Non-technical summary
Acute intermittent hypoxia elicits a serotonin-dependent form of respiratory plasticity known as phrenic long-term facilitation via synthesis of brain-derived neurotrophic factor (BDNF) and activation of its receptor TrkB. Recently we demonstrated that spinal activation of Gs protein-coupled adenosine A2A receptors ‘trans-activates’ the BDNF receptor, TrkB, and induces phrenic motor facilitation (PMF). Therefore we hypothesized that perhaps other Gs protein-coupled serotonin receptors elicit PMF, specifically serotonin receptor type-7 (5-HT7) which are expressed in phrenic motor neurons and underlie multiple forms of spinal respiratory plasticity. We demonstrate that spinal 5-HT7 receptor activation in anaesthetized rats elicits long-lasting PMF. 5-HT7 agonist-induced PMF appears to require TrkB receptor activity and/or new synthesis of TrkB protein, in addition to activity of the signalling molecule PI3K. A more complete understanding of signalling mechanisms involved in PMF may guide development of novel therapeutic strategies to treat ventilatory control disorders.
Abstract
Abstract
Acute intermittent hypoxia elicits a form of serotonin-dependent respiratory plasticity known as phrenic long term facilitation (pLTF). Episodic spinal serotonin-2 (5-HT2) receptor activation on or near phrenic motor neurons is necessary for pLTF. A hallmark of pLTF is the requirement for serotonin-dependent synthesis of brain-derived neurotrophic factor (BDNF), and activation of its high affinity receptor, TrkB. Activation of spinal Gs protein-coupled adenosine 2A receptors (GsPCRs) elicits a unique form of long-lasting phrenic motor facilitation (PMF), but via unique mechanisms (BDNF independent TrkB trans-activation). We hypothesized that other GsPCRs elicit PMF, specifically serotonin-7 (5-HT7) receptors, which are expressed in phrenic motor neurons. Cervical spinal (C4) injections of a selective 5-HT7 receptor agonist, AS-19 (10 μm, 5 μl; 3 × 5 min), in anaesthetized, vagotomized and ventilated male Sprague–Dawley rats elicited long-lasting PMF (>120 min), an effect prevented by pretreatment with a 5-HT7 receptor antagonist (SB 269970; 5 mm, 7 μl). GsPCR activation ‘trans-activates’ TrkB by increasing synthesis of an immature TrkB isoform. Spinal injection of a TrkB inhibitor (k252a) and siRNAs that prevent TrkB (but not BDNF) mRNA translation both blocked 5-HT7 agonist-induced PMF, confirming a requirement for TrkB synthesis and activity. k252a affected late PMF (≥90 min) only. Spinal inhibition of the PI3K/AKT pathway blocked 5-HT7 agonist-induced PMF, whereas MEK/ERK inhibition delayed, but did not block, PMF. An understanding of signalling mechanisms giving rise to PMF may guide development of novel therapeutic strategies to treat ventilatory control disorders associated with respiratory insufficiency, such as spinal injury and motor neuron disease.
Introduction
Exposure to repetitive low oxygen elicits respiratory plasticity (Mitchell & Johnson, 2003). Following acute intermittent hypoxia (3–10 hypoxic episodes), a long-lasting increase in respiratory activity is observed known as long term facilitation (LTF; Mitchell et al. 2001; Feldman et al. 2003; Mahamed & Mitchell 2007). LTF is most frequently studied in phrenic nerve activity (pLTF). pLTF is a central neural mechanism that requires spinal serotonin receptor activation (Baker-Herman & Mitchell, 2002). In our working cellular model, pLTF requires activation of spinal Gq protein-coupled serotonin-2 (5-HT2; Fuller et al. 2001) receptors and new protein synthesis (Baker-Herman & Mitchell, 2002), specifically new synthesis of brain-derived neurotrophic factor (BDNF; Baker-Herman et al. 2004). Newly synthesized BDNF activates its endogenous high affinity receptor tyrosine kinase (TrkB), which is required for pLTF expression (Baker-Herman et al. 2004).
Recently we reported a unique mechanism of long-lasting phrenic motor facilitation (PMF) without intermittent hypoxia (Golder et al. 2008). Specifically, spinal injections of a selective agonist for the Gs protein-coupled adenosine 2A (A2A) receptor (CGS21680) elicit long-lasting PMF by a BDNF-independent mechanism (Golder et al. 2008). Spinal A2A receptor agonist injections induce new synthesis of an immature TrkB isoform that becomes phosphorylated and signals from inside phrenic motor neurons (Golder et al. 2008); the new TrkB signals via increased phosphorylation and activation of protein kinase B (pAkt; Chao, 2003; Golder et al. 2008). A2A agonists also improve motor neuron survival after facial nerve transection (Wiese et al. 2007). Improved survival required TrkB receptor activity and Akt activation, similar to A2A agonist-induced PMF (Wiese et al. 2007; Golder, 2008).
The ability to elicit PMF with molecules that bypass the requirement for intermittent hypoxia has considerable potential for therapeutic applications, particularly in cases of inadequate respiratory motor output (e.g. spinal injury, motor neuron disease; Mitchell, 2007). Although A2A agonist-induced PMF is of considerable interest, A2A receptor activation elicits untoward side effects including neural and cardiovascular morbidity (Pedata et al. 2001; Mojsilovic-Petrovic et al. 2006; Minghetti et al. 2007). Thus, alternative pharmacological strategies to elicit PMF may be of benefit.
Other Gs protein coupled receptors (GsPCRs) trans-activate TrkB receptors (Golder, 2008). For example, pituitary adenylate cyclase activating polypeptides (PACAP) exerts trophic effects on hippocampal neurons by increasing TrkB levels and Akt activity (i.e. trans-activation; Lee et al., 2002a). In the developing cortex, endocannabinoids trans-activate TrkB receptors, guiding interneuron migration (Berghuis et al. 2005). Hence, multiple GPCRs have the capacity to trans-activate TrkB receptors and exert trophic effects on neurons.
