Abstract
There exists a growing demand for new technology that can take over the function of the human lung, from assisting an injured or recently transplanted lung to completely replacing the native organ. Many obstacles must be overcome to achieve the lofty goals and expectations of such a device. An artificial lung must be able to sustain the gas exchange requirements of a normal functioning lung. Pursuant to this purpose, the device must maintain appropriate blood pressure, decrease injury to blood cells and minimize clotting and immunologic response. Attachment methods vary, and, ideally, researchers want to find a way that minimizes bodily trauma, maximizes gas exchange and utilizes the inherent properties of the native lung. The currently proposed methods include the parallel, in-series and venous double-lumen cannula configurations. For the time being, current research focuses on the extracorporeal (i.e., outside the body) placement, but ultimate long-term goals look toward total implantation.
Key words: artificial lung, paracorporeal lung, lung transplant, mechanical ventilation, pulmonary function, organ replacement
Meeting the Need
The classic medical drama would be incomplete without the suspenseful mouth-to-mouth rescue scene, one person breathing for another to prolong life. Hardly a ventilator device in itself, the concept still plays an essential role in current thoracic studies: develop a means of respiration, outside of an individual's own power, that can assist or completely take over breathing for an impaired individual. Currently in use is the mechanical ventilator with a wide variety of techniques, calculations and adjustments aimed at improving its success. On the horizon is the mechanical or artificial lung, a device that is implantable in a human for respiratory support.
The number of individuals requiring a lung transplant is on the rise. Between 1997 and 2007 there was an 11% increase in the number of candidates on the lung transplant list.1 Coupled with the vast gap between those requiring lung transplants and the number of lungs available, the demand clearly outweighs the supply. Additionally, only 18% of the 13,154 lungs from organ donors were transplanted in 2006; 81% were not recovered.1 The reason for this discrepancy was cited as “poor organ function,” leading to an even greater disparity between needed and available lungs.1 As such, research has focused not merely on an artificial lung as a replacement organ, but rather an artificial lung as a bridge to transplantation.2,3 Additionally, a successful artificial lung could be used as a support device following transplant or as supplemental support to mechanical ventilation.2,4
Current Devices
Historically, artificial lung technology has been limited to its use in cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO). ECMO, however, has inherent complications. It is very time-consuming, needing large amounts of monitoring and personnel, and the device itself consists of tubes and cannulae that can rupture and lead to fatal results.5 ECMO therapy is nonambulatory and requires multiple transfusions; additional complications include infection, erythrocyte and platelet damage and embolization of tube fragments.2,5,6 For these reasons and others, the latest advances have diverted from ECMO therapy.
The intravascular oxygenator, IVOX, is one such alternative. Gas enters and leaves the system via conduits outside a small skin incision. The device, a membrane oxygenator, is placed in the vena cava and a vacuum pump pulls oxygen through the device fibers.6 Another version has replaced the vacuum pump with a pulsating balloon that allows for optimal blood mixing across the gas exchange fibers.7 Yet, IVOX does not have the surface area or the gas exchange capacity to meet the needs of a pre- or post-operative lung transplant patient.3,8
Another alternative choice is a high-flow cannula placed in the femoral artery and vein that creates a pumpless shunt. This method, deemed interventional lung assist, allows for complete CO2 removal, and, due to the device's location in the peripheral vasculature, the natural lung can still provide oxygenation within its capabilities.6 Conversely, the device will receive only partial cardiac output, which will limit its effectiveness, as both adequate cardiac output and arterial pressure are necessary. Additionally, interventional lung assist devices require patients with a stable hemodynamic condition.9 To increase hemodynamic stability, alternative cannulae locations have been proposed. Studies of the use of the axillary vessels show that, despite decreased blood flow, the gas exchange is comparable to that of traditional placement and improves hemodynamic stability.10
Some research has focused on repairing lung function not through technology but through cells. Much of the focus has been on the lung epithelium, the conduction pathway for air and the major defense system against inhaled irritants and toxins.11 During injury to the lung epithelium, repairs are made by existing cells that proliferate and differentiate to reconstitute the epithelium.11,12 Recent studies have focused on inducing this repair effect by introducing bone marrow or embryonic stem cells.12 In fact, researchers have been able to use special signaling molecules to derive lung epithelia from in vitro and in vivo embryonic stem cells.12 The goal in cellular studies is to repair lung injury not by mechanical means but by stimulating lung self-repair or re-growth through the use of stem cells.
