Abstract
Ipomoea batatas, Agaricus blazei and Smallanthus sonchifolius are known to favorably influence diabetes mellitus. To clarify their antidiabetic efficacy and hypoglycemic mechanisms, we treated streptozotocin-induced diabetic rats with daily oral feeding of powdered Ipomoea batatas (5 g kg−1 d−1), Agaricus blazei (1 g kg−1 d−1) or Smallanthus sonchifolius (4 g kg−1 d−1) for 2 months. Treatments with Ipomoea batatas or Agaricus blazei, but not Smallanthus sonchifolius, significantly suppressed the increases of fasting plasma glucose and hemoglobin A1c levels, and restored body weight loss during diabetes. Serum insulin levels after oral glucose administration tests increased along the treatments of Ipomoea batatas or Agaricus blazei. Moreover, Ipomoea batatas and Agaricus blazei reduced superoxide production from leukocytes and vascular homogenates, serum 8-oxo-2'-deoxyguanosine, and vascular nitrotyrosine formation of diabetic rats to comparable levels of normal control animals. Stress- and inflammation-related p38 mitogen-activated protein kinase activity and tumor necrosis factor-α production of diabetic rats were significantly depressed by Ipomoea batatas administration. Histological examination also exhibited improvement of pancreatic β-cells mass after treatments with Ipomoea batatas or Agaricus blazei. These results suggest that hypoglycemic effects of Ipomoea batatas or Agaricus blazei result from their suppression of oxidative stress and proinflammatory cytokine production followed by improvement of pancreatic β-cells mass.
Keywords: hypoglycemic effect, oxidative stress, pancreatic islet β-cells, Ipomoea batatas (Caiapo), Agaricus blazei (Agaricus)
Introduction
Nowadays, diabetes mellitus in the industrialized countries has spread to younger generation and the number of patients is prospected to increase to 300 million or more worldwide by the year 2025. Diabetes mellitus is a serious complex chronic condition that is a major source of ill health worldwide. Even optimal control of blood glucose can not prevent clinical diabetic complications, so that diabetes therapy has revolved around dietary measures including the use of traditional antihyperglycemic medicines. Moreover, insulin or oral hypoglycemic drugs have serious side effects on overdose medication. Therefore, there is a real need today for further pharmacological study on the effectiveness and side effects caused by the alternative medicines.
In fact, about 800 species of plants and mushrooms have been reported to improve the metabolism of carbohydrates and to be effective against diabetes mellitus.(1–3) However, only a few compounds of antihyperglycemic plants and mushrooms have shown the efficacy on the management of diabetes in randomized trials.(3,4) Their hypoglycemic mechanisms in chronic treatment are not clear and scientific evidences are very poor. In the present study, we selected the three of plants and mushroom based on the previous reports, Ipomoea batatas L. (Convolvulaceae), i.e., Caiapo, Agaricus blazei Murill (Agaricaceae), i.e., Agaricus, and Smallanthus sonchifolius Poepp. & Endl. (Compositae), i.e., Yacon, which may improve on the balance of glucose and insulin through a safe and effective program.
Ipomoea batatas is a white-skinned sweet potato originating in Amazonis Brazil, and it has been used in Shikoku region of Japan, as a folk medicine for the treatments of diabetes and other metabolic diseases.(5,6) In concerning diet therapy of Ipomoea batatas, the treatment for 3 months has been reported to lower the plasma glucose and cholesterol levels in patients with type 2 diabetes.(7,8) Oral administration of Ipomoea batatas to diabetic animal models for longer than 6 weeks was shown to prevent and improve the symptoms of diabetes and hypoglycemia in streptozotocin (STZ)-induced diabetic and obese Zucker rats.(9,10) Further, Ipomoea batatas has abundant phenolic compounds, such as caffeic acid and its derivatives,(11) whose efficacious functions(12) are expected to prove a number of health benefits.
Agaricus blazei is a medical mushroom that grows in North America and Brazil, and it is widely taken, and described in the world.(13) There are many reports on the immunological beneficial properties such as anti-tumor,(14,15) anti-viral,(16) and disinfectant(17) activities. Moreover, it is recently reported that β-glucan extracts from Agaricus blazei could reduce blood glucose, triglyceride, and cholesterol levels(18) as well as insulin-like action.(19)
Smallanthus sonchifolius roots are a rich source of fractooligosacharides and have a long use tradition as food in the Andean region. Expansion to other counties including New Zealand, Japan and Brazil has been stimulated further by presumed medical properties of both roots and leaves.(20) The water-soluble extract of its leaves has been recognized to have hypoglycemic effect,(21,22) antioxidant property,(23) and enhancement of liver metabolism.(24) Natural and neutraceutical products including the above three materials are highly expected on diabetes control.
In the present study, Ipomoea batatas, Agaricus blazei, or Smallanthus sonchifolius were fed with diet for 2 months to STZ-induced type 2 diabetes rats, and their efficacy against diabetes and diabetes-associated pathophysiologic changes including pancreatic β-cells, insulin secretion, oxidative stress, and inflammatory cytokine production were investigated.
Materials and Methods
Preparation of experimental materials
Ipomoea batatas (Caiapo) was grown in Kagawa Prefecture (Japan). Whole extract was obtained including the peel, because Ipomoea batatas contains more phenolic compounds in the outer part than in the center,(25) and then lyophilized to prepare a uniform powder.(26) The powder was purchased from Fuji Sangyo Co., Ltd. (Kagawa, Japan). All ingredients were measured by Japan Food Research Laboratories (Tokyo, Japan) using the standard protocols recommended by the Resources Council, the Science and Technology Agency of Japan. The main composition was as follows: protein, 5.2%; carbohydrate, 78.3%; fiber, 11.0%. Ipomoea batatas had also abundant phenolic compounds such as chlorogenic acid, caffeic acid and its derivatives,(27) and high levels of vitamins including ascorbic acid and tochopherol. It contained many minerals including zinc, copper, manganese and selenium.
Agarics blazei (Agarics) fruit body was cultivated outdoors in Brazil. Fruit bodies were air dried by ventilator with a blowing temperature low than 60°C to maintain their enzyme activities, powdered, and imported by Toei Pharmaceutical Co., Ltd. (Tokyo, Japan). The main composition was as follows: protein, 38.5%; carbohydrate, 27.7%; fiber, 20.6%; β-glucan, 12.4%. It had also enzymes such as polyphenol oxidase, peroxidase, glucanase, and laccase. It contained many minerals including large amounts of zinc and copper, and certain amounts of manganese and selenium.(28–30)
Smallanthus sonchifolius (Yacon) plants were grown in Brazil. The tuberous roots and stems of Smallanthus sonchifolius were peeled, sliced and dried at 40°C in a forced air circulation oven. Partially dried slices were subjected to 60°C for 2 h to stop rapid degradation of fructooligosaccharides,(31) milled, and imported by Toei Pharmaceutical Co., Ltd. The main composition was as follows: protein, 9.6%; carbohydrate, 66.0%; fiber, 11.6%. The major proportion of carbohydrates was in the form of inulin-type oligofructans and β-fructooligosaccharides.(20) The tubers had chlorogenic acid and caffeic acid derivatives.(32,33) It also contained ascorbic acid, and many minerals including zinc, copper, manganese and selenium.
