Abstract
Resveratrol, a phytoalexin present in the skin of grapes and red wine, has been demonstrated to possess a wide range of health promoting activities including anti-diabetic properties. In the present study, we investigated the effect of resveratrol in both type 2 diabetic mice and cell culture systems. In cultured L6 myotubes, we studied the effect of resveratrol on glucose uptake and translocation of glucose transporter 4 to plasma membrane from the aspects of insulin signaling and AMP-activated protein kinase signaling. In cultured RIN-5F cells, we examined whether resveratrol would protect the pancreas-derived β-cells from oxidative stress. Resveratrol significantly suppressed the elevation in the fasting blood glucose level and the serum triglyceride and lipid peroxide levels in db/db mice. Resveratrol stimulated glucose uptake and glucose transporter 4 translocation by activating both insulin signaling and AMP-activated protein kinase signaling. Moreover, resveratrol could protect pancreatic β-cells from advanced glycation end products-induced oxidative stress and apoptosis. From these results, resveratrol is suggested to show anti-diabetic effect by stimulating both insulin-dependent and -independent glucose uptake in muscles and by protecting pancreatic β-cells from advanced glycation end products-induced oxidative stress and apoptosis.
Keywords: AMPK signaling, myotube, pancreatic β-cell, resveratrol, type 2 diabetic db/db mouse
Introduction
The total number of people with diabetes is increasing worldwide. To reduce the hyperglycemia, several trials have been conducted; for instance, inhibition of α-glucosidase to interfere with glucose absorption from the intestine, stimulation of pancreatic islet cells to secret insulin, reduction of hepatic glucose production, and augmentation of glucose utilization.(1,2) Type 2 (non-insulin-dependent) diabetes is associated with metabolic syndrome and a marked increase in the risk of cardiovascular morbidity and mortality.(3–7) Under diabetic condition, chronic hyperglycemia and subsequent augmentation of reactive oxygen species (ROS) increase insulin resistance and deteriorate β-cell functions which lead to the aggravation of type 2 diabetes.(8) The skeletal muscles which account for the majority (~80%) of insulin-mediated glucose uptake in the post-prandial state play an important role in maintaining glucose homeostasis.(9)
In skeletal muscles, insulin increases glucose uptake via a signaling that leads to activation of phospatidylinositol-3 kinase (PI3K) and Akt, resulting in increased translocation of glucose transporter 4 (GLUT4) to the plasma membrane.(9) Therefore, skeletal muscle tissue implies an attractive therapeutic target for the treatment of insulin resistance and type 2 diabetes. There is another GLUT4 translocation promoter, the AMP-activated protein kinase (AMPK) which is composed of three subunits, α, β and γ.(10) In mammalian cells, AMPK activated by an increase in AMP/ATP ratio acts as an energy sensor,(10) and has at least two upstream kinases, LKB1 and calmodulin-dependent protein kinase kinase (CaMKK).(10) AMPK is activated by exercise/contraction(11) and numerous compounds including metformin(12) and thiazolidinedione,(13) resulting in stimulation of glucose uptake in skeletal muscles. The study of novel compounds that activate AMPK and increase skeletal muscle glucose uptake could be used for the development of new treatment of insulin resistance and type 2 diabetes. In the hyperglycemic state, glucose reacts nonenzymatically with protein amino groups to initiate post-translational modification process known as nonenzymatic glycosylation, or glycation. This process begins with the conversion of reversible Schiff base adducts to more stable, covalently bound Amadori rearrangement products.(14) Over the course of weeks to months, the Amadori products undergo further rearrangement reactions to form irreversibly bound moieties called advanced glycation end products (AGEs).(14,15) Increased oxidative stress and AGEs may result in the overexpression of receptor of AGEs (RAGE),(16,17) which is minimally expressed in normal vasculature.(18) The RAGE gene promoter contains NF-κB binding sites, which positively control RAGE expression and link RAGE to the inflammatory response.(19) Up-regulation of RAGE has been shown to occur in endothelial cells, smooth muscle cells, and mononuclear phagocytes in the diabetic vasculature.(20,21) The binding of AGEs to RAGE activates multiple signaling cascades, including extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase (MAPK), and the generation of ROS.(22) ROS augmentation reportedly deteriorates β-cell functions, because gene expression of antioxidant enzymes such as glutathione peroxidase and catalase was very low in pancreatic cells.(23,24) Thus, β-cells are vulnerable to oxidative stress, leading to the reduction of insulin secretion.
