Non-technical summary
Saturated free fatty acids (FFAs) have been shown to exert initial stimulatory actions on insulin-producing cells, followed by a gradual impairment of insulin release. In this study we have investigated the effects of FFAs on the electrical properties of rat insulin-secreting cells. We have demonstrated an initial stimulation followed by a gradual and prolonged inhibition of electrical activity by FFAs, actions that could account for the effects of FFAs on insulin release. We have also explored the underlying changes in ionic currents in insulin-producing cells in response to FFAs. The results of the study could be relevant to the development of metabolic abnormalities brought about by high levels of FFAs in the circulation.
Abstract
Abstract
Free fatty acids (FFAs) exert complex actions on pancreatic β-cells. Typically, an initial potentiation of insulin release is followed by a gradual impairment of β-cell function, the latter effect being of possible relevance to hyperlipidaemia in type 2 diabetes mellitus. The molecular actions of FFAs are poorly understood. The present study investigated the acute effects of saturated FFAs on electrophysiological responses of rat pancreatic β-cells. Membrane potential and KATP channel activity were recorded using the perforated patch technique. Volume-regulated anion channel (VRAC) activity was assessed from conventional whole-cell recordings. Cell volume regulation was measured using a video-imaging technique. Addition of octanoate caused a transient potentiation of glucose-induced electrical activity, followed by a gradual hyperpolarisation and a prolonged inhibition of electrical activity. Octanoate caused an initial increase in VRAC activity followed by a secondary inhibition coinciding with increased KATP channel activity. Similar effects were observed with palmitate and 2-bromopalmitate whereas butyrate was virtually ineffective. Octanoate and palmitate also exerted a dual effect on electrical activity evoked by tolbutamide. Octanoate significantly attenuated cell volume regulation in hypotonic solutions, consistent with VRAC inhibition. It is concluded that medium and long chain FFAs have a dual action on glucose-induced electrical activity in rat pancreatic β-cells: an initial stimulatory effect followed by a secondary inhibition. These effects appear to be the result of reciprocal actions on VRAC and KATP channel currents, and could contribute towards the stimulatory and inhibitory actions of FFAs on pancreatic β-cell function.
Introduction
The pancreatic β-cell functions as a ‘fuel sensor’, being activated by increased levels of circulating nutrients. β-Cell activation is associated with a ‘spiking’ pattern of electrical activity, consisting of Ca2+-dependent action potentials which represent the gating of voltage-sensitive calcium channels. Electrical activity is therefore accompanied by Ca2+ entry into the cell, leading to the release of insulin by exocytosis. The initial event leading to electrical activity is depolarisation of the β-cell membrane potential. In the case of glucose, the principal stimulus for insulin release, depolarisation depends on metabolism of the hexose in the β-cell, and is thought to involve closure of ATP-sensitive K+ (KATP) channels (see Ashcroft & Rorsman, 1989 for review). There is also increasing evidence that activation of volume-regulated anion channels (VRACs) contributes to the coupling of glucose metabolism to β-cell depolarisation (see Best & McLaughlin, 2004; Best et al. 2010a for reviews). In addition to hexoses, several non-carbohydrate nutrients are known to activate the β-cell. A number of amino acids (notably leucine) depolarise the cell as a result of their metabolism (Panten et al. 1972) whilst others, such as alanine and arginine, undergo electrogenic transport into the β-cell (Lambert et al. 1969; Smith et al. 1997).
The effects of free fatty acids (FFAs) on pancreatic β-cell function are complex and subject to considerable confusion and uncertainty (see Grill & Qvigstad, 2000; Poitout, 2003; Nolan et al. 2006; Morgan & Dhayal, 2009 for reviews). The majority of reports indicate that acute exposure to FFAs enhances insulin secretion in vitro (Malaisse & Malaisse-Lagae, 1968; Elks, 1993; Conget et al. 1994; Warnotte et al. 1999; Gravena et al. 2002; Parker et al. 2003) and in vivo (Crespin et al. 1969; Boden et al. 1995; Stein et al. 1997). This effect appears to be dependent on fatty acid chain length and degree of saturation, long-chain saturated FFAs being most effective (Warnotte et al. 1999; Stein et al. 1997; Gravena et al. 2002). Several molecular mechanisms have been proposed to be involved in the activation of β-cells by fatty acids, including transport into the cell and subsequent metabolism (see Poitout, 2003; Nolan et al. 2006 for reviews), protein kinase C activation (Alcazar et al. 1997) and an increase in cytosolic [Ca2+] ([Ca2+]i; Remizov et al. 2003). This latter effect could be the result, at least in part, of the binding of FFAs to the cell surface G-protein coupled receptor GPR40, leading to phospholipase C activation, IP3 production and Ca2+ release from intracellular pools (Crespin et al. 1969; Warnotte et al. 1994; Boden et al. 1995; Stein et al. 1997). However, it is interesting to note that the rise in [Ca2+]i in insulin-secreting cells evoked by FFAs is sensitive to inhibition by omission of Ca2+ from the incubation medium and by blockers of voltage-sensitive Ca2+ channels (VSCCs: Warnotte et al. 1994; Stein et al. 1997; Remizov et al. 2003). This raises the possibility that β-cell activation by FFAs might involve increased Ca2+ entry into the cell. In support of this possibility, Warnotte and co-workers (1994) found evidence that palmitate increased Ca2+ influx and also caused a small mobilisation of intracellular Ca2+. Furthermore, injection of palmitate in mice was found to enhance β-cell electrical activity in vivo, with no increase in blood glucose concentration (Fernandez & Valdeolmillos, 1998), again indicating that FFAs could influence Ca2+ entry via VSCCs. This enhanced Ca2+ entry was suggested to be due to a direct activation of VSCCs (Warnotte et al. 1994; Stein et al. 1997) or a consequence of KATP channel closure coupled to FFA metabolism, leading to depolarisation and hence activation of VSCCs (Remizov et al. 2003).
