Abstract
The hormone erythropoietin (Epo) maintains red blood cell mass by promoting the survival, proliferation and differentiation of erythrocytic progenitors. Circulating Epo originates mainly from fibroblasts in the renal cortex. Epo production is controlled at the transcriptional level. Hypoxia attenuates the inhibition of the Epo promoter by GATA-2. More importantly, hypoxia promotes the availability of heterodimeric (α/β) hypoxia-inducible transcription factors (predominantly HIF-2) which stimulate the Epo enhancer. The HIFs are inactivated in normoxia by enzymatic hydroxylation of their α-subunits. Three HIF-α prolyl hydroxylases (PHD-1, -2 and -3) initiate proteasomal degradation of HIF-α, while an asparaginyl hydroxylase (‘factor inhibiting HIF-1’, FIH-1) inhibits the transactivation potential. The HIF-α hydroxylases contain Fe2+ and require 2-oxoglutarate as co-factor. The in vivo response is dynamic, i.e. the concentration of circulating Epo increases initially greatly following an anaemic or hypoxaemic stimulus and then declines despite continued hypoxia. Epo and angiotensin II collaborate in the maintenance of the blood volume. Whether extra-renal sites (brain, skin) modulate renal Epo production is a matter of debate. Epo overproduction results in erythrocytosis. Epo deficiency is the primary cause of the anaemia in chronic kidney disease and a contributing factor in the anaemias of chronic inflammation and cancer. Here, recombinant analogues can substitute for the hormone.
Wolfgang Jelkmann (University of Lübeck, Germany) received his medical degree from the Medical School of Hannover. His first professorship was at the Department of Physiology at the University of Bonn. Since 1995 he has been a Professor and Chairman of the Institute of Physiology at Lübeck. His research focuses on haematopoietic growth factors. He has authored >140 original publications and >100 review articles/book chapters, and (co-)edited 5 books. He is currently on the editorial boards of African Journal of Biotechnology, American Journal of Hematology, Annals of Hematology, Current Medicinal Chemistry, Journal of Interferon and Cytokine Research and Open Journal of Hematology.
 |
Introduction
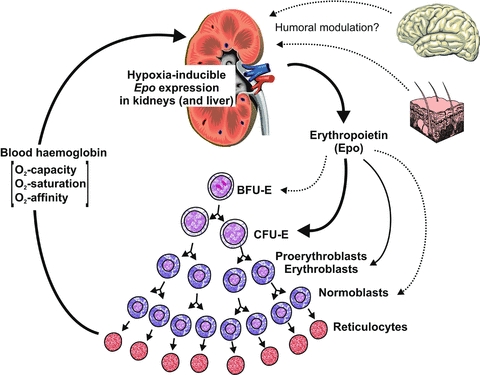
The hormone erythropoietin (Epo) is essential for red blood cell (RBC) production. The relationship between the O2 content of the blood and erythropoiesis was first described by the French anatomist Francois-Gilbert Viault in 1890 (Viault, 1890), who observed a rise in RBC numbers on a journey to the highlands of Peru (Morococha, about 4500 m). Indeed, the specific stimulus for Epo expression is a fall in tissue O2 pressure (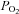 ). Epo production increases under hypoxic conditions in the kidneys and, in minor amounts, in distinct other organs such as the liver and the brain. This article summarises the present understanding of the control of Epo production.
). Epo production increases under hypoxic conditions in the kidneys and, in minor amounts, in distinct other organs such as the liver and the brain. This article summarises the present understanding of the control of Epo production.
Physiology of Epo
Epo is primarily expressed by hepatocytes during the fetal state. After birth, peritubular fibroblasts in the renal cortex become the main production site. Epo synthesis is regulated at the transcriptional level. Epo mRNA is also detectable in brain, liver, spleen, lung and testis, but these organs are not able to substitute for renal Epo in chronic kidney disease (CKD). Brain-derived Epo acts locally as a neuroprotective factor (see Noguchi et al. 2007). Epo is an acidic glycoprotein of about 30 kDa and comprises 165 amino acids and four glycans. Circulating Epo exhibits several glycosylation isoforms that differ in electrical charge and biological activity. Epo amounts are usually expressed in international units (IU), with one IU exerting the same erythropoiesis stimulating activity in rodents as 5 μmol cobaltous chloride (see Jelkmann, 2007).