All diets for animals were prepared by mixing powdered test materials and powdered standard diet (SP; Funabashi Farm, Funabashi, Japan). The doses of Ipomoea batatas, Agarics blazei, or Smallanthus sonchifolius were kept at 5 g/kg of body weight/day, 1 g/kg of body weight/day, or 4 g/kg of body weight/day, respectively. This is a dose likely to be in the upper limit of what can be tolerated in long-term experiments in human as indicated by the manufacturers.
Animals
Male Wistar rats weighing 130 g to 150 g (6 weeks of age, Japan SLC, Inc., Shizuoka, Japan) were housed in an air-conditioned room at 22 ± 2°C with 60 ± 5% humidity, under 12 h light-dark cycle in the center for animal experiments of Kinki University School of Medicine. Rats were given laboratory diets as mentioned above and water ad labium. Rats were treated according to the ethical guideline of Kinki University School of Medicine Animal Committee.
Experimental procedure and sample collection
After initial determination of fasting blood glucose levels at 7 weeks of age, rats were intravenously injected the streptozotocin fluid (STZ, Sigma Chemical Co., St. Louis, MO) at the dose of 45 mg/kg (freshly dissolved in 3 mM citrate buffer pH 4.5). Blood glucose levels were monitored on the fifth day after STZ injection and thereafter at a fasting period of 18 h prior to the monitoring once a week. Animals showing above 250 mg/dl of blood glucose levels on the fifth day after STZ injection were selected as diabetic rats for this study and randomly divided into 4 groups: (i) normal diet (n = 5), (ii) Ipomoea batatas diet (n = 5), (iii) Agarics blazei diet (n = 5), (iv) Smallanthus sonchifolius diet (n = 5). The treatments with Ipomoea batatas, Agarics blazei or Smallanthus sonchifolius were performed for 8 weeks during 8 to 15 weeks of age. For normal reference, non-diabetes control (n = 5) was also examined.
Body weight of normal and diabetic rats was weekly recorded in fasting state. Food and water intake, and urine output for 24 h were measured every other week (1, 3, 5, and 7th week after treatments) individually in metabolic cages. Blood samples were collected from the tail vein. Urine and serum samples were stored at −80°C until the assay. Animals were sacrificed under pentobarbital anesthesia in the eighth week after treatments, and the pancreas, kidney and aorta were collected for the pathophysiological examination.
Plasma glucose and HbA1c
Plasma glucose values were obtained by the electric tip using glucose oxidase method(34) (MediSense Xtra; Abbott Laboratories Inc., Tokyo, Japan). Levels of glycosylated hemoglobin A1c (HbA1c) and total hemoglobin(35) after 7 weeks of treatments were determined according to manufacture’s instruction (Liquitech HbA1cII; Roche Diagnostic Systems, Branchburg, MO).
Serum insulin
Serum insulin levels 20 min after oral administration of 20% glucose solution were determined by radioimmunoassay(36) using Rat Insulin radioimmunoassay (RIA) kit (Linco Research Inc., St. Charles, MO) 1, 3, 5 and 7 weeks after treatments. Fasting insulin levels were also measured in rats fasted overnight in the eighth week after treatments using Ultrasensitive Rat Insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden).
Superoxide production by peripheral leukocytes
Superoxide production by polymorphonuclear leukocytes (PMNs) and monocytes (MNCs) was measured using a gated flow cytometry according to the methods described by Perticarari et al.(37) with some modifications. Seven weeks after treatments fresh blood was collected and erythrocytes were removed by Tris-bufferd ammonium chloride lysis. White blood cells were preincubated for 15 min in a shaking water bath in 37°C with 500 ng/ml hydroethidium (HE) (Invitrogen Life Technologies, Carlsbad, CA) in Hank’s Balanced Salt Solution containing 1% bovine serum albumin (Sigma Chemical Co.), and then the reaction was immediately stopped on ice. HE, a nonfluorescent compound which can diffuse through cell membrane, is rapidly oxidized to ethidium bromide by oxidative products, giving red fluorescence emission. Superoxide levels produced by PMNs and MNCs were evaluated by mean fluorescence intensity. Superoxide levels in stimulated leukocytes in the presence of 1 µg/ml phorbol mirystate acetate (PMA; Sigma-Aldrich Co.) were also measured.
Lucigenin-enhanced chemiluminescence detection of vascular superoxide
Vascular superoxide production was measured using lucigenin (bis-N-methyl acridinium; Sigma-Aldrich Co.)-enhanced chemiluminescence as described by Guzik et al.(38) Chemiluminescence in the vascular homogenates 8 weeks after treatments was measured in Krebs-HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid) buffer containing lucigenin (100 µM) using luminometer analyzer (PICO-LITE 6100; Packard Instrument Co. Inc., Downers Grove, IL). Specificity for superoxide was determined by coincubation with tiron (20 mM) (4,5-dihydroxy-1,3-benzene disulfonic acid; Sigma-Aldrich Co.). Superoxide production was expressed as chemiluminescence counts/10 min/30 mg vessel wet weight.
Serum 8-oxo-2'-deoxyguanosine (8-OHdG)
Serum samples of 8 weeks after treatments of the experimental diets were filtrated to remove high molecular weight products above 10,000 MW (Ultrafree-MC; Millipore Co., Bedford, MA). Thereafter, 8-OHdG levels of the samples were determined(39) by high sensitive commercial ELISA kit (Japan Institute for The Control of Aging, Nikken SEIL Co., Shizuoka, Japan).
Urinary Tumor necrosis factor-α (TNF-α)
Urine samples of 5 and 7 weeks after treatments of the experimental diets were centrifuged at 5,000 × g for 10 min. TNF-α level in the supernatants was measured(40) by BD Opt EIA Rat TNF ELISA kit II (BD biosciences, San Jose, CA).
Western blot analysis
Protein from aortas were extracted in a lysis buffer containing 0.1% sodium dodecylsulfate (SDS), 2% Triton X-100, 1% dithiothreitol and 9 M urea (Wako Pure Chemical Industries, LTD., Osaka, Japan). For extraction from tissues, aortas were excised and snap-frozen in liquid nitrogen before homogenization in lysis buffer. Equal amounts of protein (30 µg) were separated by SDS-polyacrylamide gel. Anti-nitrotyrosine (Upstate biotechnology, Lake Placid, NY),(41) anti-p38 mitogen-activated protein (MAP) kinase, and anti-phospho p38 MAP kinase (Cell Signaling Technology, Danvers, MA)(42) antibodies were used for western blot analysis. Bands were visualized by enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK) and were quantified by using luminescent image analyzer (LAS-1000, Fuji Photo Film Co., LTD, Tokyo, Japan) and Image Gauge software version 3.12 (Fuji Photo Film). All antibodies were used as indicated by the manufacturers.