Resveratrol (3,5,4'-trans-trihydroxystilbene), a phytoalexin polyphenol that is found naturally in such as grapes, peanuts and mulberries. Resveratrol has a number of health benefits including anticarcinogenic,(25) cardiovascular protective(26) and estrogenic activities(27) as well as anti-inflammatory,(28) free-radical scavenging(29) activities, inhibition/induction of apoptosis,(30) inhibition of platelet aggregation,(31) life-extension,(32) and neuroprotection.(33) We have reported that resveratrol shows anti-invasive effect against hepatoma cells,(34–36) and hypolipidemic effect in glomerulonephritic(37) and hepatoma-bearing rats.(38)
Although a number of studies concerning the effect of resveratrol on diabetes or hyperglycemia have been reported, little has been validated in type 2 diabetes model animals. Hence, the present study is intended to investigate comprehensive effect of resveratrol on metabolic functions in type 2 diabetic model animals (db/db mice) as well as myotubes and pancreatic β-cells in culture. The db/db mice are characterized by obesity, insulin resistance, severe hyperglycemia, pancreatic injury and cardiovascular complications, and therefore regarded as a useful model of heavy human type 2 diabetes.(39,40)
Materials and Methods
Materials
L6 myoblasts derived from a rat(41) and RIN-5F cells derived from rat pancreatic β-cells(42) were purchased from American Type Culture Collection (Manassas, VA; ATCC® numbers: CRL-1458 and CRL-2058, respectively), Dulbecco’s modified Eagle medium (DMEM) and RPMI 1640 medium were from Nissui Pharmaceutical Co., (Tokyo, Japan), fetal bovine serum (FBS) was from JRH Biosciences, (Lenexa, KS) and streptomycin and penicillin G were from Nacalai Tesque, Inc., (Kyoto, Japan). Resveratrol, bovine serum albumin (BSA, fatty acid free) and Triton X-100 were purchased from Sigma Chemical Co., (St. Louis, MO). Glucose, total cholesterol (T-Ch) and triglyceride (TG) assay kits (Glucose CII Test Wako, Cholesterol E-Test Wako and Triglyceride E-Test Wako, respectively) were from Wako Pure Chemical Industries, Ltd., (Osaka, Japan). Serum lipid peroxide was estimated as thiobarbituric acid-reactive substances (TBARS) with a commercial kit (TBARS Assay Kit, ZeptoMetrix Corporation, Buffalo, NY). Insulin assay kit was from Morinaga Institute of Biological Science, Inc., (Yokohama, Japan). The anti-phospho-Akt (Ser473) antibody was obtained from Upstate Biotechnology (Lake Placid, NY). The anti-Akt1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-phospho-AMPKα (Thr172) and anti-AMPK antibodies were obtained from Cell Signaling Technology, Inc., (Beverly, MA). Anti-Na+/K+ ATPase α-1 antibody from Millipore (Billerica, MA), anti-GLUT4 antibody from AbD Serotec (Oxford, UK), horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG antibodies from Invitrogen (San Diego, CA). All other chemicals were of the best grade commercially available, unless otherwise noted. Plastic multiwell plates and tubes were obtained from Nunc A/S (Roskilde, Denmark) or Iwaki brand (Asahi Glass Co., Ltd., Tokyo, Japan).
Effect of resveratrol on blood glucose levels in db/db mice
All animal experiments were conducted in accordance with guidelines established by the Animal Care and Use Committee of Tokyo University of Agriculture and Technology and were approved by this committee. To determine the effect of resveratrol on fasting blood glucose levels, db/db mice were used as a model of type 2 diabetes. Male db/db and its misty (m/m) control (normal) mice (5 weeks of age) were obtained from Charles River Japan, Kanagawa, Japan. Animals were individually housed in stainless-steel cages with wire bottoms in an air-conditioned room with a temperature of 22 ± 2°C, a relative humidity of 60 ± 5%, and an 8:00–20:00 light cycle. All mice were maintained on a stock CE-2 pellet diet (CLEA Japan, Tokyo, Japan) for 3 days and thereafter a basal 20% casein diet (20C) for 4 days. The composition of the 20C diet was as follows (dry weight basis): 20% casein (Oriental Yeast Co., Tokyo, Japan), 7% corn oil (Hayashi Chemicals Co., Tokyo, Japan), 13.2% α-cornstarch (Nihon Nosan Kogyo Co., Yokohama, Japan), 49.75% β-cornstarch (Nihon Nosan Kogyo Co.), 3.5% mineral mixture (AIN-93G composition, Nihon Nosan Kogyo Co.), 1% vitamin mixture (AIN-93 composition, Nihon Nosan Kogyo Co.), 0.25% choline bitartrate (Wako Pure Chemical Industries), 0.3% l-cystine (Wako Pure Chemical Industries), and 5% cellulose powder (Oriental Yeast Co.). After preliminary feeding for 1 week, mice were deprived of their diet at 9:00 a.m. but allowed free access to water until blood collection from tail vein 4 h later. Blood (10 µl) was burst in water (40 µl), 20% (w/v) trichloroacetic acid aqueous solution (50 µl) was added and test tubes containing the mixture were kept in ice-cold water. The mixture was then centrifuged at 13,000 × g and 4°C for 5 min. The resultant supernatant (80 µl) was subjected to glucose determination with a commercial kit and a spectrophotometer (Model U-1100, Hitachi Science Systems, Ltd., Ibaraki, Japan) at 505 nm,(43) and db/db mice (6 weeks of age at the moment) were divided into two groups of similar fasting blood glucose levels and body weights (0 week). Diabetic mice of each of the two groups were given either the 20C as the control group or the 20C supplemented with 0.04% resveratrol as the test group for 5 weeks. Resveratrol was supplemented to the 20C at the expense of β-cornstarch. Likewise, misty control mice were given the 20C as the normal group for 5 weeks.