The artificial lung seeks to be an even more viable alternative, a pumpless device attaching to pulmonic circulation that can support the metabolic functions of the lung, provide adequate gas exchange and be perfused entirely by the right ventricle. Key to the device is its use in an ambulatory setting, allowing for virtually no limitations on patient movement and action.13 Yet there are several functional obstacles that must be accounted for in order for the device to operate as intended.
Potential Obstacles
The gas exchange requirements in an adult can vary. Light exercise can increase the average 240 ml/min of required oxygen to 800 ml/min. Thus, even minor variations need to be accounted for when creating an artificial respiratory system. Current technology for gas exchange in artificial lung prototypes uses porous fibers.2 Blood flows over the fibers while the gas flows in the lumen of the fibers, allowing for exchange. Imperative to the arrangement of the fibers is the need to maintain a constant pressure. Artificial lungs use the right ventricle as a pump, as opposed to an external device. The pressure must, therefore, not drop significantly, as there is no external pump to help maintain the pressure of the system and ensure proper blood flow. The latest devices use a variety of shapes and arrangements to restrict pressure decreases and increase blood access to the fibers.2,3,14
Another consideration in artificial lung design is the minimization of injury to the blood cells. Injured cells could potentially lead to clotting and immunologic response. The shear stress within the system must be kept within defined parameters. If the shear stress is too high it can stimulate platelets and white cells, and it can also cause hemolysis.8 Conversely, if the shear stress is too low, it is suggested that the artificial lung will become a depository for thromboses.8,13 Additional problems include the attachment of phospholipids to the fiber surfaces, which can alter surface tension and promote plasma leakage.15 Remedies include additions to the surface of the artificial lung. Fibers with surface coatings, such as silicon, could inhibit phospholipid adsorption, reduce activation of the coagulation cascade and decrease the inflammatory response.2,3,13
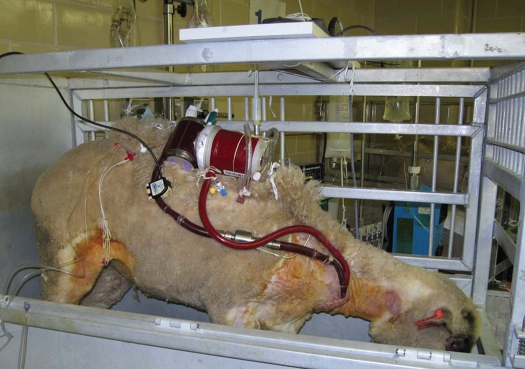
As the artificial lung relies on the right ventricle as its pump, the artificial lung itself must not cause undue stress on the heart. Right heart failure could result in and has been a major consideration of artificial lung design. The power needed to drive blood through the artificial lung and the native lungs is affected by impedance, which is dependent on the structure of the artificial lung and the way it is connected to circulation.8 The artificial lung must have a similar impedance to that of the native lung; it must mimic the opposition to pulsing blood flow, which in turn affects cardiac load.16,17 To create a matching impedance and to help decrease the workload of the right ventricle, a compliance chamber is often used with the artificial lung. In 2001, it was reported that the use of a compliance chamber along with the use of an enlarged diameter outlet and an inflow separator to better distribute blood flow through the artificial lung improved heart function and initial survival in a sheep model (Fig. 1).14 Later studies introduced an active compliance chamber that synchronized with the pulsations of the femoral artery to provide coordinated mechanical assistance for the right ventricle.18 Although this study showed an improvement in right heart function, additional complications were introduced due to bleeding at anastomosis sites caused by an increase in pulmonary artery pressure.18 Thus, for an effective artificial lung, a compatible compliance chamber must exist that decreases the workload of the right heart while at the same time preserving the integrity of the device and its connections.
Figure 1.
An experimental sheep standing freely and eating/drinking normally with dark black blood out of drainage lumen in clear contrast against the vivid red blood in the infusion lumen.