Systemic Epo is an anti-apoptotic agent for erythrocytic progenitors, predominantly the colony-forming units-erythroid (CFU-Es). In response to Epo the CFU-Es proliferate and differentiate to generate cohorts of proerythroblasts and normoblasts (Fig. 1). The human haematopoietic Epo receptor (Epo-R) is a 484 amino acid glycoprotein of about 60 kDa, which belongs to the cytokine class I receptor family and forms homodimers. On the binding of Epo to the Epo-R dimer, cytoplasmic Janus kinases 2 (JAK2) catalyse the phosphorylation of tyrosine residues of the Epo-R and of various intracellular proteins (enzymes and transcription factors). Erythropoiesis is a slow-acting process. Following a rise in plasma Epo it takes 3–4 days before reticulocytosis becomes apparent.
Figure 1. Diagram of the feedback regulation of erythropoiesis.
Lack of O2 (hypoxia) is a stimulus for the synthesis of erythropoietin (Epo), primarily in the kidneys. Epo is a survival, proliferation and differention factor for the erythrocytic progenitors, particularly the colony-forming units-erythroid (CFU-Es). The O2 capacity of the blood increases with the enhanced release of reticulocytes. The role of extra-renal sites (brain, skin) in the control of the renal Epo synthesis is still incompletely understood. BFU-E, burst-forming unit-erythroid.
Epo is essential for erythropoiesis. However, the action of Epo is augmented by several other hormones, namely testosterone, somatotropin and insulin-like growth factor 1. The higher RBC counts and haemoglobin concentrations [Hb] in men compared to women result from the stimulation of erythropoiesis by androgens and its inhibition by oestrogens.
A role has been proposed for Epo as a cytoprotective agent for several non-haematopoietic tissues, including the brain, the heart, blood vessels and the kidneys (Brines & Cerami, 2006). Some investigators believe that the effects of Epo on non-erythrocytic cells are mediated by a heterotrimeric receptor consisting of one Epo-R molecule in complex with a dimer of the common cytokine β receptor (βcR). The Epo-R/βc-R concept is mainly based on the observation that erythropoietically inactive Epo derivatives and analogues provide tissue protection in animal models (Leist et al. 2004). However, the physiological function of Epo outside the bone marrow of healthy creatures has been questioned based on molecular biology studies. First, transgene-rescued Epo-R-null mutant mice expressing Epo-R exclusively in the haematopoietic lineage develop normally and are fertile (Suzuki et al. 2002). Second, Epo-R protein is not generally detectable in cells of non-haematopoietic origin, if appropriate anti-Epo-R antibody is used for study (Sinclair et al. 2010).
Molecular mechanism of the hypoxia-induced Epo expression
Most of the present knowledge of the O2-sensing mechanism in control of Epo production has been based on in vitro studies utilising human hepatoma cells (lines Hep3B and HepG2). Noteworthily, the mechanisms of the renal and the hepatic Epo expression differ. (i) Renal cells respond in an all-or-nothing fashion to hypoxia (Koury et al. 1989), whereas hepatoma cells respond in a graded way. (ii) The hypoxia-response elements (HREs) in control of the Epo gene are located upstream in the kidney (between 9.5 and 14 kb 5′ to Epo) but downstream in the liver (within 0.7 kb 3′ to Epo) according to studies in transgenic mice (Kochling et al. 1998). In both tissues Epo expression is under the control of distinct transcription factors. The Epo promoter is suppressed by GATA-2 in normoxia (Tsuchiya et al. 1997). GATA-2 levels decrease in hypoxia (Imagawa et al. 2003). More importantly, the Epo enhancer is activated by hypoxia-inducible transcription factors (HIFs). These are composed of an O2-labile α-subunit (120 kDa; isoforms 1α, 2α or 3α) and a constitutive β-subunit (90–95 kDa). Although the prototype HIF-1 was discovered in studies of Epo (Wang & Semenza, 1995), later investigations have identified HIF-2 (also called EPAS1 for endothelial PAS domain protein 1) as the primary transcription factor inducing Epo expression (Warnecke et al. 2004; see Haase, 2010). HIF-2α is activated by the stress-responsive deacetylase Sirtuin 1 (Dioum et al. 2009). On using Cre-loxP recombination to ablate renal HIF-2α,Kapitsinou et al. (2010) have shown that hepatic HIF-2 takes over as the main regulator of the serum Epo level. Interestingly, hepatocyte-derived HIF-2 is also involved in the regulation of iron metabolism genes, supporting a role for HIF-2 in the coordination of Epo synthesis with iron homeostasis (Kapitsinou et al. 2010).