Syntheses of advanced glycation end products (AGEs)
AGEs were generated from co-incubation of BSA with either D-glucose (AGE1) or D-glyceraldhyde (AGE2) according to the method of Kume et al.(44) AGE1 and AGE2 were incubated at 37°C for 8 weeks and 2 weeks, respectively. BSA alone was incubated at 37°C for 2 weeks under conditions without any carbohydrates, and employed as the control for AGE1 and AGE2. This BSA was designated as CNT.
Determination of glucose uptake by cultured L6 myotubes
Stock cultures of L6 myoblasts were maintained in DMEM supplemented with 10% (v/v) FBS, streptomycin (100 µg/ml), and penicillin G (100 U/ml) (10% FBS/DMEM) under an atmosphere of 5% CO2/95% humidified air at 37°C as described previously.(45) Effect of resveratrol was examined by the procedure described previously(43,46) with slight modifications. Briefly, the L6 myoblasts (5 × 104 cells/well) were subcultured into Nunc 24-place multiwell plates and grown for 11 days to form myotubes in 0.4 ml of 10% FBS/DMEM. The medium was renewed every 3 days. Later, the 11-day-old myotubes were kept for 2 h in filter-sterilized Krebs-Henseleit buffer (pH 7.4, 141 mg/l MgSO4, 160 mg/l KH2PO4, 350 mg/l KCl, 6900 mg/l NaCl, 373 mg/l CaCl2-2H2O and 2100 mg/l NaHCO3) containing 0.1% bovine serum albumin, 10 mM Hepes and 2 mM sodium pyruvate (KHH buffer). The myotubes were thereafter cultured in KHH buffer containing 11 mM glucose without or with resveratrol (1–100 µM) for another 4 h. Glucose concentrations in KHH buffer were determined with a glucose assay kit and a microplate reader (Appliskan, Thermo Fisher Scientific Inc., MA) at 508 nm, and the amounts of glucose consumed were calculated from the differences in glucose concentrations between before and after culture.
Preparation of plasma membrane from L6 myotubes
The L6 myoblasts (5 × 105 cells) were subcultured into Nunc 60 mm dishes and grown for 11 days to form myotubes in 3 ml of 10% FBS/DMEM. The medium was renewed every 3 days. Later, the 11-day-old myotubes were kept for 2 h in KHH buffer. Then, the myotubes were cultured in KHH buffer containing 11 mM glucose without or with resveratrol (10, 100 µM) for appropriate time intervals. Then, plasma membrane fractions were obtained by the method described by Nishiumi and Ashida.(47) Briefly, to prepare the plasma membrane and post-plasma membrane fraction, L6 myotubes were harvested with buffer A (50 mM Tris-HCl (pH 8.0), 0.5 mM DTT, protease inhibitor cocktail and 1 mM Na3VO4 (Nacalai Tesque, Inc., Kyoto, Japan) containing 0.1% (v/v) IGEPAL CA-630 (Sigma Chemical Co.), and homogenated with 21-gauge needle. Each homogenate was centrifuged at 1,000 × g for 10 min at 4°C, and the precipitate was suspended in IGEPAL CA-630-free buffer A, and recentrifuged at 1,000 × g for 10 min at 4°C. The precipitate obtained was suspended again in buffer A containing 1.0% (v/v) IGEPAL CA-630, stood on ice for 1 h with occasional mixing and centrifuged at 16,000 × g for 20 min at 4°C. The supernatant was collected and stored as the plasma membrane fraction at −80°C until analyses. The supernatant from the first 1,000 × g centrifugation was gathered and centrifuged again at 16,000 × g for 20 min at 4°C. The supernatant obtained was collected and used as a post-plasma membrane fraction.
Preparation of cell lysate
The 11-day-old myotubes were kept for 2 h in KHH buffer. Then, the myotubes were cultured in KHH buffer containing 11 mM glucose with resveratrol (10 µM) for appropriate time intervals. The cells were scraped from the plates into ice-cold RIPA lysis buffer (Nacalai Tesque) and centrifuged for 20 min at 4°C and 16,000 × g. The supernatant was collected and stored at −80°C until use.
Western blotting
Protein samples were separated by SDS–PAGE, transferred to a PVDF membrane which was blocked for 1 h with 5% (w/v) dry milk in Tris-buffered saline, and incubated overnight at 4°C with the primary antibody. The primary antibody was detected with HRP-conjugated anti-rabbit or anti-mouse secondary antibody and visualized by using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fischer, Rockford, IL).