Device Placement
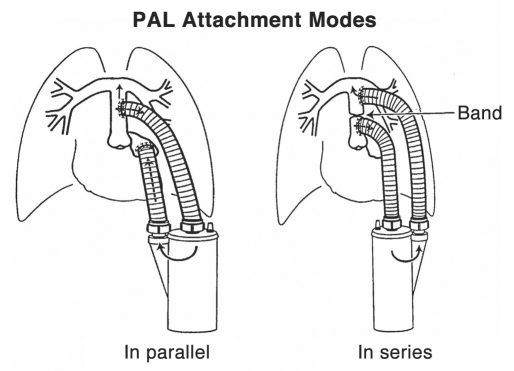
The placement of the device is crucial to its function. Currently two modes of placement predominate, in-series and in-parallel (Fig. 2), although alternative configurations have also been considered. A third configuration has recently shown great promise, combining the in-series method through the venous system There are advantages and disadvantages to each, with the patient and his/her disease state being the ultimate deciding factor.
Figure 2.
PAL modes of attachment. In-parallel configuration: inflow attached to pulmonary artery and outflow attached to left atrium. In-series configuration: inflow attached to proximal pulmonary artery and outflow attached to distal pulmonary artery (pulmonary artery ligated with band between inflow and outflow cannulae). (From Perfusion 2002; 17:253–268; Fig. 4)
For the in-parallel configuration, the blood inlet to the artificial lung is anastomosed surgically to the pulmonary artery. The blood outlet of the artificial lung is then similarly connected to the left atrium. The blood will flow into the device as a result of the high resistance in the patient's own pulmonary system and a much lower resistance in the artificial lung pathway. This can lower the total amount of resistance encountered by the right ventricle. Alternatively, a band can be placed on the pulmonary artery distal to the anastomosis in order to convert flow to the artificial lung. The in-parallel configuration is the least stressful for the right ventricle and is likely to be used in patients with diseases in which the work of the heart is a major consideration, such as pulmonary hypertension.2
Problems with the in-parallel configuration stem from the complete bypass of the native lungs. Providing more than just gas exchange, the lungs also perform metabolic functions within the body and act as a blood filter. Metabolically, the lungs produce prostacyclin and angiotensin II as well as deactivate vasoactive compounds.4 Currently there are no attempts to duplicate these actions in an artificial lung device. In addition to its role in the metabolic pathway, the native lung also acts as a filter, capturing small emboli and preventing them from entering systemic circulation.3 Therefore, an artificial lung in the in-parallel configuration increases the risk of systemic thromboemboli.
For the in-series configuration, the blood inlet of the artificial lung is again anastomosed to the pulmonary artery. The blood outlet, however, is also anastomosed to the pulmonary artery distal from the blood inlet anastomosis. A band is placed between the two anastomoses in order to direct blood flow from the proximal pulmonary artery into the artificial lung and back to the distal pulmonary artery before proceeding through the lungs and the ensuing normal circulatory pathway. This configuration causes the greatest amount of stress for the right ventricle, yet developments for an effective compliance chamber hope to alleviate this stress. Compliance chamber removal is also optional and can be coordinated based on the individual patient's varying pulmonary artery resistance.18 Additionally, since the blood will circulate through the native pulmonary bed, the metabolic functions and filtering properties of the lungs are not lost. Metabolic pathways can continue normally, and, if necessary, the device can be replaced without the additional risk of systemic thromboemboli. Finally, all of the cardiac output is being oxygenated by the artificial lung and then run through the native lung. This property of the in-series configuration might be useful for post-transplantation in which the donor lungs are not in optimum condition, with the artificial lung serving as a bridge to recovery. The in-series configuration is not without its problems. The aforementioned right heart stress, despite alleviating design alterations, is still an important consideration for researchers. Another complication to this setup is the short pulmonary artery in humans. Placement is limited, and the outflow graft competes for space in the mediastinum with the superior vena cava and the curve of the ascending aorta.18,19
While research continues to improve upon these configurations, alternative configurations are still being proposed and considered. One method is to attach the artificial lung to the right atrium with flow returning to the pulmonary artery. Under development is a centrifugal pump with a spinning disc that produces a forward flow while incorporating gas exchange.19 Another alternative is the hybrid configuration, which serves as a middle ground between the in-series and in-parallel configurations. In the hybrid configuration, the blood inlet is anastomosed with the pulmonary artery. The blood outlet has dual outlets: one anastomosing with the distal pulmonary artery and one anastomosing with the left atrium. A band can be placed between each pulmonary artery anastomosis, and up to 100% of the blood can be shunted to the artificial lung. The hybrid configuration is a compromise, reducing the stress on the right ventricle while maintaining the nonrespiratory functions of the native lung.8 While the hybrid configuration assumes some of the advantages of both in-series and in-parallel, it does not fully overcome the disadvantages associated with these configurations. Problems include more anastomosis sites, risk for thromboemboli if the device is replaced and not all of the blood going through native pulmonary bed. Instead of trying to overcome the milder but multiple disadvantages of the hybrid configuration, researchers instead have focused on amending the in-parallel or in-series configuration to perform at a more optimal level, and studies using both modes of attachment can be found.