The HIFs cooperate with hepatocyte nuclear factor 4 (HNF-4) and the transcriptional co-activators p300 and cAMP response element-binding protein (CREB)-binding protein (CBP) (Bunn et al. 1998). Using short interfering RNAs (siRNAs) and chromatin immunoprecipitation (ChIP) analysis, Wang et al. (2010) studied in more detail the roles of p300, CBP and p160 steroid receptor coactivator (SRC) in Epo expression in hypoxic Hep3B cells. Knocking down p300 resulted in a great reduction of Epo expression, negated recruitment of RNA polymerase II to the gene's promoter, and eliminated hypoxia-stimulated acetylation at the promoter and recruitments of SRC-1 and SRC-3 to the enhancer. Knocking down CBP only slightly decreased the hypoxia-induced Epo transcription (Wang et al. 2010). The directly repeated (DR2) DNA element neighbouring the HIF-binding site (Blanchard et al. 1992) is probably responsible for the effects of thyroid hormone (Fandrey et al. 1994) and retinoic acid (Kambe et al. 2000), which stimulate Epo expression in a tissue-specific but O2-independent manner.
The C-terminus of the HIF-α subunits comprises O2-dependent degradation domains (O-DDD) that are prolyl hydroxylated (at Pro402 and Pro564 in HIF-1α, and Pro405 and Pro531 in HIF-2α) in the presence of O2 (Epstein et al. 2001; Bruick & McKnight, 2001; Jaakkola et al. 2001; Ivan et al. 2001; Yu et al. 2001). The reaction is catalysed by specific Fe2+-containing prolyl-4-hydroxylases (PHD-1, -2 and -3), which transfer one O-atom to the proline and the other to 2-oxoglutarate yielding CO2 and succinate (see Bruegge et al. 2007). The prolyl hydroxylated HIF-α combines with von Hippel-Lindau tumour suppressor protein (VHL)/E3 ligase und promptly undergoes proteasomal degradation (Pugh et al. 1997; Huang et al. 1998; Maxwell et al. 1999). As PHD-2 and PHD-3 are themselves HIF-target genes, their expression increases and HIF-α levels decline during long-term hypoxic periods (del Peso et al. 2003; Marxsen et al. 2004; Aprelikova et al. 2004; Stiehl et al. 2006). This feedback regulation may explain the declining Epo production during chronic anaemia or prolonged stay at high altitude (see below). Furthermore, the transcriptional activity of the HIFs is suppressed by HIF-α asparaginyl hydroxylation (at Asn803 in HIF-1α and Asn847 in HIF-2α), which prevents the binding of the transcriptional co-activator CBP/p300. This reaction is catalysed by the ‘factor inhibiting HIF-1’, FIH-1 (Mahon et al. 2001; McNeill et al. 2002), another Fe2+-containing and 2-oxoglutarate-requiring dioxygenase. According to in vitro assays, the Km values of the three PHDs for O2 are above the arterial 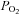 (∼170 mmHg), whereas FIH-1 operates at a lower
(∼170 mmHg), whereas FIH-1 operates at a lower 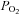 (∼60 mmHg) (Koivunen et al. 2004). Thus, it is likely that the HIF-α PHDs are the primary O2 sensors in control of Epo production.
(∼60 mmHg) (Koivunen et al. 2004). Thus, it is likely that the HIF-α PHDs are the primary O2 sensors in control of Epo production.
The discovery that Fe2+ is required for HIF-α degradation may provide an explanation for the increased plasma Epo level in patients treated with the iron chelator deferoxamine (Kling et al. 1996). Furthermore, cobalt is thought to enhance Epo expression by replacing the essential Fe2+ in the HIF-α dioxygenases, which results in HIF-α stabilisation. 2-Oxoglutarate competitors (clinical jargon: ‘HIF stabilisers’) likewise inhibit the hydroxylation of HIF-α. HIF stabilisers have been shown to stimulate Epo production in vitro and in mice (Hsieh et al. 2007). Chronic oral dosing of compound FG-2216 in rhesus macaques proved to increase erythropoiesis and to prevent anaemia induced by weekly phlebotomy (Hsieh et al. 2007). Epo mRNA and HIF-2α have been co-localised in renal fibroblasts of rats treated with the 2-oxoglutarate competitor FG-4497 (Paliege et al. 2010).