In contrast to the acute stimulatory effects of FFAs on insulin release, prolonged exposure to raised levels of FFAs has been shown to have detrimental effects on β-cell function and viability (Elks, 1993; Zhou & Grill, 1994; Liang et al. 1997; Lupi et al. 2002), effects that could contribute towards impaired glucose homeostasis associated with type 2 diabetes mellitus (Grill & Qvigstad, 2000; Morgan & Dhayal, 2009). Again, the underlying mechanisms of the inhibitory actions of fatty acids on the β-cell are poorly understood, but could include impaired glucose oxidation in the β-cell (Grill & Qvigstad, 2000). However, altered glucose metabolism may not be sufficient to account for the degree of impairment of insulin release (Liang et al. 1997). In common with the acute stimulatory actions of FFAs, GPR40 could also play a role in the adverse effects of chronic exposure (Steneberg et al. 2005).
In an attempt to understand further the mechanisms of interaction of fatty acids with insulin-secreting cells, we have investigated the electrophysiological effects of acute exposure to saturated FFAs on rat pancreatic β-cells.
Methods
All experiments were carried out in accordance with local and national guidelines for animal welfare. Pancreatic islets were isolated from Sprague–Dawley rats (350–400 g, either sex, killed by stunning and cervical dislocation) by collagenase digestion (Worthington type 4, Cambridge BioScience, Cambridge, UK). Islets were dispersed into single cells and small clusters by brief exposure to Ca2+-free medium containing (mmol l−1): NaCl (130), KCl (5), MgCl2 (1), glucose (5), EGTA (1) and Hepes-NaOH (20; pH 7.4). Islet cells were centrifuged (500 g for 5 min), re-suspended in Hepes-buffered minimal essential medium (Invitrogen, Paisley, UK) supplemented with 5% (v/v) fetal calf serum and 50 μg ml−1 gentamycin and cultured in 30 mm diameter polystyrene dishes for 2–12 days in humidified air at 37°C. Human embryonic kidney (HEK 293) cells were kindly provided by Dr Jason Bruce, University of Manchester. For experimental procedures, cells were superfused at approximately 2 ml min−1 with a bath solution consisting of (mmol l−1): NaCl (130), KCl (4), MgCl2 (1), CaCl2 (1.2), glucose (5) and Hepes-NaOH (20; pH 7.4). Sodium octanoate and butyrate were added at the required concentration. In the case of palmitate and 2-bromopalmitate, a fine emulsion of fatty acid micelles was formed by heating the medium to approximately 60°C followed by sonication (Stein et al. 1997). In some experiments, palmitate was bound to fatty acid free bovine serum albumin (1% w/v).
β-Cells were identified by their size (larger than non β-cells), their granular appearance and, where possible, their electrophysiological response to glucose or tolbutamide. Membrane potential was recorded by means of the perforated patch technique using a List EPC-7 amplifier in current clamp mode (zero current) essentially as described previously (Best, 2005). The pipette solution contained KCl (130), NaCl (4), MgCl2 (1), Hepes-NaOH (10; pH 7.2) and gramicidin D (50 μg ml−1) as perforating agent. This antibiotic does not alter intracellular [Cl−] and therefore allows membrane potential recordings under minimally invasive conditions. All membrane potential recordings were repeated in a minimum of four cells with similar results.
Activity of the VRAC was recorded at the whole-cell level using the conventional whole-cell recording technique. A hypertonic pipette solution consisting of CsCl (60), MgCl2 (2), ATP (1), EGTA (1), mannitol (220) and Hepes (10, pH 7.2) was used to induce cell swelling and hence VRAC activation. CsCl (1 mmol l−1) was added to the bath solution to block inward K+ currents. Currents were recorded in response to 50 ms voltage pulses of ±100 mV at 2 s intervals from a holding potential of 0 mV. Cell capacitance was measured by nulling the capacitance transients, and current density expressed as pA pF−1.
Whole-cell KATP channel activity was assessed by measuring input resistance (Ginput), to which KATP channels make a major contribution (Ashcroft & Rorsman, 1989). The pipette solution contained (mmol l−1): KCl (130), NaCl (4), MgCl2 (1), Hepes-NaOH (10; pH 7.2) and amphotericin B (240 μg ml−1). This antibiotic was used in this case since it provides improved electrical access (<30 MΩ) for voltage-clamp recording compared to gramicidin D. The cells were subjected to 50 ms pulses of ±10 mV from a holding potential of −70 mV.
Relative cell volume (RCV) was measured using a video-imaging technique essentially as described previously (Miley et al. 1997). These experiments were limited to the use of octanoate and butyrate since the adhesion of palmitate micelles to the cells was found to frequently interfere with clear imaging of the cells. Cell swelling was induced by exposure of the cells to a 33% hypotonic solution. All data are expressed as means ±s.e.m. and statistical significance was ascribed using Student's paired t test.
Sodium palmitate, octanoate, butyrate and 2-bromopalmitate, tolbutamide, gramicidin D and amphotericin B were purchased from Sigma-Aldrich, Poole, UK.