One GsPCR implicated in phrenic motor plasticity (McGuire et al. 2004; Zhang et al. 2004) and expressed in spinal motor neurons (Doly et al. 2005; Liu et al. 2009) is the serotonin-7 (5-HT7) receptor. Thus, our primary objective was to test the hypothesis that episodic spinal 5-HT7 receptor activation elicits PMF via TrkB trans-activation. Our initial experiment was designed to investigate PMF after spinal 5-HT7 receptor activation in anaesthetized rats. We then explored the role of TrkB receptor activity in 5-HT7 receptor-induced PMF. We further tested the hypothesis that new TrkB (not BDNF) protein synthesis is necessary for 5-HT7-induced PMF utilizing translation inhibition via RNA interference (RNAi) targeting TrkB and BDNF mRNA. Finally, spinal injections of PI3K (phosphatidylinositol 3-kinase; PI-828) and MEK (extracellular regulated kinase, ERK; UO126) inhibitors were used to characterize signalling cascades downstream from TrkB.
Here we demonstrate that spinal 5-HT7 receptor activation elicits prolonged PMF, and this effect is blocked by inhibition of TrkB synthesis and TrkB and/or PI3K activity. Although exogenous BDNF is sufficient to elicit PMF (Baker-Herman et al. 2004), it has a short plasma half-life (<1 min) and poor blood–brain barrier penetration, which limit its therapeutic potential (Poduslo & Curran, 1996). Molecules that permeate the blood–brain barrier, activate 5-HT7 receptors (Perez-Garcia & Meneses, 2005) and mimic BDNF effects on respiratory motor output are of interest as an approach to ‘harness’ respiratory plasticity in the treatment of severe ventilatory control disorders (Mitchell, 2007; Golder, 2008).
Methods
The primary objective of this study was to test whether selective (episodic) activation of spinal 5-HT7 receptors elicits a long-lasting PMF. To test this hypothesis, anaesthetized adult male Sprague–Dawley rats (3–6 months; 280–450 g; Harlan colony 218a; Harlan Inc., Indianapolis, IN, USA) received episodic (3, 5 min intervals) intrathecal injections (C4) of a selective 5-HT7 agonist (AS-19; Perez-Garcia & Meneses, 2005), while recording phrenic neural activity. Experimental group size was deemed sufficient once assumptions of the ANOVA were satisfied and statistical significance was established with sufficient statistical power. All procedures were approved by the Institutional Animal Care and Use Committee of the School of Veterinary Medicine, University of Wisconsin, Madison and are in compliance with the policies and regulations outlined by The Journal of Physiology (Drummond, 2009).
Experimental series
The initial experiment was designed to investigate PMF after spinal 5-HT7 receptor activation in anaesthetized rats. The second experiment explored the role of TrkB receptor activity (tyrosine kinase inhibitor, k252a) in 5-HT7-induced PMF. The third experimental series tested the hypothesis that new TrkB (not BDNF) protein synthesis is necessary for 5-HT7-induced PMF. In the final experimental series, spinal injections of PI3K (PI-828) and MEK (UO126) inhibitors were used to characterize signalling cascades downstream from TrkB receptor activation.
Surgical preparation
Anaesthesia was induced with isoflurane (2.5–3.5% in 50% O2, balance N2), and isoflurane was continued throughout surgical preparations. Once surgical procedures were completed, rats were converted to urethane anaesthesia over 15 min (1.75 μg kg−1, i.v.). Adequate anaesthetic depth was tested by lack of a pressor or respiratory neural response to toe pinch with a haemostat. After conversion to urethane anaesthesia, a continuous infusion (4–6.5 ml kg−1 h−1) of a 1:4 mixture of 6% Hetastarch and lactated Ringer solution was implemented to maintain fluid balance and acid–base status. A tracheal cannula was placed in the neck to enable artificial ventilation (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA, USA; tidal volume = 2.5 ml). A rapidly responding flow-through carbon dioxide analyser (Capnogard, Novametrix, Wallingford, CT, USA) was placed on the expired limb of a Y-tube connected to the tracheal cannula to enable measurements of end-tidal carbon dioxide partial pressures. The vagus nerves were cut in the mid-cervical region to prevent entrainment of respiratory neural activity with the ventilator. During ventilation, rats were subject to neuromuscular bloackade with pancuronium bromide (2.5 mg kg−1, i.v.). A polyethylene catheter (PE-50, Intramedic PE tubing; Becton Dickinson, Sparks, MD) was placed in the right femoral artery and blood pressure was monitored with a pressure transducer (Gould, P23ID). A three-way stop-cock, attached to the arterial catheter, was used to withdraw blood samples (0.2–0.4 ml) for blood gas analysis (ABL-500, Radiometer; Copenhagen, Denmark). During each experiment, blood gases were assessed during baseline, and at 30, 60, 90 and 120 min post-5-HT7 receptor agonist injections. Body temperature was monitored with rectal thermister (Fisher Scientific, Pittsburgh, PA, USA) and maintained (37.5 ± 1°C) with a heated surgical table.
The left phrenic nerves (and hypoglossal nerves for dose–response studies) were isolated using a dorsal approach, cut distally, desheathed and placed on bipolar silver electrodes to record respiratory neural activity. Nerve signals were amplified (100,000×), band-pass filtered (300–10,000 Hz Model 1800, A-M Systems, Carlsborg, WA, USA), rectified and integrated (Paynter filter; time constant, 50 ms, CWE Inc., MA-821; Ardmore, PA, USA). The resulting integrated nerve bursts were digitized (8000 Hz) and analysed using a WINDAQ data acquisition system (DATAQ Instruments, Akron, OH, USA). Following preparation and conversion to urethane anaesthesia, rats were allowed a minimum of one hour to stabilize before beginning an experimental protocol.