Histochemical analysis of pancreatic-β cells
Whole pancreatic tissues were fixed in 15% formalin neutral buffer solution and embedded in paraffin. Sections were stained by hematoxylin and eosin, and aldehyde fuchsin(43) for pancreatic β-cells population. To quantify the contents of pancreatic β-cells, the 12 randomly selected sections with the maximal lesion in each animal were chosen. A computer-assisted morphometric analysis was performed with a semiautomatic image computer system (Lumina Vision version 1.5; Mitani Co., Maruoka, Japan) and software for image analysis (Mac SCOPE version 2.5; Mitani Co.). The numbers of islets per field were manually counted.
Statistical analysis
Values were expressed as mean ± standard error of the mean (SEM). Data were analyzed by one-way analysis of variance (ANOVA), followed by post hoc Scheffé test. Differences were considered statistically significant at p<0.05.
Results
Body weight
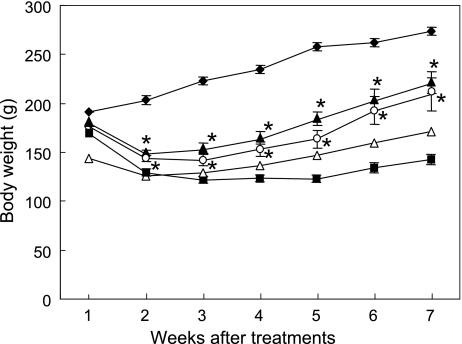
Body weight significantly lowered in all the diabetic groups compared with normal control (NC) group (p<0.05, all diabetic groups vs NC) (Fig. 1). Two weeks after treatments with the experimental diets, the body weight of Ipomoea batatas-treated diabetic (Caiapo) and Agarics blazei-treated diabetic (Agarics) groups was significantly increased compared with that of untreated diabetic control (DC) group. The weight of Caiapo group was 221.5 ± 11.2 g and that of Agaricus group was 208.8 ± 16.6 g (p<0.05, Caiapo or Agarics group vs DC 142.5 ± 6.1 g) 7 weeks after treatments. On the other hand, Smallanthus sonchifolius-treated diabetic (Yacon) rats showed no significant restoration of the body weight.
Fig. 1.
Effects of treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon) on body weight in STZ-induced diabetic rats. Streptozotocin-induced diabetic rats were treated with daily oral feeding of powdered Ipomoea batatas (5 g/kg body weight/day), Agaricus blazei (1 g/kg body weight/day) or Smallanthus sonchifolius (4 g/kg body weight/day) for 2 months. Treatments with Caiapo or Agaricus significantly restored weight loss during diabetes, but Yacon had no effect on body weight loss. Normal control (filled diamond), untreated diabetic control (filled square), Ipomoea batatas (Caiapo)-treated diabetic (filled triangle), Agaricus blazei (Agarics)-treated diabetic (open circle), and Smallanthus sonchifolius (Yacon)-treated diabetic (open triangle) rats. Values are mean ± SEM (n = 5). *p<0.05 vs untreated diabetic control.
NC showed greater daily food intake than those of all the diabetic groups for 3 weeks after the start of treatments, and thereafter there were no significant differences observed among any groups (data not shown). There were also no significant differences in food intakes among DC, Caiapo, Agaricus and Yacon groups during the experimental period.
Plasma glucose and HbA1c levels
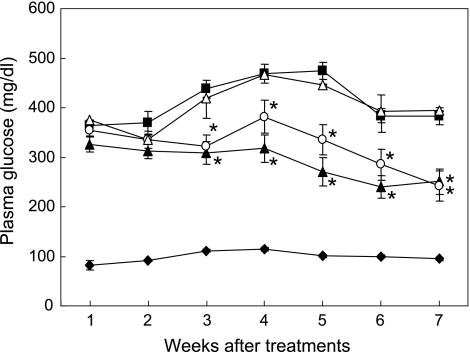
Fasting blood glucose levels of NC ranged from 82.5 ± 9.4 mg/dl to 111.2 ± 2.6 mg/dl during the experiment. On the other hand, blood glucose levels of DC were increased after injection of STZ, and 5 weeks later those reached to the peak level (474.2 ± 34.1 mg/dl). In comparison with DC, Caiapo and Agaricus groups showed significant suppression on blood glucose levels after 3 weeks of treatments (p<0.05, Caiapo or Agarics group vs DC). In contrast, Yacon showed no significant hypoglycemic effect during the experimental period (Fig. 2).
Fig. 2.
Effects of treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon) on fasting blood glucose levels in STZ-induced diabetic rats. Blood glucose levels were monitored on the fifth day after STZ injection and thereafter at a fasting period of 18 h prior to the monitoring once a week. Hyperglycemia was significantly suppressed by the treatments with Caiapo or Agarics, but not with Yacon. Normal control (filled diamond), untreated diabetic control (filled square), Ipomoea batatas (Caiapo)-treated diabetic (filled triangle), Agaricus blazei (Agarics)-treated diabetic (open circle), and Smallanthus sonchifolius (Yacon)-treated diabetic (open triangle) rats. Values are mean ± SEM (n = 5). *p<0.05 vs untreated diabetic control.
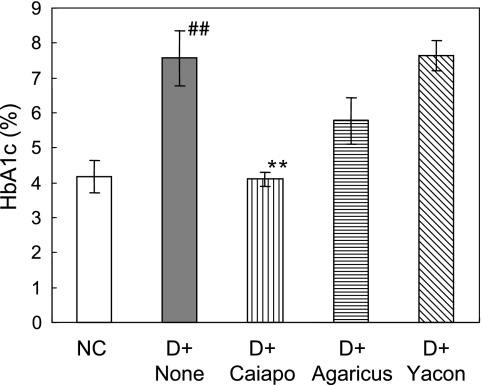
There was a significant increase in the level of glycosylated HbA1c in DC after 7 weeks of STZ injection compared with that of NC (p<0.01). Of note, the value of Caiapo group was suppressed to 4.1 ± 0.2%, which was approximately the same level as NC (p<0.01 vs DC, 7.6 ± 0.8%). Suppressive tendency was also observed in Agaricus group (5.8 ± 0.7%). In contrast, HbA1c level of Yacon group did not differ from that of DC (Fig. 3).
Fig. 3.
Levels of HbA1c in STZ-induced diabetic rats treated with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon) in the seventh week after treatments. HbA1c values were reduced with treatment with Caiapo. Values are mean ± SEM (n = 5). ##p<0.01 vs normal control, **p<0.01 vs untreated diabetic control. NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei-treated diabetic group, D + Yacon; Smallanthus sonchifolius-treated diabetic group.
Furthermore, urinary ketone levels were increased in all of the diabetic groups compared with NC after 5 weeks of STZ injection. The levels were suppressed in Caiapo and Agaricus groups compared with DC. On the other hand, Yacon had no effect on urinary ketone (data not shown).
