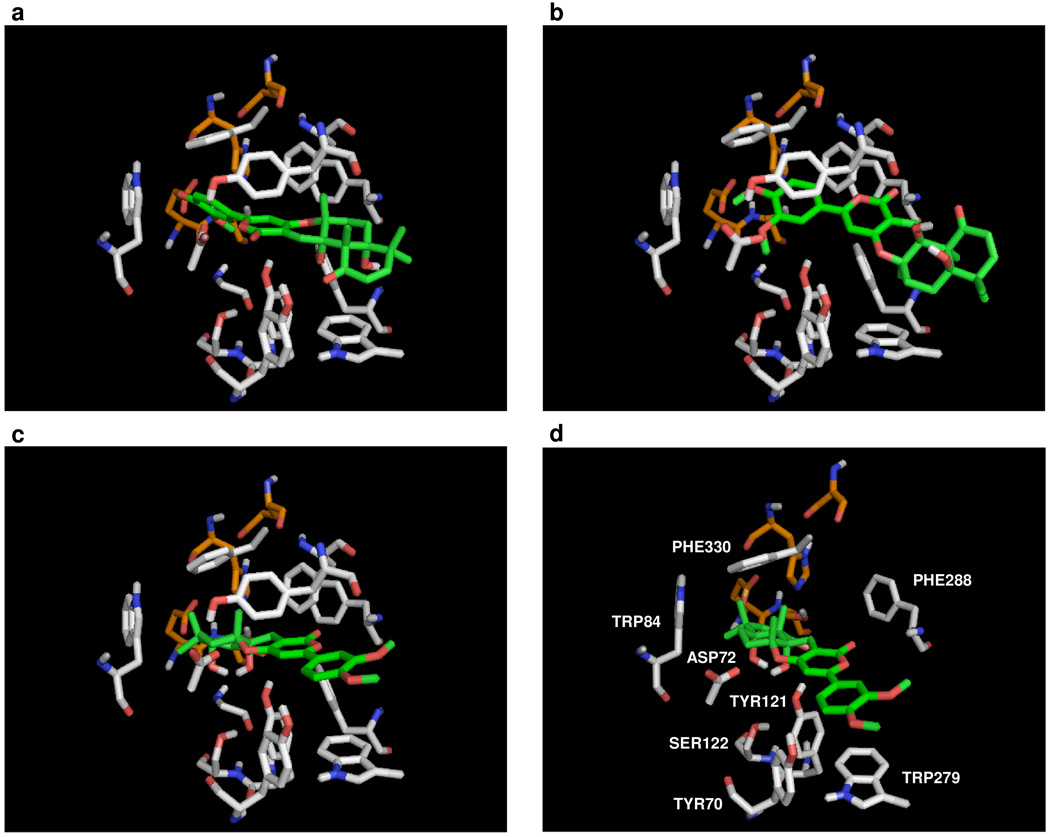
Consequently, computational docking studies of (+)-arisugacin A with AChE yielded three major binding modes at the enzyme’s binding/active site given that (+)-arisugacin A is a competitive inhibitor. As shown in Figure 6, the three most favored binding modes are presented along with the first shell of residues surrounding (+)-arisugacin A. Mode-I and Mode-II [a and b] place the A-ring enone moiety near the opening of the binding pocket. Mode-III places the enone deep into the binding pocket [c and d], next to the residues known to be involved in catalysis. The classical catalytic triad of serine proteases is shaded in orange in all four panels. Although Mode-I and Mode-II remain distinct possibilities, we chose to focus on binding Mode-III because of the following observations:
Figure 6.
Three major binding modes of (+)-arisugacin A at the active site of AChE: Mode-I [a]: 1st of 100 binding modes. Binding affinity = −11.8 kcal mol−1; Mode-II [b]: 2nd of 100 binding modes. Binding affinity = −10.7 kcal mol−1; Mode-III [c and d]: 3rd of 100 binding modes. Binding affinity = −10.4 kcal mol−1.
