Abstract
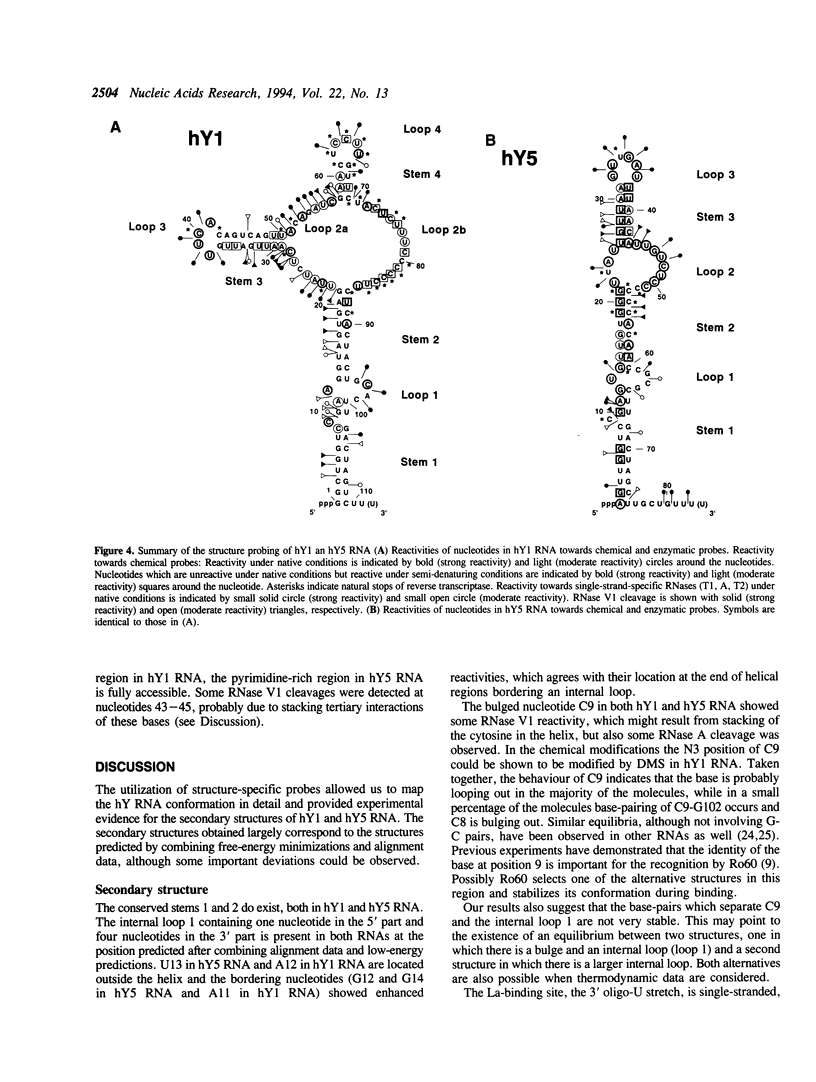
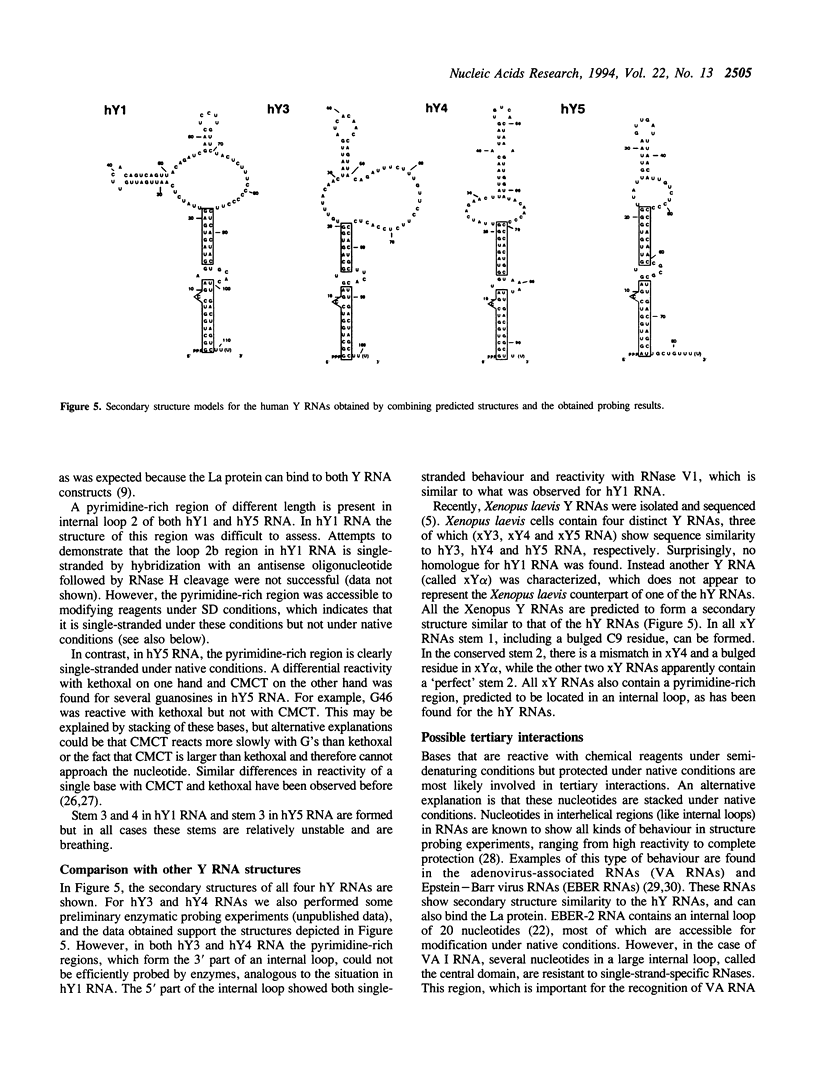
The secondary structures of human hY1 and hY5 RNAs were determined using both chemical modification techniques and enzymatic structure probing. The results indicate that both for hY1 and for hY5 RNA the secondary structure largely corresponds to the structure predicted by sequence alignment and computerized energy-minimization. However, some important deviations were observed. In the case of hY1 RNA, two regions forming a predicted helix appeared to be single-stranded. Furthermore, the pyrimidine-rich region of hY1 RNA appeared to be very resistant to reagents under native conditions, although it was accessible to chemical reagents under semi-denaturing conditions. This may point to yet unidentified tertiary interactions for this region of hY1 RNA. In the case of hY5 RNA, two neighbouring internal loops in the predicted structure appeared to form one large internal loop.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudin F., Ehresmann C., Romby P., Mougel M., Colin J., Lempereur L., Bachellerie J. P., Ebel J. P., Ehresmann B. Higher-order structure of domain III in Escherichia coli 16S ribosomal RNA, 30S subunit and 70S ribosome. Biochimie. 1987 Oct;69(10):1081–1096. doi: 10.1016/0300-9084(87)90008-3. [DOI] [PubMed] [Google Scholar]
- Brunel C., Romby P., Westhof E., Ehresmann C., Ehresmann B. Three-dimensional model of Escherichia coli ribosomal 5 S RNA as deduced from structure probing in solution and computer modeling. J Mol Biol. 1991 Sep 5;221(1):293–308. doi: 10.1016/0022-2836(91)80220-o. [DOI] [PubMed] [Google Scholar]
- Clemens M. J. The small RNAs of Epstein-Barr virus. Mol Biol Rep. 1993 Feb;17(2):81–92. doi: 10.1007/BF00996215. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington A. D. RNA ligands: out of shape but fir for recognition. Curr Biol. 1993 Jun 1;3(6):375–377. doi: 10.1016/0960-9822(93)90206-4. [DOI] [PubMed] [Google Scholar]
- Glickman J. N., Howe J. G., Steitz J. A. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus-infected cells. J Virol. 1988 Mar;62(3):902–911. doi: 10.1128/jvi.62.3.902-911.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Knapp G. Enzymatic approaches to probing of RNA secondary and tertiary structure. Methods Enzymol. 1989;180:192–212. doi: 10.1016/0076-6879(89)80102-8. [DOI] [PubMed] [Google Scholar]