To test whether spinal mechanisms underlie 5-HT7-induced PMF, we utilized intrathecal cervical drug delivery. The spinal column was exposed dorsally, followed by laminectomy at cervical level 2 (C2), where a small incision was made in the dura. A soft silicone catheter (2 French; Access Technologies, Skokie, IL, USA) was inserted caudally through the incision until the tip was located at approximately C4. The catheter was attached to a 50 μl Hamilton syringe filled with drug solutions to allow localized drug injections into the cervical spinal region. In experiments using a 5-HT7 receptor antagonist and kinase inhibitors or siRNAs, an identical second catheter was placed to allow administration of two drugs at different times without disturbing the preparation.
Experimental protocol
At least 1 h after conversion to urethane anaesthesia, apnoeic and recruitment CO2 thresholds for phrenic nerve activity were determined by increasing ventilation and lowering end-tidal 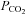 until rhythmic nerve bursts could no longer be detected (i.e. apnoeic threshold). After approximately 1 min, the ventilator rate was slowly decreased, or inspired carbon dioxide increased, until rhythmic nerve bursts resumed (i.e. recruitment threshold). Baseline conditions were then established by holding end-tidal
until rhythmic nerve bursts could no longer be detected (i.e. apnoeic threshold). After approximately 1 min, the ventilator rate was slowly decreased, or inspired carbon dioxide increased, until rhythmic nerve bursts resumed (i.e. recruitment threshold). Baseline conditions were then established by holding end-tidal 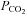 ∼2 mmHg above the recruitment threshold and allowing sufficient time to establish stable neural activity (≥15 min). At this point an arterial blood sample was taken to document baseline blood gas levels. Arterial
∼2 mmHg above the recruitment threshold and allowing sufficient time to establish stable neural activity (≥15 min). At this point an arterial blood sample was taken to document baseline blood gas levels. Arterial 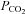 was maintained isocapnic (± 1.5 mmHg) with respect to baseline by manipulation of inspired carbon dioxide and/or ventilation rate and baseline oxygen levels (∼51% inspired oxygen,
was maintained isocapnic (± 1.5 mmHg) with respect to baseline by manipulation of inspired carbon dioxide and/or ventilation rate and baseline oxygen levels (∼51% inspired oxygen, 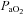 ≥ 150 mmHg) were maintained for duration of an experiment.
≥ 150 mmHg) were maintained for duration of an experiment.
To test the hypothesis that spinal 5-HT7 receptor activation induces PMF, a selective agonist (AS-19; Perez-Garcia & Meneses, 2005) was injected via an intrathecal catheter over the cervical spinal cord (C3–5). In these experiments, AS-19 was dissolved in saline to an effective concentration determined from preliminary dose–response studies (10 μm, three 5 μl injections) delivered slowly (>1 min) with 5 min intervals. In some rats, SB269970 (5 mm, 7 μl), a selective 5-HT7 receptor antagonist, was administered intrathecally 25 min before AS-19 to confirm that the effects of AS-19 were caused by C4 spinal 5-HT7 receptor activation. k252a, a tyrosine kinase inhibitor, prevents Trk receptor phosphorylation in response to neurotrophin binding (Lee & Chao, 2001). k252a (10 μl, 100 μm dissolved in 20% DMSO; Sigma-Aldrich) was administered intrathecally to some rats 25 min before intrathecal AS-19 to determine whether 5-HT7-induced changes in phrenic motor output require C4 spinal tyrosine kinase activity, consistent with a requirement for TrkB receptor phosphorylation and activation. The dose of k252a required to block TrkB phosphorylation was determined from preliminary studies as the minimal k252a concentration needed to block pLTF following acute intermittent hypoxia (Baker-Herman et al. 2004).
In vivo RNAi
Some rats received pools of four small interfering RNAs (siRNAs) to inhibit BDNF or TrkB mRNA translation, as shown previously (Baker-Herman et al. 2004; Golder et al. 2008). TrkB siRNAs were obtained as a pool of four, 21-nucleotide duplexes (OnTarget plus, Dharmacon, Lafayette, CO, USA, gene NTRK2; GenBank accession number, NM 012731). siRNAs targeting BDNF were also used to verify that 5-HT7-induced PMF did not require new BDNF synthesis and to provide a negative control for siTrkB. siRNAs were reconstituted with siRNA Universal Buffer (Dharmacon; final concentration, 5 μm) and stored at −20°C. The concentration of TrkB siRNA used for these experiments was the same as that used to inhibit A2A-induced PMF (Golder et al. 2008); the concentration of BDNF siRNA used for these experiments was the same as that used to inhibit pLTF following acute intermittent hypoxia (Baker-Herman et al. 2004). siRNAs (100 nm, 4 μl of 5 μm solution) were combined with Oligofectamine (16 μl; Invitrogen, Carlsbad, CA, USA), a lipophyllic transfection reagent, and RNase-free water (180 μl); they were incubated at room temperature for 20 min. siRNAs were injected at C4 via an intrathecal catheter (two 10 μl injections, separated by 5 min). Two hours were allowed before initiating the protocol with intrathecal AS-19. At the end of each experiment, rats were humanely killed by urethane overdose in accordance with our animal protocol approval.