Measurement of intracellular ROS
RIN-5F cells derived from rat pancreatic β-cells were maintained in RPMI 1640 supplemented with 10% (v/v) FBS, streptomycin (100 µg/ml), and penicillin G (100 U/ml) (10% FBS/RPMI 1640) under an atmosphere of 5% CO2/95% humidified air at 37°C. The medium was renewed every 3 days. The cells (5 × 105 cells/well) were cultured into Nunc 12-place multiwell plates. After being cultured for 72 h in 1 ml of 10% FBS/RPMI 1640, the medium in each well was removed. Thereafter, RIN-5F cells received 1 ml of fresh medium (1% FBS/RPMI 1640) without or with resveratrol and AGEs for another 3 h. Effect of resveratrol on oxidative stress was examined by measurement of intracellular ROS based on ROS-mediated conversion of non-fluorescent 2',7'-DCFH-DA into DCFH.(48) The intensity of fluorescence reflects enhanced oxidative stress. After the 3 h incubation, RIN-5F cells were incubated with DCFH-DA (final 25 µM) in 1% FBS/RPMI 1640 at 37°C for 20 min. At the end of the incubation, DCFH fluorescence of the cells from each well was measured at an emission wavelength of 530 nm and an excitation wavelength of 488 nm using a flow cytometer (Becton Dickinson, San Jose, CA).
Flow cytometric evaluation of apoptosis
RIN-5F cells received 1 ml of fresh medium (1% FBS/RPMI 1640) containing 200 µg BSA alone (CNT) or AGE2 without or with 50 µM resveratrol for another 6 h. Effect of resveratrol on oxidative stress-induced apoptosis was examined by using an Annexin V-FITC apoptosis detection kit (Becton Dickinson, San Jose, CA). Annexin V has a strong, Ca2+-dependent affinity for phosphatidylserine (PS), which translocates from the internal to the external surface of the plasma membrane as a probe for detecting apoptosis.(49) Samples were incubated on ice for 10 min in the dark with Annexin V and propidium iodide (PI) and analyzed with a flow cytometer.
Statistical analysis
Data are expressed as means ± standard errors of means (SEM). Multigroup comparisons were carried out by one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test (Instat ver 2.00, GraphPad Software Inc., San Diego, CA). Values of p < 0.05 were considered statistically significant.
Results
Effect of resveratrol on blood glucose level and other parameters in db/db mice
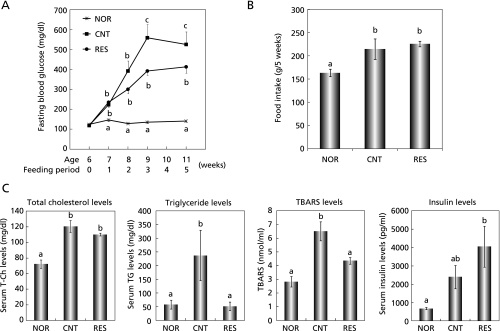
To study the in vivo effect of resveratrol, we employed db/db mice, a model for type 2 diabetes. Fig. 1A shows the effect of resveratrol on the fasting blood glucose level. The blood glucose level linearly increased to the third week of feeding and thereafter maintained the high value up to fifth week in diabetic control mice, while it was unchanged and constant in normal mice for this period. Resveratrol (0.04% in diet) tended to suppress until the second week of feeding and significantly suppressed the rise in the blood glucose level at the third week and thereafter. As shown in Fig. 1B, food intake of the resveratrol group for 5 weeks was almost the same as that of the control group, suggesting that the suppressive effect of resveratrol on fasting blood glucose level was not due to reduced food intake but due to its pharmacological action. As seen in Fig. 1C, T-Ch, TG and TBARS concentrations in serum of the diabetic control group significantly increased as compared with those of the normal group. Resveratrol, however, significantly suppressed the rises in the TG and TBARS concentrations. Serum insulin concentration in the control group tended to increase as compared with that in the normal group. The concentration in the resveratrol group also tended to increase as compared with that in the control group. However, these differences in insulin concentrations between the normal and diabetic control groups and between the diabetic control and resveratrol groups were not statistically significant (Fig. 1C).
Fig. 1.
Effect of resveratrol on blood glucose level and other parameters in db/db mice. A: Effect of resveratrol on the fasting blood glucose level. Mice were fasted for 4 h before blood collection. B: Food intake per mouse for 5 weeks. C: Serum levels of T-Ch, TG, TBARS and insulin were determined at fifth week of feeding. NOR, CNT and RES indicate normal, diabetic control and resveratrol-treated groups, respectively. Each value represents the mean ± SEM for 5 (NOR, RES,) and 4 (CNT) mice. Values not sharing a common letter are significantly different at p < 0.05 by Tukey-Kramer multiple comparisons test.