Animal studies, focused on sheep, have found good success rates in different configurations. In one study, acute respiratory distress syndrome was induced in sheep and the artificial lung in-series was compared to volume-controlled mechanical ventilation.4 In the five day study, six of the eight artificial lung sheep survived versus one of the six mechanically ventilated sheep. This study showed the viability of the artificial lung for use in severe lung injury. In a separate study, the artificial lung was attached for thirty days in an in-parallel configuration to eight healthy sheep.13 Five sheep survived the trials, and it was found that one of the greatest complications arose from the long-term use of the device as device replacement was common in order to maintain function. This study indicates the artificial lung is capable of thirty days of respiratory support but needs some design changes to increase biocompatibility.
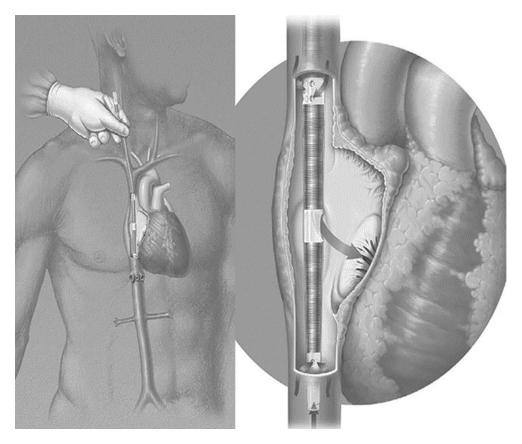
As researchers continued to improve upon the in-series and in-parallel configurations, a newly proposed third configuration came to the forefront as a highly restructured in-series type of attachment. Many of the problems encountered with the in-series configuration are eliminated with this new method. This third configuration centers around a newly designed cannula, which is based on the double lumen cannula used in neonatal and pediatric venovenous ECMO.20 This new cannula, deemed the Wang-Zwische Double Lumen Cannula, or W-Z DLC (Fig. 3), replaces the in-series and in-parallel anastomoses with a single cannula. The device consists of two pathways: a drainage pathway and an infusion pathway. The cannula is placed percutaneously through the internal jugular vein; the drainage lumen is open to both the superior vena cava and the inferior vena cava, while the infusion lumen is open to the right atrium.20 The blood from systemic circulation flows through the superior vena cava and inferior vena cava into the drainage lumen to the artificial lung device. The blood is oxygenated and returned via the infusion lumen into the right atrium. This oxygenated blood will then be pumped through the native circulation, and thus the native pulmonary bed, receiving the full metabolic and filtering capacities of the native lungs. This third configuration eliminates the required major surgery of the other configurations in order to properly anastamose the artificial lung.20 As there are no anastamoses to the pulmonary artery, there is no direct pumping action from the right ventricle. This has two-fold consequences. First, the artificial lung must now have a pump device associated with it in order to ensure blood flow and circulation. Secondly, the stress on the right ventricle is completely eliminated and thus avoids any coinciding heart damage and/or strain.
Figure 3.
