Abstract
This study quantitatively assesses the effectiveness of retrospective beat-to-beat respiratory motion correction (B2B-RMC) at near 100% efficiency using high-resolution coronary artery imaging. Three-dimensional (3D) spiral images were obtained in a coronary respiratory motion phantom with B2B-RMC and navigator gating. In vivo, targeted 3D coronary imaging was performed in 10 healthy subjects using B2B-RMC spiral and navigator gated balanced steady-state free-precession (nav-bSSFP) techniques. Vessel diameter and sharpness in proximal and mid arteries were used as a measure of respiratory motion compensation effectiveness and compared between techniques. Phantom acquisitions with B2B-RMC were sharper than those acquired with navigator gating (B2B-RMC vs. navigator gating: 1.01±0.02 mm−1 vs. 0.86±0.08 mm−1, P<.05). In vivo B2B-RMC respiratory efficiency was significantly and substantially higher (99.7%±0.5%) than nav-bSSFP (44.0%±8.9%, P<.0001). Proximal and mid vessel sharpnesses were similar (B2B-RMC vs. nav-bSSFP, proximal: 1.00±0.14 mm−1 vs. 1.08±0.11 mm−1, mid: 1.01±0.11 mm−1 vs. 1.05±0.12 mm−1; both P=not significant [ns]). Mid vessel diameters were not significantly different (2.85±0.39 mm vs. 2.80±0.35 mm, P=ns), but proximal B2B-RMC diameters were slightly higher (2.85±0.38 mm vs. 2.70±0.34 mm, P<.05), possibly due to contrast differences. The respiratory efficiency of B2B-RMC is less variable and significantly higher than navigator gating. Phantom and in vivo vessel sharpness and diameter values suggest that respiratory motion compensation is equally effective.
Keywords: Respiratory motion, Motion correction, Coronary, Quantitative comparison, Spiral, Efficiency
1. Introduction
For high-resolution applications, the majority of cardiovascular magnetic resonance studies are performed with respiratory gating during free-breathing using diaphragmatic navigators [1,2]. The accept/reject algorithm [3,4], used to limit respiratory motion to a small (typically 5 mm) gating window around end expiration, is inherently inefficient and unpredictable particularly in the presence of respiratory drift [5]. A number of techniques including motion adaptive gating [6] and phase encode ordering methods [7–9] reduce the effects of respiratory motion within the navigator acceptance window, enabling improved image quality or greater respiratory efficiency. Alternatively, navigator information may be used to both gate and provide input to respiratory motion models which relate the motion of the diaphragm to that of the heart. The most basic of these models uses a fixed superior–inferior factor to perform slice tracking [1,10], but tracking factors vary considerably between subjects [11,12], and calculating accurate subject-specific values is both difficult and time consuming. More complex models, often derived from multiple navigators, include three-dimensional (3D) translational [13] and affine transformations [14–16] which take into account the nonrigid deformation of the heart and its hysteretic relationship with the diaphragm. Such methods have enabled increases in the acceptance window from 5 to 10 mm without loss of image quality, resulting in improved respiratory efficiency (from ∼40% [4] to ∼70% [17]). These models, however, are derived from a prescan and do not adapt to changes that may occur over subsequent long acquisitions. Several novel non-model-based alternatives have been developed which derive respiratory motion information directly from the anatomy of interest. Self-gated techniques use respiratory information obtained from a repeated superior–inferior projection within the acquisition to gate [18] or perform one-dimensional translational corrections [19], while other methods reconstruct heavily aliased subimages from a subset of the full high-resolution acquisition on every cardiac cycle for respiratory gating [20] or to obtain 3D affine corrections [21]. Alternatively, simultaneously acquired additional low-resolution images have been used to obtain two-dimensional (2D) in-plane translational corrections [22] and rotations [23].
Recently, a beat-to-beat respiratory motion correction (B2B-RMC) technique [24] was developed whereby unaliased 3D low-resolution images of the epicardial fat surrounding the heart are acquired on every cardiac cycle. The low-resolution fat images from each cardiac cycle are used to derive beat-to-beat 3D localized translations for the coronary arteries. The motion information obtained is used to correct the corresponding 3D high-resolution data acquired immediately afterwards in the same cardiac cycle. This technique was initially [24] demonstrated for black blood 3D spiral right coronary artery wall imaging with 100% respiratory efficiency. In this manuscript, we present the first quantitative assessment of the efficacy of this motion correction technique. Three-dimensional high-resolution imaging of the right coronary artery was chosen as the imaging application as its small size and substantial motion with both the cardiac and respiratory cycles make it a particularly challenging target. The efficacy of the technique is verified with comparison to an identical navigator gated sequence in 3D spiral acquisitions of a coronary artery test object moving with realistic respiratory motion. Subsequently, a full in vivo evaluation in 10 healthy subjects comparing 3D spiral imaging using B2B-RMC to a widely used navigator-gated coronary artery imaging technique is presented.
2. Methods
All imaging was performed on a Siemens 1.5 T Avanto MRI scanner (Siemens Medical Systems, Erlangen, Germany) with maximum gradient amplitude 40 mT/m and maximum slew rate 170 mT/m/s, using an anterior phased array coil. In vivo acquisitions were gated using an electrocardiographic system which was designed in-house.