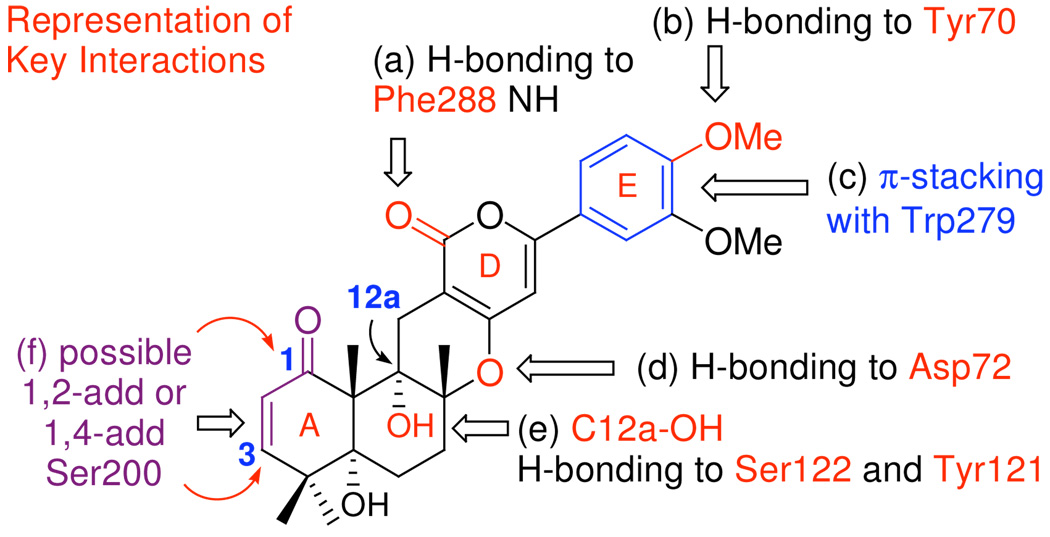
Firstly, close examinations of the first shell of residues surrounding (+)-arisugacin A in Mode-III reveal a subset of residues concluded to share in non-covalent interactions [Figure 6-d]. The signature interactions leading to high binding affinity of (+)-arisugacin A for AChE may include: i). H-bonding between the D-ring α–pyrone carbonyl and the Phe288-NH [for clarity see (a) in Figure 7]. ii). H-bonding between Tyr70 and the 4-OMe group of E-ring [(b)]. iii). A key π -stacking interaction between the E-ring and Trp279 [(c)]. iv). Potential H-bonding between residue Asp72 and the C-ring pyranyl oxygen [(d)]. v). Ser122, Tyr121, and C12-OH group may be involved in a H-bonding network bridged by water [(e)].
Figure 7.
(+)-Arisugacin A-AChE interactions seen in docking Mode-III.
All of these key interactions in Mode-III are consistent with the only SAR data available from Peng25 [Figure 4]. Furthermore, it is noteworthy that although Mode-III supports our preliminary assertion regarding the importance of the CDE-ring of (+)-arisugacin A, the π-stacking interaction is not with Trp84 and Phe330 as proposed or often seen in smaller molecules such as tacrine binding to AChE, but with Trp279.
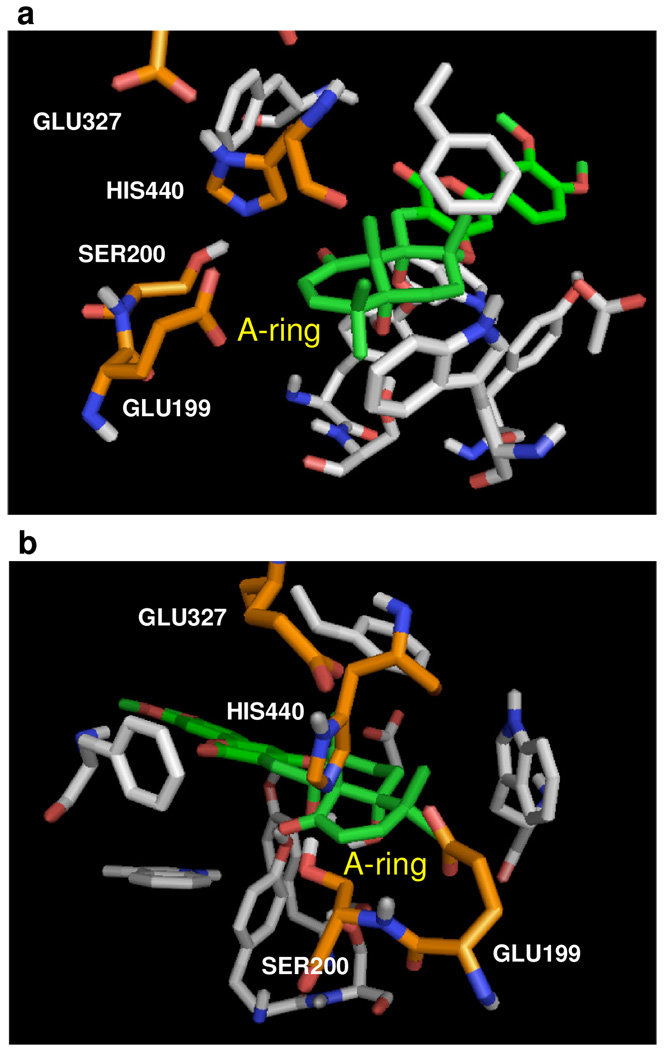
Secondly, and more significantly, a close up of Mode-III in the vicinity of the catalytic triad, His440, Ser200, and Glu327, uncovers a new and likely critical interaction also consistent with Peng’s observation that the enone in the A-ring is significant.25
