Abstract
Chronic moderate hypoxia results in systemic and central nervous system adaptations that allow acclimatization. Long-term responses to hypoxia involve systemic physiological changes, metabolic regulation, and vascular remodeling. To investigate whether aging affects systemic and cerebral angiogenic adaptational changes in response to prolonged hypoxia, the present study assessed the responses of 4 month old (“young”) C57BL/6 mice and 24 month old (“aged”) C57BL/6 mice to chronic hypobaric hypoxia of 0.4 ATM (290 torr). Compared to young mice, delayed body weight-loss recovery and a lag in polycythemic response were observed in aged mice. As previously shown, Hypoxia Inducible Factor-1α (HIF-1α) accumulation was attenuated and vascular endothelial growth factor (VEGF) expression was decreased in the cerebral cortex of aged mice. Conversely, Cyclooxygenase-2 (COX-2), angiopoietin-2 (Ang-2) and Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) protein upregulation were not affected in the aged mice. Despite an initial delay in cerebral angiogenic response in aged mice in the first week of hypoxia, no significant differences were observed in microvascular density between young and aged mice in normoxia and at 2 and 3 weeks of hypoxia. Taken together, these observations indicate that even though the HIF-1 response to hypoxia is greatly attenuated, HIF-1 independent compensatory pathways are eventually able to maintain baseline and cerebral angiogenic adaptational changes to chronic hypoxia in aged mice. The delayed adaptive response, however, may result in decreased survival in the aged cohort.
Keywords: HIF-1, VEGF, Ang-2, COX-2, brain capillary density
1. Introduction
In order to maintain normal cellular function, mammalian tissue oxygen concentration must be tightly regulated within a narrow physiological range (Haddad and Harb, 2005; James and Murphy, 2002; Semenza, 2004; Vasquez-Vivar et al., 2000; Wiesener et al., 2003). The immediate responses to oxygen deprivation include hyperventilation and transient increase in blood flow to vital organs. In contrast, long-term responses to hypoxia involve physiological changes, metabolic regulation and vascular remodeling (Dore-Duffy and LaManna, 2007; LaManna et al., 2004). Systemic physiological adaptational changes in response to prolonged hypoxia include polycythemia (increase in hematocrit or red blood cell volume), body weight loss and decreased metabolism (LaManna et al., 2004; Pichiule and LaManna, 2002). Another more dramatic adaptation to chronic hypoxia in the mammalian tissue is structural change of the microvasculature through induction of angiogenesis (i.e., the process of formation of new vascular networks from preexisting capillaries) (Bosch-Marce et al., 2007; Eklund and Olsen, 2006). In a chronic hypoxic challenge to the central nervous system, the cerebral cortex is known to undergo a significant cerebrovascular remodeling, in order to preserve tissue oxygen and energy supply needed to support optimal neuronal function (Boero et al., 1999; Dore-Duffy and LaManna, 2007; LaManna et al., 2004; Pichiule and LaManna, 2003). The microvascular changes occur relatively late compared to the physiological adaptations (Xu et al., 2004; Xu and LaManna, 2006).
Angiogenesis is a highly complex and coordinated process requiring multiple angiogenic and regulatory factors, receptors and intracellular signaling pathways (Benelli et al., 2006; Carmeliet, 2003; Dore-Duffy and LaManna, 2007; Eklund and Olsen, 2006; Semenza, 2003). There appears to be at least two major pathways and likely a number of other redundant pathways responsible for brain angiogenesis: Hypoxia-inducible factor-1 (HIF-1) dependent upregulation of vascular endothelial growth factor (VEGF) (Ferrara et al., 2003; Semenza, 2003), and HIF-1 independent, COX-2 dependent, upregulation of angiopoietin-2 (Ang-2) (Feng et al., 2008; Pichiule and LaManna, 2002). These angiogenesis mechanisms have been previously reviewed (LaManna et al., 2004). HIF-1 is known to be a major transcription factor during hypoxic angiogenesis that activates many downstream genes with a hypoxic response element (HRE) in their promoter regions; which include VEGF, erythropoietin (EPO), glucose transporter-1 (GLUT-1) and the glycolytic enzymes (Dore-Duffy and LaManna, 2007; Semenza, 2003).
There are also other HIF isoforms, HIF-2α and HIF-3α, but HIF-1α is known to be widely expressed and plays a major role in brain during hypoxia (Chavez et al., 2000; LaManna et al., 2004; Yoon et al., 2006). The role of HIF-3α is less clear and its short splice variant, termed as inhibitory PAS protein (IPAS), functions as a transcriptional repressor (Wenger, 2002). Although HIF-2 is suspected as an important regulator of EPO but not VEGF (Chavez et al., 2006), the precise differential contributions of HIF-1 and HIF-2 remain, for the most part, unknown. In addition, it has been shown that despite elevated HIF-2 levels in aged rat brain, VEGF was not significantly upregulated (Ndubuizu et al., 2010), probably due to the action of a HIF-2α specific repressor which would makes it transcriptionally inactive (Hu et al., 2006).
The HIF-1 induction of VEGF gene expression often represents a critical rate-limiting step in angiogenesis (Bates and Curry, 1997; Ferrara et al., 2003; Ferrara and vis-Smyth, 1997; Gerber et al., 1998; Yamagishi et al., 1999). Acting on its two endothelial receptor tyrosine kinases, Flt-1 and Flk-1, VEGF is known to regulate the endothelial cell proliferation and migration that are essential elements of the angiogenic process (Ferrara et al., 2003; Yamagishi et al., 1999). In rats, HIF-1α is detected in the brain, in all cell types, shortly after the onset of hypoxia and persists for at least two weeks, until cerebral angiogenesis is completed within three weeks of exposure to hypoxia (Chavez et al., 2000; Chavez and LaManna, 2003).
Recent studies have also shown involvement of Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) signaling as an auxiliary pathway to induce VEGF expression and suggested its involvement in regulation of angiogenesis in response to deprivation of oxygen and nutrient in rat brain and skeletal muscle cell culture (Arany et al., 2008; Chinsomboon et al., 2009; Ndubuizu et al., 2010; Shoag and Arany, 2010). It was also demonstrated that PGC-1α induction is not regulated by HIF-1 activities and its mechanism of action is independent of the HIF-1 pathway (Arany et al., 2008).
Brain angiogenesis also requires additional proangiogenic factors such as Ang-2. Ang-2, which is not constitutively expressed under normoxic conditions, is upregulated in rat and mouse endothelial cells following hypoxia (Pichiule and LaManna, 2002; Ward et al., 2007). Ang-2 induction during hypoxia is known to occur independently of HIF-1 and is due to cyclooxygenase-2 (COX-2) enzyme activity (LaManna et al., 2006; Pichiule et al., 2004). The effect of Ang-2 on angiogenesis is context-dependent. In the presence of proangiogenic factors such as VEGF, Ang-2 signaling via Tie-2 (tyrosine kinase that contains immunoglobulin-like loops and epidermal-growth-factor-similar domain 2) receptor promotes endothelial cell growth, proliferation, and formation of capillary sprouts to form new vessels (Eklund and Olsen, 2006; LaManna et al., 2004; Pichiule and LaManna, 2002). In contrast, in the absence of proangiogenic factors, Ang-2 induces microvascular apoptotic regression (Eklund and Olsen, 2006; Feng et al., 2008; Pichiule and LaManna, 2002). There are reports from mice, rats, rabbits, and human studies indicating attenuated accumulation of HIF-1α in response to hypoxia which may result in diminished VEGF expression in old age (Bosch-Marce et al., 2007; Chang et al., 2007; Chavez and LaManna, 2003; Rivard et al., 2000a; Rivard et al., 2000b; Rohrbach et al., 2005). But it is not yet known whether Ang-2 expression is affected by aging.