2.1. Phantom acquisitions
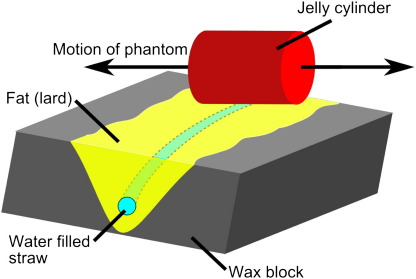
A test object was constructed to imitate the proximal and mid right coronary artery surrounded by epicardial fat in the atrioventricular groove. This was achieved, as shown in Fig. 1, by positioning a curved water-filled straw (diameter 3 mm) in a V-shaped groove in a wax block and surrounding the straw with fat (lard). Air bubbles within the straw provided additional structural detail for visual assessment of the effects of motion. A gel cylinder was placed adjacent to the coronary artery test object and was used for monitoring displacement with a standard navigator [2]. Both objects were placed on the trolley of a mechanical respiratory motion phantom, driven by a stepper motor system with microstepping capabilities. The phantom was programmed to follow respiratory traces obtained from six healthy subjects using a diaphragmatic navigator (repeat time [TR]=250 ms, acquisition duration=∼5 min). The first five respiratory traces had mean amplitudes in the range 8–17 mm and mean respiratory periods in the range 3–6 s. The sixth volunteer had a respiratory trace with an unusually large amplitude (36 mm) and long mean period (11 s). The test object was orientated so that motion along the axis of the magnet bore resulted in translation (without deformation) of the vessel test object both in and through the imaging plane which was orientated in the plane of the vessel. Imaging of the phantom was performed using a 3D spiral acquisition, as described below. Three acquisitions were performed: (a) with no motion, (b) with motion (reconstructed with and without B2B-RMC) and (c) with motion and a standard navigator accept/reject algorithm (5-mm window). In this way, the performance of B2B-RMC is directly compared to accept/reject navigator gating and motion free acquisitions using an identical sequence.
Fig. 1.
The coronary artery test object consisted of a water-filled straw within a fat-filled groove in a wax block which was rotated with respect to the direction of motion. A jelly-filled cylinder was placed adjacent to the test object and orientated parallel to the direction of motion in order to monitor the phantom location with a standard navigator.
2.2. In vivo acquisitions
A right coronary artery imaging protocol was performed on 10 healthy subjects (5 female, 22–53 years old) recruited with informed consent according to local ethics procedures. The longest right coronary rest period was first determined from a cine acquisition in a plane showing the four-chamber view [25]. All subsequent high-resolution imaging was performed in this rest period. In-plane high-resolution right coronary acquisitions were planned from a 3D balanced steady-state free-precession (bSSFP) whole-heart study with navigator-based respiratory gating. The imaging plane was planned by selecting three points on the right coronary artery in the whole-heart volume and verified by acquiring a rapid, 2D navigator gated bSSFP image. A targeted 3D high-resolution acquisition was then performed using the 3D spiral B2B-RMC acquisition. In addition, a standard 3D navigator gated bSSFP (nav-bSSFP) acquisition with T2 preparation [26] was also performed with the same spatial resolution. While a three-way comparison between the 3D spiral with B2B-RMC, the 3D spiral with navigator gating and the standard nav-bSSFP acquisition would have been preferable, two navigator gated 3D acquisitions could not be acquired within a reasonable duration. Consequently, we chose to compare 3D spiral B2B-RMC with nav-bSSFP as this is currently the most widely used MR coronary artery imaging technique. Both techniques are described below.
2.3. B2B-RMC 3D spiral
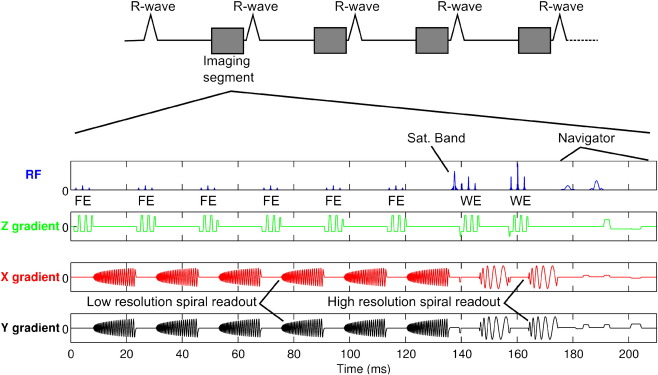
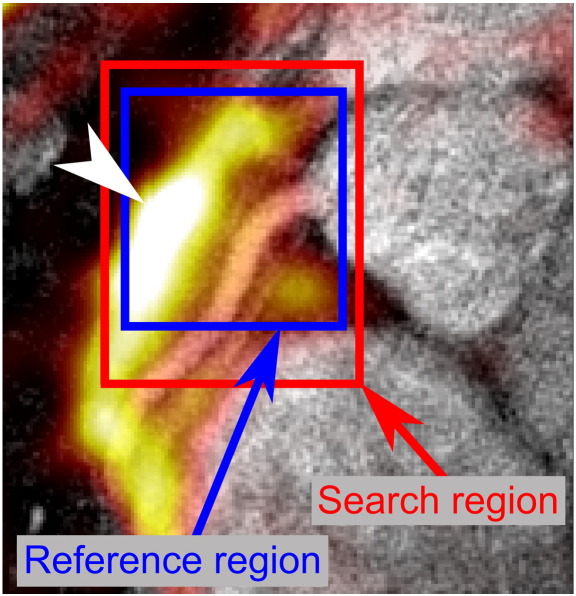
The B2B-RMC technique that was used in this study is similar to that described by Keegan et al. [24] and is shown in Fig. 2. In each cardiac cycle, a low-resolution acquisition consisting of a 3D stack of spirals with binomial fat selective excitation (FE) was acquired immediately before a segment of a high-resolution 3D stack of spirals acquisition with binomial water selective excitation (WE). A traditional crossed-pair diaphragmatic navigator immediately followed the high-resolution segment. Navigator information was used to reject data acquired at only very extreme respiratory locations. These were defined as those diaphragm positions falling more than 10 mm outside of the normal tidal respiratory range which was determined in a ∼30 s navigator scout acquisition. The low-resolution 3D acquisition consisted of eight single-shot center-out spirals with through-plane (kz) phase encoding and six-eighths partial Fourier in kz, resulting in six acquired spirals. The order of acquisition was reverse-centric so that data closest to the center of kz-space were acquired temporally close to the start of the high-resolution data acquisition. Each low-resolution single-shot spiral consisted of 6144 points acquired over 15 ms (echo time [TE]=3.9 ms and TR=23 ms) preceded by a 15° FE pulse, resulting in a 135-ms low-resolution acquisition window. The resolution was 4.8×4.8×3 mm at 261×261×24 mm field of view, reconstructed to 0.5×0.5×1.5 mm. Each high-resolution segment consisted of two interleaves of a 75-interleave 3D center-out spiral acquisition with eight through-plane phase encode steps. The first interleaf of each segment was acquired with a 45° WE pulse and the second with a 90° WE pulse. Each interleave consisted of 4096 points acquired over 10 ms (TE=3.4 ms and TR=1 RR interval). A spatial saturation pulse was applied to the chest wall immediately prior to the high-resolution imaging segment in order to minimize artifacts from structures not moving with the coronary artery. The high-resolution data were temporally located in the subject-specific right coronary rest period. Where possible, the low-resolution data were also acquired during this period of minimal motion, but the timing of the high-resolution data