Serum insulin levels after glucose loading
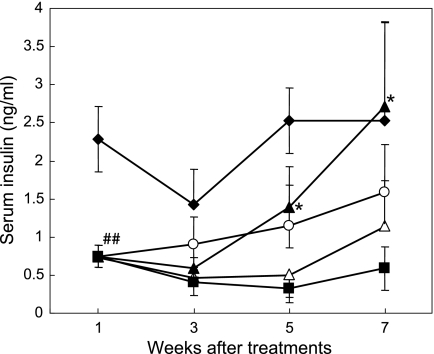
Changes of serum insulin levels 20 min after 400 mg/rat glucose loading were shown in Fig. 4. In the first week of experiment, mean serum insulin levels in all of the diabetic groups were remarkably decreased to 0.74 ng/ml (p<0.01 vs NC, 2.3 ± 0.4 ng/ml). After 5 weeks of treatments with the experimental diets, the increases in insulin levels were observed in Caiapo and Agaricus groups after glucose loading. The levels of Caiapo groups was significantly higher compared with DC (p<0.05). In the seventh week after treatment, the mean value of Caiapo group was 2.7 ng/ml, and thus recovered to the level comparable to that of NC, although the error range was great (p<0.05 vs DC). By contrast, the levels of serum insulin in Yacon group did not differ from those of DC during the experiment. On the other hand, fasting insulin levels remained unchanged in all the diabetic groups.
Fig. 4.
Serum insulin levels after oral glucose administration in Ipomoea batatas-treated diabetic (Caiapo), Agaricus blazei-treated diabetic (Agarics) or Smallanthus sonchifolius-treated diabetic (Yacon) rats. The levels were determined 20 min after oral administration of 20% glucose solution by radioimmunoassay. Treatment with Caiapo for 2 months restored to serum insulin level comparable to that of normal control. Normal control (filled diamond), untreated diabetic control (filled square), Caiapo-treated diabetic (filled triangle), Agaricus-treated diabetic (open circle), and Yacon-treated diabetic (open triangle) rats. Values are mean ± SEM (n = 5). ##p<0.01 vs normal control, *p<0.05 vs untreated diabetic control.
Oxidative stress
One major mechanism underlying STZ toxicity is cytokine-mediated β-cell destruction in which oxidative stress plays a key role.(44) First, we examined the changes of superoxide production from leukocytes in normal or diabetic rats with or without the treatments of Caiapo, Agarics, or Yacon for 8 weeks. Mean fluorescence intensity, which is equivalent to the ability of superoxide production by a single monocyte or neutrophil, under phorbol mirystate acetate stimulation (1 µg/ml) showed in DC group 1.6 times as high as that of NC groups 7 weeks after experiment (arbitrary units, mean fluorescence intensity; DC 773.1 vs NC 474.8). Of note, mean intensity of Caiapo group was suppressed by 495.7, approximately the same level as the NC. Agarics or Yacon treatment did not significantly influence on superoxide production from blood cells (arbitrary units, mean fluorescence intensity; Agarics 634.9, Yacon 801.6). There were also no differences in superoxide production without stimulation between the groups.
Next, the levels of 8-OHdG, an additional oxidative stress maker, were measured in the serum from normal or diabetic rats. Level of serum 8-OHdG was 240.8 ± 58.3 pg/ml in untreated DC. Only Yacon-treated rats showed significant increase of 8-OHdG and the value was 104.0 ± 40 pg/ml 8 weeks after treatments. In contrast, 8-OHdGs of Caiapo and Agaricus groups were suppressed below the detectable limit (0.1 ng/ml).
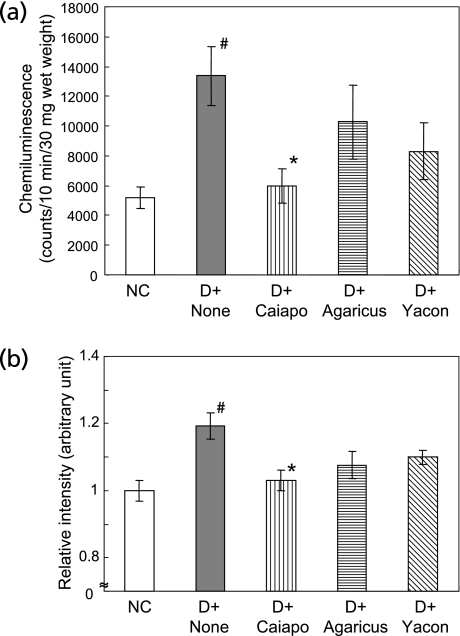
Finally, in order to investigate the effects on oxidative stress in the tissue under high blood glucose, lucigenin-enhanced chemiluminescence was measured in aortas of diabetic rats of the eighth week after treatments with Caiapo, Agarics, or Yacon. Fluorescence level in aortas of DC was approximately 2.5 times as high as that of NC, showing a significant increase in superoxide production (p<0.05, DC 13,376.8 ± 1,994.9 counts vs NC 5,186.3 ± 715.8 counts). Caiapo group significantly reduced the levels of superoxide production of tissue (5,985.3 ± 1,162.5 counts, p<0.05 vs DC) (Fig. 5a). In addition, formation of nitrotyrosine, another oxidative stress marker, was assessed in aortas by western blotting. Nitrotyrosine formation was also accompanied with the development of diabetes. Treatment of Caiapo for 8 weeks significantly attenuated the formation (p<0.05, Fig. 5b).
Fig. 5.
(a) Lucigenin-enhanced chemiluminescence intensity in aortas after the treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon). Chemiluminescence in the vascular homogenates 8 weeks after the treatments was measured by lucigenin-enhanced chemiluminescence using luminometer analyzer. Values were shown as counts/10 min/30 mg wet tissue weight. Superoxide production was increased in diabetic rats. Treatment with Caiapo significantly suppressed the oxidative stress in aorta. Values are mean ± SEM (n = 4–5). #p<0.05 vs normal control, *p<0.05 vs untreated diabetic control. NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei-treated diabetic group, D + Yacon; Smallanthus sonchifolius-treated diabetic group.
(b) Nitrotyrosine formation in aortas after the treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon). The values were measured by western blotting in the vascular homogenates 8 weeks after the treatments. Data are expressed as the relative ratio to normal control, which were assigned as value of 1. Nitrotyrosine formation was significantly increased in diabetic rats. Treatment with Caiapo significantly suppressed the oxidative stress in aorta. Values are mean ± SEM (n = 5). #p<0.05 vs normal control, *p<0.05 vs untreated diabetic control. NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei-treated diabetic group, D + Yacon; Smallanthus sonchifolius-treated diabetic group.
Urinary TNF-α
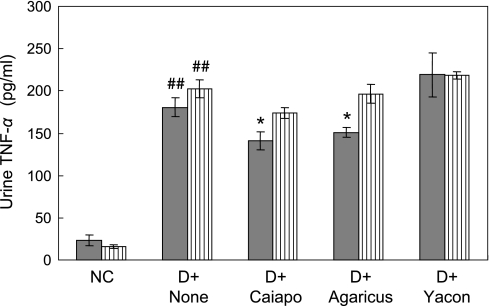
Urinary TNF-α level 5 and 7 weeks after treatments was illustrated in Fig. 6. In the fifth week of treatment, urinary TNF-α level of the diabetic rats was increased by 6 to 9 times as high as that of NC (p<0.01, NC 23.6 ± 6.3 pg/ml vs DC 180.4 ± 11.2 pg/ml). The levels of Caiapo (141.1 ± 10.6 pg/ml) and Agarics (150.9 ± 6.2 pg/ml) groups were significantly less than that of DC (p<0.05 vs DC). In contrast, Yacon group remained unchanged. In the seventh week, TNF-α levels showed similar tendencies with those of the fifth week, although significant differences between the groups were not observed. We also measured the levels of serum TNF-α in the experimental groups. The values were low (range of the mean from 9 pg/ml to 13 pg/ml) and were not significantly different between the groups.