In previous studies we have demonstrated that HIF-1α accumulation in hypoxia is attenuated in the brain of aged rats and this attenuation is most likely due to increased post transcriptional degradation by upregulation of prolyl hydroxylase (Chavez and LaManna, 2003; Ndubuizu et al., 2009). However, baseline capillary density and hypoxic angiogenesis appeared to be unimpeded, possibly due to preserved hypoxic upregulation of the VEGF inducer PGC1α (Arany et al., 2008; Chinsomboon et al., 2009; Ndubuizu et al., 2010).
The objective of this study was to determine if capillary density and hypoxic angiogenesis are also maintained in aged mouse brain, and to determine the relative time course of the hypoxic adaptation pathways in aging. The results demonstrated that although the hypoxic capillary response in aged mice was preserved when measured after 3 weeks of hypoxia, there was a significant delay in the response resulting in a period of vulnerability during the first week of exposure.
2. Results
2.1. Survival rate
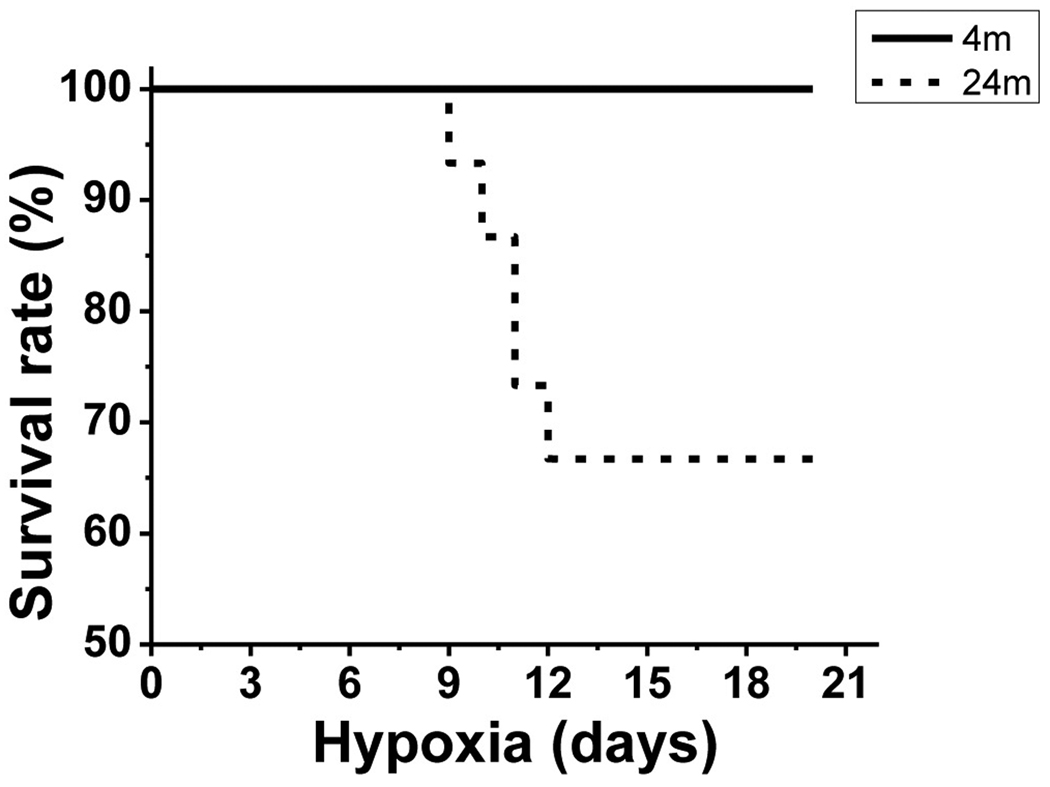
All 4 month old (young) mice (n=16) kept in 290 torr hypoxia for 3 weeks survived throughout hypoxic exposure (Fig.1). In contrast, only 10 of 15 aged mice (66.7%) exposed to hypoxia for 3 weeks survived the hypoxic exposure (n=15). In this and previous studies, the younger mouse cohorts have no difficulty adapting to this hypoxic exposure, with only rare examples of mortality. In this study however, 1/3 of the older mice died, with all of the deaths occurring between 8–12 days of hypoxia. The deaths were from unknown causes, but the mice were found dead the next morning, indicating that they probably died during their nocturnal active phase. The difference in survival rate between young and aged mice was statistically significant (p < 0.05).
Fig.1.
Survival rate. Survival rate in 4 month and 24 month old mice exposed chronic hypoxia, showing 16 of 16 (100%) survival in young mice in 3 weeks of hypoxia, whereas only 10 of 15 (66.7%) of aged mice survived similar hypoxic condition of 3 weeks.
2.2. Systemic physiological changes in response to chronic hypoxia
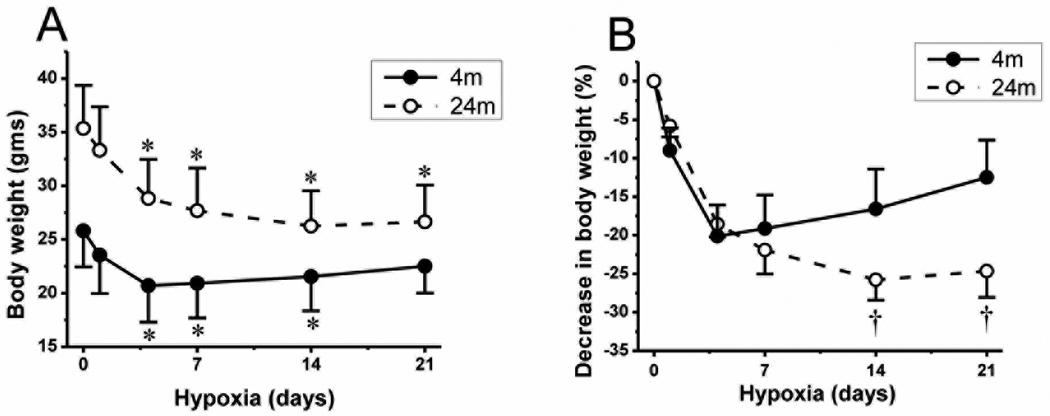
All mice exposed to chronic hypoxia showed rapid and significant body weight loss of about 20% in the first 4 days (Fig. 2A). The young mice recovered from weight loss quickly and started to gain weight after 1 week of hypoxic exposure. The average normoxic (non-hypoxic) body weight of young mice (25.8 ± 3.4 g) decreased to 20.7 ± 4.0 at 4 days of hypoxia. After that, weight recovery was observed in young mice, weighing 20.9 ± 3.2 g at 7 days, 21.5 ± 3.2 g at 14 days and 22.5 ± 2.5 g at 21 days of hypoxia (n = 16). Whereas the aged mice did not recover from the loss of body weight until the end of the second week and only stabilized their weight in the third week. The normoxic body weight of aged mice (35.3 ± 4.0 g) decreased to 28.8 ± 3.6 at 4 days, 27.7 ± 4.0 g at 7 days and 26.3 ± 3.3 g at 14 days of hypoxia. Then, we observed a slight gain in weight in aged mice (26.7 ± 3.4 g) only at 21 days of hypoxia (n = 10). Fig. 2B shows that the relative percent weight gain in young mice starts in the first week of hypoxic exposure, whereas there is a delay in the relative weight gain in the aged mice until the end of the third week. The difference in the relative percent decrease of the body weight between the young and aged mice in 14 and 21 days of hypoxia was statistically significant (p < 0.05).