Systemic Epo response
The primary functions of Epo are (i) to keep RBC mass and [Hb] constant day by day, and (ii) to hasten RBC recovery after haemorrhage. Little Epo is needed to maintain the steady state in healthy persons. The basal plasma concentration of Epo ranges from 6 to 32 IU l−1 (about 10−11 mol l−1). The levels vary greatly between individuals, with the result that significant sex- or age-specific differences cannot be detected. Of note is the diurnal fluctuation, with a nadir in the morning. An acute loss of about 0.5 l blood does not induce a major rise in circulating Epo in men (Miller et al. 1982), yet the plasma Epo concentration increases exponentially when [Hb] falls below ∼125 g l−1 in humans not suffering from renal disease or inflammation. The response is dynamic, with initially very high Epo values that drop towards the normal ones before [Hb] normalises. The mechanism of the rapid decrease is not fully understood, but it may in part be caused by lowered HIF-α levels during long-term hypoxia (Stiehl et al. 2006). Furthermore, one has to remember that the plasma Epo level is not only dependent on the rate of Epo production, but also on its removal. In vitro studies have demonstrated that Epo is internalised and degraded by its target cells (Gross & Lodish, 2006). Accordingly, anaemic patients with bone marrow hypoplasia exibit extremely high plasma Epo levels (10,000 IU l−1 or more) compared with subjects suffering from haemolytic anaemia. Independently of changes in tissue oxygenation, the level of circulating Epo increases when the erythrocytic progenitors are arrested by the administration of chemotherapeutics (Birgegard et al. 1989).
Because Epo production depends on the tissue 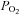 , Epo expression is also activated when the arterial
, Epo expression is also activated when the arterial 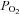 declines or when the O2 affinity of the blood increases. On ascent to altitude, Epo levels reach peak values after 1–2 days and then fall to a new plateau at about twice that present at sea-level (Abbrecht & Littell, 1972). As noted above, HIF-α levels decline during long-term hypoxic periods. In addition, the decrease in Epo production at continued hypoxia may be associated with the decrease in O2-affinity of the blood resulting from an increase in the intraerythrocytic concentration of 2,3-bisphosphoglycerate (Klausen, 1998). Nutritional factors, such as low protein intake, were excluded as a reason for the rapid fall in circulating Epo in a controlled high-altitude study in the Chilean Andes, which was performed on shift workers and Caucasian lowlanders (Gunga et al. 1996). Since Epo expression studies cannot be performed in humans and there is incomplete knowledge of the mechanisms in control of the degradation of the hormone, the question remains as to how much the increased number of Epo-consuming erythrocytic progenitors contribute to the decline of circulating Epo.
declines or when the O2 affinity of the blood increases. On ascent to altitude, Epo levels reach peak values after 1–2 days and then fall to a new plateau at about twice that present at sea-level (Abbrecht & Littell, 1972). As noted above, HIF-α levels decline during long-term hypoxic periods. In addition, the decrease in Epo production at continued hypoxia may be associated with the decrease in O2-affinity of the blood resulting from an increase in the intraerythrocytic concentration of 2,3-bisphosphoglycerate (Klausen, 1998). Nutritional factors, such as low protein intake, were excluded as a reason for the rapid fall in circulating Epo in a controlled high-altitude study in the Chilean Andes, which was performed on shift workers and Caucasian lowlanders (Gunga et al. 1996). Since Epo expression studies cannot be performed in humans and there is incomplete knowledge of the mechanisms in control of the degradation of the hormone, the question remains as to how much the increased number of Epo-consuming erythrocytic progenitors contribute to the decline of circulating Epo.
The location of the Epo-expressing cells in the kidney cortex is well suited for the regulated production of the hormone: there is a constant ratio of blood flow rate and O2 consumption, and the O2 extraction is small. The renal cortical 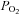 is barely affected by changes in cardiac output and blood flow because the renal O2 consumption decreases with the glomerular filtration rate. A renal flow reduction by 50% of normal was necessary to elicit at least some Epo formation in rats (Pagel et al. 1989). By comparison, the production of Epo increases much more during systemic hypoxia due to anaemia or hypoxaemia.
is barely affected by changes in cardiac output and blood flow because the renal O2 consumption decreases with the glomerular filtration rate. A renal flow reduction by 50% of normal was necessary to elicit at least some Epo formation in rats (Pagel et al. 1989). By comparison, the production of Epo increases much more during systemic hypoxia due to anaemia or hypoxaemia.