Results
The first series of experiments examined the effects on β-cell membrane potential of octanoate, a medium chain FFA (C8:0) readily soluble in aqueous media. As shown in Fig. 1A, addition of octanoate (0.5 mmol l−1) in the presence of a substimulatory concentration of glucose (5 mmol l−1) caused a modest, transient depolarisation of β-cell membrane potential. In 3/5 cells, this was insufficient to evoke action potentials (left panel) but in the remaining two cells tested, a brief period of electrical activity was observed (right panel). Raising the glucose concentration to 15 mmol l−1 evoked a characteristic pattern of electrical activity, depolarising the cells to a mean plateau potential of –51.7 ± 0.6 mV (n = 6) leading to the generation of action potentials at a mean frequency of 28 ± 3 min−1 (n = 5; Fig. 1B). Under such conditions, the application of octanoate was found to have a biphasic action. Initially, a further depolarisation was observed to a plateau potential of –42.7 ± 1.6 mV (n = 6, P < 0.02) with a brief period of more intense electrical activity (mean action potential frequency of 67 ± 10 min−1, n = 5, P < 0.01). This transient potentiation of electrical activity, lasting 0.5–4 min, was followed by a repolarisation of the membrane potential and termination of action potentials. A similar dual effect was observed with a lower concentration of octanoate (0.1 mmol l−1; Fig. 1B), although in this case, a prolonged stimulatory period was observed (approximately 5 min) and the secondary hyperpolarisation delayed. The inhibitory action of octanoate was prolonged or, particularly after several minutes exposure to the FFA, irreversible for up to 30 min following withdrawal (for example, see Fig. 1B). Consistent with this finding, a prior 5 min exposure of β-cells to octanoate completely prevented a subsequent response to a high concentration of glucose (not shown).
Figure 1. Effect of octanoate (OA; 0. or 0.1 mmol l−1) on membrane potential in isolated rat pancreatic β-cells.
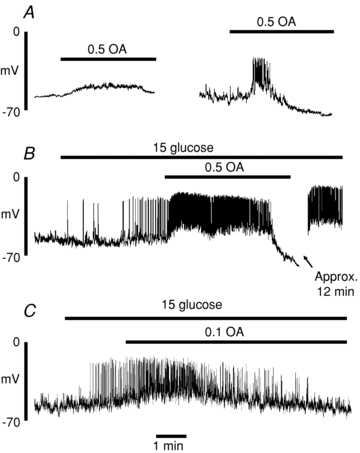
Gramicidin perforated patch recordings showing typical responses to octanoate in the presence of 5 (A) or 15 (B and C) mmol l−1 glucose. The recordings are representative of those from a total of 5 cells in each case.
The next series of experiments investigated the ionic mechanisms underlying the effects of octanoate on β-cell electrical activity. The effects of octanoate on VRAC activity are illustrated in Fig. 2. In conventional whole-cell recordings, a hypertonic pipette solution was used resulting in cell swelling and a characteristic outwardly rectifying pattern of VRAC activation. Addition of 0.5 mmol l−1 octanotate resulted in an initial, transient increase in the amplitude of both outward and inward currents, followed by a gradual reduction in current amplitudes. Again, as was apparent from membrane potential recordings, the inhibitory effect of octanoate on VRAC activity was very slowly reversible for 15–30 min following withdrawal of the fatty acid.
Figure 2. Effects of octanoate (OA; 0. mmol l−1) on whole-cell VRAC currents.
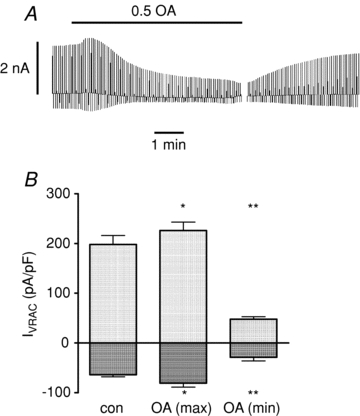
A, conventional whole-cell recording using a hypertonic pipette solution. The gap in the trace is a period of approximately 15 min following washout of OA. B, mean ±s.e.m. values from 5 cells showing maximum and minimum currents following application of OA. *P < 0.05, **P < 0.02.
Figure 3 illustrates the effect of octanoate on β-cell input conductance (Ginput), commonly used as an index of whole-cell KATP channel activity. In the presence of 15 mmol l−1 glucose, Ginput was 1.12 ± 0.07 nS (n = 5). This value was increased significantly (P < 0.01) to 3.75 ± 0.14 nS following the removal of glucose from the medium, consistent with KATP channel activation (Smith et al. 1990; Best, 2000). The application of octanoate (0.5 mmol l−1) in the presence of 15 mmol l−1 glucose produced a similar gradual increase in Ginput, reaching a peak value of 3.92 ± 0.75 nS (P < 0.01). The increase in Ginput evoked by octanoate was not apparently preceded by any initial reduction in conductance, suggesting that the transient stimulatory effect of octanoate on glucose-induced electrical activity was not related to KATP channel inhibition. The increase in Ginput evoked by octanoate was completely reversed by tolbutamide (100 μmol l−1), consistent with an activation of KATP channel activity by octanoate. Time courses of relative changes in VRAC and KATP conductances following the addition of octanoate are shown in Fig. 4. Following the initial increase, a progressive reduction in VRAC conductance occurred in parallel with a rise in KATP conductance. The dynamics of these changes were broadly comparable with the effects of octanoate on β-cell membrane potential.
Figure 3. Effects of octanoate (OA; 0. mmol l−1) on input conductance (Ginput).
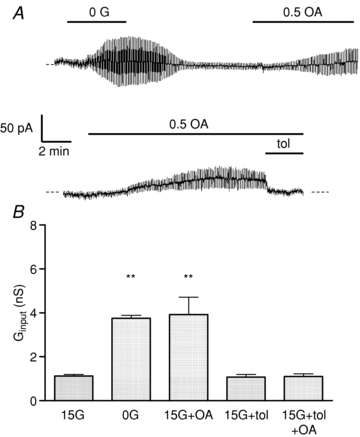
A, Ginput was increased by withdrawal of glucose (15 mmol l−1) and by octanoate in the presence of 15 mmol l−1 glucose. The effect of octanoate was reversed by tolbutamide (tol; 100 μmol l−1). B, corresponding mean ±s.e.m. values from 4–5 cells. **P < 0.01.