Fig.2.
Change in body weight during chronic hypoxia. A: change in gross body weight during 21 days of hypoxia in 4 month old (4m) mice (n=16) and 24 month old (24m) mice (n=10). Error bars are indicated at normoxia (0), and 1, 4, 7, 14, and 21 days of hypoxia. B: relative % decrease in body weight in 4m and 24m old mice during hypoxic exposure. Values are means ± standard deviation (SD). *p < 0.05 compared with corresponding age normoxic control value. †p < 0.05 compared with corresponding duration 4 month old mice values.
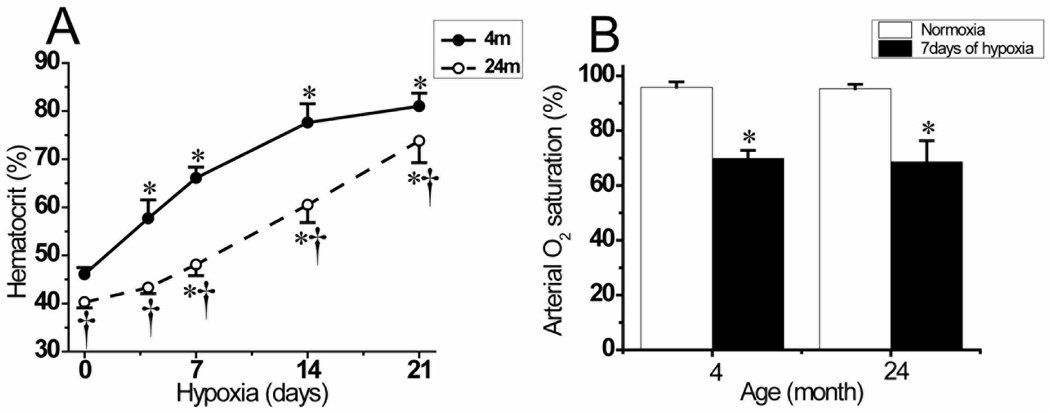
All mice exposed to chronic hypoxia developed polycythemia. In young mice, there was a significant and progressive increase in hematocrit in the first and second week which stabilized at the high level in the third week of hypoxia. In aged mice, however, increase in the relatively reduced normoxic hematocrit was slower in the first week but significantly elevated in the second and third week of hypoxia (Fig. 3A). The differences in the hematocrit values between young and aged mice in normoxic control (46.1 ± 1.4% vs 40.3 ± 1.2%) as well as throughout the end of third week of hypoxic exposure (81.0 ± 2.7% vs 73.8 ± 4.6%) were significant (p < 0.05). The arterial oxygen saturation (SaO2), which is hemoglobin oxygen saturation in arterial blood, measured in separate groups of mice was: 95.8 ± 2.0% and 69.8 ± 3.0% in normoxia and at 7 days of hypoxia respectively in young mice, and 95.3 ± 1.6% and 68.6 ± 7.7% in normoxia and at 7 days of hypoxia respectively in aged mice (Fig.3B). The difference in arterial oxygen saturation between age groups was not significant but the difference between normoxia and hypoxia exposed mice in both age groups was statistically significant (p < 0.05).
Fig.3.
Hematocrit and arterial oxygen saturation during chronic hypoxia. A: hematocrit values in 4 month old mice under normoxic control (0) (n=19), and 4 (n=10), 7 (n=10), 14 (n=10), and 21 (n=16) days of hypoxia; and 24 month old mice under normoxic control (0) (n=11), and 4 (n=10), 7 (n=11), 14 (n=10), and 21 (n=10) days of hypoxia. B: arterial oxygen saturation in 4month and 24 month old mice in normoxic and 7 days hypoxia exposed mice (n=6 mice in each groups). Values are means ± standard deviation (SD). *p < 0.05 compared with corresponding age normoxic control value. †p < 0.05 compared with corresponding duration 4 month old mice values.
2.3. HIF-1α accumulation in cerebral cortex
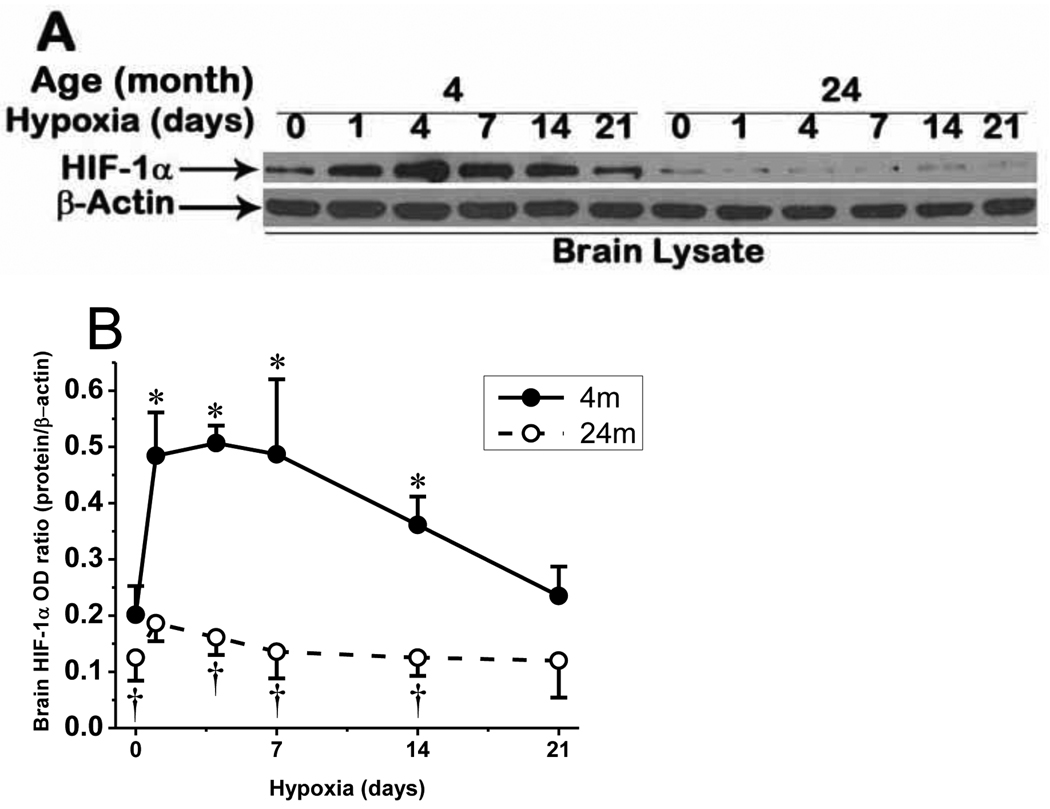
Since HIF-1 is the main transcription factor for induction of many genes involved in erythropoiesis and angiogenesis during hypoxia (Carmeliet, 2003; Semenza, 2003), we compared its accumulation in young and aged mouse cerebral cortex. The Western blot analyses showed the characteristic HIF-1α protein band with the molecular weight of 120 kDa in brain cortical samples (Fig. 4A). We observed rapid and significant accumulation of HIF-1α in the first week of hypoxia in young mice which gradually returned to baseline level in prolonged hypoxia. HIF-1α protein accumulation was found to be attenuated in aged mice, in normoxia and persistently throughout hypoxic periods, demonstrating an impared HIF-1α accumulation in the aged brain (Fig. 4A and 4B).
Fig. 4.