Resveratrol accelerated glucose uptake ability and effectively translocated GLUT4 to plasma membrane
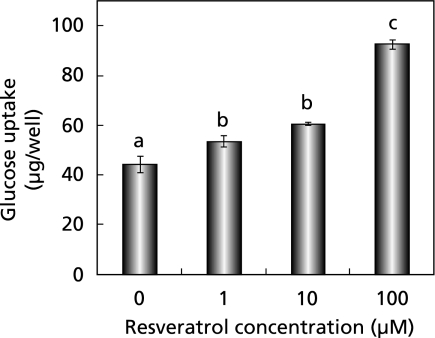
To investigate mechanisms for resveratrol actions in vivo, we first examined its effect on glucose uptake by L6 myotubes in vitro (Fig. 2). Resveratrol dose-dependently increased glucose uptake at concentrations of 1–100 µM in the absence of insulin.
Fig. 2.
Effect of resveratrol on glucose uptake in cultured L6 myotubes. Each value represents the mean ± SEM for 6 wells. Values not sharing a common letter are significantly different at p < 0.05 by Tukey-Kramer multiple comparisons test.
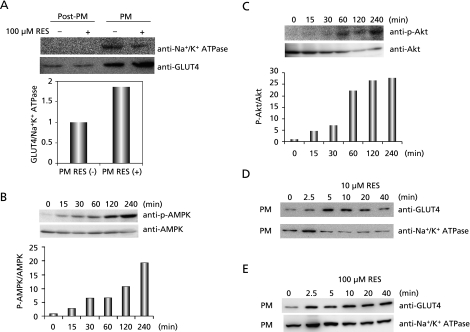
Myotubes take glucose by mainly using GLUT4. Its recruitment to the surface of muscle cells is induced by both insulin and exercise.(50,51) Thus, we examined whether or not resveratrol would promote GLUT4 translocation to plasma membrane by western blotting analyses using antibodies to GLUT4 and Na+/K+ ATPase, the latter being a plasma membrane marker enzyme. Treatment of L6 myotubes with 100 µM resveratrol for 15 min promoted GLUT4 translocation (Fig. 3A). Phosphorylation ratios of AMPK and Akt commenced to increase 15 min after resveratrol (10 µM) treatment and thereafter further increased up to 240 min (Fig. 3 B and C). Time-dependent changes of GLUT4 translocation after resveratrol treatment are shown in Fig. 3 D and E. While maximum translocation of GLUT4 was attained 5–10 min after treatment and thereafter GLUT4 gradually diminished at the low concentration of resveratrol (10 µM), GLUT4 rapidly translocated to plasma membrane 2.5 min after treatment and maintained the promotive effect up to 40 min at the high concentration of resveratrol (100 µM).
Fig. 3.
Effect of resveratrol on translocation of GLUT4 to plasma membrane and phosphorylation of AMPK and Akt. A: Translocation of GLUT4 to plasma membrane of L6 myotubes which were exposed to 100 µM resveratrol for 15 min. GLUT4 and plasma membrane were detected by western blotting analyses with anti-GLUT4 and anti-Na+/K+ ATPase antibodies. Post-PM and PM indicate post plasma membrane and plasma membrane fractions, respectively. B, C: Effect of resveratrol on phosphorylation of AMPK (B) and Akt (C). L6 myotubes were exposed to resveratrol (10 µM) for indicated time intervals. Western blotting analyses were carried out with anti-AMPK, anti-phospho-AMPK, anti-Akt and anti-phospho-Akt antibodies. D, E: Time-dependent changes of GLUT4 translocation in L6 myotubes at 10 µM (D) or 100 µM (E) resveratrol. L6 myotubes were exposed to resveratrol for indicated time intervals. Plasma membrane fractions of L6 myotubes were subjected to western blotting analyses.
Resveratrol protects pancreatic β-cells from oxidative stress
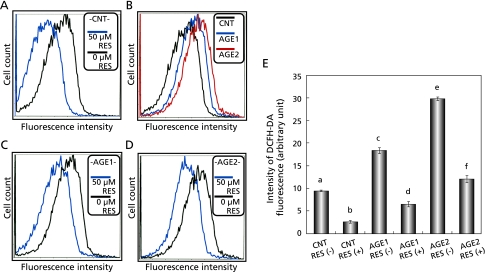
As above-mentioned, β-cells are vulnerable to oxidative stress. Resveratrol is one of polyphenols with antioxidative property. Thus, the effect of resveratrol on the AGEs-induced oxidative stress was investigated in RIN-5F cells. RIN-5F cells that had been adhered and pre-cultured with medium alone for 72 h were treated with resveratrol or medium alone for another 3 h. At the end of the culture, the intracellular peroxide level was measured with DCFH-DA fluorescent probe. The fluorescence intensity of the resveratrol-treated group was significantly lower than that of the control (0 µM resveratrol) group (Fig. 4A). This result suggested that resveratrol might reduce oxidative stress in RIN-5F cells. To verify this hypothesis, RIN-5F cells were given oxidative stress by adding AGE1 and AGE2, BSA conjugated with glucose and glyceraldehyde, respectively, to experimental media. Control cells were incubated with BSA alone (CNT) (Fig. 4B). After 3 h treatment with AGEs, fluorescence intensity of AGE1 and AGE2-treated cells was significantly higher than that of the CNT-treated cells (Fig. 4 B and E). In the same experiments with AGEs, treatment of RIN-5F cells with resveratrol for 3 h resulted in dramatic reductions in oxidative stress with significant differences (Fig. 4 C, D and E). These results clearly defined that resveratrol was capable to protect pancreatic β-cells from AGEs-induced oxidative stress.