Role of angiotensin II
The signal to produce more erythrocytes following haemorrhage is apparently linked to the signals to retain salt and water by means of the renin–angiotensin system (Dunn et al. 2007). Angiotensin II (Ang II) is thought to stimulate erythropoiesis by two means. First, Ang II increases Epo production. Second, Ang II is a growth factor for the myeloid erythrocytic progenitors (Dunn et al. 2007; Vlahakos et al. 2010). The infusion of Ang II has been shown to raise the plasma Epo level to 17 IU l−1 (vs. 11 IU l−1 in controls) in healthy men (Gossmann et al. 2001). Furthermore, Ang II treatment at blood pressure-increasing doses (1.3 μg min−1 for 6 h) was found to raise the Epo concentration in men subjected to a haemorrhage of 750 ml as a basic physiological stimulus (Freudenthaler et al. 1999). The effect of Ang II on Epo production is prevented by Ang II type 1 receptor blockers (Freudenthaler et al. 1999; Gossmann et al. 2001). Transgenic mice harbouring the human renin and angiotensinogen genes exhibit erythrocytosis as well as hypertension (Kato et al. 2005). Bone marrow transplantation experiments have shown that Ang II-1a receptors on bone marrow-derived cells are dispensable for the Ang II-dependent erythrocytosis. Plasma Epo levels and renal Epo mRNA expression in the double transgenic mice were increased compared with those of the wild-type control, while the elevated plasma Epo levels were attenuated in the compound mice. Thus, the renin–Ang II system enhances erythropoiesis mainly through the Ang II-1a receptor of the Epo-producing renal cells. The question still needs to be answered whether Ang II stimulates Epo synthesis directly or indirectly by decreasing the renal O2 supply/O2 demand ratio (Dunn et al. 2007).
A feedback regulation of red cell mass and blood volume by means of Epo and Ang II seems to exist. Treatment of healthy men with recombinant human Epo (rhEpo) produces an increase in red cell mass. However, the increase in haematocrit (Hct) is accompanied by a decrease in plasma volume, which is probably due to a downregulation of the renin–angiotensin–aldosterone system and results in a constancy of the blood volume. Thus, Epo treatment in healthy humans raises [Hb] by two mechanisms: (i) an increase in red cell mass, and (ii) a decrease in plasma volume (Lundby et al. 2007).
Extra-renal sites affecting renal Epo production
The question arises as to how far renal Epo synthesis is influenced by extra-renal sites under hypoxic conditions (Fig. 1). One hypothesis suggests that the brain modulates O2-dependent Epo expression in the kidney. Local hypoxia of the brain stem was associated with an increase in renal Epo production in experimental animals (von Wussow et al. 2005). Both astrocytes and neurons express Epo. Moreover, evidence suggests that glial cells contribute to circulating Epo following the induction of hypoxia (Weidemann et al. 2009). Renal (but not hepatic) Epo mRNA levels are suppressed in transgenic mice lacking VHL or both VHL and HIF-1α in astrocytes (Weidemann et al. 2009). The mechanism by which astrocytes influence renal Epo expression still needs to be explored. In addition, the O2 supply to the skin has been implicated in the control of renal Epo expression (Boutin et al. 2008). According to this concept, an increased blood flow to the skin causes a reduction in the renal O2 supply. Mice with an epidermal deletion of VHL have increased Epo synthesis and develop erythrocytosis (Boutin et al. 2008). However, the concept of the dermal control of renal Epo production is not generally accepted (Paus et al. 2009), amongst other things because blood flow is not a major parameter in renal Epo synthesis (Pagel et al. 1989). Also, dermal blood flow depends on body heat, which has not been shown to affect Epo production. Note, here, that renal nerve inputs appear to be less relevant for O2-dependent Epo expression in the kidney (Eckardt et al. 1992). Frankly speaking, the evidence assigning extra-renal sites a major role in the control of renal Epo production is far from convincing.
Information on the various other humoral factors that have been implicated in the control of renal Epo production, such as adenosine and prostanoids, has been published elsewhere (Fisher, 2003).
