Abstract
We measured the energy expenditure weekly in patients undergoing a pylorus preserving pancreatoduodenectomy for bile duct cancer or pancreatic tumors. Twelve patients (5 women and 7 men; mean age 70.1 years) were enrolled in this study, and their resting energy expenditure levels were determined by indirect calorimetry. In these patients, a significant correlation was observed between the measured resting energy expenditures and the predicted resting energy expenditures calculated by the Harris-Benedict equation. The resting energy expenditures measured before surgery were almost the same as the predicted resting energy expenditures (measured resting energy expenditure: 22.4 ± 3.9 kcal/kg/day vs predicted resting energy expenditure: 21.7 ± 2.0 kcal/kg/day). The measured resting energy expenditure/predicted resting energy expenditure ratio, which reflects the stress factor, was 1.02 ± 0.10. After the pylorus preserving pancreatoduodenectomy, a significant increase in energy expenditure was observed, and the measured resting energy expenditure was 25.7 ± 3.5 kcal/kg/day on postoperative day 7 and 25.4 ± 4.9 kcal/kg/day on postoperative day 14. The measured resting energy expenditure/predicted resting energy expenditure ratio was 1.16 ± 0.14 on postoperative day 7, and 1.16 ± 0.18 on postoperative day 14 respectively. In conclusion, patients undergoing a pylorus preserving pancreatoduodenectomy showed a hyper-metabolic status as evaluated by their measured resting energy expenditure/predicted resting energy expenditure ratio. From our observations, we recommend that nutritional management based on 30 kcal/body weight/day (calculated by the measured resting energy expenditure×activity factor 1.2–1.3) may be optimal for patients undergoing a pylorus preserving pancreatoduodenectomy.
Keywords: resting energy expenditure, indirect calorimetry, pylorus preserving pancreatoduodenectomy (PPPD)
Introduction
It has been widely recognized that marked metabolic changes occur after a major operation.(1,2) Therefore, the energy expenditure must be markedly changed during the perioperative period.(3) Nutritional support is essential for patients undergoing major surgery, and nutritional therapies such as total parenteral nutrition or enteral nutrition are integral to the management of these patients. However, surgery causes a state of insulin resistance, and changes in glucose metabolism occur after surgery, and overfeeding with high loads of carbohydrates has been associated with clinical complications.(4,5) Therefore, knowledge of the optimal energy requirements is very important in the nutritional management of the patients undergoing surgery.
An evaluation of the energy requirements of patients undergoing surgery is critical in planning optimal nutritional therapy. Typically, the total energy of the parenteral or enteral nutrition is determined by using the predicted resting expenditure (pREE) calculated by the Harris-Benedict equation,(4) and the total energy requirement is calculated by the pREE × activity factor × stress factor.(5) On the other hand, the mREE can be determined by indirect calorimetry.
There have been several studies reporting that the energy expenditure of patients undergoing surgery changes to a hyper-metabolic status.(1–3,6) However, there have not been any reports on the energy metabolism of patients undergoing a pancreatoduodenectomy. Currently, the pylorus-preserving pancreatoduodenectomy (PPPD) is generally accepted as the preferred procedure for pancreatic head cancer or bile duct tumors.(7–11) In this study, we evaluated the change in the energy metabolism of patients undergoing PPPD, and determined the optimal energy requirements for their nutritional management.
Subjects and Methods
Patients
Twelve patients with bile duct cancer or pancreatic tumors (5 women and 7 men, median age 70.1 years old) were enrolled in this study. The patients were admitted to the Gastrointestinal Surgery Unit of Shiga University of Medical Science Hospital. The ethics committee of the Shiga University of Medical Science approved this study.
Four patients had pancreatic cancers, 6 patients had bile duct carcinomas, and 2 patients had intraductal papillary mucinous neoplasms (IPMN). All patients underwent PPPD without major complications. Parenteral nutrition was started before surgery in three patients, and parenteral nutrition and enteral nutrition were started on postoperative day (POD) 1 in other patients. Oral diets were started on POD 8. The total energy of the parenteral nutrition (TPN), enteral nutrition (EN) and oral diet was 33.2 ± 7.6 kcal/kg/day before surgery, 29.5 ± 7.8 kcal/kg/day on POD 7 and 24.3 ± 8.4 kcal/kg/day on POD 14. Fat emulsions were not used for TPN during this period.