Based on the two perspectives of (+)-arisugacin A bound in the active site [Mode-III, Figure 8], there is potential for the engagement of Ser200 with the A-ring enone through either 1,2-addition of Ser200-OH to the C1-carbonyl or 1,4-addition to C3, thereby strongly suggesting the mode of action involves reversible covalent inhibition [(f) in Figure 7]. This possibility may be dependent on the ability of (+)-arisugacin A to move within the binding pocket leading to C1- or C3 being closer to Ser200. Another interaction involving the A-ring to consider would be between the nitrogen lone pair on His440 with the C1-carbonyl with possible formation of the hemi-aminal.
Figure 8.
Close ups of Mode-III examining the vicinity of the catalytic triad (His440, Ser200, and Glu327) and Glu199 highlighted in orange.
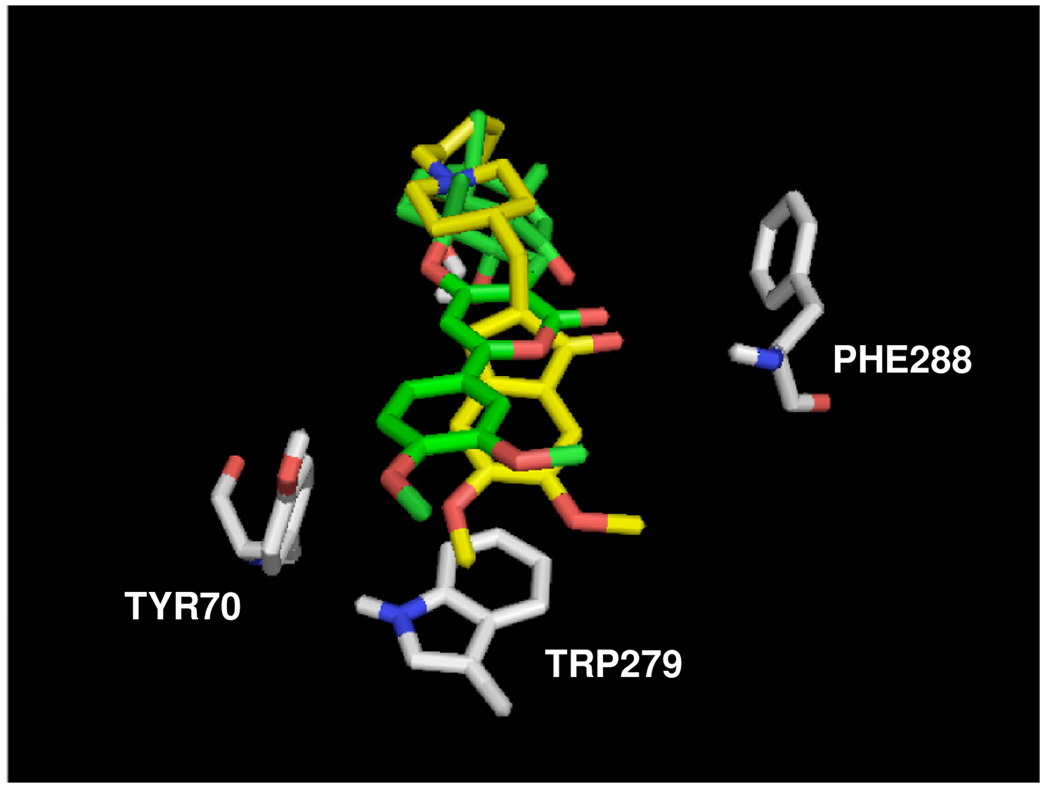
Lastly, we examined overlays of Mode-III of (+)-arisugacin A [in green] with crystal structures of inhibitor-bound AChE, an example of which comparing donepezil [in yellow] is shown here [Figure 9].28a The overlay reveals similarities between these docking events. They share an H-bond interaction between the Phe288 amide proton and the respective ligand carbonyls, a π-stack interaction between Trp279 and aromaticity in the respective ligands, and an H-bonding interaction between Tyr70 and the respective methoxy-aryl groups. These comparisons further support the choice of Mode-III being worthy of consideration in the understanding of (+)-arisugacin A's ability to inhibit AChE.