W-Z DLC is inserted from right jugular vein into superior vena cava (SVC), traversing the right atrium (RA) to inferior vena cava (IVC). It drains venous blood from both SVC and IVC and delivers oxygenated blood in RA toward tricuspid valve to achieve minimal to no recirculation and potential total gas exchange. (From ASAIO J 2008; 54:606–611; Fig. 1)
There are some potential drawbacks to this W-Z DLC that need to be taken into account. Many cannulae are often plagued with structural problems, including kinking, lack of flexibility and traumatic insertion. The W-Z DLC has attempted to overcome these obstacles through its unique design. The infusion lumen is an ultra-thin membrane that collapses during insertion to allow room for an atraumatic introducer.20 The atraumatic introducer allows for easy placement, while the ultra-thin membrane not only allows for better insertion but also provides minimal blood resistance.20 To counter cannula kinking and promote flexibility, the cannula wall is also ultra-thin but is reinforced with anti-kink stainless steel wire wound around it.20 Another obstacle is the prevention of recirculation. The use of a double lumen cannula is only successful if recirculation can be properly prevented. The W-Z DLC has shown recirculation as low as 2%, and the developers recommend placement under fluoroscopic guidance along with transthoracic echocardiography to achieve minimal recirculation and prevent injury through optimal placement.20 This cannula can also achieve 2 L/min blood flow, which meets the minimal 1 L/min blood flow required for total CO2 removal.20 In the initial animal study of this new cannula, two acute sheep and one 15-day performance study sheep were evaluated. The results showed that the W-Z DLC is a viable percutaneously-placed alternative with minimal recirculation and optimal blood flow.20 Further long-term animal studies, however, must be conducted to verify the efficacy of the device on a larger scale.
Device Location
Widespread use of an artificial lung will most likely come first as an extracorporeal device existing outside of the body, also termed paracorporeal. External ventricular assist devices are currently in use, and protocols for such paracorporeal technology are already in place.3 The external location also makes the device easy to access, allowing for repair and/or replacement of the device and its parts. Additionally, a paracorporeal artificial lung eliminates the design restrictions placed on an intracorporeal, or inside the body, device. With an intracorporeal device, specific care must be taken to design the artificial lung so that it fits within the body cavity provided, does not impede the function of any surrounding tissues and is able to function in its own capacity in the space provided.3 Additionally, the entirety of the device must be implantable, requiring special coatings and a demonstrable consideration of the device's shape, avoiding sharp angles, tubing kinks and bacterial breeding grounds. From an engineering and maintenance perspective, the paracorporeal device is the easier of the two devices to create and maintain, yet an intracorporeal artificial lung would provide patients with the advantages of a true organ transplant: freedom of movement, no restriction in clothing or outward appearance, less fear of accidental physical damage and a feeling of incorporation.
As the design restrictions on the intracorporeal device greatly outweigh that of the paracorporeal device, the first widely used artificial lung will likely be paracorporeal in nature, with advances and research paving the way for a future with the intracorporeal artificial lung.19 Due to biocompatibility, the first devices to appear in humans will most likely arise as a bridge to transplant, with an increasingly lengthier duration of use as designs are modified and improved.4,19 The artificial lung might also see a future in rehabilitation for patients with lung injury.4 Ultimately, the artificial lung might become an alternative to lung transplantation, replacing the need for highly functional donor lungs with a fully functional, man-made model incorporated into the human respiratory and circulatory system.