was prioritized. As the low-resolution data are acquired in a reverse-centric kz phase order, the effect of any motion during the low-resolution acquisition is expected to be minimal. The total acquisition duration was 300 cardiac cycles (assuming 100% respiratory efficiency) or 5 min (with a heart rate of 60 beats/min). The acquired resolution was 0.7×0.7×3 mm over a 570×570×24 mm field of view which was reconstructed to a 0.7×0.7×1.5 mm pixel size. The high field of view was used to bolster signal to noise ratio (SNR) in the images and to move any characteristic spiral artifacts away from the anatomy of interest. The high-resolution acquisition window was 35 ms. All images were reconstructed and processed offline using in-house software written in MATLAB 2009a (The Mathworks, Natick, MA). Beat-to-beat 3D respiratory displacement of the right coronary artery was determined using a 3D local normalized subpixel cross-correlation of the low-resolution volumes acquired in each cardiac cycle. An end expiratory volume was chosen as a reference using the diaphragmatic navigator information. A cuboid-shaped reference region around the coronary origin was defined on the reference volume, aided by a colored overlay of the fat image on the uncorrected high-resolution water image, as seen in Fig. 3. A search region was also defined on this volume and copied to the other low-resolution volumes for the subsequent beat-to-beat cross-correlation. In order to determine the appropriate dimensions for the search region, the cross-correlation was initially performed on a subset of 20 of the low-resolution volumes before performing the full procedure. The two high-resolution spiral interleaves acquired in each cardiac cycle were corrected [2] for respiratory motion using the 3D beat-to-beat translations obtained, and high-resolution images were reconstructed using a standard gridding [27] and fast Fourier transform technique.
Fig. 2.
Sequence diagram for the B2B-RMC technique used for bright blood acquisition with 3D stack of spirals readouts. In every cardiac cycle, a full fat selective 3D low-resolution volume is acquired before the two interleaves from the high-resolution acquisition.
Fig. 3.
To aid positioning of the search and reference regions for the localized 3D cross-correlation of the fat images, the reference low-resolution fat volume was transparently overlaid (“hot” color scale - an arrow head indicates the center of the largest region of fat for clarity (in the black and white print version of this figure)) on the uncorrected high-resolution water images (“gray” scale). This enables accurate positioning of the regions around the right coronary origin which is otherwise challenging using the fat images alone. Both reference and search regions are defined in three dimensions, extending into multiple slices (not shown here for clarity).
2.4. Navigator gated balanced steady-state free-precession imaging
Three-dimensional navigator-gated balanced steady-state free-precession (nav-bSSFP) imaging with T2 preparation is the most commonly used technique for MR coronary angiography [17,26,28–31]. Nav-bSSFP data sets with T2 preparation [26] were acquired in the same image plane and with the same spatial resolution as the B2B-RMC acquisition. The sequence used a flip angle of 70°, TE=1.78 ms, TR=4.1 ms, 17–25 phase encode lines per cardiac cycle (depending on the length of the cardiac rest period), 512 readout points, 512 phase encode lines, 8 through-plane phase encode steps, 360×360×24 mm field of view, acquired resolution 0.7×0.7×3 mm and reconstructed resolution 0.7×0.7×1.5 mm. Phase oversampling (equivalent to increasing the field of view and subsequently cropping after reconstruction), by a factor of 20% in the phase encode direction and by a factor of 25% in the through-plane direction, was used to bolster SNR and generate a similar slice profile to that used for the B2B-RMC technique. Accept/reject navigator gating was performed with a 5-mm navigator acceptance window using the same standard crossed-pair navigator used for the B2B-RMC acquisition. The respiratory gating was performed without slice tracking but with automatic updates of the acceptance window position to follow the end expiratory position [32,33]. Acquisition duration was 246 cardiac cycles (assuming 100% respiratory efficiency and 25 phase encode lines per cardiac cycle) or 4 min (at 60 beats/min).
2.5. Image processing and analysis
The efficacy of the B2B-RMC technique was assessed by comparing quantitative measures of vessel sharpness and vessel diameter in the proximal and mid coronary arteries with those obtained using the standard navigator gating technique. Signal and contrast to noise ratios were not compared as these are inherently different between the spiral and T2-prepared bSSFP techniques.
All images were postprocessed using in-house MATLAB software. Parallel plane maximum intensity projections (MIPs) were generated from all in vivo slices containing the right coronary artery (typically five slices) with anatomy overlying the coronary artery (such as the right atrial appendage) selected in a region of interest in each slice and zeroed to show the maximum length of artery. Average vessel sharpness and diameter were obtained from a length of the proximal (0–20 mm from the coronary origin) and mid (20–40 mm from origin) right coronary artery. Vessel sharpness was defined as the inverse of the distance from the 20% to 80% of the maximum intensity in a profile drawn perpendicular to the vessel and averaged over both vessel edges [34]. Vessel diameter was defined as the full width half maximum of the intensity profiles [34]. Respiratory efficiency and both proximal and mid vessel sharpness and vessel diameter were compared between the B2B-RMC and nav-bSSFP techniques using a two-tailed paired Student's t test and a 5% significance level. Vessel sharpness and diameter were similarly obtained from MIPs (four slices) of the phantom data in a bubble-free 10-mm section of the test object.