HIF-1α accumulation in cerebral cortex of mouse in chronic hypoxia. A: attenuated HIF-1α accumulation in aged mice in normoxia and chronic hypoxia compared to the young mice. B: Optical density ratio of HIF-1α normalized to β-actin in normoxia (0), and 1, 4, 7, 14, and 21 days of hypoxia. *p < 0.05 compared with corresponding age normoxic control. †p < 0.05 compared with corresponding 4 month old mice values. Each value represents the mean ± SD from 5 mice.
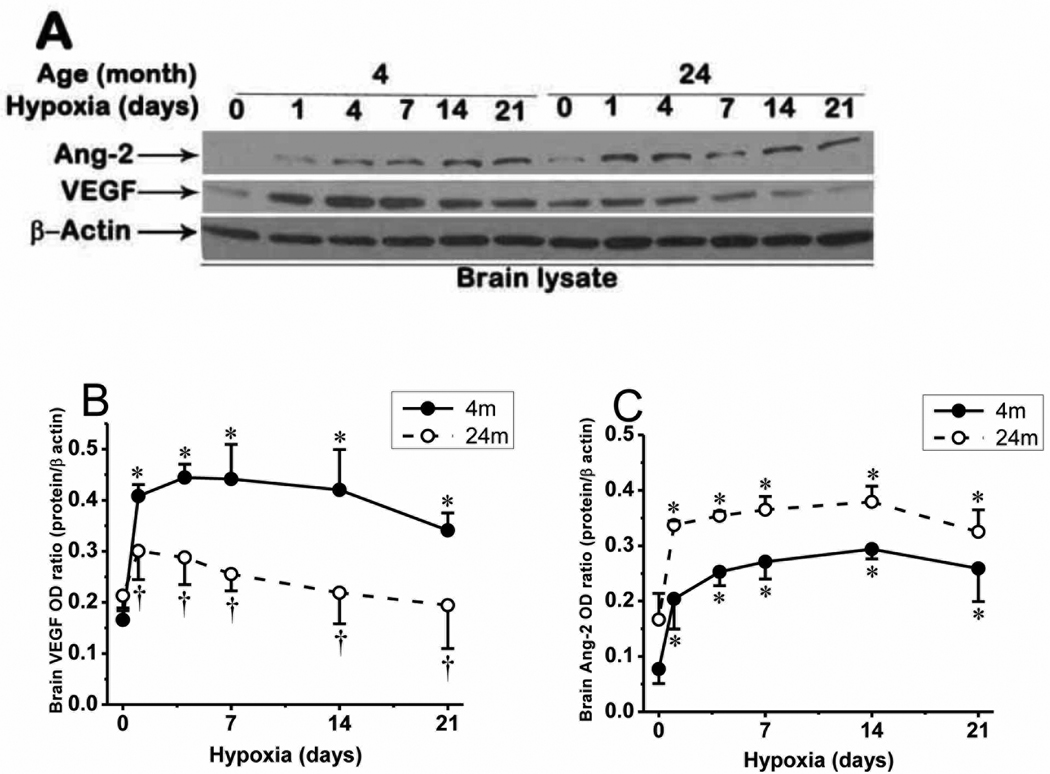
2.4. VEGF and Ang-2 expression in cerebral cortex
Since angiogenesis requires the coordinated expression and signaling between proangiogenic proteins and factors involved in the process (Dore-Duffy and LaManna, 2007; Pichiule and LaManna, 2002), we investigated the expression of the two main angiogenic proteins, VEGF (molecular weight of about 42 kDa) and Ang-2 (molecular weight of about 68 kDa), in young and aged mice cerebral cortex (Fig. 5). Ang-2 protein expression which was barely detected during normoxia was significantly upregulated throughout chronic hypoxia of 3 weeks, in both young and aged mice, being slightly higher in aged mice. As expected, VEGF expression was upregulated during chronic hypoxia in young mice, but hypoxic upregulation of VEGF was diminished, but not completely attenuated in aged mice compared to its expression in the young mice. The difference in VEGF expression in cerebral cortex between young and aged mice during similar hypoxic time point is statistically significant (p < 0.05).
Fig. 5.
Expression of VEGF and Ang-2 in cerebral cortex of 4 month and 24 month old mice. A: VEGF and Ang-2 protein expression in normoxic control and hypoxia in young and aged mice. B: VEGF protein expression optical density ratio in young and aged mice in normoxia (0), and 1, 4, 7, 14, and 21 days of hypoxia and C: Ang-2 protein expression optical density ratio in young and aged mice in normoxia (0), and 1, 4, 7, 14, and 21 days of hypoxia. *p < 0.05 compared with normoxic control values of each category. †p < 0.05 aged mice vs corresponding young mice values. Values are mean ± SD, n = 5 mice per time point.
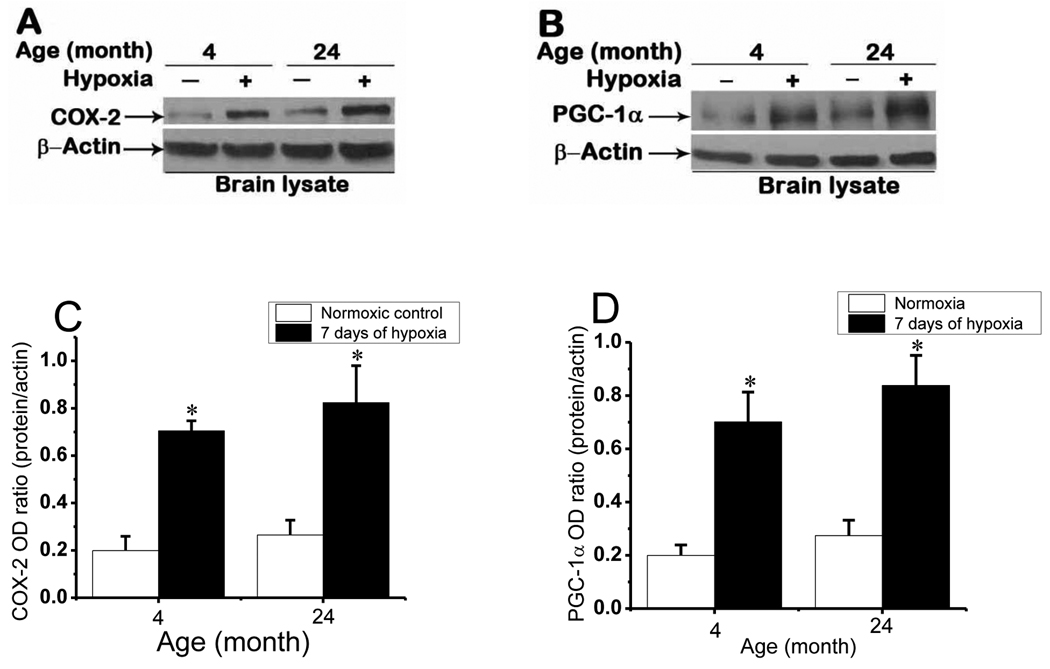
2.5. COX-2 and PGC-1α expression in cerebral cortex
COX-2 signaling is the main pathway for Ang-2 induction during hypoxia (LaManna et al., 2006; Pichiule et al., 2004). To assess whether COX-2 expression is affected with aging we analyzed COX-2 protein expression in brain cortical samples from normoxic and 7 days hypoxic mice. Diminutive baseline normoxic expression of COX-2 was significantly upregulated (band near 72 kDa) at 7 days of chronic hypoxia in both age groups (Fig. 6A). COX-2 expression in normoxic control as well as at 7 days of hypoxia was observed to be slightly higher in aged mice compared to the young mice, but the difference was not statistically significant. This implies that the COX-2 and corresponding Ang-2 (Fig.5A) signaling pathway is not impaired with aging. PGC-1α expression was also unaffected by aging. Normoxic cortical samples showed a faint PGC-1α band at about 91 kDa that was strongly induced at 7 days of hypoxia in both age groups (Fig. 6B). The difference in PGC-1α protein expression between young and aged mice in normoxic control as well as at 7 days of hypoxia was not statistically significant. Since PGC-1α is known to be involved as auxiliary signaling cofactor for induction of VEGF during hypoxia in the absence of HIF-1, strong PGC-1α expression in aged mice may explain the intact expression of VEGF in aged mice, despite blunted expression of HIF-1α.