Indirect calorimetry
We performed weekly indirect calorimetry on patients undergoing PPPD. The mREEs and non protein respiratory quotients (npRQ) were measured by computed open-circuit indirect calorimetry (AE-300S; Minato Medical Science Co., Osaka, Japan).(12,13) Indirect calorimetry (IC) was performed in the hospital room on the morning after a 10-h overnight fast before surgery. However, the infusion of parenteral nutrition was maintained on POD 7 and POD 14. The period flow and gas calibration were performed prior to all measurements. After resting for a minimum of 30 min, the patients were assessed in the supine position with a facemask. A pump drew ambient air through a facemask at a constant rate. After equilibrium was reached for 10 min, the respiratory exchange was performed continuously over 30 min. The mREE and npRQ data were obtained every minute.
The mREE was calculated from the oxygen consumption (VO2) and carbon dioxide production (VCO2) by the Weir equation.(14):
| mREE = (3.94 × VO2 + 1.11 × VCO2) × 1.44 |
Measurement of the non protein RQ was calculated as RQ = VCO2/VO2. The measured mREE was then compared with the pREE (predicted resting energy expenditure) calculated by the Harris and Benedict equation.(15):
| Men: pREE = 66.47 + 13.75 × W [weight (kg)] + 5.0 × H [height (cm)] – 6.75 × A [age (year)]. |
| Women: pREE = 665.09 + 9.56 × W [weight (kg)] + 1.84 × H [height (cm)] – 4.67 × A [age (year)]. |
Statistical analyses
Statistical analyses were performed using Student’s paired t tests when appropriate. The correlations were investigated with Spearman rank correlation tests. The results are presented as mean ± SD, and a p value <0.05 was considered to be statistically significant.
Results
The characteristics of the patients are shown on Table 1. The BMI of these patients was 21.4 ± 3.8 kg/m2, and the average total protein, serum albumin, total cholesterol, prealbumin and retinol-binding protein levels were normal. The total lymphocyte counts were 1571.5 ± 285.9/mm3.
Table 1.
Background
| No of patients | 12 | ||
| pancreas head cancer | 4 | ||
| bile duct cancer | 6 | ||
| IPMN | 2 | ||
| Female/Male | 5/7 | ||
| Age (yrs) | 70.1 ± 7.1 | ||
| Height (cm) | 159.6 ± 7.0 | ||
| Body weight (kg) | 54.8 ± 11.3 | ||
| BMI (kg/m2) | 21.4 ± 3.8 | ||
| Laboratory data | |||
| Total Protein (g/dl) | 6.2 ± 0.6 | ||
| Albumin (g/dl) | 3.5 ± 0.4 | ||
| Total Lymphocyte cell counts (/mm3) | 1571.5 ± 285.9 | ||
| Total Cholesterol (mg/dl) | 169.3 ± 32.8 | ||
| C-reactive protein (mg/dl) | 1.3 ± 2.1 | ||
| Prealbumin (mg/dl) | 20.1 ± 7.2 | ||
| Retinol-binding protein (mg/dl) | 2.6 ± 1.3 | ||
| Energy expenditure | |||
| measured resting energy expenditure (kcal/day) | 1198.0 ± 191.7 | ||
| predicted resting energy expenditure (kcal/day) | 1174.5 ± 175.2 | ||
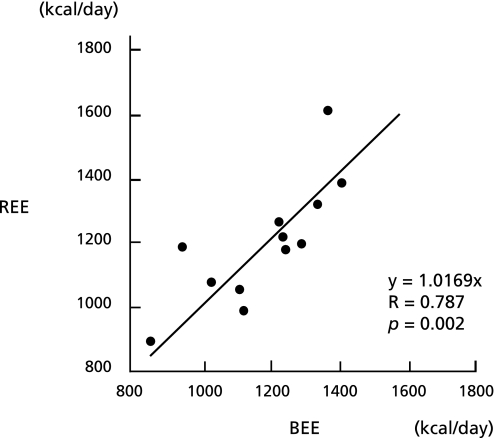
The mREE in these patients determined by indirect calorimetry before the surgery was 1198.0 ± 191.7 kcal/day. On the other hand, the pREE calculated by the Harris-Benedict equation was 1174.5 ± 175.2 kcal/day. There was a positive correlation between the pREE and mREE (p<0.05) (Fig. 1).