Figure 4. Time courses of changes in VRAC conductance (filled circles) and KATP channel conductance (Ginput; open circles) following addition of 0. mmol l−1 octanoate (added at time zero).
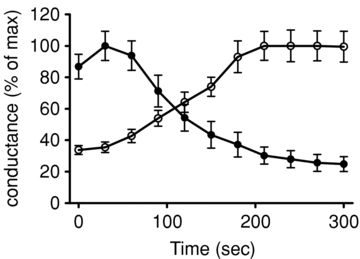
Values are means ±s.e.m. from 6 and 5 cells, respectively.
A major FFA component of serum is palmitate (C16:0). Therefore, similar experiments were conducted to examine the acute effects of palmitate on isolated β-cells. An essentially similar dual action on β-cell membrane potential, VRAC activity and Ginput was observed in response to 0.5 mmol l−1 palmitate (Fig. 5). As was the case with octanoate, similar though more delayed responses were observed with a lower concentration of palmitate (0.1 mmol l−1; Fig. 6A) or when palmitate was bound to 1% (w/v) bovine serum albumin (Fig. 6B). The calculated concentration of unbound FFA under the latter conditions was likely to be in the region of 26 nm, based on previous estimates (Olofsson et al. 2004). Since FFAs are predominantly bound to plasma proteins in vivo, this situation therefore represents a closer approximation to physiological conditions. The finding that palmitate bound to BSA also exerted rapid effects on the β-cell suggests that FFAs can readily partition between the protein – bound and free form.
Figure 5. Effects of palmitate (PA; 0. mmol l−1) on electrical activity (A), whole-cell VRAC currents (B) and input conductance (Ginput; C).
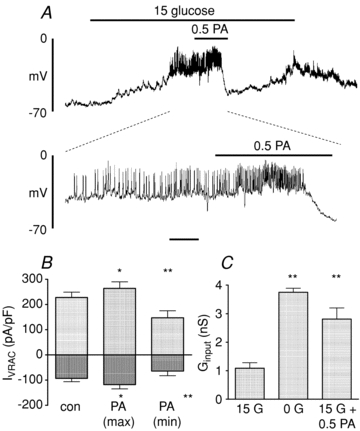
Time bar: 1 min (upper trace) or 10 s (lower expanded trace). The bars represent mean ±s.e.m. values from 5 and 4 cells, respectively. *P < 0.05, **P < 0.01.
Figure 6. Effect of palmitate (PA) on glucose-induced electrical activity.
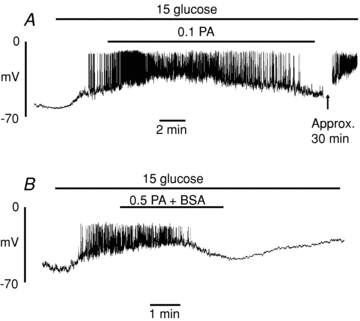
Cells were superfused with solutions containing 0. mmol l−1 PA (A) or 0.5 mmol l−1 bound to 1% BSA (B). The recordings are representative of those from a total of 4 cells in each case.
In order to establish whether any of the above actions of FFAs involved their esterification or oxidation, we next carried out similar experiments using the non-metabolisable analogue 2-bromopalmitate. Figure 7 shows that the electrophysiological effects of 2-bromopalmitate were essentially similar to those observed with palmitate. Thus, 2-bromopalmitate transiently depolarised β-cells in the presence of 15 mmol l−1 glucose, resulting in enhanced electrical activity (Fig. 7A). This was followed after 1–2 min by a repolarisation and cessation of electrical activity, and eventually a hyperpolarisation to approximately –70 mV. In common with palmitate, the 2-bromo derivative caused an initial transient activation followed by a marked inhibition of the whole-cell VRAC current (Fig. 7B). The latter effect was paralleled by a pronounced increase in Ginput (Fig. 7C). These findings indicate that neither the initial stimulatory nor the subsequent inhibitory actions of FFAs required their metabolism in the β-cell.
Figure 7. Effects of 2-bromopalmitate (BP; 0. mmol l−1) on electrical activity (A), whole-cell VRAC currents (B) and input conductance (Ginput; C).
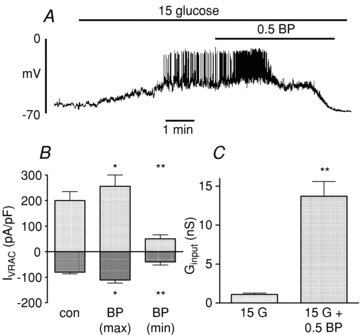
The bars represent mean ±s.e.m. values from 4 cells in each case. *P < 0.05, **P < 0.01
In contrast to the effects of octanoate and palmitate, butyrate (C4:0) was found to have little or no effect on β-cell electrical activity (Fig. 8A) and no significant effect on either VRAC currents (Fig. 8B) or Ginput (Fig. 8C).
Figure 8. Effects of butyrate (BA; 0. mmol l−1) on electrical activity (A), whole-cell VRAC currents (B) and input conductance (Ginput; C).
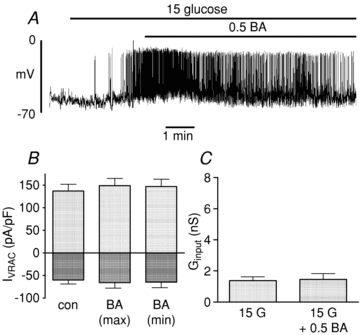
The bars represent mean ±s.e.m. values from 4 cells in each case.