Fig. 6.
COX-2 and PGC-1α expression in 4 and 24 month old mice cerebral cortex in normoxia and 7 days of chronic hypoxia. Representative Western blot analysis of Cox-2 (A) and PGC-1α (B) during normoxic control and 7 days of hypoxic exposure in the young and aged mice. C and D: Optical density ratios of COX-2 and PGC-1α respectively, normalized to β-actin in normoxia and 7 days of hypoxia as a function of age. *p < 0.05 compared with normoxic control values of each age group. Values are mean ± SD, n = 5 mice in each category.
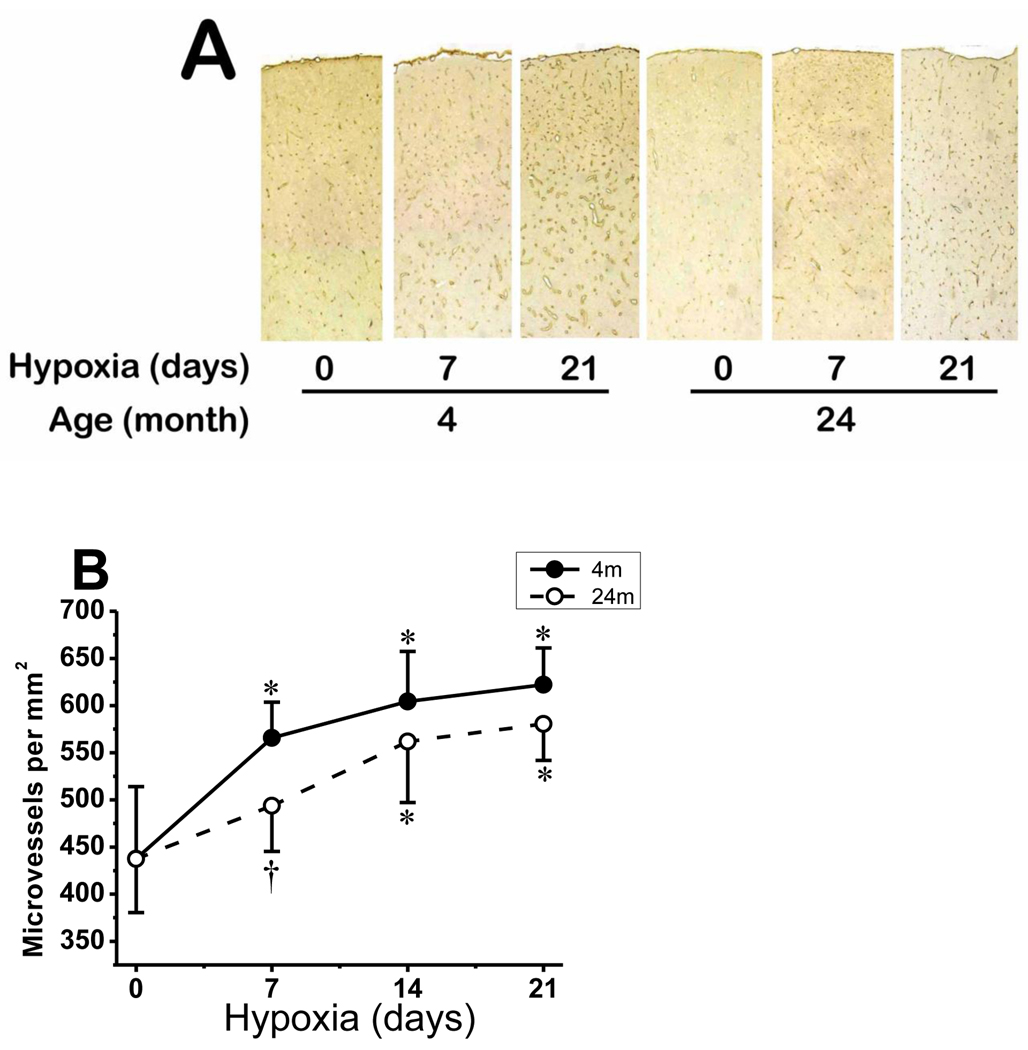
2.6. Microvascular density in cerebral cortex
Cerebral capillaries were identified by GLUT-1 immunostaining and their density was quantified by counting the number of positive capillaries per unit area. In young mice, as expected, there was robust capillary sprouting, 29% increase, in the first week of hypoxia, which remained significantly increased in the second and third weeks of hypoxia (42% increase in cortical microvessels after 21 days of hypoxic exposure). The average normoxic capillary count, per mm2, of young mice (438 ± 76) increased to 566 ± 38 at 7 days, 604 ± 53 at 14 days and 622 ± 39 at 21 days of hypoxia (Fig. 7). On the other hand, there was initial lag in capillary sprouting in aged mice in the first week of hypoxia (increase in 13% only), which then showed significant increase in second and third weeks of hypoxia (33% increase in cortical microvessels after 21 days of hypoxic exposure) (Fig. 7B). The average normoxic capillary count, per mm2, of aged mice (437 ± 57) increased to 494 ± 49 at 7 days, 562 ± 65 at 14 days and 580 ± 39 at 21 days hypoxia. Compared to young mice, there was a significant difference (p < 0.05) in capillary density in aged mice at the 7 days of hypoxia. However, the differences in capillary density between young and aged mice in normoxia as well as 14 and 21 days of hypoxia did not reach statistical significance.
Fig. 7.
Microvascular density in young and aged mice cerebral cortex in normoxic control and prolonged hypoxia. A: composite photomicrograph of GLUT-1–stained sections spanning part of the parietal cortex of 4 and 24 month old mice at normoxia (0), and 7 and 21 days of hypoxia. B: Capillary density count (number per mm2) of GLUT-1-stained sections of 4 month old and 24 month old mice during normoxia (0) and 7, 14 and 21 days of hypoxia. *p < 0.05 compared with corresponding normoxic control. †p < 0.05 compared with corresponding 4 month old mice value. Values are mean ± SD, n = 5 mice per time point in each group.
3. Discussion
Prolonged moderate hypoxia, such as occurs with exposure to altitude, results in systemic and central nervous system adaptational changes that allow successful acclimatization (LaManna et al., 2004; Lenfant and Sullivan, 1971). Weight loss, decreased metabolism and body temperature, increased hematocrit and brain microvascular remodeling are important compensatory mechanisms responsible for this adaptation (LaManna et al., 2004; Mortola et al., 1994). Older animals have decreased ability to acclimatize to prolonged hypoxia, but the specific defects underlying this increased vulnerability are not known. In this investigation we found significant differences in some of these adaptive mechanisms between young and aged mice.