Pathophysiology
Normochromic normocytic anaemia due to insufficient Epo synthesis develops in patients with CKD (‘renal anaemia’), systemic inflammations or malignancies. The relative lack of Epo in patients with anaemia associated with chronic disease has been related to the negative effects of the cytokines interleukin-1 (Il-1) and tumour necrosis factor-α on Epo expression (Jelkmann, 1998). In vitro, Il-1 inhibits HNF-4α mRNA formation and causes proteasome-dependent degradation of HNF-4α protein, thereby suppressing the hypoxic inducibility of the Epo enhancer (Krajewski et al. 2007). The anaemias of patients with CKD or cancer in combination with chemotherapy can be corrected by replacement therapy with rhEpo or its analogues (see Macdougall & Ashenden, 2009).
Erythrocytosis is due to persistent over-stimulation of erythropoiesis (see Hodges et al. 2007). Hct, RBC counts and [Hb] are abnormally high. Primary erythrocytosis is generally a myeloproliferative disorder. Secondary erythrocytosis is due to Epo over-production, most commonly caused by hypoxaemia. Theoretically, an increase in [Hb]– and thus in the O2 capacity of the blood – should be beneficial for tissue oxygenation (Brauner & Wang, 1997). However, blood viscosity increases with Hct, which raises the cardiac afterload and impedes the flow in microvessels. Hence, the erythrocytosis in high-altitude residents can be considered a maladaptive reaction, bearing risks for thromboembolic events and mortality. There have been distinct genetic adaptations in the evolution of tolerance of humans to high altitude (Hochachka et al. 1998). Tibetans living at about 4000 m altitude have relatively low [Hb] (Winslow et al. 1989), while South-American high-altitude natives often suffer from erythrocytosis and chronic mountain sickness (Leon-Velarde et al. 1991). Cobalt exposure may contribute to the excessive erythrocytosis in high-altitude miners (Jefferson et al. 2002). Beall et al. (2010) have recently identified 31 EPAS1 (encoding HIF-2α) single-nucleotide polymorphisms that correlate with low [Hb] in Tibetans residing at high altitude. This pioneering work may be taken as a good example of how information from molecular research can improve understanding at the systemic physiological level.
Glossary
Abbreviations
- Ang
angiotensin
- CBP
CREB-binding protein
- CFU-E
colony-forming unit-erythroid
- CKD
chronic kidney disease
- CREB
cAMP response element-binding protein
- Epo
erythropoietin
- Epo-R
Epo receptor
- Hb
haemoglobin
- Hct
haematocrit
- HIF
hypoxia-inducible factor
- HNF
hepatocyte nuclear factor
- HRE
hypoxia-response element
- PHD
prolyl hydroxylase
- RBC
red blood cell
- rhEpo
recombinant human Epo
- VHL
von Hippel-Lindau
References
- Abbrecht PH, Littell JK. Plasma erythropoietin in men and mice during acclimatization to different altitudes. J Appl Physiol. 1972;32:54–58. doi: 10.1152/jappl.1972.32.1.54. [DOI] [PubMed] [Google Scholar]
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhaxi P, Zheng Y. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgegard G, Wide L, Simonsson B. Marked erythropoietin increase before fall in Hb after treatment with cytostatic drugs suggests mechanism other than anaemia for stimulation. Br J Haematol. 1989;72:462–466. doi: 10.1111/j.1365-2141.1989.tb07733.x. [DOI] [PubMed] [Google Scholar]
- Blanchard KL, Acquaviva AM, Galson DL, Bunn HF. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992;12:5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner CJ, Wang T. The optimal oxygen equilibrium curve: A comparison between environmental hypoxia and anemia. Amer Zool. 1997;37:101–108. [Google Scholar]
- Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl- 4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Gu J, Huang LE, Park JW, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso L, Castellanos MC, Temes E, Martin-Puig S, Cuevas Y, Olmos G, Landazuri MO. The von Hippel Lindau/hypoxia inducible factor (HIF) pathway regulates the transcription of the HIF-proline hydroxylase genes in response to low oxygen. J Biol Chem. 2003;278:48690–48695. doi: 10.1074/jbc.M308862200. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Dunn A, Lo V, Donnelly S. The role of the kidney in blood volume regulation: the kidney as a regulator of the hematocrit. Am J Med Sci. 2007;334:65–71. doi: 10.1097/MAJ.0b013e318095a4ae. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, LeHir M, Tan CC, Ratcliffe PJ, Kaissling B, Kurtz A. Renal innervation plays no role in oxygen-dependent control of erythropoietin mRNA levels. Am J Physiol Renal Physiol. 1992;263:F925–F930. doi: 10.1152/ajprenal.1992.263.5.F925. [DOI] [PubMed] [Google Scholar]
- Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fandrey J, Pagel H, Frede S, Wolff M, Jelkmann W. Thyroid hormones enhance hypoxia-induced erythropoietin production in vitro. Exp Hematol. 1994;22:272–277. [PubMed] [Google Scholar]
- Fisher JW. Erythropoietin: Physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- Freudenthaler SM, Schreeb K, Korner T, Gleiter CH. Angiotensin II increases erythropoietin production in healthy human volunteers. Eur J Clin Investig. 1999;29:816–823. doi: 10.1046/j.1365-2362.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- Gossmann J, Burkhardt R, Harder S, Lenz T, Sedlmeyer A, Klinkhardt U, Geiger H, Scheuermann EH. Angiotensin II infusion increases plasma erythropoietin levels via an angiotensin II type 1 receptor-dependent pathway. Kidney Int. 2001;60:83–86. doi: 10.1046/j.1523-1755.2001.00773.x. [DOI] [PubMed] [Google Scholar]
- Gross AW, Lodish HF. Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP) J Biol Chem. 2006;281:2024–2032. doi: 10.1074/jbc.M510493200. [DOI] [PubMed] [Google Scholar]
- Gunga HC, Röcker L, Behn C, Hildebrandt W, Koralewski E, Rich I, Schobersberger W, Kirsch K. Shift working in the Chilean Andes (> 3,600 m) and its influence on erythropoietin and the low-pressure system. J Appl Physiol. 1996;81:846–852. doi: 10.1152/jappl.1996.81.2.846. [DOI] [PubMed] [Google Scholar]
- Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Gunga HC, Kirsch K. Our ancestral physiological phenotype: an adaptation for hypoxia tolerance and for endurance performance? Proc Natl Acad Sci U S A. 1998;95:1915–1920. doi: 10.1073/pnas.95.4.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol Hematol. 2007;64:139–158. doi: 10.1016/j.critrevonc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa S, Nakano Y, Obara N, Suzuki N, Doi T, Kodama T, Nagasawa T, Yamamoto M. A GATA-specific inhibitor (K-7174) rescues anemia induced by IL-1β, TNF-α, or l-NMMA. FASEB J. 2003;17:1742–1744. doi: 10.1096/fj.02-1134fje. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Escudero E, Hurtado ME, Pando J, Tapia R, Swenson ER, Prchal J, Schreiner GF, Schoene RB, Hurtado A, Johnson RJ. Excessive erythrocytosis, chronic mountain sickness, and serum cobalt levels. Lancet. 2002;359:407–408. doi: 10.1016/s0140-6736(02)07594-3. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- Kambe T, Tada-Kambe J, Kuge Y, Yamaguchi-Iwai Y, Nagao M, Sasaki R. Retinoic acid stimulates erythropoietin gene transcription in embryonal carcinoma cells through the direct repeat of a steroid/thyroid hormone receptor response element half-site in the hypoxia-response enhancer. Blood. 2000;96:3265–3271. [PubMed] [Google Scholar]
- Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ishida J, Imagawa S, Saito T, Suzuki N, Matsuoka T, Sugaya T, Tanimoto K, Yokoo T, Ohneda O, Sugiyama F, Yagami K, Fujita T, Yamamoto M, Nangaku M, Fukamizu A. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005;19:2023–2025. doi: 10.1096/fj.05-3820fje. [DOI] [PubMed] [Google Scholar]
- Klausen T. The feed-back regulation of erythropoietin production in healthy humans. Dan Med Bull. 1998;45:345–353. [PubMed] [Google Scholar]
- Kling PJ, Dragsten PR, Roberts RA, Dos Santos B, Brooks DJ, Hedlund BE, Taetle R. Iron deprivation increases erythropoietin production in vitro, in normal subjects and patients with malignancy. Br J Haematol. 1996;95:241–248. doi: 10.1046/j.1365-2141.1996.d01-1919.x. [DOI] [PubMed] [Google Scholar]