We next studied the influence of FFAs on β-cells during stimulation with the hypoglycaemic sulphonylurea tolbutamide. Application of 100 μmol l−1 tolbutamide caused a rapid ‘silent’ depolarisation of the membrane potential (Fig. 9A). The further addition of octanoate (0.5 mmol l−1) appeared to have little if any stimulatory effect but, following a delay of 6–8 min, caused a gradual hyperpolarisation of the membrane potential, suggesting that VRAC inhibition was sufficient to hyperpolarise the β-cell even in the absence of KATP channel activation. Addition of a lower submaximal concentration of tolbutamide (35 μmol l−1) induced an intermittent pattern of action potentials (Fig. 9B and C). Under such conditions, the addition of octanoate (Fig. 9B) or palmitate (Fig. 9C) caused a transient enhancement of electrical activity followed by a subsequent hyperpolarisation and suppression of electrical activity, essentially a similar pattern to that observed during stimulation by glucose.
Figure 9.
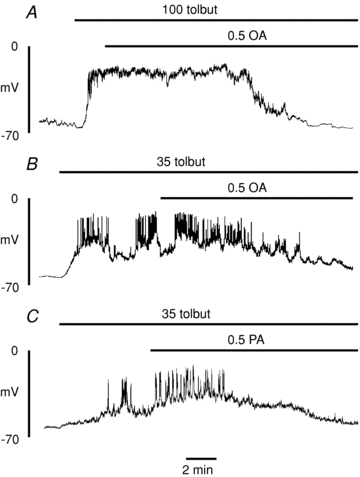
Effect of octanoate (A and B; 0. mmol l−1) and palmitate (C; 0.5 mmol l−1) on electrical activity evoked by tolbutamide (100 or 35 μmol l−1)
An important function of the VRAC in β-cells, as in other cell types, appears to be in the regulation of cell volume (Best et al. 1996). Therefore, it would be predicted that inhibition of the conductance by a medium or long chain FFA would impair volume regulation. Figure 10 shows that this was indeed the case with octanoate. Thus, whilst the addition of the FFA (at time zero) did not significantly alter cell volume, the ability of the cells to undergo regulatory volume decrease (RVD) following hypotonic cell swelling was markedly impaired. In contrast, RVD was completely unaffected by butyrate.
Figure 10. Effects of FFAs on β-cell volume regulation.
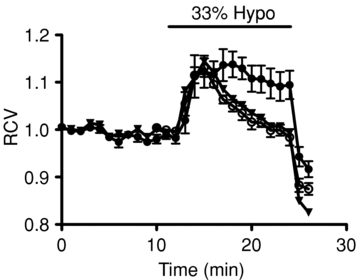
Relative cell volume (RCV) was measured in the absence (open circles) or presence of 0. mmol l−1 octanoic acid (filled circles) or butyric acid (triangles). In all cases, the cells were exposed to a 33% hypotonic bath solution during the period represented by the horizontal bar. Each point represents the mean ±s.e.m. from 5 cells.
A limited number of experiments were conducted to investigate the effects of octanoate on VRAC activity in a non-islet cell type. For this purpose, we used the HEK 293 cell line previously employed in this laboratory for such studies (Best & Brown, 2009). Prior to addition of octanoate, the outward and inward current densities from seven cells were 177 ± 19 and –91 ± 9 pA pF−1 respectively. Following application of 0.5 mmol l−1 octanoate, these values were increased to 207 ± 24 pA pF−1 (P < 0.05) and –144 ± 16 pA pF−1 (P < 0.02). In contrast to pancreatic β-cells, however, no subsequent inhibition of VRAC activity was observed for at least up to 30 min exposure to the fatty acid.
Discussion
It is well established that short-term exposure of β-cells to FFAs potentiates insulin release in the presence of a stimulatory concentration of glucose. As outlined above, the molecular mechanisms underlying this stimulatory effect remain to be elucidated, though much recent attention has focused on the role of the G-protein coupled receptor GPR40 and intracellular Ca2+ mobilisation (Itoh et al. 2003; Steneberg et al. 2005; Shapiro et al. 2005; Schnell et al. 2007). The results of the present study suggest that an acute activation of the VRAC, leading to depolarisation and enhanced electrical activity, could contribute towards the stimulatory effect of FFAs. Such a potentiation of the VRAC and electrical activity could explain why the rise in [Ca2+]i elicited by FFAs is apparently sensitive to blockers of VSCCs (Remizov et al. 2003; Shapiro et al. 2005; Schnell et al. 2007). We found no evidence that the depolarisation produced by FFAs involved a concomitant inhibition of KATP channel currents. In accordance with these findings, the stimulatory actions of palmitate were previously shown to be independent of KATP channel closure (Olofsson et al. 2004), and in fact involve an increase in 86Rb+ efflux (Warnotte et al. 1994). In common with studies of insulin release, a stimulatory effect of FFAs on electrical activity could only be consistently observed in the presence of a stimulatory concentration of glucose. In fact, at a substimulatory glucose concentration (5 mmol l−1), only octanoate was found (in 2/5 cells) to evoke a sufficient depolarisation to cause a transient period of electrical activity. Activation of the β-cell VRAC current and the enhancement of electrical activity by FFAs were both transient responses, with a duration of up to 6 min. In contrast, previous in vitro studies of the dynamics of insulin release under comparable conditions in response to FFAs indicate a more prolonged stimulatory effect, typically lasting 30–60 min (Elks, 1993; Warnotte et al. 1994, 1999). Thus, whilst enhanced VRAC and electrical activity could well contribute to the stimulation of insulin release by FFAs, there are likely to be additional mechanisms such as GPR40 activation that play a more prolonged role in the secretory response.