Data analysis
Nerve recordings were analysed using custom designed software (LabView 6.1, National Instruments, Austin, TX, USA; courtesy of Dr S. Mahamed). Briefly, integrated phrenic (∫Phr) nerve burst amplitudes and burst frequencies were averaged over 1 min bins at each experimental time point (baseline, 30, 60, 90 and 120 min). Changes in nerve burst amplitudes were normalized and reported as percentage change from baseline. Burst frequencies were also normalized to baseline, but expressed in absolute terms as a difference in bursts per minute. All other statistical comparisons between treatment groups for nerve amplitude, mean arterial pressures, 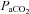 and
and 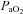 were made using a two-factor ANOVA with a repeated measures design. Individual comparisons were made using the Student–Neuman–Keuls (SNK) post hoc test (SigmaStat version 2.03; Jandel Scientific, St Louis, MO, USA). Differences between groups were considered significant if P < 0.05. All values are expressed as means ±s.e.m.
were made using a two-factor ANOVA with a repeated measures design. Individual comparisons were made using the Student–Neuman–Keuls (SNK) post hoc test (SigmaStat version 2.03; Jandel Scientific, St Louis, MO, USA). Differences between groups were considered significant if P < 0.05. All values are expressed as means ±s.e.m.
Results
Cervical 5-HT7 receptor activation elicits PMF
Since the peak amplitude of integrated inspiratory phrenic nerve bursts strongly correlates with tidal volume and respiratory muscle activity in spontaneously breathing cats (Eldridge, 1976), we used these measures as an index of changes in respiratory motor output. Typical phrenic neurograms during experimental protocols are shown in Fig. 1. To test whether spinal 5-HT7 receptor activation elicits PMF, we intrathecally injected a selective agonist (AS-19, 10 μm) into the cervical spinal cord (C4), in the vicinity of the phrenic motor nucleus (Goshgarian & Rafols, 1981). Three AS-19 (5 μl) injections, separated by 5 min, were administered slowly. During drug injections, no acute (<1 min) effects were observed on baseline phrenic burst activity. After drug injections, phrenic burst amplitude slowly and progressively increased above baseline values, an effect lasting more than 120 min after the final AS-19 injection (30 min post-injection: 24 ± 7%, P = 0.001; 60 min: 57 ± 12%, P < 0.001; 90 min: 75 ± 11%, P < 0.001; 120 min: 80 ± 11%, P < 0.001; n = 7; Fig. 2). In the time frame observed, there was no evidence that the effect was reversing. Thus, the duration of AS-19-induced PMF is unknown other than that it lasts at least 120 min. Phrenic burst frequency (fictive respiratory rate) also increased to a small extent in AS-19 treated rats (4 ± 1 burst min−1, Δbaseline at 120 min, P = 0.024), but this effect was independent of drug treatment since vehicle control rats exhibited the same ‘drift’ in burst frequency (4 ± 1 burst min−1, Δbaseline, P = 0.675; n = 8).
Figure 1. Representative phrenic neurograms from rats treated with intra-spinal solutions.
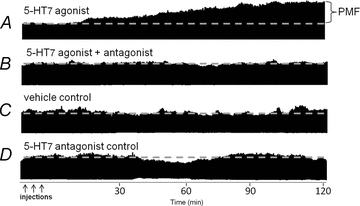
A, 5-HT7 receptor agonist (AS-19; 10 μm, 3 × 5 μl). B, 5-HT7 receptor antagonist (SB269970; 5 mm, 7 μl) prior to AS-19 injections. C, vehicle control treatment for equivalent experimental duration (i.e. vehicle time control). 5-HT7 antagonist control treatment for equivalent experimental duration (i.e. antagonist time control). Only the 5-HT7 agonist treatment elicits phrenic motor facilitation (PMF) alone.
Figure 2. Cervical spinal 5-HT7 receptor activation facilitates phrenic burst amplitudes in anaesthetized rats.
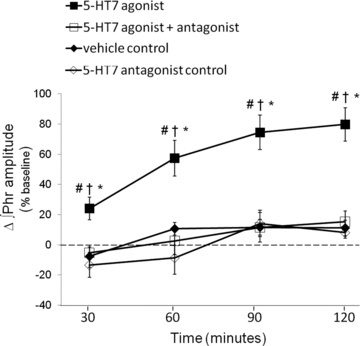
Comparisons of integrated phrenic burst amplitudes from spinally treated rats; values are expressed as a percentage change from baseline. Spinal 5-HT7 receptor agonist (▪, AS-19; 10 μm, 3 × 5 μl) significantly increased phrenic burst amplitude (80%± 11%, n = 7; P < 0.05) compared to vehicle treated rats (♦, 11%± 5%, n = 7). An injection of a 5-HT7 receptor antagonist (□, SB269970, 5 mm, 7 μl) blocked 5-HT7 agonist-induced PMF at all time points (30 min: −5 ± 4%, P = 0.007; 60 min: 3 ± 9%, P < 0.001; 90 min: 15 ± 7%, P < 0.001; 120 min: 15 ± 7%, P < 0.001; n = 6). To control for drug effects, SB269970 (⋄, 5-HT7 antagonist control, n = 4) was given alone; it had no effect on phrenic burst amplitude at any time. #Different from vehicle; †different from 5-HT7 agonist + antagonist; *different from 5-HT7 antagonist control; RMANOVA, P < 0.05.
To confirm that AS-19 was acting via spinal 5-HT7 receptors, some rats were prepared with a second intrathecal catheter and were pretreated with SB269970, a selective 5-HT7 receptor antagonist. SB269970 pretreatment (5 mm, 7 μl) abolished AS-19-induced PMF at all time points (30 min: −5 ± 4%, P = 0.007; 60 min: 3 ± 9%, P < 0.001; 90 min: 15 ± 7%, P < 0.001; 120 min: 15 ± 7%, P < 0.001; n = 6), thus confirming that AS-19 elicited PMF via 5-HT7 receptor activation; Fig. 2. SB269970 without AS-19 (n = 4) did not alter phrenic motor output, and was not different versus vehicle time controls (SB269970: 8 ± 4%; vehicle: 11 ± 5%, Δbaseline at 120 min, P = 0.819; Fig. 2).