- Kochling J, Curtin PT, Madan A. Regulation of human erythropoietin gene induction by upstream flanking sequences in transgenic mice. Br J Haematol. 1998;103:960–968. doi: 10.1046/j.1365-2141.1998.01081.x. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- Krajewski J, Batmunkh C, Jelkmann W, Hellwig-Bürgel T. Interleukin-1β inhibits the hypoxic inducibility of the erythropoietin enhancer by suppressing hepatocyte nuclear factor-4α. Cell Mol Life Sci. 2007;64:989–998. doi: 10.1007/s00018-007-6561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, Monge CC, Vidal A, Carcagno M, Criscuolo M, Bozzini CE. Serum immunoreactive erythropoietin in high altitude natives with and without excessive erythrocytosis. Exp Hematol. 1991;19:257–260. [PubMed] [Google Scholar]
- Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, Calbet JA, Robach P. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol. 2007;578:309–314. doi: 10.1113/jphysiol.2006.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall IC, Ashenden M. Current and upcoming erythropoiesis-stimulating agents, iron products, and other novel anemia medications. Adv Chronic Kidney Dis. 2009;16:117–130. doi: 10.1053/j.ackd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- McNeill LA, Hewitson KS, Claridge TD, Seibel JF, Horsfall LE, Schofield CJ. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem J. 2002;367:571–575. doi: 10.1042/BJ20021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P, Metzen E. Hypoxia-inducible factor-1 promotes its degradation by induction of HIF-α-prolyl-4-hydroxylases. Biochem J. 2004;381:761–767. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Miller ME, Cronkite EP, Garcia JF. Plasma levels of immunoreactive erythropoietin after acute blood loss in man. Br J Haematol. 1982;52:545–549. doi: 10.1111/j.1365-2141.1982.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel H, Jelkmann W, Weiss C. O2-supply to the kidneys and the production of erythropoietin. Respir Physiol. 1989;77:111–117. doi: 10.1016/0034-5687(89)90034-0. [DOI] [PubMed] [Google Scholar]
- Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, Flippin LA, Arend M, Klaus SJ, Bachmann S. Hypoxia-inducible factor-2α-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010;77:312–318. doi: 10.1038/ki.2009.460. [DOI] [PubMed] [Google Scholar]
- Paus R, Bodo E, Kromminga A, Jelkmann W. Erythropoietin and the skin: a role for epidermal oxygen sensing? Bioessays. 2009;31:344–348. doi: 10.1002/bies.200800192. [DOI] [PubMed] [Google Scholar]
- Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the α subunit. J Biol Chem. 1997;272:11205–11214. doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- Sinclair AM, Coxon A, McCaffery I, Kaufman S, Paweletz K, Liu L, Busse L, Swift S, Elliott S, Begley CG. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–4272. doi: 10.1182/blood-2009-10-248666. [DOI] [PubMed] [Google Scholar]
- Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Ohneda O, Takahashi S, Higuchi M, Mukai HY, Nakahata T, Imagawa S, Yamamoto M. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Okada M, Ueda M, Yasukochi Y. Activation of the erythropoietin promoter by a point mutation from GATA to TATA in the -30 region. J Biochem (Tokyo) 1997;121:193–196. [PubMed] [Google Scholar]
- Viault F. Sur l’augmentation considérable du nombre des globules rouges dans le sang chez les habitants des hauts plateaux de l’Amérique du Sud. C R Acad Sci Paris. 1890;111:917–918. [Google Scholar]
- Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56:558–565. doi: 10.1053/j.ajkd.2009.12.042. [DOI] [PubMed] [Google Scholar]
- von Wussow U, Klaus J, Pagel H. Is the renal production of erythropoietin controlled by the brain stem? Am J Physiol Endocrinol Metab. 2005;289:E82–E86. doi: 10.1152/ajpendo.00182.2004. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang R, Wu X, Hankinson O. Roles of coactivators in hypoxic induction of the erythropoietin gene. PLoS One. 2010;5:e10002. doi: 10.1371/journal.pone.0010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2α target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RM, Chapman KW, Gibson CC, Samaja M, Monge CC, Goldwasser E, Sherpa M, Blume FD, Santolaya R. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol. 1989;66:1561–1569. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- Yu F, White SB, Zhao Q, Lee FS. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]