The long-term inhibitory effects of FFAs on β-cell function are also well documented (Grill & Qvigstad, 2000). The progressive hyperpolarising effect of FFAs on β-cell membrane potential demonstrated in the present study could contribute to the impairment of insulin release after prolonged exposure to FFAs (Sako & Grill, 1990; Elks, 1993; Zhou & Grill, 1994). These findings are also consistent with those in a recent report using linoleic acid (Zhao et al. 2008). In agreement with this study, we observed an increase in Ginput, presumably representing activation of KATP channels by FFAs, which would contribute to their hyperpolarising action. It remains to be established how FFAs activate KATP channels, although direct actions on the channel of unesterified FFAs and/or fatty acyl CoA esters are both possibilities (Bränström et al. 1998, 2004; Zhao et al. 2008). However, we observed that 2-bromopalmitate, an inhibitor of FFA oxidation (Chase & Tubbs, 1972) which is not metabolised to a CoA ester nor oxidised (Grimaldi et al. 1992), was highly effective in increasing Ginput. This finding would suggest that FFAs can activate KATP channels directly in the absence of esterification.
In addition to KATP channel activation, the present study reveals evidence of a novel action of FFAs, namely a marked inhibition of VRAC activity following the initial, transient activation described above. In this regard, it is of interest to note that several pharmacological chloride channel inhibitors bear structural similarities to fatty acids (Wangemann et al. 1986). The inhibition of VRAC activity by FFAs, together with KATP channel activation, would clearly be expected to hyperpolarise the cells. However, the finding that FFA-induced hyperpolarisation persisted when KATP channel activation was prevented by tolbutamide suggests that VRAC inactivation alone is sufficient to cause hyperpolarisation. This conclusion is consistent with the documented hyperpolarising actions of pharmacological inhibitors of the VRAC channel (see Best & McLaughlin, 2004 for review). The pattern of KATP channel activation by FFAs in association with VRAC inhibition is also of interest, and has been noted previously with the pharmacological agents 4-(2-butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid (DCPIB; Best et al. 2004) and tenidap (Best et al. 2010b). Such a reciprocal relationship in the regulation of VRAC and KATP channels suggests the possibility of a close functional relationship between these two nutrient-sensitive conductances. A recent study has reported increased pancreatic β-cell volume in vivo in mice fed a high fat diet (Ahren et al. 2010). As noted above, the VRAC plays an important function in volume regulation, and inhibition of the channel by free fatty acids prevents regulatory volume decrease. It is therefore conceivable that the effect of a high fat diet on β-cell volume is mediated via a prolonged inhibition of VRAC activity.
Whilst there is uncertainty regarding the mechanism of KATP channel activation by FFAs, it is also unclear how VRAC activity might be influenced by FFAs. In the case of VRAC activation by a rise in glucose concentration, there is evidence that β-cell swelling, possibly as a result of intracellular accumulation of glucose metabolites, could be involved (Miley et al. 1997). However, the finding that 2-bromopalmitate was at least as effective as palmitate in its effects on VRAC activity suggests that neither activation nor subsequent inactivation of the channel requires the formation of CoA esters or oxidative metabolism. Furthermore, the addition of octanoate had no detectable effect on β-cell volume. It is therefore conceivable that FFAs could exert direct effects on the VRAC channel, or possibly indirect actions resulting from partitioning of FFA into the plasma membrane leading to changes in membrane fluidity (see Ordway et al. 1991 for a discussion of this topic). Indeed, there is evidence that VRAC activity in vascular endothelial cells can be modified by agents such as cholesterol that alter the biophysical properties of the plasma membrane (Levitan et al. 2000; Byfield et al. 2006; Lim et al. 2006). It has also been demonstrated that FFAs can inhibit epithelial chloride channels by a direct action on the channel, though this effect was limited to cis-unsaturated fatty acids (Hwang et al. 1990; Anderson & Welsh, 1990; Kubo & Okada, 1992).
It is unlikely that the effects of FFAs on VRAC and KATP currents described in the present study are mediated via the binding of FFAs to GPR40 since octanoate, which has minimal activity as a GPR40 agonist (Itoh et al. 2003), appeared the most effective FFA tested in terms of modulating β-cell VRAC, KATP and electrical activities. Incidentally, the pattern of effectiveness of FFAs in influencing these activities, octanoate and palmitate being effective whilst butyrate had little or no activity, is broadly consistent with the potentiation of insulin release by FFAs (Opara et al. 1994).
It is of interest that the dual action of FFAs on VRAC activity in pancreatic β-cells was not apparent in the HEK 293 cell line where the addition of octanoate was found to increase current amplitudes with no subsequent inhibition. A lack of inhibition of VRAC activity by saturated fatty acids has also been reported in cultured intestinal epithelial cells (Kubo & Okada, 1992). The reasons for this difference between cell types are at present unknown, though it should be noted that the VRAC in β-cells appears distinct from that in other cell types, at least on the basis of halide selectivity (Best et al. 1996). As discussed above, it is also conceivable that differences in membrane lipid composition and fluidity between different cell types could determine ion channel responses to lipophilic substances such as FFAs.
In conclusion, the results of the present study reveal a dual action of medium and long chain FFAs on glucose-induced electrical activity in rat pancreatic β-cells. The initial stimulatory action could be, at least in part, the result of a transient activation of the VRAC conductance, whilst the secondary long-term inhibition could result from KATP channel activation coupled with inhibition of VRAC currents. These effects of FFAs could contribute to the short and long term actions of FFAs on the release of insulin release and possibly other islet hormones, notably glucagon and somatostatin (Collins et al. 2008).