Figure 9.
Overlay of binding Mode-III of (+)-arisugacin A (green) and donepezil (yellow) bound to AChE.
The possibility that (+)-arisugacin A is a covalent modulator of AChE may have some important ramifications regarding the long-term efficacy of such a therapeutic. Reports emerging from clinical trials have identified a significant difference between AChE inhibitors that are rapidly reversible versus slowly reversible. Rapidly reversible inhibitors such as donepezil, tacrine, and galanthamine induce marked elevation in AChE activity and protein levels over time, whereas the slowly reversible inhibitor rivastigmine significantly decreases activity with no upregulation in protein levels.9,10 The clinical relevance of these findings is yet to be determined, however it is arguable that an escalation of AChE levels and therefore activity would lead to loss of therapeutic efficacy with regards to ameliorating the cholinergic deficit. Designs of long term neuroprotective therapies involving AChE should take this into account.
Binding Mode-III of (+)-arisugacin A strongly suggests that this entity is a dual binding site inhibitor of AChE. In the model, (+)-Arisugacin A's dimethoxyaryl group is clearly in the PAS of AChE. The key interaction with Trp279 is known to lead to the inhibition of the Aβ pro-aggregating activity of AChE.13 Furthermore, positioning of the dimethoxyaryl group at the opening of the catalytic gorge presents the opportunity to tether functionality that would add metal chelating or β-secretase (BACE-1) inhibitory activity.15,16
The combination of dual binding site modulation, and covalent inhibition by (+)-arisugacin A would be unique.14 A therapeutic designed in this way has the potential of treating the symptomatic aspects of AD, while being neuroprotective with long term efficacy. The wealth of information surrounding AChE, its biological activities, and its inhibitors presents new opportunities at tailoring molecules to satisfy both cholinergic and amyloid hypotheses. (+)-Arisugacin A has the potential of being a multi-target-directed ligand, and through our oxa-[3 + 3]24 annulation we hope to fully elucidate these modes of action as well as discover new therapeutic leads for Alzheimer's disease and related dementias.
Supplementary Material
Supplementary material presenting AChE docking model development and validation has been provided.
Acknowledgments
We thank the NIH [NS38049] for financial support, and The Scripps Research Institute for making available the AutoDock docking program and supporting literature.
References and notes
- 1.Alzheimer A. Gesamte Psychiatrie. 1907;64:1264. [Google Scholar]
- 2.For a census study on the prevalence of dimentia, see: Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Lancet. 2005;366:2112. doi: 10.1016/S0140-6736(05)67889-0.
- 3.For general reviews on therapeutic treatment of Alzheimer's disease, see: Giacobini E. In: Cognitive Enhancing Drugs. Buccafusco JJ, editor. Basel–Boston–Berlin: Birkhauser; 2004. pp. 11–36. Giacobini E, Spiegel R, Enz A, Veroff AE, Cutler RE. J. Neural Transm. 2002;109:1053. doi: 10.1007/s007020200089. Giacobini E, Becker R, editors. Alzheimer's Disease: From Molecular Biology to Therapy. Boston: Birkhauser; 1997. John V, Lieberburg I, Thorsett ED. Ann. Rep. Med. Chem. 1993;28:197.
- 4.For a review of the Amyloid Hypothesis, see: Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994.
- 5.For references on β-amyloid targeting, see: Melnikova I. Nat. Rev. Drug Discov. 2007;6:341. doi: 10.1038/nrd2314. Skovronsky DM, Lee VM-Y, Trojanowski JQ. Annu. Rev. Pathol. Mech. Dis. 2006;1:151. doi: 10.1146/annurev.pathol.1.110304.100113. Saido TC, Iwata N. Neurosci. Res. 2006;54:235. doi: 10.1016/j.neures.2005.12.015.