Fig. 4.
Effect of resveratrol on AGEs-induced oxidative stress in RIN-5F cells. Intracellular ROS in RIN-5F cells were measured with DCFH-DA fluorescent probe and a flow cytometer. A: Effect of resveratrol on basal ROS in RIN-5F cells. B: Effects of AGE1 and AGE2 on intracellular ROS levels in RIN-5F cells after 3 h treatment with AGEs. BSA alone (CNT) was used as control. C, D: Effect of resveratrol on AGEs-induced oxidative stress. E: Graph of statistically processed data. Each value represents the mean ± SEM for 4 assays. Values not sharing a common letter are significantly different at p < 0.001 by Tukey-Kramer multiple comparisons test.
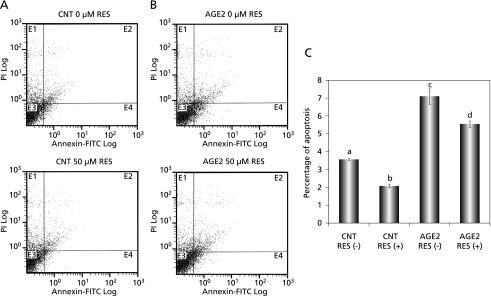
Resveratrol protects pancreatic β-cells from oxidative stress-induced apoptosis
We examined the effect of resveratrol on oxidative stress-induced apoptosis in RIN-5F cells. To quantitatively gain insight into anti-apoptotic effect of resveratrol in AGE2-treated RIN-5F cells, a display of propidium iodide (PI) versus Annexin V-FITC fluorescence was measured by flow cytometric analysis (Fig. 5). Annexin V-positive and PI-negative cells, which are shown in quadrant E4 in Fig. 5 A and B, are thought to be early apoptotic cells.(52) RIN-5F cells were exposed to 200 µg/ml CNT and AGE2 in the absence or presence of 50 µM resveratrol for 6 h (Fig. 5 A and B). In this experiment, AGE2 was selected, because it induced more notable oxidative stress in RIN-5F cells than did AGE1 (Fig. 4E). The percentage of apoptosis in AGE2-treated cells was significantly higher than that in CNT-treated cells (Fig. 5C). In the same experiments with CNT and AGE2, treatment of RIN-5F cells with resveratrol for 6 h resulted in significant reductions in the percentage of apoptosis (Fig. 5C).
Fig. 5.
Effect of resveratrol on AGE2-induced apoptosis in cultured RIN-5F cells. The cells were exposed to resveratrol and/or AGE2 for 6 h. BSA alone (CNT) (A) and AGE2 (B) were added to media at a concentration of 200 µg/ml in the absence or presence of 50 µM resveratrol. C: Graph of statistically processed data. Each value represents the mean ± SEM for 3 assays. Values not sharing a common letter are significantly different at p < 0.05 by Tukey-Kramer multiple comparisons test.
Discussion
Many kinds of phytochemicals have been reported to possess anti-diabetic potentials.(43,53,54) Resveratrol has been reported to competent to shift the physiology of mice on a high-calorie diet towards that of mice on a standard diet and improves their survival and insulin sensitivity.(32) This polyphenol also has been found to possess an insulin-mimetic effect in type 1 diabetic model rats and to ameliorate several metabolic parameters.(55) In the present study, resveratrol was demonstrated to suppress the elevation in the blood glucose level and partially improved dyslipidemia in type 2 diabetic model db/db mice. Serum lipid peroxide (TBARS) concentration was significantly lower in the resveratrol group than in the diabetic control group (Fig. 1C). This result suggests that antioxidative property of resveratrol is available in vivo as well as in vitro (Fig. 4).
AMPK is an important protein to provide energy in mammalian cells.(10) Previous reports showed that resveratrol activated AMPK signaling(32,56) or insulin signaling including Akt.(57) In the present study, we found for the first time that resveratrol promoted rapidly endogenous GLUT4 translocation through simultaneously enhancing phosphorylation of both AMPK (AMPK signaling) and Akt (insulin signaling), resulting in stimulation of glucose uptake in skeletal muscle cells. Although AMPK signaling is independent of insulin signaling, GLUT4 translocation to plasma membrane is a common final event. Thus, resveratrol is suggested to effectively improve and overcome insulin resistance.