5-HT7 receptor activation requires tyrosine kinase activity
In cell lines (PC12 cells), Gs protein-coupled receptor (GsPCR) activation (e.g. A2A, PACAP receptors; Lee & Chao, 2001; Lee et al. 2002a) trans-activates receptor tyrosine kinases, which subsequently signal via protein kinase B (i.e. Akt; Lee & Chao, 2001; Lee et al. 2002b; Rajagopal et al. 2004). A2A receptor activation in vivo also trans-activates TrkB via new synthesis and, presumably, dimerization and auto-phosphorylation of an immature TrkB isoform (Wiese et al. 2007; Golder et al. 2008). Signalling via Trk trans-activation is blocked by k252a, a non-specific tyrosine kinase inhibitor (Tapley et al. 1992). To determine whether 5-HT7-induced PMF required tyrosine kinase activity, k252a was delivered intrathecally before AS-19. The dose of k252a was selected based on its ability to inhibit A2A agonist-induced PMF (Golder et al. 2008) and acute intermittent hypoxia-induced pLTF (Baker-Herman et al. 2004). Spinal k252a injections (100 μm) prior to AS-19 (∼25 min) blocked PMF expression at 90 min post-injection, and beyond (90 min: 42 ± 11%, P = 0.009; 120 min: 29 ± 12%, P < 0.001; n = 8; Fig. 3). Thus it appears that a late phase of AS-19-induced PMF requires receptor tyrosine kinase activity, while early facilitation appears unaffected. This response is similar to A2A agonist-induced PMF (Golder et al. 2008).
Figure 3. Cervical spinal 5-HT7 agonist-induced PMF requires tyrosine kinase activity.
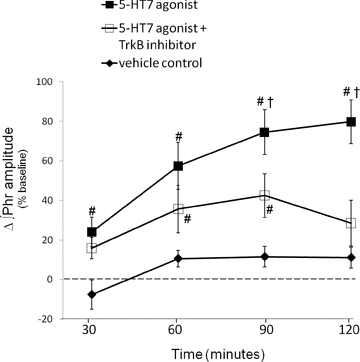
Comparisons were made of integrated phrenic burst amplitudes from spinally treated rats and expressed as a percentage change from baseline. Spinal 5-HT7 receptor agonist (▪, 10 μm, AS-19; 3×5 μl) significantly increased ∫Phr (80%± 11%, n = 7) compared to vehicle treated rats (♦, 11%± 5%, n = 7). An injection of a TrkB receptor tyrosine kinase inhibitor (□, k252a, 100 μm, 12 μl) significantly reduced phrenic burst amplitude (30%± 13%, n = 7) 120 min post-AS-19. #Different from vehicle; †different from 5-HT7 agonist+TrkB antagonist; RMANOVA, P < 0.05.
New TrkB synthesis required for 5-HT7 agonist-induced PMF
New protein synthesis contributes to multiple forms of synaptic plasticity (Martin et al. 2000; Sajikumar et al. 2005), including pLTF following acute intermittent hypoxia (Baker-Herman et al. 2004). Spinal administration of small, synthetic double-stranded RNA (small interfering RNA; siRNA) segments directed against BDNF mRNA reduced translation of new BDNF following acute intermittent hypoxia, subsequently blocking pLTF (Baker-Herman et al. 2004). Using a similar approach, siRNAs targeting TrkB mRNA prevent new TrkB synthesis and A2A agonist-induced PMF (Golder et al. 2008). Therefore we tested whether new TrkB (and not BDNF) synthesis is necessary for 5-HT7 agonist-induced PMF using siRNAs targeting TrkB and BDNF mRNA. Intrathecal administration of TrkB receptor siRNAs (siTrkB; pool of 4 duplexes) prior to AS-19 injection blocked PMF at 60 min and beyond (60 min: 9 ± 11%, P < 0.001; 90 min: 8 ± 17%, P < 0.001; 120 min: 11 ± 19%, P < 0.001; n = 7; Fig. 4). In contrast, siBDNF application had no effect on 5-HT7-induced PMF (30 min: 30 ± 9%, P = 0.585; 60 min, 42 ± 14%, P = 0.166; 90 min: 49 ± 14%, P = 0.0.089; 120 min: 65 ± 13%, P = 0.184; n = 6) (Fig. 4). Thus, siRNAs targeting TrkB mRNA are selective in their effects on 5-HT7-induced PMF, and were not dependent on new BDNF synthesis. Further, the lack of effect following BDNF siRNAs demonstrates that our results did not result from non-specific (off-target) effects of siRNA administration.
Figure 4. Cervical spinal 5-HT7 agonist-induced PMF requires new TrkB synthesis.
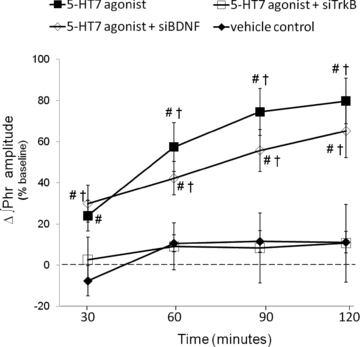
Comparisons were made of integrated phrenic burst amplitudes from spinally treated rats and expressed as a percentage change from baseline. Spinal 5-HT7 agonist (▪, 10 μm, AS-19; 3 × 5 μl) significantly increased phrenic burst amplitude (80 ± 11%, n = 7) compared to vehicle treated rats (♦, 11 ± 5%, n = 7). An injection of siTrkBs (□, 100 nm, 10 μl × 2, separated by 5 min) blocked phrenic burst amplitudes 120 min post-AS-19 (11 ± 19%, n = 6). siBDNF injections did not block AS-19-induced PMF (◊, 120 min: 65 ± 13%, n = 6). #Different from vehicle; †different from 5-HT7 agonist+siTrkB; RMANOVA, P < 0.05.