3. Results
3.1. Phantom
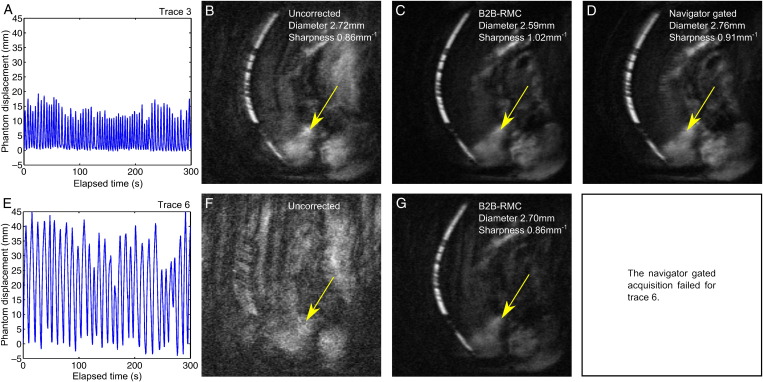
Table 1 documents the results of the phantom tests. The navigator gated acquisition using respiratory trace 6 failed due to a very low respiratory efficiency (13%) which resulted in the respiratory trace exceeding the maximum length. For the remaining five respiratory traces, B2B-RMC resulted in a significant increase in vessel sharpness compared to both uncorrected acquisitions (1.01±0.02 mm−1 vs. 0.71±0.10 mm−1, P<.01) and navigator gated acquisitions (1.01±0.02 mm−1 vs. 0.86±0.08 mm−1, P<.05). The measured vessel diameter was reduced (from 3.06±0.52 mm uncorrected) using both navigator gating (2.74±0.12 mm, P=not significant [ns]) and B2B-RMC (2.60±0.02 mm, P=ns), but the differences were not significant. The diameter obtained from the stationary images (2.60 mm) was similar to the average value using B2B-RMC. The respiratory efficiency for the B2B-RMC acquisitions was 100% in every case, and the mean respiratory efficiency for the navigator gated acquisitions was 46%±17%. Examples of the results from two of the acquisitions are shown in Fig. 4 (using trace 3 [4.A] and trace 6 [4.E]). In both cases, B2B-RMC demonstrates a substantial visual improvement (4.C and 4.G) with improved vessel diameter and sharpness over the uncorrected images. For trace 3, navigator gating also demonstrates improved visual image quality, vessel diameter and vessel sharpness compared to the uncorrected data (4.D), while the navigator gated acquisition failed for trace 6, as described above.
Table 1.
Results of the phantom tests
| Trace | Mean amplitude (mm) | Mean period (s) | Sharpness |
Diameter |
||||
|---|---|---|---|---|---|---|---|---|
| Uncorrected (mm−1) | Nav-Gated (mm−1) | B2B-RMC (mm−1) | Uncorrected (mm) | Nav-Gated (mm) | B2B-RMC (mm) | |||
| 1 | 15 | 6 | 0.69 | 0.96 | 1.01 | 2.84 | 2.60 | 2.64 |
| 2 | 17 | 5 | 0.75 | 0.79 | 1.02 | 2.64 | 2.73 | 2.58 |
| 3 | 14 | 5 | 0.86 | 0.91 | 1.02 | 2.72 | 2.76 | 2.59 |
| 4 | 9 | 3 | 0.60 | 0.76 | 0.97 | 3.91 | 2.94 | 2.60 |
| 5 | 8 | 3 | 0.63 | 0.88 | 1.02 | 3.20 | 2.68 | 2.60 |
| 6 | 36 | 11 | a | b | 0.86 | a | b | 2.70 |
| Mean (S.D.) | 17 | 6 | 0.71(0.10) | 0.86 (0.08) | 1.01⁎ (0.02) | 3.06 (0.52) | 2.74 (0.12) | 2.60 (0.02) |
Sharpness and diameter measurements in the vessel test object acquired while following respiratory traces obtained from six healthy subjects. For reference, the measured vessel sharpness in the stationary phantom was 1.35 mm−1, and the measured vessel diameter was 2.60 mm.
Not measurable.
Acquisition failed.
P<.05 compared to uncorrected and P<.01 compared to navigator gated.
Fig. 4.
Example results from the phantom tests using respiratory traces 3 (A) and 6 (E). Corresponding MIP images are shown from acquisitions without gating or correction (uncorrected) (B) and (F) and corrected using B2B-RMC (C) and (G). For respiratory trace 3, the MIP image from data acquired with a 5-mm navigator gating window is also shown (D). Respiratory efficiency of the uncorrected and B2B-RMC images (B, C, F, G) is 100% and 54% the navigator gated image (d). Some residual fat is seen in all of the images (arrows).
3.2. In vivo
High-quality right coronary artery images were obtained in 10 subjects with both the B2B-RMC and nav-bSSFP techniques. Example images from one subject using both methods are shown in Fig. 5. Respiratory efficiency of the B2B-RMC technique was near 100% and significantly higher than that of the nav-bSSFP technique (99.7%±0.5%, range 98.4%–100% vs. 44.0%±8.9%, range 33.0%–62.8%; P<.0001). Vessel diameter and sharpness were successfully measured for the proximal vessel in all 10 subjects. One subject had a particularly small and tortuous vessel which could not be accurately measured in the midsection using either technique, and as a result, midsection vessel sharpness and diameter were obtained in 9 subjects. The sharpness and diameter measurements are summarized in Table 2 together with the average respiratory efficiency of both techniques. Vessel sharpness measured in both the proximal and mid vessel was not significantly different between the two methods. Vessel diameter in the mid artery was not significantly different, and although there is a significant difference in the proximal diameter, it is not substantial (0.15 mm or ∼5%).
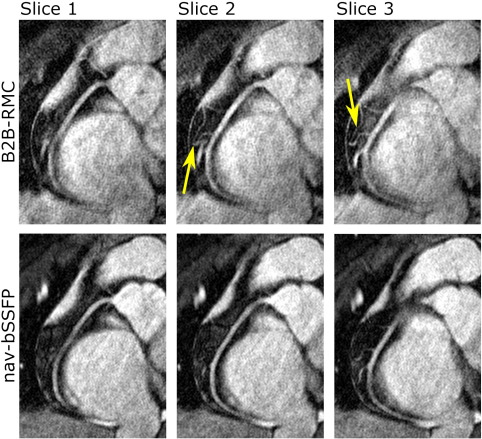
Fig. 5.