- 6.For leading references on the Cholinergic Hypothesis, see: Recanatini M, Cavalli A. Expert Opin. Ther. Pat. 2002;12:1853. Hogan DB, Patterson C. Can. J. Neurosci. 2002;29:306. doi: 10.1017/s031716710000216x. Farlow M. Int. Psychogeriatr. 2002;14:93. doi: 10.1017/s1041610203008688. Emre M. Int. J. Clin. Pract. 2002;64 For an earlier review, see: Perry EK. Br. Med. Bull. 1986;42:63. doi: 10.1093/oxfordjournals.bmb.a072100.
- 7.For recent reviews of clinical trials involving AChE inhibitors, see: Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Ann. Intern. Med. 2008;148:379. doi: 10.7326/0003-4819-148-5-200803040-00009. Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR. CMAJ. 2003;169:557.
- 8.For a comparison of the long-term effects of ChE inhibitors, see: Giacobini E. Neurochem. Res. 2003;28:515. doi: 10.1023/a:1022869222652.
- 9.For a clinical trial report comparing ChE inhibitor effects on ChE activities and protein levels, see: Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Eagle G, Lane R. Curr. Alz. Res. 2009;6:4. doi: 10.2174/156720509787313961.
- 10.For reviews of clinical trials comparing AChE inhibitor effects on AChE activity levels, see: Darreh-Shori T, Soininen H. Curr. Alz. Res. 2010;7:67. doi: 10.2174/156720510790274455. Lane RM, Kivipelto M, Greig NH. Clin. Neuropharmacol. 2004;27:141. doi: 10.1097/00002826-200405000-00011.
- 11.For a lead reference linking cholinergic activation with APP processing, see: Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Science. 1992;258:304. doi: 10.1126/science.1411529.
- 12.For lead references on AChE's ability to promote Aβ-aggregation, see: Inestrosa NC, Alvarez A, Pérez CA, Moreno RD, Vicente M, Linker C, Casanueva OI, Soto C, Garrido J. Neuron. 1996;16:881. doi: 10.1016/s0896-6273(00)80108-7. Alvarez A, Opazo C, Alarcón R, Garrido J, Inestrosa NC. J. Mol. Biol. 1997;272:348. doi: 10.1006/jmbi.1997.1245.
- 13.For reviews on dual binding site AChE inhibitors as disease-modifying therapeutics, see: Muñoz-Torrero D. Curr. Med. Chem. 2008;15:2433. doi: 10.2174/092986708785909067. Castro A, Martinez A. Curr. Pharm. Des. 2006;12:4377. doi: 10.2174/138161206778792985. Recanatini M, Valenti P. Curr. Pharm. Des. 2004;10:3157. doi: 10.2174/1381612043383313.
- 14.For a report of a covalent dual binding site inhibitor of AChE, see: Belluti F, Rampa A, Piazzi L, Bisi A, Gobbi S, Bartolini M, Andrisano V, Cavalli A, Recanatini M, Valenti P. J. Med. Chem. 2005;48:4444. doi: 10.1021/jm049515h.
- 15.For designs of AChE inhibitors also able to inhibit β-secretase (BACE-1), see: Zhu Y, Xiao K, Ma L, Xiong B, Fu Y, Yu H, Wang W, Wang X, Hu D, Peng H, Li J, Gong Q, Chai Q, Tang X, Zhang H, Li J, Shen J. Bioorg. Med. Chem. 2009;17:1600. doi: 10.1016/j.bmc.2008.12.067. Camps P, Formosa X, Galdeano C, Muñoz-Torrero D, Ramírez L, Gómez E, Isambert N, Lavilla R, Badia A, Clos MV, Bartolini M, Mancini F, Andrisano V, Arce MP, Rodriguez-Franco MI, Huertas O, Dafni T, Luque J. J. Med. Chem. 2009;52:5365. doi: 10.1021/jm900859q. Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F, Recanatini M, Andrisano V, Rampa A. Bioorg. Med. Chem. Lett. 2008;18:423. doi: 10.1016/j.bmcl.2007.09.100.