Spinal 5-HT7-induced PMF requires PI3K/Akt and MEK/ERK activity
Once activated, TrkB receptors recruit adapter proteins (e.g. Shc, Grb) that are phosphorylated by the tyrosine kinase activity, and subsequently stimulate multiple ‘downstream’ signal transduction cascades (Chao, 2003). To characterize ‘downstream’ signalling events involved in 5-HT7-induced PMF, we tested whether two predominant TrkB signalling pathways were required for 5-HT7-induced PMF: the PI3K/Akt and MEK/ERK pathways. To determine whether PI3K activity was required for PMF, a selective PI3K inhibitor (PI-828, 100 μm, 12 μl) was delivered intrathecally prior (∼20 min) to AS-19 injection. PI-828 completely blocked AS-19-induced PMF since phrenic burst amplitudes were not different from vehicle treatment 30 min post-AS-19 administration and beyond (30 min: 5 ± 4%, P = 0.225; 60 min: 14 ± 5%, P = 0.702; 90 min: 13 ± 8%, P = 0.869; 120 min: 13 ± 8%, P = 0.880, n = 7; Fig. 5). In complementary experiments, a selective MEK/ERK inhibitor (UO126, 100 μm, 12 μl) was delivered prior to AS-19. UO126 significantly decreased the early (60 min: 26 ± 14%, P = 0.008; 90 min; 48 ± 8%, P = 0.019), but not late effects of AS-19 (120 min: 60 ± 3%, P = 0.074, n = 6; Fig. 5). Compared to vehicle treatment, phrenic burst amplitude was increased in UO126 treated rats 120 min post-AS-19 injection (P < 0.001; Fig. 5) whereas PI-828 had no such effect (P = 0.880; Fig. 5). Thus, although 5-HT7 agonist-induced PMF requires TrkB receptor, PI3K/AKT and MEK/ERK activity for its full expression, MEK/ERK may be necessary only during early phases of the response.
Figure 5. Cervical spinal 5-HT7 agonist-induced PMF requires PI3K/Akt activity.
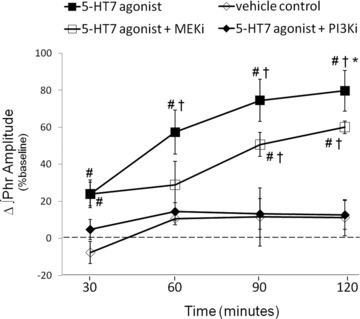
Comparisons were made of integrated phrenic burst amplitudes from spinally treated rats and expressed as a percentage change from baseline. Phrenic burst amplitude from rats spinally pretreated with an inhibitor of PI3K/Akt (PI3Ki; ♦, PI-828, 100 μm, 12 μl) blocked AS-19-induced PMF 60, 90 and 120 min post-injection (15 ± 5%, 13 ± 8%, 13 ± 8%, respectively, n = 7). Phrenic burst amplitude in rats receiving spinal injections of a MEK/ERK inhibitor (MEKi; □, UO126, 100 μm) was attenuated at 120 min post-AS-19 injection (60 ± 3%, n = 6); however, UO126 pretreated rats were significantly greater than vehicle (◊, 11 ± 5%, n = 7) and PI-828 treated rats at 120 min. #Different from vehicle; †different from 5-HT7 agonist+PI3Ki, *different from 5-HT7 agonist+MEKi; RMANOVA, P < 0.05.
Mean arterial pressures and blood gases
For studies of respiratory plasticity in vivo, it is critical to maintain precise control of arterial blood gases so that the plasticity is not obscured by changes in chemoreceptor feedback (Mitchell & Johnson, 2003). Measurements of 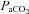 (±1.5 mmHg of baseline) and
(±1.5 mmHg of baseline) and 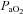 (>200 mmHg) were similar across treatment groups and did not change significantly over time during a protocol (Table 1). Mean arterial blood pressure decreased with time, but the change was similar across all experimental groups (Table 1). Previous studies have used hypoglossal nerve activity as an internal control for drug spread in the central nervous system (Baker-Herman et al. 2004; Macfarlane & Mitchell, 2007; Golder et al. 2008). To assure that 5-HT7 agonist-induced PMF was not due to unintended brainstem effects, in a subset of experiments (n = 10), respiratory activity was recorded in the hypoglossal nerve after cervical AS-19 injections. At the highest doses studied (1–10 mm in the same volume, data not shown), hypoglossal facilitation was observed. However, the standard dose of AS-19 (10 μm) was chosen based on its ability to elicit phrenic (Fig. 2) without hypoglossal facilitation (6 ± 2%, Δbaseline; n = 3). Since AS-19 elicits hypoglossal facilitation at higher doses (10 mm; 141 ± 6%, n = 2) that are likely to have spread to the brainstem, hypoglossal motor neurons are capable of expressing 5-HT7-induced motor facilitation, and are an appropriate internal control for unintended, supraspinal drug distribution.
(>200 mmHg) were similar across treatment groups and did not change significantly over time during a protocol (Table 1). Mean arterial blood pressure decreased with time, but the change was similar across all experimental groups (Table 1). Previous studies have used hypoglossal nerve activity as an internal control for drug spread in the central nervous system (Baker-Herman et al. 2004; Macfarlane & Mitchell, 2007; Golder et al. 2008). To assure that 5-HT7 agonist-induced PMF was not due to unintended brainstem effects, in a subset of experiments (n = 10), respiratory activity was recorded in the hypoglossal nerve after cervical AS-19 injections. At the highest doses studied (1–10 mm in the same volume, data not shown), hypoglossal facilitation was observed. However, the standard dose of AS-19 (10 μm) was chosen based on its ability to elicit phrenic (Fig. 2) without hypoglossal facilitation (6 ± 2%, Δbaseline; n = 3). Since AS-19 elicits hypoglossal facilitation at higher doses (10 mm; 141 ± 6%, n = 2) that are likely to have spread to the brainstem, hypoglossal motor neurons are capable of expressing 5-HT7-induced motor facilitation, and are an appropriate internal control for unintended, supraspinal drug distribution.