The rapid and significant systemic physiological changes in response to hypoxia in young mice after a few days of chronic hypoxia in this study are consistent with previous reports (Chavez et al., 2000; Pichiule and LaManna, 2002; Xu and LaManna, 2006). However, in this study we observed delayed recovery in weight loss, a lag in polycythemic adaptational response and significant diminution of hematocrit values in normoxia and throughout hypoxic durations in aged mice. Delayed weight regain and retarded polycythemic response in aged mice may be due to impairment in hypoxic stress adaptive mechanisms and molecular and cellular mechanisms responsible for production and/or longevity of the red blood cells (Anderson et al., 2009; Weed, 1975; Yoon et al., 2006).
The lag in systemic erythropoeitic and brain angiogeic mechanisms may have resulted in increased vulnerability and contributed to the death of the aged mice after the first week of hypoxia when acute compensatory mechanisms to hypoxia, such as increase of blood flow to vital organs by vascular vasodilatation, decline and has to be overtaken by long term compensatory mechanisms (Xu and LaManna, 2006). According to the acclimatization time line the most important event occurring between 8 and 12 days of hypoxic exposure is the opening of the newly formed capillaries (Xu et al., 2010; Xu and LaManna, 2006). This could suggest that mortality may result from failure of the angiogenic mechanisms. In our previous study in rats we have recorded decreased hypoxic ventilatory ratios (HVR) with aging (Xu et al., 2010), which may be due to reduced respiratory muscles strength, lung elasticity, and hypoxic ventilatory drive in older animals (Mahler et al., 1986; Sharma and Goodwin 2006; Zeleznik, 2003). However, in this study we have recorded that arterial oxygen saturation was significantly diminished after 7 days of hypoxia in both age groups but there was no significant difference in SaO2 between young and aged mice. At the molecular level exacerbation of hypoxic adaptation may be due to a decreased expression of HIF-1α target genes like VEGF, EPO or GLUT-1 (Cataldi et al., 2004; Rivard et al., 2000a; Xia et al., 1997).
Consistent with previous reports from mice, rats and humans (Anderson et al., 2009; Bosch-Marce et al., 2007; Chavez and LaManna, 2003; Ndubuizu et al., 2009) we observed increased HIF-1α accumulation in response to hypoxia which returned toward baseline normoxic level in prolonged hypoxia in young mice, and attenuated HIF-1α accumulation in aged mice in normoxia and prolonged hypoxia. Similar decreases in HIF-1α accumulation were observed in mouse brain, liver, kidney and heart as a function of age (Frenkel-Denkberg et al., 1999; Rohrbach et al., 2005). Diminished HIF-1α accumulation with aging is due to an increase in its post-translational degradation by increased accumulation of prolyl hydroxylase domain (PHD) enzymes (Katschinski, 2006; Ndubuizu et al., 2009; Rohrbach et al., 2005). Increased accumulation of PHDs with aging was reported from human heart (Rohrbach et al., 2005) rat brain (Ndubuizu et al., 2009), and mouse brain, liver, kidney and heart (Frenkel-Denkberg et al., 1999; Katschinski, 2006; Li et al., 2010; Rohrbach et al., 2005).
In young mice, both VEGF and Ang-2 protein expressions were transiently upregulated during hypoxia. However, an inverse pattern of induction between VEGF and Ang-2 was observed in aged mice during normoxia and prolonged hypoxia. This differential regulation of Ang-2 and VEGF expression may be explained by the difference in transcriptional regulation between VEGF and Ang-2. VEGF has Hypoxic response element (HRE) and its upregulation during hypoxia is mainly induced by HIF-1 (Ferrara et al., 2003; Pichiule et al., 2004; Rivard et al., 2000a; Semenza, 2003). Nevertheless, observation that severe attenuation of HIF-1α did not completely abolish expression of VEGF in normoxia and hypoxic durations in cerebral cortex of aged mice implies that there are other signaling pathways for enhancing the expression of VEGF. In this regard, PGC-1α is an accessory HIF-1 pathway independent inducer of VEGF and angiogenesis in response to hypoxia and nutrient deprivation (Arany et al., 2008; Chinsomboon et al., 2009; Ndubuizu et al., 2010; Shoag and Arany, 2010). Our observation that aging did not affect the hypoxic PGC-1α level in normoxia and hypoxia implies that this compensatory mechanism remains active in aged animals to spare the important angiogenic and neuroprotective functions of VEGF, as we found in the rat (Ndubuizu et al., 2010). In Fischer rats, VEGF protein level was reported to be very low at day 4 (Ndubuizu et al., 2009) but increased at day 10 of hypoxia (Ndubuizu et al., 2010). The secondary VEGF pathways may be quite different in rat and mouse. Reduction in VEGF expression in kidney of aged mouse (Li et al., 2010; Yamaji et al., 2010) but not in liver of aged mouse (Li et al., 2010) or rat (Cheluvappa et al., 2007) were also observed. These differences may be due to species and/or organ specific differences in expression of angiogenic proteins in rodents as noted previously (Ward et al., 2007).
Ang-2 induction in hypoxia is independent from HIF-1 in vivo and is due to COX-2 signaling mechanisms (LaManna et al., 2004; Pichiule et al., 2004). COX-2 is constitutively expressed primarily in neurons, but is upregulated in capillary endothelial cells with hypoxic exposure (LaManna et al., 2006). COX-2 catalyzes the rate limiting step in biosynthesis of prostaglandin E-2 (PGE-2) from arachidonic acid (LaManna et al., 2006; Mayahara et al., 2007; Wu et al., 2003). It has been demonstrated that PGE-2 is responsible for induction of Ang-2 during hypoxia (LaManna et al., 2006; Pichiule et al., 2004). Under normal physiological conditions, angiopoietin-1 (Ang-1) is constitutively produced by pericytes and astrocytes and binds to and phosphorylates the Tie-2 receptors to help maintain structural integrity of the capillaries (Dore-Duffy and LaManna, 2007; LaManna et al., 2004). However, following hypoxia, upregulated Ang-2 binds to the Tie-2 receptors, blocking the binding site and antagonizing the activities of Ang-1 (Dore-Duffy and LaManna, 2007; LaManna et al., 2004). This causes destabilization of microvessels, and if sufficient amount of VEGF is present, consecutive endothelial cell growth, proliferation, and formation of capillary sprouts leads to formation of new vessel or angiogenesis (Dore-Duffy and LaManna, 2007; Eklund and Olsen, 2006; LaManna et al., 2004). This results in increased capillary density, decreased intercapillary distance and increased oxygen availability in the vicinity of the cells (Boero et al., 1999; Dore-Duffy and LaManna, 2007; Eklund and Olsen, 2006; LaManna et al., 2004).
Upregulation of COX-2 during hypoxia and its involvement in physiological and pathological angiogenesis have been reported (Dore-Duffy and LaManna, 2007; LaManna et al., 2006; Manabe et al., 2004). Diminished COX-2 expression has been seen in old mouse bone tissue (Naik et al., 2008), whereas upregulated COX-2 expression was shown in mouse and human connective and glandular tissues in advanced age (Wu et al., 2003; Wu et al., 2007). However, in this study we report that aging did not significantly affect COX-2 – Ang-2 protein expression in mouse brain in normoxia and during chronic hypoxia. Although there was slightly upregulated level in COX-2 and Ang-2 expression in aged mice in normoxia and hypoxia compared to the young mice, the difference did not reach statistical significance.