Fig. 6.
Urinary TNF-α excretion after 5 weeks (filled bar) and 7 weeks (striped bar) of treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon). TNF-α levels were measured in the urine of 5 and 7 weeks after the treatments using enzyme-linked immunosorbent assay. Urinary TNF-α excretion was markedly increased in untreated diabetic rats. Caiapo and Agaricus significantly reduced urinary TNF-α excretion after 5 weeks with treatments. Values are mean ± SEM (n = 5). ##p<0.01 vs normal control, *p<0.05 vs untreated diabetic control. NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei-treated diabetic group, D + Yacon; Smallanthus sonchifolius-treated diabetic group.
Mean urinary albumin was increased to 4.02 mg/day urine albumin excretion (UAE) in DC group 5 weeks after the experiment. Caiapo, Agaricus or Yacon reduced UAE to the level comparable to that of NC group (1.5 mg/day mean UAE) (data not shown).
After 1 week of the experiment, water intakes in all of the diabetic rat groups were increased by 3-fold as much as that of NC, and their urine excretions were also increased by 5-fold, with no difference between DC and the other treated diabetic groups. In addition, the ratio of kidney/body weight 8 weeks after treatments became approximately twice in the diabetic rat groups compared with that of the NC, with no difference between DC and the other treated diabetic groups (data not shown).
Activation of p38 mitogen-activaed protein kinase
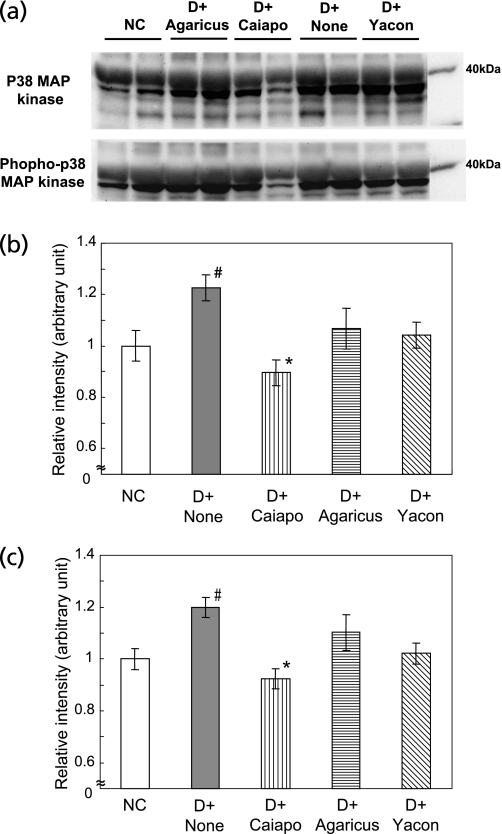
Data of the superoxide production and TNF-α level in STZ-induced diabetes rats suggest that Caiapo or Agaricus is capable of depressing the increases in oxidative stress and inflammatory responses. We examined p38 MAP kinase activation by western blotting. P38 MAP kinase is potently and preferentially activated by a variety of stress including oxidative stress and inflammatory cytokines and receptor systems of the TNF family.(45)
Increases in p38 MAP kinase expression and its phosphorylation level were noted in aortas of diabetic rats compared with normal rats (p<0.05, DC vs NC). Caiapo significantly decreased these levels (p<0.05 vs DC), but Agaricus and Yacon were not effective (Fig. 7a–c).
Fig. 7.
Activation of p38 mitogen-activated protein kinase (MAP kinase) in aortas after the treatments with Ipomoea batatas (Caiapo), Agaricus blazei (Agarics) or Smallanthus sonchifolius (Yacon). The values were measured by western blotting in the vascular homogenates 8 weeks after the treatments. (a) shows a typical western blot analysis of vascular homogenates, using p38 MAP kinase antibody (upper) and phopho-p38 MAP kinase antibody (Thr180/Thr182) (lower). P38 expression (b) and its phosphorylation (c) were significantly increased in diabetic rats. Treatment with Caiapo significantly suppressed p38 MAP kinase activation in aorta. Data are expressed as the relative ratio to normal control, which were assigned as value of 1. Values are mean ± SEM (n = 5). #p<0.05 vs normal control, *p<0.05 vs untreated diabetic control. NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei-treated diabetic group, D + Yacon; Smallanthus sonchifolius-treated diabetic group.
Pancreatic β-cells
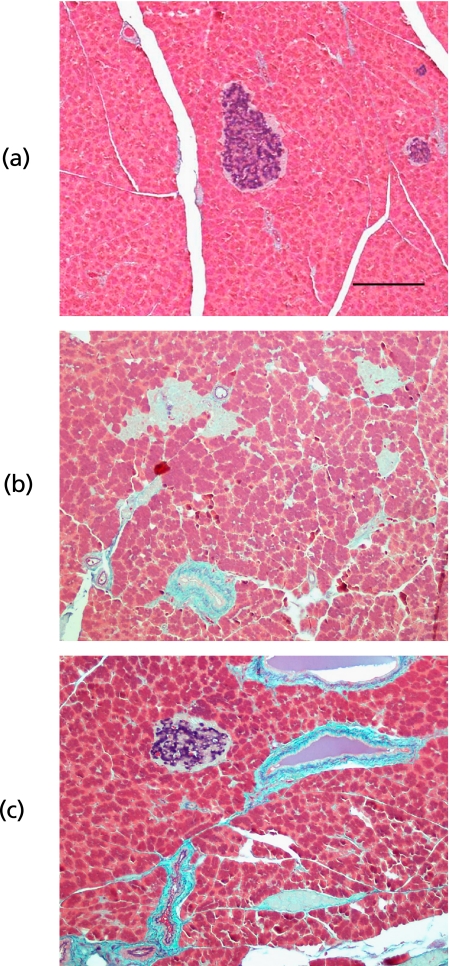
Representative light micrographs of pancreatic β-cells in NC, Agarics-treated, and DC rats were shown in Fig. 8. Area and numbers of islet of Langerhans were recorded as an index of pancreatic β-cells mass (Table 1). STZ treatment caused a remarkable degranulation of pancreatic β-cells. In the DC, the β-cells area for an islet and number of islets in pancreas were decreased by 1/6 and 1/4, respectively, as compared with those of NC (p<0.01, DC vs NC). Eight weeks after treatments, the β-cells mass in Caiapo and Agarics groups were increased by twice volume and more as compared with that of DC (p<0.05, Caiapo or Agarics group vs DC). Yacon treatment showed no effects on pancreatic β-cells recovery.
Fig. 8.
Typical pancreatic islets in (a) normal control, (b) untreated diabetic control (DC), and (c) Agaricus blazei (Agarics)-treated diabetic rats. Pancreatic β-cells showed blue or purple by aldehyde fuchsin staining. Following STZ injection, remarkable degranulation of pancreatic β-cells was observed. The remaining β-cells of DC or Smallanthus sonchifolius (Yacon)-treated diabetic rats were interspersed between the pancreatic cells and exhibited little to no cytoplasmic staining. Treatments with Agaricus blazei (Agarics) or Ipomoea batatas (Caiapo) tended to increase β-cells mass in STZ-induced diabetic rats. Scale bar; 200 µm.