Activation of rennin-angiotensin-aldosterone system has been reported to increase ROS and impaired insulin signaling. Transgenic Ren2 rats manifest increased tissue rennin-angiotensin system activity, hypertension and insulin resistance.(58) In the soleus muscle of Ren2 rats, along with increased NADPH oxidase activity and ROS, there was systemic insulin resistance and reduced IRS-1 tyrosine phosphorylation, Akt phosphorylation/activation, and GLUT4 expression,(58) suggesting oxidative stress disturbs insulin signaling in muscle tissues. In isolated cardiomyocytes, elevated levels of lipid peroxidation product 4-hydroxy-2-nonenal (HNE) have been reported to result in the formation of HNE-LKB1 adducts that inhibit LKB1 and hence AMPK activity.(59) Treatment of cardiomyocytes with resveratrol prevents HNE modification of the LKB1/AMPK signaling.(59) If this be true of L6 myotubes and skeletal muscle tissues of db/db mice, oxidative stress is also suggested to disturb AMPK signaling in muscle tissues and to be improved by resveratrol treatment. These two findings are not inconsistent with the results shown in Fig. 3.
In the diabetic state, glycation reaction is observed in various tissues and organs, and various kinds of glycated proteins such as glycosylated hemoglobin, albumin, and lens crystalline are produced in a nonenzymatical manner through the glycation reaction. The reaction produces Schiff base, Amadori product, and finally AGEs.(8) In the present study, the resveratrol group was still in the high blood glucose state in db/db mice, although the polyphenol lowered the level of blood glucose significantly as compared with that of the diabetic control group. Therefore, syntheses of AGEs might occur in both the control and resveratrol groups. Nonetheless, our data demonstrated that resveratrol protected RIN-5F cells from AGEs-induced oxidative stress (Fig. 4) and AGEs-induced apoptosis (Fig. 5), which is followed by decreasing insulin gene expression and secretion. Although not statistically significant, the serum insulin level of the resveratrol group tended to increase as compared with that of the diabetic control (Fig. 1C), suggesting that resveratrol might partially rescue exhausted pancreatic β-cells of db/db mice from further AGEs-induced oxidative stress and apoptosis. It is necessary to analyze common signals in vivo such as levels of AGEs, AMPK phosphorylation and GLUT4 translocation in the skeletal muscle of db/db mice to tie the results obtained in vitro in the present study. Further intensive studies from these aspects are required to clarify the precise mechanisms for anti-diabetic action of resveratrol. In a recent study, resveratrol alleviated diabetes mellitus induced vasculopathy through attenuation of RAGE for AGE-NF-κB signaling pathway.(60) These previous and present experimental results raise the possibility that resveratrol has health benefits as both forceful protector of pancreatic β-cells and soothing agent of hyperglycemia as well as stimulator of glucose uptake by muscle.
In summary, resveratrol was suggested to suppress the elevation in the blood glucose level in db/db mice. This polyphenol showed antioxidative property both in vitro and in vivo. Results obtained also suggested that resveratrol might enhance glucose uptake in the muscle via both AMPK and insulin signaling pathways and reduce the exhaustion of pancreatic β-cells in db/db mice.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (B) to KY from the Japan Society for the Promotion of Science (JSPS) (No. 19380072).
Abbreviations
- AGEs
advanced glycation end products
- AMPK
AMP-activated protein kinase
- BSA
bovine serum albumin
- CaMKK
calmodulin-dependent protein kinase kinase
- DMEM
Dulbecco’s modified Eagle medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GLUT4
glucose transporter 4
- HNE
4-hydroxy-2-nonenal
- IRS-1
insulin receptor substrate-1
- LKB1
liver kinase B1
- MAPK
mitogen-activated protein kinase
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate oxidase
- PI
propidium iodide
- PI3K
phospatidylinositol-3 kinase
- PS
phosphatidylserine
- RAGE
receptor of AGEs
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid-reactive substances
- T-Ch
total cholesterol
- TG
triglyceride
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 2.Banskota AH, Nguyen NT, Tezuka Y, Nobukawa T, Kadota S. Hypoglycemic effects of the wood of Taxus yunnanensis on streptozotocin-induced diabetic rats and its active components. Phytomedicine. 2006;13:109–114. doi: 10.1016/j.phymed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999;33:1130–1134. doi: 10.1161/01.hyp.33.5.1130. [DOI] [PubMed] [Google Scholar]
- 4.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 6.Verdecchia P, Reboldi G, Angeli F, et al. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43:963–969. doi: 10.1161/01.HYP.0000125726.92964.ab. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 8.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. DOI: 10.115/2010/453892. Mediators Inflamm. 2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 10.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 12.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 13.Konrad D, Rudich A, Bilan PJ, et al. Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia. 2005;48:954–966. doi: 10.1007/s00125-005-1713-7. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee MA, Cerami A, Vlassara H. Advanced glycosylation endproducts in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi M, Makita Z, Yanagisawa K, Kameda Y, Koike T. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol Med. 1999;5:393–405. [PMC free article] [PubMed] [Google Scholar]