Abbreviations
- ECMO
extracorporeal membrane oxygenator
- IVOX
intravascular oxygenator
- W-Z DLC
Wang-Zwische double lumen cannula
Note
With regards to reference 1, the data and analyses reported in the 2007 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients have been supplied by UNOS and Arbor Research under contract with HHS. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the U.S. Government
References
- 1.Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant data 1997–2006. Rockville, MD: Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2007. [Google Scholar]
- 2.Zwischenberger JB, Alpard SK. Artificial lungs: A new inspiration. Perfusion. 2002;17:253–268. doi: 10.1191/0267659102pf586oa. [DOI] [PubMed] [Google Scholar]
- 3.Zwischenberger JB, Anderson CM, Cook KE, Lick SD, Mockros LF, Bartlett RH. Development of an implantable artificial lung: Challenges and progress. ASAIO J. 2001;47:316–320. doi: 10.1097/00002480-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Zwischenberger JB, Wang D, Lick SD, Deyo DJ, Alpard SK, Chambers SD. The paracorporeal artificial lung improves 5-day outcomes from lethal smoke/burn-induced acute respiratory distress syndrome in sheep. Ann Thorac Surg. 2002;74:1011–1018. doi: 10.1016/s0003-4975(02)03896-1. [DOI] [PubMed] [Google Scholar]
- 5.Kopp R, Dembinski R, Kuhlen R. Role of extracorporeal lung assist in the treatment of acute respiratory failure. Minerva Anestesiol. 2006;72:587–595. [PubMed] [Google Scholar]
- 6.Matheis G. New technologies for respiratory assist. Perfusion. 2003;18:171–177. doi: 10.1191/0267659103pf684oa. [DOI] [PubMed] [Google Scholar]
- 7.Hattler BG, Lund LW, Golob J, Russian H, Lann MF, Merrill TL, et al. A respiratory gas exchange catheter: in vitro and in vivo tests in large animals. J Thorac Cardiovasc Surg. 2002;124:520–530. doi: 10.1067/mtc.2002.123811. [DOI] [PubMed] [Google Scholar]
- 8.Boschetti F, Perlman CE, Cook KE, Mockros LF. Hemodynamic effects of attachment modes and device design of a thoracic artificial lung. ASAIO J. 2000;46:42–48. doi: 10.1097/00002480-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Hoeper MM, Bein T, Simon A, Gottlieb J, Wisser W, et al. Interventional lung assist: a new concept of protective ventilation in bridge to lung transplantation. ASAIO J. 2008;54:3–10. doi: 10.1097/MAT.0b013e318161d6ec. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias M, Jungebluth P, Sibila O, Aldabo I, Matute MP, Petit C, et al. Experimental safety and efficacy evaluation of an extracorporeal pumpless artificial lung in providing respiratory support through the axillary vessels. J Thorac Cardiov Sur. 2007;133:339–345. doi: 10.1016/j.jtcvs.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotton DN, Fine A. Lung stem cells. Cell Tissue Res. 2008;331:145–156. doi: 10.1007/s00441-007-0479-2. [DOI] [PubMed] [Google Scholar]
- 13.Sato H, Hall CM, Lafayette NG, Pohlmann JR, Padiyar N, Toomasian JM, et al. Thirty-day in-parallel artificial lung testing in sheep. Ann Thorac Surg. 2007;84:1136–1143. doi: 10.1016/j.athoracsur.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Lick SD, Zwischenberger JB, Wang D, Deyo DJ, Alpard SK, Chambers SD. Improved right heart function with a compliant inflow artificial lung in series with the pulmonary circulation. Ann Thorac Surg. 2001;72:899–904. doi: 10.1016/s0003-4975(01)02842-9. [DOI] [PubMed] [Google Scholar]
- 15.Montoya JP, Shanley CJ, Merz SI, Bartlett RH. Plasma leakage through microporous membranes. Role of phospholipids. ASAIO J. 1992;38:399–405. doi: 10.1097/00002480-199207000-00064. [DOI] [PubMed] [Google Scholar]
- 16.Haft JW, Bull JL, Rose R, Katsra J, Grotberg JB, Bartlett RH, et al. Design of an artificial lung compliance chamber for pulmonary replacement. ASAIO J. 2003;49:35–40. doi: 10.1097/00002480-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ha RR, Wang D, Zwischenberger JB, Clark JW. Hemodynamic analysis and design of a paracorporeal artificial lung device. Cardiovasc Eng. 2006;6:11–30. doi: 10.1007/s10558-006-9000-x. [DOI] [PubMed] [Google Scholar]
- 18.Alpard SK, Wang D, Deyo DJ, Smolarz CM, Chambers S, Zwischenberger JB. Optional active compliance chamber performance in a pulmonary artery-pulmonary artery configured paracorporeal artificial lung. Perfusion. 2007;22:81–86. doi: 10.1177/0267659107078483. [DOI] [PubMed] [Google Scholar]
- 19.Lick SD, Zwischenberger JB. Artificial lung: bench toward bedside. ASAIO J. 2004;50:2–5. doi: 10.1097/01.mat.0000107282.22793.49. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB. Wang-Zwische Double lumen-cannula—toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J. 2008;54:606–611. doi: 10.1097/MAT.0b013e31818c69ab. [DOI] [PubMed] [Google Scholar]