- 16.For designs of metal ion chelating or chelator releasing AChE inhibitors, see: Zheng H, Youdim MBH, Fridkin M. Chem. Neurosci. 2010;1:737. doi: 10.1021/cn100069c. Zheng H, Youdim MBH, Fidkin M. Chem. Bio. 2010;5:603. doi: 10.1021/cb900264w. Zheng H, Youdim MBH, Fidkin M. J. Med. Chem. 2009;52:4095. doi: 10.1021/jm900504c. Bolognesi ML, Cavalli A, Valgimigli L, Bartolini M, Rosini M, Andrisano V, Recanatini M, Melchiorre C. J. Med. Chem. 2007;50:6446. doi: 10.1021/jm701225u.
- 17.For leading references on arisugacins, see: Otoguro K, Shiomi K, Yamaguchi Y, Arai N, Sunazuka T, Masuma R, Iwai Y, Õmura S. J. Antibiot. 2000;53:50. doi: 10.7164/antibiotics.53.50. Otoguro K, Kuno F, Õmura S. Pharmacol. Ther. 1997;76:45. doi: 10.1016/s0163-7258(97)00093-4. and leading references cited within. For biological activities of arisugacin, see: Kuno F, Otoguro K, Shiomi K, Iwai Y, Õmura S. J. Antibiot. 1996;49:742. doi: 10.7164/antibiotics.49.742. Õmura S, Kuno F, Otoguro K, Sunazuka T, Shiomi K, Masuma R, Iwai Y. J. Antibiot. 1995;48:745. doi: 10.7164/antibiotics.48.745.
- 18.For tacrine, see: Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. N. Engl. J. Med. 1986;315:1241. doi: 10.1056/NEJM198611133152001. For donepezil, see: Inoue A, Kawai T, Wakita M, Iimura Y, Sugimoto H, Kawakami Y. J. Med. Chem. 1996;39:4460. doi: 10.1021/jm950596e. For huperzine A, see: Kozikowski AP, Campiani G, Nacci V, Sega A, Saxena A, Doctor BP. J. Chem. Soc., Perkin Trans. I. 1996;1287 Bai DL, Tang XC, He XC. Curr. Med. Chem. 2000;7:355. doi: 10.2174/0929867003375281. For total syntheses of huperzine A, see: Xia Y, Kozikowski AP. J. Am. Chem. Soc. 1989;111:4116. Qian L, Ji R. Tetrahedron Lett. 1989;30:2089. For rivastigimine, see: Ogura H, Kosasa T, Kuriya Y, Yamanishi Y. Meth. Find. Exp. Clin. Pharmacol. 2000;22:609. doi: 10.1358/mf.2000.22.8.701373. For galanthamine, see: Fulton B, Benfield P. Drugs Aging. 1996;60 doi: 10.2165/00002512-199609010-00006.
- 19.For lead references on cation-π interactions and how they relate to AChE, see: Harel M, Quinn DM, Nair HK, Silman I, Sussman JL. J. Am. Chem. Soc. 1996;118:2340. Gallivan JP, Dougherty DA. J. Am. Chem. Soc. 2000;122:870. Gallivan JP, Dougherty DA. Proc. Natl. Acad. Sci. (USA) 1999;96:9459. doi: 10.1073/pnas.96.17.9459. Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, Dougherty DA. Proc. Natl. Acad. Sci. (USA) 1998;95:12088. doi: 10.1073/pnas.95.21.12088. Ma JC, Dougherty DA. Chem. Rev. 1997;97:1324. doi: 10.1021/cr9603744. Dougherty DA. Science. 1996;271:163. doi: 10.1126/science.271.5246.163.
- 20. Douglas CJ, Sklenicka HM, Shen HC, Golding GM, Mathias DS, Degen SJ, Morgan CD, Shih RA, Mueller KL, Seurer LM, Johnson EW, Hsung RP. Tetrahedron. 1999;55:13683. Also see: Degen SJ, Mueller KL, Shen HC, Mulder JA, Golding GM, Wei L-L, Zificsak CA, Neeno-Eckwall A, Hsung RP. Bioorg. Med. Chem. Lett. 1999;9:973. doi: 10.1016/s0960-894x(99)00115-8.