Glossary
Abbreviations
- FFA
free fatty acid
- Ginput
input conductance
- RCV
relative cell volume
- VRAC
volume-regulated anion channel
- VSCC
voltage-sensitive calcium channel
Author contributions
L. B.: Conception and design of study, analysis and interpretation of data, experimental work, drafting and revision of manuscript, final approval of version to be published. E.J.: Experimental work, final approval of version to be published. P.D.B.: Design, analysis and interpretation of data, experimental work, revising manuscript critically for important intellectual content, final approval of the version to be published.
References
- Ahren J, Ahren B, Wierup N. Increased β-cell volume in mice fed a high-fat diet: A dynamic study over 12 months. Islets. 2010;2:1–4. doi: 10.4161/isl.2.6.13619. [DOI] [PubMed] [Google Scholar]
- Alcazar O, Qiu-yue Z, Giné E, Tamarit-Rodriguez J. Stimulation of islet protein kinase C translocation by palmitate requires metabolism of the fatty acid. Diabetes. 1997;46:1153–1158. doi: 10.2337/diab.46.7.1153. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Welsh MJ. Fatty acids inhibit apical membrane chloride channels in airway epithelia. Proc Natl Acad Sci U S A. 1990;87:7334–7338. doi: 10.1073/pnas.87.18.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β-cell. Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose-sensitive conductances in rat pancreatic β-cells: contribution to electrical activity. Biochim Biophys Acta. 2000;1468:311–319. doi: 10.1016/s0005-2736(00)00272-8. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose-induced electrical activity in rat pancreatic β-cells: dependence on intracellular chloride concentration. J Physiol. 2005;568:137–144. doi: 10.1113/jphysiol.2005.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L, Brown PD. Studies of the mechanism of activation of the volume-regulated anion channel in rat pancreatic β-cells. J Membr Biol. 2009;230:83–91. doi: 10.1007/s00232-009-9189-x. [DOI] [PubMed] [Google Scholar]
- Best L, Brown PD, Sener A, Malaisse WJ. Electrical activity in pancreatic islet cells: the VRAC hypothesis. Islets. 2010a;2:59–64. doi: 10.4161/isl.2.2.11171. [DOI] [PubMed] [Google Scholar]
- Best L, Brown PD, Sener A, Malaisse WJ. Opposing effects of tenidap on the volume-regulated anion channel and KATP channel activity in rat pancreatic β-cells. Eur J Pharmacol. 2010b;629:159–163. doi: 10.1016/j.ejphar.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Best L, McLaughlin J. Nutrients as regulators of endocrine and neuroendocrine secretion. In: Taylor P, Winderickx J, editors. Topics in Current Genetics. Vol. 7. New York: Springer-Verlag; 2004. pp. 79–111. [Google Scholar]
- Best L, Sheader EA, Brown PD. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch. 1996;431:363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Best L, Yates AP, Decher N, Steinmeyer K, Nilius B. Inhibition of glucose-induced electrical activity in rat pancreatic β-cells by DCPIB, a selective inhibitor of volume-sensitive anion currents. Eur J Pharmacol. 2004;489:13–19. doi: 10.1016/j.ejphar.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes. 1995;44:1239–1242. doi: 10.2337/diab.44.10.1239. [DOI] [PubMed] [Google Scholar]
- Bränström R, Aspinwall CA, Välimäki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, Larsson O. Long-chain CoA esters activate human pancreatic β-cell KATP channels: potential role in Type 2 diabetes. Diabetologia. 2004;47:277–283. doi: 10.1007/s00125-003-1299-x. [DOI] [PubMed] [Google Scholar]
- Bränström R, Leibiger IB, Leibiger B, Corkey BE, Berggren PO, Larsson O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6.2) of the ATP-regulated potassium channel. J Biol Chem. 1998;273:31395–31400. doi: 10.1074/jbc.273.47.31395. [DOI] [PubMed] [Google Scholar]
- Byfield FJ, Hoffman BD, Romanenko VG, Fang Y, Crocker JC, Levitan I. Evidence for the role of cell stiffness in modulation of volume-regulated anion channels. Acta Physiol. 2006;187:285–294. doi: 10.1111/j.1748-1716.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Chase JF, Tubbs PK. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972;129:55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SC, Salehi A, Eliasson L, Olofsson CS, Rorsman P. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia. 2008;51:1689–1693. doi: 10.1007/s00125-008-1082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget I, Rasshaert J, Sener A, Leclercq-Meyer V, Villanueva-Penacarrillo M, Valverde I, Malaisse WJ. Secretory, biosynthetic, respiratory, cationic and metabolic responses of pancreatic islets to palmitate and oleate. Biochem Med Metab Biol. 1994;51:175–184. doi: 10.1006/bmmb.1994.1023. [DOI] [PubMed] [Google Scholar]
- Crespin SR, Greenough WB, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969;48:1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks ML. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993;133:208–214. doi: 10.1210/endo.133.1.8319569. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Valdeolmillos M. Increased levels of free fatty acids in fasted mice stimulate in vivo β-cell electrical activity. Diabetes. 1998;47:1707–1712. doi: 10.2337/diabetes.47.11.1707. [DOI] [PubMed] [Google Scholar]
- Gravena C, Mathias PC, Ashcroft SJH. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol. 2002;173:73–80. doi: 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- Grill V, Qvigstad E. Fatty acids and insulin secretion. Br J Nutrition. 2000;83:S79–S84. doi: 10.1017/s0007114500000994. [DOI] [PubMed] [Google Scholar]