Example images from one subject showing corrected images from the B2B-RMC technique in three consecutive slices (top line) and the corresponding slices from the nav-bSSFP technique (bottom line). Note the similar appearance using both techniques of the right coronary artery in the proximal and midsections, but increased blurring in the B2B-RMC images in the distal vessel. Also note the improved depiction of the branch vessels in the midsection in the B2B-RMC images, highlighted by the arrows.
Table 2.
Results of the in vivo tests
| Respiratory efficiency (%) | Proximal sharpness (mm−1) | Mid sharpness (mm−1) | Proximal diameter (mm) | Mid diameter (mm) | |
|---|---|---|---|---|---|
| B2B-RMC (S.D.) | 99.7 (0.5) | 1.00 (0.14) | 1.01 (0.11) | 2.85 (0.38) | 2.85 (0.39) |
| nav-bSSFP (S.D.) | 44.0 (8.9) | 1.08 (0.11) | 1.05 (0.12) | 2.70 (0.34) | 2.80 (0.35) |
| P value | <.0001 | .15 | .24 | .026 | .60 |
Comparison of respiratory efficiency and both proximal and distal vessel sharpness and diameter obtained from images acquired with spiral B2B-RMC technique and nav-bSSFP technique. Proximal and mid results were obtained in 10 and 9 healthy subjects, respectively. All values are mean±S.D.
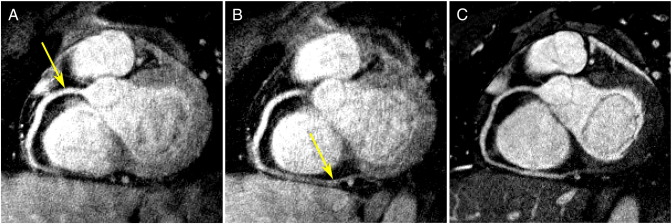
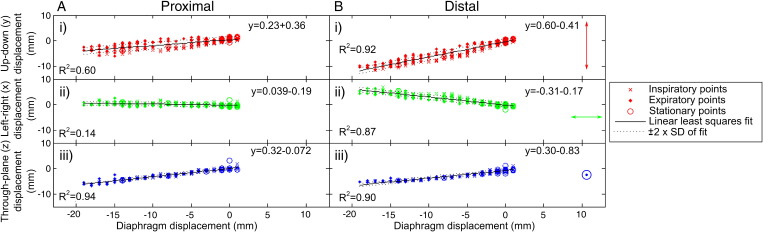
The nonrigid motion of the heart and the localized nature of the cross-correlation method used in the B2B-RMC technique mean that, as expected, the 3D respiratory translations obtained using reference and search regions around the coronary artery origin do not provide optimal respiratory correction in the distal artery. This is demonstrated in Fig. 6 where a curved-plane reformat of a B2B-RMC image corrected for proximal coronary motion (as performed for the comparisons in Table 2) (a) and corrected for distal motion (b) are compared to the equivalent curved-plane reformat of the nav-bSSFP acquisition (c). For the B2B-RMC images, it is apparent that the distal vessel is sharpest in (b) while the proximal vessel is sharpest in (a). In comparison, the nav-bSSFP image (c) is sharp over both proximal and distal regions, although at the expense of a 2.3-fold decrease in respiratory efficiency. This need for different respiratory motion corrections in the proximal and distal regions is emphasized in Fig. 7 which shows the beat-to-beat in-plane (x and y) and through-plane (z) respiratory translations relating to the corrected images shown in Fig. 6 (A) and (B) plotted against the corresponding diaphragm displacements, as measured with the following navigator. In this instance, the slope of the y in-plane correction vs. the superior–inferior diaphragm displacement was 0.23 in the proximal region and 0.60 in the distal region. Similarly, the corresponding slope for the in-plane x corrections was 0.039 in the proximal region and –0.31 in the distal region. An initial attempt to combine the B2B-RMC images corrected for both proximal and distal motion was performed by selectively replacing data in the vicinity of the distal artery in the proximally corrected data set with equivalent data from the distally corrected data set. Voxels in the border region between the two corrected data sets were linearly combined, resulting in a fading effect. The result of this is shown in Fig. 8 and demonstrates high clarity along the entire length of the vessel.
Fig. 6.
Curved planar reformat of 3D spiral images acquired with the B2B-RMC technique (0.7×0.7×3-mm resolution) in 302 cardiac cycles (99.3% efficient) over 20 mm of diaphragm motion, corrected for optimal proximal (A) and distal (B) vessel quality. For comparison, the equivalent curved planar reformat of the 3D nav-bSSFP with identical resolution acquired in 576 cardiac cycles (43.6% efficient) is shown (C).
Fig. 7.
The localized in-plane (bottom to top–y [i] and right to left–x [ii]) and through-plane (z [iii]) beat-to-beat displacements obtained from the low-resolution images using a cross-correlation technique around the (A) proximal and (B) distal artery, plotted against the diaphragm displacement obtained from the navigator. Inspiratory, expiratory and stationary points are differentiated using the difference between successive points in the navigator trace. The increased slope for the in-plane translations observed in the distal artery highlights the need for a localized correction.
Fig. 8.
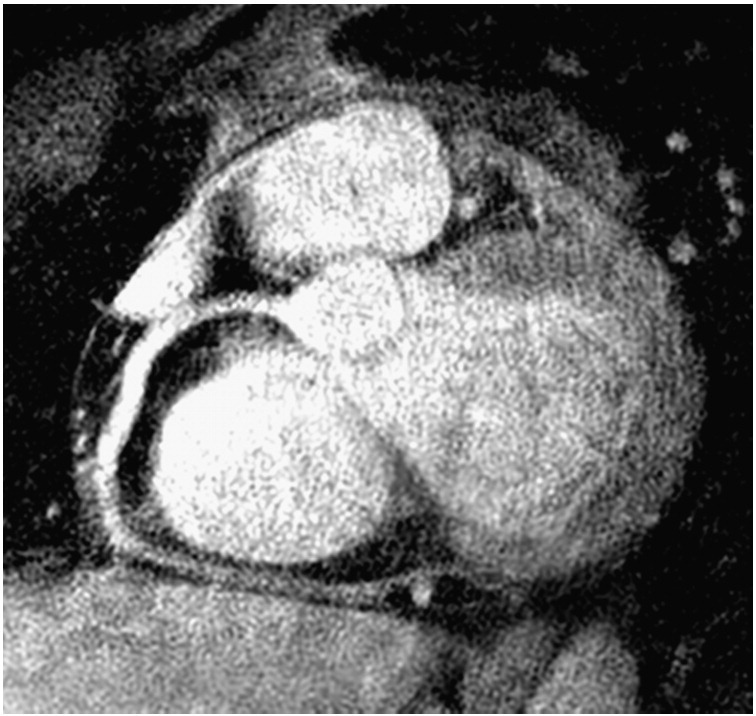
Curved plane reformat of the data sets shown in Fig. 6A and B combined to correct for both proximal and distal motion. The data sets were combined in a basic manner, and further work will address more sophisticated methods.