Table 1.
Measurements of 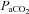 ,
, 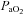 and mean arterial pressure (MAP) during baseline, 30, 60, 90 and 120 min time points
and mean arterial pressure (MAP) during baseline, 30, 60, 90 and 120 min time points
| Time (min) | 5-HT7 agonist | 5-HT7 agonist + SB 269970 | vehicle control | 5-HT7 antagonist control | 5-HT7 agonist + k252a | 5-HT7 agonist + siTrkB | 5-HT7 agonist + siBDNF | 5-HT7 agonist + UO126 | 5-HT7 agonist + PI-828 | |
|---|---|---|---|---|---|---|---|---|---|---|
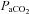 (mmHg) (mmHg) |
0 | 45.8 ± 1.0 | 44.6 ± 1.2 | 47.0 ± 1.0 | 45.0 ± 0.3 | 46.5 ± 0.7 | 47.7 ± 0.8 | 47.3 ± 0.7 | 48.3 ± 1.3 | 46.6 ± 1.2 |
| 30 | 45.9 ± 1.2 | 44.8 ± 0.9 | 47.0 ± 0.8 | 45.1 ± 0.6 | 45.6 ± 0.8 | 48.3 ± 1.0 | 46.8 ± 1.4 | 47.7 ± 1.2 | 46.4 ± 1.3 | |
| 60 | 46.9 ± 0.9 | 44.9 ± 1.4 | 46.5 ± 0.7 | 43.6 ± 0.9 | 45.6 ± 0.6 | 48.3 ± 1.2 | 47.7 ± 1.2 | 48.1 ± 1.1 | 45.9 ± 1.1 | |
| 90 | 46.7 ± 0.9 | 45.4 ± 1.5 | 48.7 ± 1.6 | 44.6 ± 0.2 | 46.3 ± 0.6 | 47.7 ± 1.1 | 48.4 ± 0.8 | 48.8 ± 1.5 | 45.7 ± 0.9 | |
| 120 | 45.9 ± 1.1 | 44.4 ± 1.1 | 47.9 ± 0.9 | 45.8 ± 0.6 | 46.7 ± 0.7 | 48.0 ± 0.8 | 47.4 ± 1.0 | 48.6 ± 1.2 | 45.7 ± 1.1 | |
 (mmHg) (mmHg) |
0 | 258 ± 23 | 283 ± 8 | 266 ± 12 | 296 ± 4 | 259 ± 7 | 283 ± 16 | 291 ± 7 | 275 ± 9 | 254 ± 10 |
| 30 | 255 ± 14 | 268 ± 8 | 261 ± 13 | 297 ± 3 | 258 ± 5 | 269 ± 13 | 268 ± 9† | 268 ± 12 | 250 ± 7 | |
| 60 | 262 ± 13 | 271 ± 6 | 260 ± 13 | 291 ± 4 | 260 ± 6 | 270 ± 13 | 269 ± 6† | 263 ± 7 | 248 ± 8 | |
| 90 | 264 ± 10 | 270 ± 6 | 264 ± 13 | 297 ± 3 | 258 ± 7 | 276 ± 11 | 274 ± 8† | 258 ± 10† | 244 ± 5 | |
| 120 | 259 ± 11 | 269 ± 7 | 261 ± 12 | 291 ± 4 | 257 ± 6 | 277 ± 10 | 268 ± 7† | 257 ± 6† | 252 ± 5 | |
| MAP (mmHg) | 0 | 114 ± 4 | 116 ± 4 | 115 ± 6 | 113 ± 4 | 119 ± 3 | 114 ± 3 | 103 ± 2 | 119 ± 9 | 115 ± 6 |
| 30 | 120 ± 6 | 117 ± 6 | 109 ± 6 | 103 ± 3 | 111 ± 3 | 107 ± 4 | 100 ± 3 | 119 ± 9 | 104 ± 6† | |
| 60 | 123 ± 5† | 120 ± 4 | 113 ± 7 | 110 ± 4 | 115 ± 3 | 109 ± 3 | 97 ± 4# | 114 ± 10† | 105 ± 6† | |
| 90 | 121 ± 5 | 122 ± 5 | 116 ± 8 | 113 ± 6 | 118 ± 4† | 112 ± 3† | 94 ± 5#† | 109 ± 10† | 107 ± 6† | |
| 120 | 118 ± 5 | 113 ± 3 | 107 ± 6 | 105 ± 3 | 109 ± 3† | 105 ± 3† | 90 ± 4#† | 106 ± 9† | 102 ± 5† |
Discussion
Here we demonstrate that episodic spinal administration of a selective 5-HT7 receptor agonist induces a long-lasting phrenic motor facilitation, consistent with our hypothesis that multiple spinal Gs protein-coupled receptors located on or near phrenic motor neurons elicit PMF. Although episodic 5-HT7 agonist administration elicited PMF, it is not yet clear if this form of respiratory plasticity is pattern sensitive similar to AIH-induced respiratory plasticity. Cumulative evidence supports a role for Gq protein-coupled 5-HT2 receptors in phrenic LTF following acute intermittent hypoxia (Lindsay & Feldman, 1993; Fuller et al. 2001). The present experiments suggest that spinal Gs protein-coupled serotonin receptors have the potential to make a unique contribution to serotonin-dependent pLTF following acute intermittent hypoxia. If true, 5-HT7 receptors contribute via a unique signalling pathway since Gq-coupled metabotropic receptors signal through phospholipase C and protein kinase C, whereas Gs-protein coupled receptors signal through cyclic AMP and protein kinase A. The hypothesis that 5-HT7 receptors contribute to AIH-induced pLTF remains to be tested. However, another Gs protein-coupled receptor, the adenosine 2A receptor, appears to inhibit (versus contribute to) AIH-induced pLTF (Hoffman et al. 2010). Regardless, 5-HT7 receptor-induced PMF is a unique finding that may inspire novel pharmacological strategies to trans-activate Trk receptors in vivo, possibly harnessing respiratory plasticity to treat impaired respiratory (and somatic) motor function (Mitchell, 2007; Golder, 2008).