- Grimaldi PA, Knobel SM, Whitesell RR, Abumrad NA. Induction of aP2 gene expression by nonmetabolized long-chain fatty acids. Proc Natl Acad Sci U S A. 1992;89:10930–10934. doi: 10.1073/pnas.89.22.10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T-C, Guggino SE, Guggino WB. Direct modulation of secretory chloride channels by arachidonic acid and other cis unsaturated fatty acids. Proc Natl Acad Sci U S A. 1990;87:5706–5709. doi: 10.1073/pnas.87.15.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Kubo M, Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J Physiol. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AE, Jeanrenaud B, Junod A, Renold AE. Organ culture of fetal rat pancreas. II. Insulin release induced by amino and organic acids, by hormonal peptides, by cationic alterations of the medium and by other agents. Biochim Biophys Acta. 1969;184:540–553. doi: 10.1016/0304-4165(69)90268-2. [DOI] [PubMed] [Google Scholar]
- Levitan I, Christian AE, Tulenko TN, Rothblat GH. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Buetteger C, Berner D, Matschinsky FM. Chronic effect of fatty acids on insulin release is not through the alteration of glucose metabolism in a pancreatic B-cell line (BHC9) Diabetologia. 1997;40:1018–1027. doi: 10.1007/s001250050783. [DOI] [PubMed] [Google Scholar]
- Lim CH, Schoonderwoerd K, Kleijer WJ, de Jonge HR, Tilly BC. Regulation of cell swelling-activated chloride conductance by cholesterol-rich membrane domains. Acta Physiol. 2006;187:295–303. doi: 10.1111/j.1748-1716.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patanè G, Boggi U, Mosca F, Piro S, Del Prato S, Marchetti P. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51(Suppl 1):S134–S137. doi: 10.2337/diabetes.51.2007.s134. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Malaisse-Lagae F. Stimulation of insulin secretion by non-carbohydrate metabolites. J Lab Clin Med. 1968;72:438–448. [PubMed] [Google Scholar]
- Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic β-cell. Biochem Pharmacol. 2009;78:1419–1427. doi: 10.1016/j.bcp.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Miley HE, Sheader EA, Brown PD, Best L. Glucose-induced swelling in rat pancreatic islets. J Physiol. 1997;504:191–198. doi: 10.1111/j.1469-7793.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CJ, Madiraju MSR, Delghingaro-Augusto V, Peyot M-L, Prentki M. Fatty acid signalling in the β-cell and insulin secretion. Diabetes. 2006;55(suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic β-cells. J Physiol. 2004;557:935–948. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opara EC, Garfinkel M, Hubbard VS, Burch WM, Akwari OE. Effect of fatty acids on insulin release: role of chain length and degree of unsaturation. Am J Physiol Endocrinol Metab. 1994;266:E635–E639. doi: 10.1152/ajpendo.1994.266.4.E635. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Singer JJ, Walsh JV. Direct regulation of ion channel by fatty acids. Trends Neurosci. 1991;14:96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Panten U, Kriegstein E, Poser W, Schonborn J, Hasselblatt A. Effects of L-leucine and α-ketoisocaproic acid upon insulin secretion and metabolism of isolated pancreatic islets. FEBS Lett. 1972;20:225–228. doi: 10.1016/0014-5793(72)80801-9. [DOI] [PubMed] [Google Scholar]
- Parker SM, Moore PC, Johnson LM, Poitout V. Palmitate potentiation of glucose-induced insulin release: a study using 2-bromopalmitate. Metabolism. 2003;52:1367–1371. doi: 10.1016/s0026-0495(03)00279-8. [DOI] [PubMed] [Google Scholar]
- Poitout V. The ins and outs of fatty acids on the pancreatic β-cell. Trends Endocrinol Metab. 2003;14:201–203. doi: 10.1016/s1043-2760(03)00086-9. [DOI] [PubMed] [Google Scholar]
- Remizov O, Jakubov R, Düfer M, Krippeit Drews P, Drews G, Waring M, Brabant G, Wienbergen A, Rustenbeck I, Schöfl C. Palmitate-induced Ca2+-signaling in pancreatic β-cells. Mol Cell Endocrinol. 2003;212:1–9. doi: 10.1016/j.mce.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Sako Y, Grill V. A 48 h lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B-cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- Schnell S, Schaefer M, Schöfl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from β-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263:173–180. doi: 10.1016/j.mce.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the β cell line INS-1E. Biochem Biophys Res Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Smith PA, Ashcroft FM, Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K+ currents in isolated mouse pancreatic β-cells. FEBS Lett. 1990;261:187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Smith PA, Sakura H, Coles B, Gummerson N, Proks P, Ashcroft FM. Electrogenic arginine transport mediates stimulus–secretion coupling in mouse pancreatic β-cells. J Physiol. 1997;499:625–635. doi: 10.1113/jphysiol.1997.sp021955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, McGarry JD. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Wittner M, Di Stefano A, Englert HC, Lang HJ, Schlatter E, Greger R. Cl− channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407(Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Warnotte C, Gilon P, Nenquin M, Henquin J-C. Mechanisms of the stimulation of insulin release by saturated fatty acids. Diabetes. 1994;43:703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]
- Warnotte C, Nenquin M, Henquin J-C. Unbound rather than total concentration and saturation rather than unsaturation determine the potency of fatty acids on insulin secretion. Mol Cell Endocrinol. 1999;153:147–153. doi: 10.1016/s0303-7207(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Zhao YF, Pei J, Chen C. Activation of ATP-sensitive potassium channels in rat pancreatic β-cells by linoleic acid through both intracellular metabolites and membrane receptor signalling pathway. J Endocrinol. 2008;198:533–540. doi: 10.1677/JOE-08-0105. [DOI] [PubMed] [Google Scholar]
- Zhou Y-P, Grill V. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93:870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]