Fig. 1.
Correlation between the measured resting energy expenditure (mREE) and the predicted resting energy expenditure (pREE). The mREE was measured by indirect calorimetry, and the pREE was calculated by the Harris-Benedict equation. There was a positive correlation between the mREE and pREE in patients with bile duct carcinoma or pancreatic head tumors.
The mREE/body weight measured before the operation was almost the same as the pREE/body weight (mREE: 22.4 ± 3.9 kcal/kg/day vs pREE: 21.7 ± 2.0 kcal/kg/day), and the mREE/pREE ratio, which reflects the stress factor, was 1.02 ± 0.11. There were no significant differences among mREEs of the patients with pancreas cancer, bile duct carcinoma and IPMN. The mREE/body weight of these patients was almost the same as that of the controls reported previously.(12,13)
The npRQ of these patients measured by indirect calorimetry before surgery was 0.87 ± 0.06, and was almost the same as that of the controls reported previously.(12,13) There was no significant correlation between the npRQ and mREE.
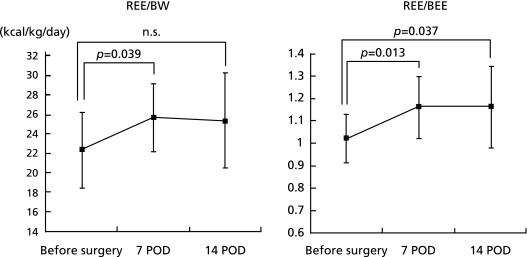
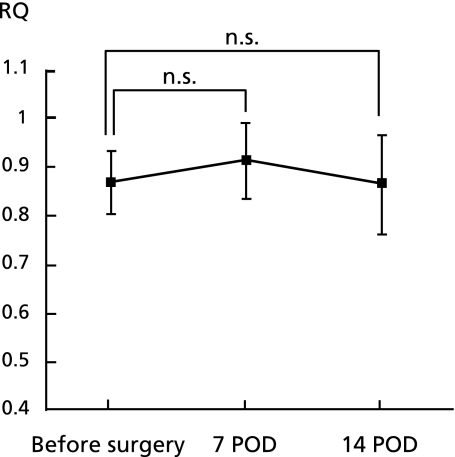
As shown in Fig. 2, a significant increase in energy expenditure was observed after PPPD. The mREE of the patients undergoing a PPPD was 25.7 ± 3.5 kcal/kg/day on POD 7 and 25.4 ± 4.9 kcal/kg/day on POD 14. The mREE/pREE ratio was 1.16 ± 0.14 on POD 7, and 1.16 ± 0.18 on POD 14 respectively. However, the npRQ did not change significantly during the perioperative period (Fig. 3).
Fig. 2.
Changes in the measured resting energy expenditure (mREE), and the mREE/the predicted resting energy expenditure REE (pREE) ratio. The pREE was calculated by Harris-Benedict equation. Significant increases were observed in the mREE and mREE/pREE ratio on POD 7 or POD 14 after the PPPD.
Fig. 3.
Changes in the respiratory quotient in patients undergoing PPPD. No significant changes were observed in the npRQ after surgery.
Discussion
This is the first report on the resting energy expenditure in patients undergoing a PPPD. In this study, we showed that the mREE measured by IC in patients with bile duct carcinomas or pancreatic tumors was not elevated before surgery. Their mREE was the same as the pREE calculated by Harris-Benedict equation, and was the same as the mREE of healthy controls. Previous studies have suggested that the mREE may be elevated in patients with specific types of tumors. It has been reported that patients with lung cancers or sarcomas have increased energy expenditure. Falconer et al.(15) also reported that the REE was significantly elevated in pancreatic cancer patients who were losing weight. This elevated REE was reported to be associated with some proinflammatory cytokines such as IL-6 or TNF-α. A progressive increase in tumor size is associated with an increase in oxygen consumption.(16) In our study, however, the patients with bile duct cancer or pancreatic tumors enrolled in this study had an operative indication for PPPD, and hence cachexic patients were not included in this study. The BMIs and other nutritional parameters of these patients were within the normal range, and had a good nutritional condition. This must be the reason why the preoperative REE in these patients was not elevated in this study.