- Krol A., Carbon P. A guide for probing native small nuclear RNA and ribonucleoprotein structures. Methods Enzymol. 1989;180:212–227. doi: 10.1016/0076-6879(89)80103-x. [DOI] [PubMed] [Google Scholar]
- Krol A., Westhof E., Bach M., Lührmann R., Ebel J. P., Carbon P. Solution structure of human U1 snRNA. Derivation of a possible three-dimensional model. Nucleic Acids Res. 1990 Jul 11;18(13):3803–3811. doi: 10.1093/nar/18.13.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakman J. H., Konings D. A., Hogeweg P., Pel H. J., Grivell L. A. Structural analysis of a group II intron by chemical modifications and minimal energy calculations. J Biomol Struct Dyn. 1990 Oct;8(2):413–430. doi: 10.1080/07391102.1990.10507813. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Silver S. L. Alternative tertiary structure attenuates self-cleavage of the ribozyme in the satellite RNA of barley yellow dwarf virus. Nucleic Acids Res. 1991 Oct 11;19(19):5313–5320. doi: 10.1093/nar/19.19.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moine H., Romby P., Springer M., Grunberg-Manago M., Ebel J. P., Ehresmann C., Ehresmann B. Messenger RNA structure and gene regulation at the translational level in Escherichia coli: the case of threonine:tRNAThr ligase. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7892–7896. doi: 10.1073/pnas.85.21.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Harley J. B. A subset of hY RNAs is associated with erythrocyte Ro ribonucleoproteins. EMBO J. 1990 Nov;9(11):3683–3689. doi: 10.1002/j.1460-2075.1990.tb07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C. A., Margelot K., Wolin S. L. Xenopus Ro ribonucleoproteins: members of an evolutionarily conserved class of cytoplasmic ribonucleoproteins. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7250–7254. doi: 10.1073/pnas.90.15.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'ery T., Mellits K. H., Mathews M. B. Mutational analysis of the central domain of adenovirus virus-associated RNA mandates a revision of the proposed secondary structure. J Virol. 1993 Jun;67(6):3534–3543. doi: 10.1128/jvi.67.6.3534-3543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R., Pruijn G. J., van der Kemp A. J., van Venrooij W. J. Subcellular distribution of Ro ribonucleoprotein complexes and their constituents. J Cell Sci. 1993 Nov;106(Pt 3):929–935. doi: 10.1242/jcs.106.3.929. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., Van Venrooij W. J. Structure and function of La and Ro RNPs. Mol Biol Rep. 1990;14(2-3):43–48. doi: 10.1007/BF00360410. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., van Venrooij W. J. Analysis of protein--RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991 Oct 11;19(19):5173–5180. doi: 10.1093/nar/19.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., Wingens P. A., Peters S. L., Thijssen J. P., van Venrooij W. J. Ro RNP associated Y RNAs are highly conserved among mammals. Biochim Biophys Acta. 1993 Dec 14;1216(3):395–401. doi: 10.1016/0167-4781(93)90006-y. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., van Venrooij W. J., Dathan N. A., Hamm J., Mattaj I. W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989 Dec 20;8(13):4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett D., Richer C., Deragon J. M., Labuda D. Alu RNA secondary structure consists of two independent 7 SL RNA-like folding units. J Biol Chem. 1991 May 15;266(14):8675–8678. [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Sturchler C., Westhof E., Carbon P., Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNA(Sec). Nucleic Acids Res. 1993 Mar 11;21(5):1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Secondary structure of the RNA component of a nuclear/mitochondrial ribonucleoprotein. J Biol Chem. 1990 Aug 5;265(22):13254–13262. [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Jaeger J. A., Turner D. H. A comparison of optimal and suboptimal RNA secondary structures predicted by free energy minimization with structures determined by phylogenetic comparison. Nucleic Acids Res. 1991 May 25;19(10):2707–2714. doi: 10.1093/nar/19.10.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stevenson I. L., Romby P., Baudin F., Brunel C., Westhof E., Ehresmann C., Ehresmann B., Romaniuk P. J. Structural studies on site-directed mutants of domain 3 of Xenopus laevis oocyte 5 S ribosomal RNA. J Mol Biol. 1991 May 20;219(2):243–255. doi: 10.1016/0022-2836(91)90565-n. [DOI] [PubMed] [Google Scholar]
- van Gelder C. W., Gunderson S. I., Jansen E. J., Boelens W. C., Polycarpou-Schwarz M., Mattaj I. W., van Venrooij W. J. A complex secondary structure in U1A pre-mRNA that binds two molecules of U1A protein is required for regulation of polyadenylation. EMBO J. 1993 Dec 15;12(13):5191–5200. doi: 10.1002/j.1460-2075.1993.tb06214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Slobbe R. L., Pruijn G. J. Structure and function of La and Ro RNPs. Mol Biol Rep. 1993 Aug;18(2):113–119. doi: 10.1007/BF00986765. [DOI] [PubMed] [Google Scholar]