Table 1.
Histochemical analysis of pancreatic islets in diabetic rats
| Treatment |
|||||
|---|---|---|---|---|---|
| NC | D + None | D + Caiapo | D + Agarics | D + Yacon | |
| Islet number | 3.77 ± 0.09 | 1.06 ± 0.12## | 1.46 ± 0.12* | 1.52 ± 0.11* | 0.96 ± 0.08 |
| β-cells area (mm2, ×1/100) | 3.58 ± 0.23 | 0.56 ± 0.17## | 1.03 ± 0.30 | 1.25 ± 0.35 | 0.68 ± 0.21 |
Values are mean ± SEM (n = 5). ##p<0.01 vs NC, *p<0.05 vs DC.
Twelve fields were examined in each animal. Islet number is count of pancreatic islets per field. Positive staining area by aldehyde fuchsin for an islet is calculated as β-cells area.
NC; normal control, D + None; untreated diabetic control, D + Caiapo; Ipomoea batatas-treated diabetic group, D + Agarics; Agaricus blazei -treated diabetic group, D + Yacon; Smallanthus sonchifolius -treated diabetic group.
Twelve fields were examined in each animal. Islet number is count of pancreatic islets per field. Positive staining area by aldehyde fuchsin for an islet is calculated as β-cells area.
Discussion
Ipomoea batatas L. (Caiapo), Agaricus blazei Murill (Agaricus), and Smallanthus sonchifolius Poepp. & Endl. (Yacon) are known to favorably influence diabetes mellitus. However, their antidiabetic efficacy and hypoglycemic mechanisms are not fully determined. In the present study, we have shown that the treatments of Ipomoea batatas and Agaricus blazei suppress the increase in oxidative stress and TNF-α production in STZ-induced diabetes, and are useful in pancreatic β-cells recovery.
There has been much recent attention given to the relationship between diabetes and oxidative stress. Excessive superoxide production is likely to injury pancreatic β-cells and to weaken the insulin production. In this study, superoxide production by peripheral blood monocytes and neutrophils was increased in diabetic rats, accompanied by an increase in the levels of serum 8-OHdG, which displays cellular DNA damage as described in the text. We also demonstrated that the levels of plasma glucose and HbA1c were correlated with increased oxidative stress in diabetic rats. These findings are coincident with the reports that superoxide production and 8-OHdG levels from peripheral blood monocytes were significantly higher in diabetic patients than in healthy people,(46) and that there was a correlation between the rise of HbA1c and oxidative stress levels from peripheral blood monocytes in diabetic patients.(47) Here, it is of note that long-term treatments with Ipomoea batatas or Agaricus blazei significantly suppressed the increase in HbA1c and oxidative stress in diabetes animals (Figs. 1 and 3).
Furthermore, Sheetz and King(48) and Aslan et al.(49) have reported that free radicals induced oxidative stress-related gene expressions and peroxidization of plasma membrane, and that contributed to the development of various diabetic complications. Consistently, the present study showed that oxidative stress levels in the aortas of diabetic rats were markedly increased, as shown in the results in superoxide production (Fig. 5a) and nitrotyrosine formation (Fig. 5b). Nitrotyrosine is a footprint of increased peroxynitrite presence, which is the reactive product of superoxide and nitric oxide, and is a highly reactive radical species. Lakey et al.(50) have reported that peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet β-cells. Of profound importance, treatments with Ipomoea batatas or Agaricus blazei significantly reduced the production of reactive oxygen species in vascular cells as well as peripheral blood cells.
Recent study shows the evidence for evaluation of nitrotyrosine and TNF-α as potential biomarkers of the presence, severity and progress of diabetes and its complication.(51) TNF-α, a proinflammatory cytokine, is mainly secreted from monocytes and macrophages, and also produced in various other tissues. It has reported that TNF-α affects not only peripheral insulin resistance,(52) but also directly pancreatic β-cells, followed by their apoptosis and reduction of insulin secretion.(53) It has also demonstrated that high glucose participate in the production of inflammatory cytokines including TNF-α through the activation of nuclear transcription factor.(54)
Here we shows that TNF-α production remarkably increased in STZ-induced diabetic rats and that treatments of Ipomoea batatas or Agaricus blazei had significant suppressive effect on urinary TNF-α level (Fig. 6). Additionally, Ipomoea batatas depressed p38 MAP kinase expression and activation (Fig. 7). P38 MAP kinase is potently and preferentially activated by a variety of stress including oxidative stress and inflammatory cytokines and receptor systems of the TNF family.(45) Excretion of urinary albumin was also significantly increased in diabetic rats and suppressed after 5 weeks of treatments with Ipomoea batatas, Agaricus blazei, and Smallanthus sonchifolius. It supports our result that increase in urinary TNF-α level is associated with the onset of diabetic nephropathy.(40)
Ipomoea batatas-treated and Agaricus blazei-treated rats exhibited the recovery tendency on serum insulin levels along the treatments (Fig. 4). In the histochemical examination of pancreatic islets (Table 1, Fig. 8), a recovery tendency of islet β-cells mass was also observed in Ipomoea batatas or Agaricus blazei groups. These results suggest that there is a possibility that Ipomoea batatas or Agaricus blazei has a protective effect on islet cell destruction and increases the secretion of insulin. On the other hand, Kusano and Abe(10) have reported that Ipomoea batatas extract directly secretes insulin and improves insulin resistance. Gray and Flatt(19) have found the insulin-like activity and the insulin-releasing effect of Agaricus blazei, although the nature or the mechanisms of action was not determined.
Taken together, hypoglycemic effects of Ipomoea batatas or Agaricus blazei are likely to be their antioxidant and anti-inflammatory action. The leaves and roots of Ipomoea batatas used in this study have abundant phenolic compounds, such as chlorogenic acid, caffeic acid, and its derivatives,(11) which exhibit high radical-scavenging activity, antimutagenic, and antidiabetic effects.(55,56) Ipomoea batatas is rich in ascorbic acid, a well-known antioxidant molecule. The plant also contains large amounts of dietary fibers, which may interfere with glucose absorption and further reduce blood glucose levels.
On the other hand, the fruit body of Agaricus blazei has highly branched 1,3-glucan as the major carbohydrate component.(57) β-glucan is a well-known biological response modifier which is widely distribution in nature and used as an alternative medicine. Although the previous reports concerning the oxidative/anti-oxidative activity of β-glucan are controversial, β-glucan treatment was found to be effective against oxidative injury through the inhibition of TNF-α response.(58–60) In addition, Agaricus blazei contains polyphenol oxidase, an antioxidant enzyme.(28,29) Cupper, zinc, and selenium contained richly in Ipomoea batatas and Agaricus blazei may be beneficial for the protection against radical generation, because the minerals have radical-scavenging activities and are contained in antioxidant enzymes. It is important that neither significant adverse effects nor biochemical changes of blood were observed in our other experiment using Ipomoea batatas, Agaricus blazei, or Smallanthus sonchifolius.