4. Discussion
The B2B-RMC technique can compensate for respiratory motion with near 100% respiratory efficiency using in vivo and phantom measures of vessel diameter and vessel sharpness in coronary artery imaging as quantitative markers of performance.
Data acquired in a respiratory motion phantom following respiratory traces obtained from healthy volunteers have demonstrated that the B2B-RMC technique can correct for a large range of translational motion. Vessel sharpness measurements are better than those obtained using conventional navigator gating with a 5-mm window, and the diameter measurements are very similar to those obtained from a stationary phantom. Even in the case of extreme respiratory motion (trace 6, Fig. 4E), the B2B-RMC technique performed well with 100% respiratory efficiency. In this instance, the respiratory efficiency using navigator gating was so low (13%) that the acquisition failed. The underestimation of the vessel diameter obtained in these experiments (2.60 mm in the stationary phantom compared to the 3.0 mm actual diameter) is a result of using the full width half maximum of a 2D projection of a 3D image of a cylindrical object and demonstrates a limitation of this method of measurement.
For the in vivo acquisitions, 3D spiral acquisitions with B2B-RMC were performed together with nav-bSSFP acquisitions, which are conventionally used for MR coronary artery imaging. All acquisitions resulted in high-quality images. Although ideally an additional 3D spiral acquisition with navigator gating would have been acquired, time constraints prohibited this. The efficiency of the nav-bSSFP technique was more variable as well as being significantly and considerably lower (44.0%±8.9% vs. 99.5%±0.5%, P<.0001) than the B2B-RMC technique in the healthy subjects studied. The variability of the respiratory efficiency using navigator gating leads to uncertainty regarding the acquisition duration. As B2B-RMC is able to correct for >99% of respiratory motion, this uncertainty is greatly reduced. Although the nav-bSSFP images had inherently different contrast characteristics to the 3D spiral images acquired with the B2B-RMC technique, there was no disparity in vessel sharpness. A statistically significant difference in proximal vessel diameter was observed between the techniques, but the magnitude of this was small (∼5%) and may possibly be due to the use of a T2 preparation pulse with the nav-bSSFP technique which reduces the signal from the coronary vessel wall. The values obtained for vessel sharpness are higher than those obtained in other studies [34,35], which is most likely due to the higher spatial resolution used in this study, while the vessel diameters obtained fall within the range of values obtained in previous studies [31,36–39].
This is the first time that this B2B-RMC technique has been applied to bright blood coronary artery imaging, and the work clearly demonstrates the expected differences in the motion of the proximal and distal right coronary artery. The proximity of the distal artery to the diaphragm results in a larger range of motion at this level than at the proximal artery which is separated from the diaphragm by a large volume of soft deformable tissue. This nonrigid deformation is highlighted by the increased magnitude of the slope of the linear fit of the in-plane (x and y) beat-to-beat displacements vs. the diaphragm displacement in the distal correction when compared to the proximal results. The spread of the points around the linear fit emphasizes the need for a beat-to-beat correction, and the previously reported inspiratory–expiratory hysteresis [40] was also observed in the corrections for several subjects as a loop-like trend in the data. As has been demonstrated, it is possible to combine multiple data sets corrected optimally for different sections of the vessel. Further work will consider combining data sets from more than two corrections, assess the optimal way of doing this and perform these corrections both rapidly and automatically.
This study has a number of limitations. For the phantom studies, the motion of the coronary arteries was modeled as a rigid 3D translation based on the motion of the diaphragm. In reality, the heart is deformable and the motion is therefore more complex. All in vivo B2B-RMC acquisitions to date have been acquired in healthy volunteers, but we are now actively recruiting patients. In general, breathing patterns are more erratic in the patient population with greater respiratory drift than for healthy subjects, and we therefore might expect the benefits of B2B-RMC to be more pronounced. In our study group, we have only targeted the right coronary artery as it is the more mobile and therefore the more challenging imaging target. However, preliminary attempts in imaging the left coronary artery system have also been successful despite a generally reduced volume of fat surrounding these arteries. Also, vessel diameter and sharpness were only measured in the first 40 mm of the artery. This is partly due to the localized nature of the cross-correlation method which was used to selectively correct for the respiratory motion of the proximal/mid artery, but these measurements also become increasingly difficult around the escalating number of branch points more distally. Nonetheless, we have qualitatively demonstrated that the B2B-RMC may be used to correct for respiratory motion in the distal right coronary artery by selecting appropriate regions of interest to cross-correlate. In the future, nonrigid implementations will be investigated in order to correct whole-heart 3D coronary artery acquisitions. A further limitation of this study is that although SNR and contrast to noise ratio are important determinants of image quality, the inherently different image contrast between the 3D spiral and nav-bSSFP techniques used in the in vivo studies meant that such measures were inappropriate for comparing the performance of respiratory compensation strategies in this context. While the ideal solution would have been to perform an additional identical 3D spiral acquisition with a 5-mm navigator gating window, this was not possible due to time constraints. One potential alternative would have been to acquire a navigator gated 3D spiral acquisition with B2B-RMC and a 5-mm gating window to enable gated and corrected images to be reconstructed from the same data set. It is also possible to implement the bSSFP with the B2B-RMC technique. However, both of these options require considerable modifications to the pulse sequence and image reconstruction software which were not possible at the time of this study.