The resting energy expenditure (pREE), calculated by the Harris-Benedict equation,(17) has been widely used to evaluate the energy status of patients. The total energy requirement is calculated by pREE × activity factor × stress factor.(18) Theoretically, the pREE is expected to be equal to the mREE in healthy humans, and the mREE/pREE ratio is a marker for a hyper-metabolic status.(12,19) In this study, the mREE was increased after PPPD, and was significantly higher than the pREE, and was also higher than the preoperative mREE (p<0.05). The mREE/pREE, which reflects the stress factor, was elevated to 1.16 on POD 7 and POD 14. From these results, it is apparent that the stress factor for bile duct cancer or pancreatic tumors surgery should be 1.2 in patients without major complications.
Surgical trauma is generally considered to produce an increase in energy metabolism, and surgical stress, as well as burns or sepsis, causes an increase in the mREE. Systemic inflammatory response syndrome (SIRS) describes a clinical response arising from a non-specific origin. SIRS may be initiated by a major operation, as well as by infection. The elevated mREE after surgery was associated with operative SIRS, and the mREE was further increased in patients with infectious complications.(2)
The increased rates of mREE in patients undergoing abdominal surgery are controversial. Previously, it has been reported that the REE was significantly increased after abdominal surgery, and TPN therapy consisting 45 kcal/kg day was optimal for these patients. This means that measurement of energy requirement can be calculated by the pREE × 1.75. Rutten et al. also reported that pREE × 1.75–2.0 kcal/day should be optimal for TPN in the patients undergoing major surgery. However, Federix et al.(3) showed a 10% increase in the mREE in patients undergoing gastrointestinal surgery without major complication. Shaw et al.(20) also reported that the infusion of 110% of REE may be suitable for TPN in patients with gastrointestinal surgery.
Our results showed that the mREE was almost 25.5 kcal/kg/day just after PPPD surgery, and the mREE/pREE ratio was 1.16 on POD 7 and POD 14. The measurement of energy requirement is very critical for nutritional management in patients undergoing major surgery such as pancreatic cancer or esophageal cancer, because over feeding has been associated with some clinical complications.(4) From our findings, the stress factor of patients undergoing PPPD should be set at 1.2 in patients without major complications. This factor was compatible to that of patients undergoing esophageal cancer surgery reported by Sato et al.(21) Although one would be more likely to find stress-induced hypermetabolism on the earlier postoperative days, the increase in the mREE of patients undergoing an esophagectomy was almost the same as during the first seven days.(21) Recently, perioperative management has been dramatically improved. In particular, infection control has been advanced, and may prevent infection induced SIRS in the patients undergoing major surgery.
In this study, a significant change in the npRQ after PPPD was not observed. In previous reports, the npRQ was reduced in patients after a liver resection.(22) This fact means that the energy substrate of the remnant liver is principally fatty acids rather than glucose after a liver resection. In patients undergoing PPPD, however, glucose is mainly utilized after the surgery as well as in patients undergoing an esophagectomy.(23,24)
In conclusion, patients undergoing PPPD have a hyper-metabolic status just after surgery, and the mREE/pREE ratio was significantly increased to 1.16. From our results, the daily energy requirements for patients undergoing PPPD are recommended at 30 kcal/body weight (mREE 25.7 kcal ± 3.5 kcal/kg/day × active factor 1.2–1.3).