In opposition to previous reports,(21,22) Smallanthus sonchifolius did not improve in glucose balance, oxidative stress or inflammatory response. We used primarily dried tuber and stem powder of Smallanthus sonchifolius in this study, and there may be a possibility of discrepancy between our results and the results of other reports which utilized the decoction from the leaves.
Collectively, we provide that cellular oxidative is a critical step in STZ-mediated islet β-cells injury, and that Ipomoea batatas and Agaricus blazei protect the organs from oxidative damage by those immunomodulatory (inhibition of TNF-α formation and its signaling pathway) and antioxidant properties. Excessive NO production is recently thought to be involved in the pathogenesis of metabolic disorders such as atherosclerosis and obesity-linked type 2 diabetes.(61,62) Proinflammatory cytokines including TNF-α increase nitric oxide (NO) production through the expression of inducible NO synthase in adipocytes and in skeletal muscle of obese human subjects and several diabetic animal models.(62,63) In the present study using STZ-induced diabetic rats, rise of plasma or urine NOx levels was not detected. However, we do not exclude the possible mechanism, because nitrotyrosine formation was markedly increased in vascular cells of untreated diabetic rats (Fig. 5b).
In conclusion, treatments with Ipomoea batatas or Agaricus blazei suppressed the increases of blood glucose and HbA1c levels in streptozotocin-induced type 2 diabetes model rats, and restored insulin release and their body weight loss. The results suggest that the antidiabetic effects result from the suppression of oxidative stress and proinflammatory cytokine, TNF-α, and improvement in β-cells mass. Further precise mechanism of suppression on oxidative stress and/or TNF-α production by the components of Ipomoea batatas or Agaricus blazei remains to be elucidated.
Acknowledgments
The authors are grateful to Kana Ooshima, Chihiro Matsufusa, Misa Satoh and Yasumitsu Akahoshi for technical assistance. The authors thank Toei Pharmaceutical Co., Ltd. for the gift of the powder of Agaricus blazei and Smallanthus sonchifolius.
Abbreviations
- ANOVA
analysis of variance
- DC
untreated diabetic control
- ELISA
enzyme-linked immunosorbent assay
- HbA1c
hemoglobin A1c
- HE
hydroethidium
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid
- MAP kinase
mitogen-activated protein kinase
- MNCs
monocytes
- NADPH
nicotinamide adenine dinucleotide phosphate
- NC
normal control
- NO
nitric oxide
- 8-OHdG
8-oxo-2'-deoxyguanosine
- PMA
phorbol mirystate acetate
- PMNs
polymorphonuclear leukocytes
- RIA
radioimmunoassay
- SDS
sodium dodecylsulfate
- SEM
standard error of the mean
- STZ
streptozotocin
- TNF-α
tumor necrosis factor-α
- UAE
urine albumin excretion
References
- 1.Baily CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 2.Gray AM, Flatt PR. Nature’s own pharmacy: the diabetes perspective. Proc Nutr Soc. 1997;56:507–517. doi: 10.1079/pns19970051. [DOI] [PubMed] [Google Scholar]
- 3.Modak M, Dixit P, Londhe J, Ghaskadbi S, Paul A, Devasagayam T. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 5.Kajimoto O, Yamamoto T, Kajimoto Y, Takahashi R, Tamura H, Aki O. Long-term administration of white skinned sweet potato-containing food for drug-free individuals with NIDDM. Health Natr Food Res. 1999;2:1–12. [Google Scholar]
- 6.Kusano S, Abe H, Tamura H. Isolation of antidiabetic components from white-skinned sweet potato. Biosci Biotechnol Bioch. 2001;65:109–114. doi: 10.1271/bbb.65.109. [DOI] [PubMed] [Google Scholar]
- 7.Ludvik B, Waldhäusl W, Prager R, Kautzky-Willer A, Pacini G. Mode of action of Ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism. 2003;52:875–880. doi: 10.1016/s0026-0495(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Ludvik B, Neuffer B, Pacini G. Efficacy of Ipomea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care. 2004;27:436–440. doi: 10.2337/diacare.27.2.436. [DOI] [PubMed] [Google Scholar]
- 9.Kusano S, Abe H, Okada A. Study of antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.); comparison of normal and streptozotocin induced diabetic rats and hereditary diabetic mice. Nippon Nogeikagaku Kaishi. 1998;72:1045–1052. [Google Scholar]
- 10.Kusano S, Abe H. Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rat. Biol Pharm Bull. 2000;23:23–26. doi: 10.1248/bpb.23.23. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DP. Chlorogenic acid and other phenolic compounds in fourteen sweet potato cultivars. J Food Sci. 1981;46:738–740. [Google Scholar]
- 12.Huang DJ, Lin CD, Chen HJ, Lin YH. Antioxidant and antiproliferative activities of sweet potato (Ipomoea batatas L.) constituents. Bot Bull Acad Sinica. 2004;45:179–186. [Google Scholar]
- 13.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae T. Antitumor β-gulucan from the cultured fruit body of Agarics blazei. Biol Pharm Bull. 2001;24:820–828. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 14.Kawagishi H, Inagaki R, Kanao T, et al. Fractionation and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydr Res. 1989;186:267–273. doi: 10.1016/0008-6215(89)84040-6. [DOI] [PubMed] [Google Scholar]
- 15.Sorimachi K, Akimoto K, Ikehara Y, Inafuku K, Okubo A, Yamazaki S. Secretion of TNF-alpha, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Struct Funct . 2001;26:103–108. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- 16.Sorimachi K, Ikehara Y, Maezato G, et al. Inhibition by Agaricus blazei Mulill fractions of cytopathic effect induced by western equine encephalitis virus on VERO cells in vitro. Biosci Biotechnol Biochem. 2001;65:1645–1647. doi: 10.1271/bbb.65.1645. [DOI] [PubMed] [Google Scholar]
- 17.Osaki Y, Kato T, Yamamoto K, Okubo J, Miyazaki T. Antimutagenic and bactericidal substances in the fruit body of a Basidiomycete Agaricus blazei. Yakugaku Zasshi. 1994;114:342–350. doi: 10.1248/yakushi1947.114.5_342. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Kim KH, Choi HJ, Lee DS. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol Lett. 2005;27:483–487. doi: 10.1007/s10529-005-2225-8. [DOI] [PubMed] [Google Scholar]
- 19.Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom) J Endocrinol. 1998;157:259–266. doi: 10.1677/joe.0.1570259. [DOI] [PubMed] [Google Scholar]
- 20.Genta SB, Cabrera WM, Grau A, Sánchez SS. Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem Toxicol. 2005;43:1657–1665. doi: 10.1016/j.fct.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Ayber MJ, Sánchez Riera, Grau A, Sánchez SS. Hypoglycemic effect of water extract of Smallantus sonchifokius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74:125–132. doi: 10.1016/s0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Itoh Y, Ishida T. Hypoglycemic and hypolipidemic activity of the leaf of Smallantus sonchifolius in genetically type 2 diabetic mice. Journal of Traditional Medicines. 2004;21:275–277. [Google Scholar]