In conclusion, the B2B-RMC technique can be used to correct for respiratory motion with 99.7% respiratory efficiency as well as a navigator-based technique with a 5-mm gating window (44.0% efficient), using vessel sharpness and vessel diameter from phantom and right coronary artery imaging to quantitatively compare the methods.
Footnotes
Grant support: Andrew D. Scott is funded by the British Heart Foundation (grant number FS/07/027). This project was undertaken at the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London.
References
- 1.Danias P.G., McConnell M.V., Khasgiwala V.C., Chuang M.L., Edelman R.R., Manning W.J. Prospective navigator correction of image position for coronary MR angiography. Radiology. 1997;203(3):733–736. doi: 10.1148/radiology.203.3.9169696. [DOI] [PubMed] [Google Scholar]
- 2.Ehman R.L., Felmlee J.P. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173(1):255–263. doi: 10.1148/radiology.173.1.2781017. [DOI] [PubMed] [Google Scholar]
- 3.Sachs T.S., Meyer C.H., Hu B.S., Kohli J., Nishimura D.G., Macovski A. Real-time motion detection in spiral MRI using navigators. Magn Reson Med. 1994;32(5):639–645. doi: 10.1002/mrm.1910320513. [DOI] [PubMed] [Google Scholar]
- 4.Oshinski J.N., Hofland L., Mukundan S., Dixon W.T., Parks W.J., Pettigrew R.I. Two-dimensional coronary MR angiography without breath holding. Radiology. 1996;201(3):737–743. doi: 10.1148/radiology.201.3.8939224. [DOI] [PubMed] [Google Scholar]
- 5.Taylor A.M., Jhooti P., Wiesmann F., Keegan J., Firmin D.N., Pennell D.J. MR navigator-echo monitoring of temporal changes in diaphragm position: implications for MR coronary angiography. J Magn Reson Imaging. 1997;7(4):629–636. doi: 10.1002/jmri.1880070404. [DOI] [PubMed] [Google Scholar]
- 6.Weiger M., Börnert P., Proksa R., Schäffter T., Haase A. Motion-adapted gating based on k-space weighting for reduction of respiratory motion artifacts. Magn Reson Med. 1997;38(2):322–333. doi: 10.1002/mrm.1910380223. [DOI] [PubMed] [Google Scholar]
- 7.Sachs T.S., Meyer C.H., Irarrazabal P., Hu B.S., Nishimura D.G., Macovski A. The diminishing variance algorithm for real-time reduction of motion artifacts in MRI. Magn Reson Med. 1995;34(3):412–422. doi: 10.1002/mrm.1910340319. [DOI] [PubMed] [Google Scholar]
- 8.Jhooti P., Gatehouse P.D., Keegan J., Bunce N.H., Taylor A.M., Firmin D.N. Phase ordering with automatic window selection (PAWS): a novel motion-resistant technique for 3D coronary imaging. Magn Reson Med. 2000;43(3):470–480. doi: 10.1002/(sici)1522-2594(200003)43:3<470::aid-mrm20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Jhooti P., Wiesmann F., Taylor A.M., Gatehouse P.D., Yang G.Z., Keegan J. Hybrid ordered phase encoding (HOPE): an improved approach for respiratory artifact reduction. J Magn Reson Imaging. 1998;8(4):968–980. doi: 10.1002/jmri.1880080428. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Riederer S.J., Ehman R.L. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med. 1995;33(5):713–719. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A.M., Jhooti P., Firmin D.N., Pennell D.J. Automated monitoring of diaphragm end-expiratory position for real-time navigator echo MR coronary angiography. J Magn Reson Imaging. 1999;9(3):395–401. doi: 10.1002/(sici)1522-2586(199903)9:3<395::aid-jmri6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Keegan J., Gatehouse P., Yang G.Z., Firmin D. Coronary artery motion with the respiratory cycle during breath-holding and free-breathing: implications for slice-followed coronary artery imaging. Magn Reson Med. 2002;47(3):476–481. doi: 10.1002/mrm.10069. [DOI] [PubMed] [Google Scholar]
- 13.Manke D., Rösch P., Nehrke K., Börnert P., Dössel O. Model evaluation and calibration for prospective respiratory motion correction in coronary MR angiography based on 3-D image registration. IEEE Trans Med Imaging. 2002;21(9):1132–1141. doi: 10.1109/TMI.2002.804428. [DOI] [PubMed] [Google Scholar]
- 14.Manke D., Nehrke K., Börnert P. Novel prospective respiratory motion correction approach for free-breathing coronary MR angiography using a patient-adapted affine motion model. Magn Reson Med. 2003;50(1):122–131. doi: 10.1002/mrm.10483. [DOI] [PubMed] [Google Scholar]
- 15.Nehrke K., Börnert P. Prospective correction of affine motion for arbitrary MR sequences on a clinical scanner. Magn Reson Med. 2005;54(5):1130–1138. doi: 10.1002/mrm.20686. [DOI] [PubMed] [Google Scholar]
- 16.Jahnke C., Nehrke K., Paetsch I., Schnackenburg B., Gebker R., Fleck E. Improved bulk myocardial motion suppression for navigator-gated coronary magnetic resonance imaging. J Magn Reson Imaging. 2007;26(3):780–786. doi: 10.1002/jmri.21078. [DOI] [PubMed] [Google Scholar]
- 17.Jahnke C., Paetsch I., Nehrke K., Schnackenburg B., Gebker R., Fleck E. Rapid and complete coronary arterial tree visualization with magnetic resonance imaging: feasibility and diagnostic performance. Eur Heart J. 2005;26(21):2313–2319. doi: 10.1093/eurheartj/ehi391. [DOI] [PubMed] [Google Scholar]
- 18.Uribe S., Muthurangu V., Boubertakh R., Schaeffter T., Razavi R., Hill D.L.G. Whole-heart cine MRI using real-time respiratory self-gating. Magn Reson Med. 2007;57(3):606–613. doi: 10.1002/mrm.21156. [DOI] [PubMed] [Google Scholar]
- 19.Stehning C., Börnert P., Nehrke K., Eggers H., Stuber M. Free-breathing whole-heart coronary MRA with 3D radial SSFP and self-navigated image reconstruction. Magn Reson Med. 2005;54(2):476–480. doi: 10.1002/mrm.20557. [DOI] [PubMed] [Google Scholar]