- 16.Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130–143. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- 20.Hudson BI, Wendt T, Bucciarelli LG, et al. Diabetic vascular disease: It’s all the rage. Antioxid Redox Signal. 2005;7:1588–1600. doi: 10.1089/ars.2005.7.1588. [DOI] [PubMed] [Google Scholar]
- 21.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 23.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 24.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 25.Bhardwaj A, Sethi G, Vadhan-Raj S, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 26.Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFκB. Free Radic Res. 2006;40:1066–1075. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- 27.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- 28.Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Jia Z, Zhu H, Misra BR, Mahaney JE, Li Y, Misra HP. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Mol Cell Biochem. 2008;313:187–194. doi: 10.1007/s11010-008-9756-y. [DOI] [PubMed] [Google Scholar]
- 30.Lin KH, Hsiao G, Shih CM, Chou DS, Sheu JR. Mechanisms of resveratrol-induced platelet apoptosis. Cardiovasc Res. 2009;83:575–585. doi: 10.1093/cvr/cvp139. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int J Mol Med. 2002;9:77–79. [PubMed] [Google Scholar]
- 32.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ates O, Cayli S, Altinoz E, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 34.Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001;167:151–156. doi: 10.1016/s0304-3835(01)00476-1. [DOI] [PubMed] [Google Scholar]
- 35.Miura D, Miura Y, Yagasaki K. Restoration by prostaglandins E2 and F2α of resveratrol-induced suppression of hepatoma cell invasion in culture. Cytotechnology. 2003;43:155–159. doi: 10.1023/B:CYTO.0000039903.22449.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura D, Miura Y, Yagasaki K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin Exp Metastasis. 2004;21:445–451. doi: 10.1007/s10585-004-2698-1. [DOI] [PubMed] [Google Scholar]
- 37.Nihei T, Miura Y, Yagasaki K. Inhibitory effect of resveratrol on proteinuria, hypoalbuminemia and hyperlipidemia in nephritic rats. Life Sci. 2001;68:2845–2852. doi: 10.1016/s0024-3205(01)01061-x. [DOI] [PubMed] [Google Scholar]
- 38.Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003;73:1393–1400. doi: 10.1016/s0024-3205(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 39.Coleman DL. Diabetes-obesity syndromes in mice. Diabetes. 1982;31:1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- 40.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chick WL, Warren S, Chute RN, Like AA, Lauris V, Kitchen KC. A transplantable insulinoma in the rat. Proc Natl Acad Sci USA. 1977;74:628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano A, Nakamura H, Hata S, Minakawa M, Miura Y, Yagasaki K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine. 2009;16:437–443. doi: 10.1016/j.phymed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Kume S, Kato S, Yamagishi S, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 45.Yagasaki K, Morisaki N, Kitahara Y, Miura A, Funabiki R. Involvement of protein kinase C activation in l-leucine-induced stimulation of protein synthesis in L6 myotubes. Cytotechnology. 2003;43:97–103. doi: 10.1023/B:CYTO.0000039898.44839.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doi M, Yamaoka I, Fukunaga T, Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun. 2003;312:1111–1117. doi: 10.1016/j.bbrc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 47.Nishiumi S, Ashida H. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci Biotechnol Biochem. 2007;71:2343–2346. doi: 10.1271/bbb.70342. [DOI] [PubMed] [Google Scholar]
- 48.Mendis E, Kim MM, Rajapakse N, Kim SK. An in vitro cellular analysis of the radical scavenging efficacy of chitooligosaccharides. Life Sci. 2007;80:2118–2127. doi: 10.1016/j.lfs.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Walton M, Sirimanne E, Reutelingsperger C, Williams C, Gluckman P, Dragunow M. Annexin V labels apoptotic neurons following hypoxia-ischemia. Neuroreport. 1997;18:3871–3875. doi: 10.1097/00001756-199712220-00007. [DOI] [PubMed] [Google Scholar]
- 50.Herman HA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- 52.Miura Y, Ono K, Okauchi R, Yagasaki K. Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J Nutr Sci Vitaminol. 2004;50:38–44. doi: 10.3177/jnsv.50.38. [DOI] [PubMed] [Google Scholar]
- 53.Modak M, Dixut P, Londhe J, Ghaskadbi S, Devasagyam TPA. Indian herbes and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afolayan AJ, Sunmonu TO. In vivo studies on antidiabetic plants used in South African herbal medicine. J Clin Biochem Nutr. 2010;47:98–106. doi: 10.3164/jcbn.09-126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 56.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Biophys Res Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 57.Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57:1814–1823. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lastra G, Whaley-Connell A, Manrique C, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008;295:E110–E116. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck LR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 60.Jing YH, Chen KH, Yang SH, Kuo PC, Chen JK. Resveratrol ameliorates vasculopathy in STZ-induced diabetic rats: role of AGE-RAGE signalling. Diabetes Metab Res Rev. 2010;26:212–222. doi: 10.1002/dmrr.1076. [DOI] [PubMed] [Google Scholar]