Brain vascular remodeling depends on the balance between HIF-1 dependent and HIF-1 independent pro and anti-angiogenic processes (Dore-Duffy and LaManna, 2007; Eklund and Olsen, 2006; LaManna et al., 2006). Although VEGF is known to be a rate limiting proangiogenic growth factor, it has to be co-induced with Ang-2 to bring effective angiogenic sprouting and vascular elongation (Carmeliet, 2003; Feng et al., 2008; Pichiule and LaManna, 2002). Robust angiogenic response in aged mice during prolonged hypoxia indicates that the amount of VEGF induced in HIF-1 independent pathways is sufficient to maintain brain vascular remodeling (capillary angioplasticity) in advanced age, reflecting the complexities of the brain angiogenic processes.
In conclusion, we have demonstrated for the first time that there is a delay in systemic physiological and brain angiogenic adaptational changes in response to hypoxia in aged mice in the first week or so of hypoxic exposure. However, despite the significant delay, the brain angiogenic adaptational changes to prolonged hypoxia in aged mice were eventually completed. Though HIF-1α protein accumulation is diminished in aged mice cerebral cortex stabilized VEGF and upregulated Ang-2 expressions through HIF-1 independent pathways maintain the balance in vascular proangiogenic proteins to enhance brain vascular angiogenesis in prolonged hypoxia.
4. Experimental procedures
4.1. Exposure to chronic hypobaric hypoxia
Male 4 month old C57BL/6 mice (young mice) and 24 month old C57BL/6 mice (aged mice) were purchased from National Institute for Aging (NIA) and maintained at the Case Western Reserve University Animal Care Facility for a few days before experiments. Animal housing and handling met Institutional Animal Care and Use Committee standards. The experimental protocol was approved by the Animal Care and Use committee at Case Western Reserve University. Mice that were exposed to hypoxia were placed in hypobaric chambers for periods up to 3 weeks, at a constant pressure of 0.4 ATM (290 torr, equivalent to 8% normobaric oxygen or altitude of about 23,000 feet). The littermate normoxic (normobaric control) mice were housed in the same room next to the hypobaric chamber to ensure identical ambient conditions. The hypoxic animals were returned daily to normoxia for a maximum of 30 minutes for cage cleaning, food and water replacement, and body weight measurement.
4.2. Arterial oxygen saturation measurement
Arterial oxygen saturation was assessed in a group of mice kept in normoxia or 7 days of hypoxia by using a Mouse-Ox pulse oximeter (STARR Life Sciences Corp., Oakmount, PA). These mice were anesthetized with isoflurane (2.5% isoflurane in medical air) and maintained with 1–2% isoflurane through a nasal cone during the procedure of loosely plastering the limbs to restrict movements and shaving the thigh region where the oximetry recorder is placed. Pulse oximetry data was recorded in awake animal after an hour of adaptation in normoxia or hypoxia.
4.3. Tissue Collection
Mice were weighed, anesthetized and tail blood samples were obtained for hematocrit determination before the animals were sacrificed. At least 10 mice from the 4 month and 24 month old groups under normoxia or hypobaric hypoxia of up to 21 days were either sacrificed, for collection of fresh specimen, or perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde, for in vivo fixation of the tissues. For Western blot analysis, cerebral cortex was dissected and immediately frozen in liquid nitrogen and stored at −80°C until further processing. To collect tissues for immuonhistochemistry, animals from normoxia or hypoxia were perfused transcardially with about 30–50 ml of ice cold PBS, pH 7.2, followed by 4% paraformaldehyde in PBS (about 100 ml). Brain and other organs were dissected and postfixed in 4% paraformaldehyde overnight and then stored at 4°C in Millonig’s buffer (NaH2PO4-H2O, NaOH, Sodium Azide and double distilled water (ddH2O), for cytoprotection, until embedded in paraffin wax.
4.4. Preparation of whole cell lysates
Frozen brain cortex samples from normoxic and hypoxic mice were dissected and homogenized in ice-cold lysis buffer (150mM NaCl, 50mM Tris.HCl pH 7.4, 1mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, in ddH2O) containing EDTA-free protease inhibitor tablet (Complete Mini, Roche Diagnostics, Indianapolis, IN). Homogenates were kept on ice for an hour and then centrifuged at 14,000 g for 30 minutes at 4°C. The supernatants were collected, aliquoted, and stored at −80 °C. Protein contents in the supernatants were determined by a Bradford protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as a standard.
4.5. Western blot analysis
Proteins from the whole cell lysates were separated by using SDS gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked in 5% skimmed milk in tris-buffered saline with 0.1% tween (TBS-T) for 1 hour, and incubated overnight in the same blocking solution with the primary antibodies. The specific primary antibodies of interest were: HIF-1α (1:500; R&D Systems, Minneapolis, MN), VEGF-A (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), Ang-2 (1:200; Millipore Co., Billerica, MA), COX-2 (1:150, Cayman, Ann Arbor, MI), PGC-1α (1:750; Novus, Littleton, CO) and β-actin (1:500; Santa Cruz, CA). The membranes were washed three times each for 15 minutes with TBS-T, followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies (Invitrogen, Camarillo, CA). After a series of three washes each for 15 minutes with TBS-T, immunoreactive protein bands were visualized using an enhanced chemiluminescence detection system (SuperSignal ECL Kit, Thermo Scientific, IL) and subsequent exposure of the membrane to Hyperfilm (Thermo Scientific, IL). Computer aided Sigma Scan was used to quantify densitometry of the protein bands and normalized to that of β-actin (optical density ratio).
4.6. Immunohistochemistry
Paraformaldehyde fixed brain specimens were washed in running water, dehydrated with increasing concentrations of ethanol (80%, 95%, and 100%), cleared with xylene, and embedded in paraffin. Coronal serial sections (5 µm) were cut from the paraffin embedded brains with a low-profile blade microtome, transferred to slides, and allowed to dry at room temperature. The slides were deparaffinized with xylene, rehydrated with graded ethanol (100%, 95%, 70% and 50%), and washed with PBS (3 × 5 minutes each). Tissue sections were then subjected to antigen retrieval at 90° C for 10 minutes in 0.1M sodium citrate buffer, and incubated with 3% H2O2, to quench endogenous peroxide. The sections were washed with PBS (3 × 5 minutes each), and blocked for 1 hour in 10% normal horse serum, followed by incubation in Avidin-D and Biotin-B solutions (Vector Labs, Burlingame, CA). The sections were incubated overnight (at 4° C) with goat polyclonal primary antibody against GLUT-1 (1:200; Santa Cruz, CA), diluted in the same blocking buffer. After a series of 3 washes, 5 minutes each, the sections were covered with biotinylated anti-goat (made in horse) secondary antibody (Vector Labs, Burlingame, CA). After 3 serial washes for 5 minutes each with PBS, the sections were covered with ABC solution for 30 minutes (Vector Labs, Burlingame, CA), washed again with PBS and incubated in DAB solution (Vector Labs, Burlingame, CA). Sections were dehydrated with increasing concentrations of ethanol (50%, 70%, 80%, 95% and 100%) and cleared with xylene. The slides were mounted with Eukit media, cover-slipped and visualized using a brightfield microscope.
4.7. Microvessel Density
Capillary density was computed on tissue immunohistochemically stained with antibodies against GLUT-1. A photo montage spanning the full depth of the parietal cortex was created using a SPOT digital camera connected to a Nikon E600 Eclipse microscope with a 20X objective. A computer-aided image analysis system (ImagePro Plus) was used to count marked antibody positive capillaries and determine the number per unit area of brain tissue.
4.8. Statistical analysis
Quantitative data are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using Origin Lab Data Analysis and Graphing software version 8.1. Statistical comparisons were performed by one-way ANOVA followed by Tukey’s comparison. In all cases, P < 0.05 was considered statistically significant. Statistical analysis for survival rate was performed by Wilcoxon survival analysis using SPSS v13.0 for Windows.