- 21.For Õmura's total synthesis of arisugacin A, see: Sunazuka T, Handa M, Nagai K, Shirahata T, Harigaya Y, Otoguro K, Kuwajima I, Õmura S. Tetrahedron. 2004;60:7845. Sunazuka T, Handa M, Nagai K, Shirahata T, Harigaya Y, Otoguro K, Kuwajima I, Õmura S. Org. Lett. 2002;4:367. doi: 10.1021/ol017046x.
- 22.For our total synthesis of arisugacin A, see Hsung RP. J. Org. Chem. 1997;62:7904. doi: 10.1021/jo971479p. Zehnder LR, Hsung RP, Wang J, Golding GM. Angew. Chem. Int. Ed. 2000;39:3876. doi: 10.1002/1521-3773(20001103)39:21<3876::AID-ANIE3876>3.0.CO;2-C. Wang J, Cole KP, Wei L-L, Zehnder LR, Hsung RP. Tetrahedron Lett. 2002;43:3337. Cole KP, Hsung RP, Yang X-F. Tetrahedron Lett. 2002;43:3341. Cole KP, Hsung RP. Tetrahedron Lett. 2002;43:8791. Hsung RP, Cole KP, Zehnder LR, Wang J, Wei L-L, Yang X-F, Coverdale HA. Tetrahedron. 2003;59:311.
- 23.For reviews on the synthesis of arisugacin A, see: Hsung RP, Cole KP. In: Strategies and Tactics in Organic Synthesis. Harmata M, editor. Vol. 4. Oxford, UK: Elsevier Science, Pergamon Press; 2004. pp. 41–70. Sunazuka T, Õmura S. Chem. Rev. 2005;105:4559. doi: 10.1021/cr040628i.
- 24.For reviews on [3 + 3] annulation: Buchanan GS, Feltenberger JB, Hsung RP. Curr. Org. Syn. 2010;7:363. doi: 10.2174/157017910791414490. Harrity JPA, Provoost O. Org. Biomol. Chem. 2005;3:1349. doi: 10.1039/b502349c. Hsung RP, Kurdyumov AV, Sydorenko N. Eur. J. Org. Chem. 2005;1:23.
- 25.Peng F-C. J. Nat. Prod. 1995;58:857. doi: 10.1021/np50120a006. [DOI] [PubMed] [Google Scholar]
- 26.For assaying of fragments of arisugacin A, see: Hua DH, Chen Y, Sin H-S, Robinson PD, Meyers CY, Perchellet EM, Perchellet J-P, Chiang PK, Biellmann J-P. Acta Cryst. 1999;C55:1698. doi: 10.1107/s0108270199007301. Hua DH, Chen Y, Sin H-S, Maroto MJ, Robinson PD, Newell SW, Perchellet EM, Ladesich JB, Freeman JA, Perchellet J-P, Chiang PK. J. Org. Chem. 1997;62:6888.
- 27.For references on AutoDock, see: Version 4.2 Morris G, Goodsell D, Halliday R, Huey R, Hart W, Belew R, Olson AJ. J. Comp. Chem. 1998;19:1639. Vina Trott O, Olson AJ. J. Comp. Chem. 2010;31:455. doi: 10.1002/jcc.21334.
- 28.For X-ray crystal structure data, see: Donepezil Kryger G, Silman I, Sussman JL. Structure Fold. Des. 1999;7:297. doi: 10.1016/s0969-2126(99)80040-9. Galanthamine Greenblatt HM, Kryger G, Lewis TT, Silman I, Sussman JL. FEBS Lett. 1999;463:321. doi: 10.1016/s0014-5793(99)01637-3. Huperzines A and B Dvir H, Jiang HL, Wong DM, Harel M, Chetrit M, He XC, Jin GY, Yu GL, Tang XC, Silman I, Bai DL, Sussman JL. Biochemistry. 2002;41:10810. doi: 10.1021/bi020151+. Wong DM, Greenblatt HM, Dvir H, Carlier PR, Han YF, Pang YP, Silman I, Sussman JL. J. Am. Chem. Soc. 2003;125:363. doi: 10.1021/ja021111w. Rivastigmine Bar-on P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Biochemistry. 2002;41:3555. doi: 10.1021/bi020016x. Tacrine Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Proc. Natl. Acad. Sci. USA. 1993;90:9031. doi: 10.1073/pnas.90.19.9031. Huprine X Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Muñoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. Biochemistry. 2002;41:2970. doi: 10.1021/bi011652i.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material presenting AChE docking model development and validation has been provided.