References
- 1.Rutten P, Blackburn GL, Flatt JP, Hallowell E, Cochran D. Determination of optimal hyperalimentation infusion rate. J Surg Res. 1975;18:477–483. doi: 10.1016/0022-4804(75)90121-3. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa M, Nishikioka M, Hanaki N, et al. Postoperative metabolic and circulatory responses in patients that express SIRS after major digestive surgery. Hepatogastroenterology. 2006;53:228–233. [PubMed] [Google Scholar]
- 3.Fredrix EWHM, Soeters PB, von Meyenfeldt MF, et al. Resting energy expenditure in cancer patients before and after gastrointestinal surgery. J Parenter Eneteral Nutr. 1991;15:604–607. doi: 10.1177/0148607191015006604. [DOI] [PubMed] [Google Scholar]
- 4.Sandströme R, Drott C, Hyltander A, et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in randomized study. Ann Surg. 1993;217:185–195. doi: 10.1097/00000658-199302000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander JW, Gonce SJ, Miskell PW, Miskell PW, Sax H. A new model for studying nutrition in peritonitis. The adverse effect of overfeeding. Ann Surg. 1989;209:334–340. doi: 10.1097/00000658-198903000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazzaniga AB, Polachek JR, Wilson AF, Day AT. Indirect calorimetry as a guide to caloric replacement during total parenteral nutrition. Am J Surg. 1978;136:128–133. doi: 10.1016/0002-9610(78)90212-x. [DOI] [PubMed] [Google Scholar]
- 7.Braasch JW, Deziel DJ, Rossi RL, Watkins E, Jr., Winter PF. Pyloric and gastric-preserving pancreatic resection. Experience with 87 patients. Ann Surg. 1986;204:411–418. doi: 10.1097/00000658-198610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenostomy. A clinical and physiologic appraisal. Ann Surg. 1986;204:655–664. doi: 10.1097/00000658-198612000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogata Y, Hishinuma S. The impact of pyrolus-reserving pancreatoduodenectomy on surgical treatment for cancer of pancreatic head. J Hepatobiliary Pancreat Surg. 2002;9:223–232. doi: 10.1007/s005340200023. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuka T, Tanaka M, Miyazaki K. Gastrointestinal function and quality of life after pylorus-preserving pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2006;13:218–224. doi: 10.1007/s00534-005-1067-z. [DOI] [PubMed] [Google Scholar]
- 11.Diener MK, Heukaifer C, Schwarzer G, et al. Pancreaticoduodenectomy (classic Whipple) versus pylorus-preserving versus classical pancreaticoduodenectomy (pp Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2008;16:CD006053. doi: 10.1002/14651858.CD006053.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Johtatsu T, Kurihara M, et al. Energy metabolism in Japanese patients with Crohn’s disease. J Clin Biochem Nutr. 2010;46:68–72. doi: 10.3164/jcbn.09-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki M, Johtatsu T, Kurihara M, et al. Energy expenditure in Japanese patients with severe or moderate ulcerative colitis. J Clin Biochem Nutr. 2010;47:32–36. doi: 10.3164/jcbn.10-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weir JB. New methods for calculating metabolic rate with special reference to protein matabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219:325–331. doi: 10.1097/00000658-199404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koea JB, Shaw JH. The effect of tumor bulk on the metabolic response to cancer. Ann Surg. 1992;215:282–288. doi: 10.1097/00000658-199203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long CL, Schaffel N, Geiger JW, et al. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J Par Ent Nutr. 1979;3:452–456. doi: 10.1177/014860717900300609. [DOI] [PubMed] [Google Scholar]
- 19.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27:1295–1302. doi: 10.1097/00003246-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Shaw SN, Elwyn DH, Askanazi J, Iles M, Schwarz Y, Kinney JM. Effects of increasing nitrogen intake on nitrogen balance and energy expenditure in nutrionally depleted adult patients receiving parenteral nutrition. Am J Clin Nutr. 1983;37:930–940. doi: 10.1093/ajcn/37.6.930. [DOI] [PubMed] [Google Scholar]
- 21.Sato N, Kusama A, Ohkawa A, et al. Resting energy expenditure in patients undergoing transhiatal or transthoracic oesophagectomy for carcinoma of the thoracic oesophgus. Br J Med. 1993;80:1413–1415. doi: 10.1002/bjs.1800801119. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi K, Sakai K, Matsubara S, Mikuni J, Katayose Y, Matsuno S. Fuel utilization and glucose hyperalimentation after liver resection. Nutrition. 1994;10:411–414. [PubMed] [Google Scholar]
- 23.Sane S, Baba M, Kusano C, Shirao K, Yamada H, Aikou T. Immunonutrition risk factors of respiratory complications after esophagectomy. Nutrition. 2004;20:364–367. doi: 10.1016/j.nut.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Sane S, Baba M, Kusano C, et al. Influence of exogenous fat emulsion on pulmonary gas exchange after major surgery. World J Surg. 2002;26:297–302. doi: 10.1007/s00268-001-0221-2. [DOI] [PubMed] [Google Scholar]