- 20.Hardy C.J., Zhao L., Zong X., Saranathan M., Yucel E.K. Coronary MR angiography: respiratory motion correction with BACSPIN. J Magn Reson Imaging. 2003;17(2):170–176. doi: 10.1002/jmri.10250. [DOI] [PubMed] [Google Scholar]
- 21.McLeish K., Kozerke S., Crum W.R., Hill D.L.G. Free-breathing radial acquisitions of the heart. Magn Reson Med. 2004;52(5):1127–1135. doi: 10.1002/mrm.20252. [DOI] [PubMed] [Google Scholar]
- 22.Sussman M.S., Stainsby J.A., Robert N., Merchant N., Wright G.A. Variable-density adaptive imaging for high-resolution coronary artery MRI. Magn Reson Med. 2002;48(5):753–764. doi: 10.1002/mrm.10275. [DOI] [PubMed] [Google Scholar]
- 23.Pipe J.G. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42(5):963–969. doi: 10.1002/(sici)1522-2594(199911)42:5<963::aid-mrm17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Keegan J., Gatehouse P.D., Yang G.Z., Firmin D.N. Non-model-based correction of respiratory motion using beat-to-beat 3D spiral fat-selective imaging. J Magn Reson Imaging. 2007;26(3):624–629. doi: 10.1002/jmri.20941. [DOI] [PubMed] [Google Scholar]
- 25.Plein S., Jones T.R., Ridgway J.P., Sivananthan M.U. Three-dimensional coronary MR angiography performed with subject-specific cardiac acquisition windows and motion-adapted respiratory gating. AJR Am J Roentgenol. 2003;180(2):505–512. doi: 10.2214/ajr.180.2.1800505. [DOI] [PubMed] [Google Scholar]
- 26.Shea S.M., Deshpande V.S., Chung Y.C., Li D. Three-dimensional true-FISP imaging of the coronary arteries: improved contrast with T2-preparation. J Magn Reson Imaging. 2002;15(5):597–602. doi: 10.1002/jmri.10106. [DOI] [PubMed] [Google Scholar]
- 27.Jackson J.I., Meyer C.H., Nishimura D.G., Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application] IEEE Trans Med Imaging. 1991;10(3):473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 28.Weber O.M., Martin A.J., Higgins C.B. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 29.Leiner T., Katsimaglis G., Yeh E.N., Kissinger K.V., van Yperen G., Eggers H. Correction for heart rate variability improves coronary magnetic resonance angiography. J Magn Reson Imaging. 2005;22(4):577–582. doi: 10.1002/jmri.20399. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma H., Ichikawa Y., Suzawa N., Hirano T., Makino K., Koyama N. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237(1):316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 31.Greil G.F., Desai M.Y., Fenchel M., Miller S., Pettigrew R.I., Sieverding L. Reproducibility of free-breathing cardiovascular magnetic resonance coronary angiography. J Cardiovasc Magn Reson. 2007;9(1):49–56. doi: 10.1080/10976640600897427. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande V.S., Krishnam M.S., Ruehm S.G., Finn J.P., Laub G.A. Vol. 1. ISMRM; Seattle (WA): 2006. Non-contrast MR angiography of the heart and great vessels using SSFP with nonselective excitation; p. 383. (Book of abstracts: Fourteenth Annual Meeting of the International Society for Magnetic Resonance in Medicine). [Google Scholar]
- 33.Shea S.M., Wang C., Bi X., Macedo R., Bluemke D.A., Lorenz C.H. Vol. 1. ISMRM; Berlin, Germany: 2007. Optimisation of coronary wall imaging; p. 2475. (Book of abstracts: Fifteenth Annual Meeting of the International Society for Magnetic Resonance in Medicine). [Google Scholar]
- 34.Shea S.M., Kroeker R.M., Deshpande V., Laub G., Zheng J., Finn J.P. Coronary artery imaging: 3D segmented k-space data acquisition with multiple breath-holds and real-time slab following. J Magn Reson Imaging. 2001;13(2):301–307. doi: 10.1002/1522-2586(200102)13:2<301::aid-jmri1043>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Li D., Carr J.C., Shea S.M., Zheng J., Deshpande V.S., Wielopolski P.A. Coronary arteries: magnetization-prepared contrast-enhanced three-dimensional volume-targeted breath-hold MR angiography. Radiology. 2001;219(1):270–277. doi: 10.1148/radiology.219.1.r01ap37270. [DOI] [PubMed] [Google Scholar]
- 36.Botnar R.M., Stuber M., Kissinger K.V., Manning W.J. Free-breathing 3D coronary MRA: the impact of "isotropic" image resolution. J Magn Reson Imaging. 2000;11(4):389–393. doi: 10.1002/(sici)1522-2586(200004)11:4<389::aid-jmri6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Jahnke C., Paetsch I., Schnackenburg B., Gebker R., Köhler U., Bornstedt A. Comparison of radial and Cartesian imaging techniques for MR coronary angiography. J Cardiovasc Magn Reson. 2004;6(4):865–875. doi: 10.1081/jcmr-200036176. [DOI] [PubMed] [Google Scholar]
- 38.Weber O.M., Pujadas S., Martin A.J., Higgins C.B. Free-breathing, three-dimensional coronary artery magnetic resonance angiography: comparison of sequences. J Magn Reson Imaging. 2004;20(3):395–402. doi: 10.1002/jmri.20141. [DOI] [PubMed] [Google Scholar]
- 39.Keegan J., Horkaew P., Buchanan T.J., Smart T.S., Yang G.Z., Firmin D.N. Intra- and interstudy reproducibility of coronary artery diameter measurements in magnetic resonance coronary angiography. J Magn Reson Imaging. 2004;20(1):160–166. doi: 10.1002/jmri.20094. [DOI] [PubMed] [Google Scholar]
- 40.Nehrke K., Börnert P., Manke D., Böck J.C. Free-breathing cardiac MR imaging: study of implications of respiratory motion — initial results. Radiology. 2001;220(3):810–815. doi: 10.1148/radiol.2203010132. [DOI] [PubMed] [Google Scholar]