Acknowledgments
We thank Youzhi Kuang for technical assistance during arterial oxygen saturation measurement. This study was supported by National Institute of Health grant NS-38632.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J. Neurotrauma. 1566;26:1557–1566. doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am. J. Physiol. 1997;273:H687–H694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- Benelli R, Lorusso G, Albini A, Noonan DM. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr. Pharm. Des. 2006;12:3101–3115. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J. Appl. Physiol. 1999;86:1211–1219. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of Aging and Hypoxia-Inducible Factor-1 Activity on Angiogenic Cell Mobilization and Recovery of Perfusion Following Limb Ischemia. Circ. Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Cataldi A, Bianchi G, Rapino C, Sabatini N, Centurione L, Di GC, Bosco D, Antonucci A. Molecular and morphological modifications occurring in rat heart exposed to intermittent hypoxia: role for protein kinase C alpha. Exp. Gerontol. 2004;39:395–405. doi: 10.1016/j.exger.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J. Appl. Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J. Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JC, LaManna JC. Hypoxia-inducible factor-1alpha accumulation in the rat brain in response to hypoxia and ischemia is attenuated during aging. Adv. Exp. Med. Biol. 2003;510:337–341. doi: 10.1007/978-1-4615-0205-0_55. [DOI] [PubMed] [Google Scholar]
- Cheluvappa R, Hilmer SN, Kwun SY, Jamieson HA, O'Reilly JN, Muller M, Cogger VC, Le Couteur DG. The effect of old age on liver oxygenation and the hepatic expression of VEGF and VEGFR2. Exp. Gerontol. 2007;42:1012–1019. doi: 10.1016/j.exger.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid. Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Feng Y, Pfister F, Schreiter K, Wang Y, Stock O, vom HF, Wolburg H, Hoffmann S, Deutsch U, Hammes HP. Angiopoietin-2 deficiency decelerates age-dependent vascular changes in the mouse retina. Cell Physiol. Biochem. 2008;21:129–136. doi: 10.1159/000113755. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrara N, vis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Harb HL. Cytokines and the regulation of hypoxia-inducible factor (HIF)-1alpha. Int. Immunopharmacol. 2005;5:461–483. doi: 10.1016/j.intimp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AM, Murphy MP. How mitochondrial damage affects cell function. J. Biomed. Sci. 2002;9:475–487. doi: 10.1159/000064721. [DOI] [PubMed] [Google Scholar]
- Katschinski DM. Is there a molecular connection between hypoxia and aging? Exp. Gerontol. 2006;41:482–484. doi: 10.1016/j.exger.2005.12.003. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J. Exp. Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Sun X, Ivy AD, Ward NL. Is cycloxygenase-2 (COX-2) a major component of the mechanism responsible for microvascular remodeling in the brain? Adv. Exp. Med. Biol. 2006;578:297–303. doi: 10.1007/0-387-29540-2_47. [DOI] [PubMed] [Google Scholar]
- Lenfant C, Sullivan K. Adaptation to high altitude. N. Engl. J. Med. 1971;284:1298–1309. doi: 10.1056/NEJM197106102842305. [DOI] [PubMed] [Google Scholar]
- Li X, Sutherland S, Takeda K, Fong GH, Lee FS. Integrity of the prolyl hydroxylase domain protein 2:erythropoietin pathway in aging mice. Blood Cells Mol. Dis. 2010;45:9–19. doi: 10.1016/j.bcmd.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Rosiello RA, Loke J. The aging lung. Clin. Geriatr. Med. 1986;2:215–225. [PubMed] [Google Scholar]
- Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann. Neurol. 2004;55:668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- Mayahara K, Kobayashi Y, Takimoto K, Suzuki N, Mitsui N, Shimizu N. Aging stimulates cyclooxygenase-2 expression and prostaglandin E2 production in human periodontal ligament cells after the application of compressive force. J. Periodontal. Res. 2007;42:8–14. doi: 10.1111/j.1600-0765.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Matsuoka T, Saiki C, Naso L. Metabolism and ventilation in hypoxic rats: effect of body mass. Respir. Physiol. 1994;97:225–234. doi: 10.1016/0034-5687(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O'Keefe RJ. Reduced COX-2 Expression in Aged Mice is Associated with Impaired Fracture Healing. J. Bone Miner. Res. 2009;24:251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndubuizu OI, Chavez JC, LaManna JC. Increased Prolyl 4-Hydroxylase (PHD) Expression and Differential Regulation of Hypoxia Inducible Factors (HIFs) in the Aged Rat Brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;279:R158–R165. doi: 10.1152/ajpregu.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndubuizu OI, Tsipis CP, Li A, LaManna JC. Hypoxia Inducible Factor-1 (HIF-1) Independent Microvascular Angiogenesis in the Aged Rat Brain. Brain Res. 2010;1366:101–109. doi: 10.1016/j.brainres.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 2004;279:12171–12180. doi: 10.1074/jbc.M305146200. [DOI] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J. Appl. Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Expression of angiopoietin-1 and -2 in the rat brain during chronic hypoxia and de-adaptation. Adv. Exp. Med. Biol. 2003;510:331–335. doi: 10.1007/978-1-4615-0205-0_54. [DOI] [PubMed] [Google Scholar]
- Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J. Biol. Chem. 2000a;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- Rivard A, Principe N, Andres V. Age-dependent increase in c-fos activity and cyclin A expression in vascular smooth muscle cells. A potential link between aging, smooth muscle cell proliferation and atherosclerosis. Cardiovasc. Res. 2000b;45:1026–1034. doi: 10.1016/s0008-6363(99)00385-5. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Simm A, Pregla R, Franke C, Katschinski DM. Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology. 2005;6:165–171. doi: 10.1007/s10522-005-7950-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoag J, Arany Z. Regulation of hypoxia-inducible genes by PGC-1 alpha. Arterioscler. Thromb. Vasc. Biol. 2010;30:662–666. doi: 10.1161/ATVBAHA.108.181636. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J. Biol. Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J. Appl. Physiol. 2007;102:1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- Weed RI. Membrane structure and its relation to haemolysis. Clin. Haematol. 1975;4:3–28. [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J. Biol. Chem. 2003;278:10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J. Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- Xia Y, Warshaw JB, Haddad GG. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am. J. Physiol. 1997;273:R1734–R1741. doi: 10.1152/ajpregu.1997.273.5.R1734. [DOI] [PubMed] [Google Scholar]
- Xu K, LaManna JC. Chronic hypoxia and the cerebral circulation. J. Appl. Physiol. 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, LaManna JC. Renormalization of regional brain blood flow during prolonged mild hypoxic exposure in rats. Brain Res. 2004;1027:188–191. doi: 10.1016/j.brainres.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X, LaManna JC. Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res. 2010;1328:181–189. doi: 10.1016/j.brainres.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Yonekura H, Yamamoto Y, Fujimori H, Sakurai S, Tanaka N, Yamamoto H. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab. Invest. 1999;79:501–509. [PubMed] [Google Scholar]
- Yamaji M, Bielby H, Licence D, Cheng CW, Cook E, Smith SK, Print CG, Charnock-Jones DS. VEGF-A loss in the haematopoietic and endothelial lineages exacerbates age-induced renal changes. Microvasc. Res. 2010;80:372–383. doi: 10.1016/j.mvr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J. Biol. Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- Zeleznik J. Normative aging of the respiratory system. Clin. Geriatr. Med. 2003;19:1–18. doi: 10.1016/s0749-0690(02)00063-0. [DOI] [PubMed] [Google Scholar]