- 23.Valentova K, Cvak L, Muck A, Ulrichova J, Simanek V. Antioxidant activity of extracts from the leaves of Smallanthus sonchifolius. Eur J Nutr. 2003;42:61–66. doi: 10.1007/s00394-003-0402-x. [DOI] [PubMed] [Google Scholar]
- 24.Valentová K, Moncion A, De Waziers, Ulrichová J. The effect of Smallanthus sonchifolius leaf extracts on rat hepatic metabolism. Cell Biol Toxicol. 2004;20:109–120. doi: 10.1023/b:cbto.0000027931.88957.80. [DOI] [PubMed] [Google Scholar]
- 25.Schadel WE, Walter WM. Localization of phenol and polyphenol oxidase in “Jewel” sweet potatoes (Ipomoea batatas “Jewel”) Can J Bot. 1981;59:1961–1967. [Google Scholar]
- 26.Miyazaki Y, Kusano S, Doi H, Aki O. Effects on immune response of antidiabetic ingredients from white-skinned sweet potato (Ipomoea batatas L.) Nutrition. 2005;21:358–362. doi: 10.1016/j.nut.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Takenaka M, Nanayama K, Isobe S, Murata M. Changes in caffeic acid derivatives in sweet potato (Ipomoea batatas L.) during cooking and processing. Biosci Biotechnol Biochem. 2006;70:172–177. doi: 10.1271/bbb.70.172. [DOI] [PubMed] [Google Scholar]
- 28.Akanuma AM, Yamaguchi A, Motoi M, Ohno N. Cloning and characterization of polyphenoloxidase DNA from Agaricus brasiliensis S. Wasser et al. (Agaricomycetideae) Int J Med Mushrooms. 2006;8:67–76. [Google Scholar]
- 29.Hashimoto S, Akanuma AM, Motoi M, Imai N, Rodrignes CA. Effect of culture conditions on chemical composition and biological activities of Agaricus braziliensis. Int J Med Mushrooms. 2006;8:329–342. [Google Scholar]
- 30.Liu Y, Fukuwatari Y, Okumura K, et al. Immunomodulating activity of Agaricus brasiliensis KA21 in mice and in human volunteers. Evidence-based Complementary and Alternative Medicine. 2008;5:205–219. doi: 10.1093/ecam/nem016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graefe S, Hermann M, Manrique I, Golombek S, Buerkert A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crops Res. 2004;86:157–165. [Google Scholar]
- 32.Simonovska B, Vovk I, Andrensek S, Valentova K, Ulrichova J. Investigation of phenolic acids in yacon (Smallanthus sonchifolius) leaves and tubers. J Chromatogr A. 2003;1016:89–98. doi: 10.1016/s0021-9673(03)01183-x. [DOI] [PubMed] [Google Scholar]
- 33.Takenaka M, Yan S, Ono H, Yoshida M, Nagata T, Nakanishi T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius) J Agric Food Chem. 2003;51:793–796. doi: 10.1021/jf020735i. [DOI] [PubMed] [Google Scholar]
- 34.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- 35.Karf J, Burns G, Engel WD, et al. Development and standardization of a new immunoturbidimetric HbA1c assay. Klin Lab. 1993;39:991–996. [Google Scholar]
- 36.Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perticarari S, Presani G, Mangiarott MA, Banfi E. Stimultaneous flow cytometric method to measure phagocytosis and oxidative products by neutrophils. Cytometry. 1991;12:687–693. doi: 10.1002/cyto.990120713. [DOI] [PubMed] [Google Scholar]
- 38.Guzik TJ, West NEJ, Black E, et al. Vascular superoxide production by NAD(P)H oxidase association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 39.Shimoike T, Inoguchi T, Umeda F, Nawata H, Kawano K, Ochi H. The meaning of serum levels of advanced glycosylation and products in diabetic nephropathy. Metabolism. 2000;49:1030–1035. doi: 10.1053/meta.2000.7738. [DOI] [PubMed] [Google Scholar]
- 40.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113–F121. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- 41.MacMillan-Crow LA, Crow JP, Kerby JD, Beckmann JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not hydrogen peroxide. J Biol Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 43.Gomori G. Aldehyde-fuchsin: A new stain for elastic tissue. Am J Clin Pathol. 1950;20:665–666. [PubMed] [Google Scholar]
- 44.Fukuda K, Tesch GH, Nikolic-Paterson DJ. c-Jun amino terminal kinase I deficient mice are protected from streptozotocin-induced islet injury. Biochem Biophys Res Commun. 2008;366:710–716. doi: 10.1016/j.bbrc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 47.Yasunari K, Maeda K, Nakamura M, Yoshikawa J. Oxidative stress in leukocyte is a possible link between blood pressure, blood glucose, and C-reactive protein. Hypertension. 2002;39:777–780. doi: 10.1161/hy0302.104670. [DOI] [PubMed] [Google Scholar]
- 48.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 49.Aslan M, Orhan DD, Orhan N, Sezik E, Yesilada E. In vivo antidiabetic and antioxidant potential of Helichrysum plicantaum ssp. Plicatum capitulums in streptozotocin-induced-diabetic rats. J Ethonopharmacol. 2007;109:54–59. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Lakey JR, Suarez-Pinzon WL, Strynadka K, et al. Peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet β cells. Lab Invest. 2001;81:1683–1692. doi: 10.1038/labinvest.3780381. [DOI] [PubMed] [Google Scholar]
- 51.Drel VR, Lupachyk S, Shevalye H, et al. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PAPR inhibitor, nitrotyrosine, and tumor necrosis factor-α. Endochrinology. 2010;151:2547–2555. doi: 10.1210/en.2009-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 53.Eizirik DJ, Mandrup-Poulsen T. A choice of death-the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 54.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 55.Oki T, Masuda M, Furuta S, Nishiba Y, Terahara N, Suda I. Involvement of anthocyanins and other phenolic compounds in radical scavenging activity of purple-fleshed sweet potato cultivars. J Food Sci. 2002;67:1752–1756. [Google Scholar]
- 56.Suda I, Oki T, Masuda M, Kobayashi M, Nishiba Y, Furuta S. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. Jpn Agric Res. 2003;37:167–173. [Google Scholar]
- 57.Ohno N, Akanuma AM, Miura NN, Adachi Y, Motoi M. (1-3)-beta-glucan in the fruit bodies of Agaricus blazei. Pharm Pharmacol Lett. 2001;11:87–90. [Google Scholar]
- 58.Soltys J, Quinu MT. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vitro treatment with β-(1,6)-branched β-(1,3)-glucan. Infect Immun. 1999;67:244–252. doi: 10.1128/iai.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa Y, Ohno N, Murai T. Suppression by Candida albicans β-glucan of cytokine release from activated human monocyte and from T cells in the presence of monocytes. J Infect Dis. 2003;187:710–713. doi: 10.1086/368334. [DOI] [PubMed] [Google Scholar]
- 60.Şener G, Ekşioğlu-Demiralp E, Çetiner M, Ercan F, Yeğen BC. β-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immunomodulatory effect. Eur J Pharmacol. 2006;542:170–178. doi: 10.1016/j.ejphar.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 61.Shimabukuro M, Ohneda M, Lee Y, Unger RH. Role of nitric oxide in obesity-induced β cell disease. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 63.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]