Location of relevant 5-HT7 receptors
Spinal mechanisms elicit 5-HT7-induced PMF because agonist injections were localized to the cervical spinal cord. It is doubtful that supraspinal mechanisms played any meaningful role in the response observed since (lower dose) intrathecal AS-19 injections had no effects on phrenic burst frequency or on activity of cranial hypoglossal motor neurons (data not shown). At higher doses of AS-19, hypoglossal facilitation is observed, demonstrating the capacity for motor facilitation when sufficient drug concentrations reach the brainstem. While we propose that spinal 5HT7 receptor activation increases synaptic strength between descending, bulbo-spinal respiratory pre-motor neurons and phrenic motor neurons, effects of 5-HT7 receptor activation on other cell types (e.g. spinal interneurons and/or glia) cannot be ruled out (Landry et al. 2006; Liu et al. 2009).
New TrkB synthesis differentiates GsPCR-induced PMF from AIH-induced pLTF
New protein synthesis is a hallmark of many forms of synaptic plasticity (Davis & Squire, 1984; Martin et al. 2000). Plasticity in respiratory motor control is no exception, as new protein synthesis is necessary for spinal A2A agonist-induced PMF and acute intermittent hypoxia (AIH)-induced pLTF; both forms of plasticity are blocked by translational inhibitors (Baker-Herman & Mitchell, 2002; Golder et al. 2008). However, the specific proteins required in these forms of spinal, respiratory plasticity are different. siRNAs that target and inhibit BDNF translation block AIH-induced pLTF (Baker-Herman et al. 2004) whereas siRNAs targeting TrkB (not BDNF) block A2A agonist-induced PMF (Golder et al. 2008). Consistent with a mechanism similar to A2A-induced PMF, siTrkB blocked 5-HT7 agonist-induced PMF whereas siBDNF was without effect (Fig. 4). One difference from A2A agonist-induced PMF is that siTrkB blocked both early and late phase spinal 5-HT7-induced PMF versus the late (but not early) phase of A2A agonist-induced PMF. Although differences were observed in sensitivity of 5-HT7-induced PMF to siTrkB when compared to A2A receptor activation, these data suggest that similar mechanisms give rise to GsPCR-induced PMF, and that this mechanism is distinct from that arising from Gq coupled metabotropic receptors (e.g. 5-HT2 dependent pLTF). Among other factors, these pathways can be distinguished by differences in their dependence on new TrkB versus BDNF synthesis.
Spinal A2A receptor activation stimulated new synthesis and auto-phosphorylation of an immature intracellular TrkB isoform (Golder et al. 2008). In this study we did not address subcellular localization of new TrkB receptors. Thus, 5-HT7 activation may stimulate production of immature and/or mature TrkB isoforms, although this aspect of the mechanism remains to be experimentally verified.
Kinases required for 5-HT7 agonist-induced PMF
The cellular effects of A2A receptor activation require tyrosine kinase activity, both in vitro and in vivo (Lee & Chao, 2001; Wiese et al. 2007; Golder et al. 2008). Spinal k252a pretreatment blocked late phase A2A agonist-induced facilitation, but not early phase (Golder et al. 2008). k252a also inhibits 5-HT7-induced PMF at 90 min post-AS-19 injection, and beyond (i.e. late phase). One possible interpretation is that PMF induced by GsPCR activation may arise from new synthesis of an immature TrkB isoform that auto-dimerizes and initiates tyrosine kinase catalytic activity, signalling from within motor neurons (Golder et al. 2008). An important caveat limiting interpretation of this experimental series is that k252a inhibits multiple kinases (Lin et al. 2003); consequently, some k252a effects may reflect inhibition of kinases other than TrkB.
Exogenous BDNF activates MAP-kinases (MEK/ERK) and PI3K/Akt in spinal motor neurons, a response also observed in the hippocampus (Gottschalk et al. 1999; Kishino & Nakayama, 2003). ERK MAP kinases in ventral cervical spinal tissue also increase phosphorylation following acute and repetitive acute intermittent hypoxia, suggesting an important role in pLTF and enhanced pLTF (Wilkerson & Mitchell, 2009). On the other hand, spinal A2A receptor activation preferentially phosphorylated Akt, suggesting that this form of PMF required activation of the PI3K pathway (Golder et al. 2008). Thus, we evaluated whether 5-HT7 agonist-induced PMF required PI3K/Akt and/or MEK/ERK. Spinal application of a PI3K inhibitor (PI-828) abolished PMF whereas spinal MEK/ERK (UO126) inhibition blocked only the early facilitation (<90 min) (Fig. 5). Thus, full expression of spinal 5-HT7 agonist-induced PMF appears to involve (1) an early phase that is TrkB independent, but ERK and Akt dependent, and (2) a late phase that is TrkB and PI3K dependent, but ERK independent. These findings suggest that early signalling cascades associated with 5-HT7 receptor activation preferentially involve MEK/ERK activity similar to some reports (Errico et al. 2001; Lee et al. 2002b), whereas the late phase is more reliant on signalling from newly synthesized TrkB (Rajagopal et al. 2004; Golder, 2008). In PC12 cells, A2A receptor activation (CGS21680) elicits a rapid increase (∼10 min) in ERK1/2 phosphorylation that quickly returns to baseline levels (Lee & Chao, 2001), suggesting MAP-kinase activation is not required for TrkB trans-activation.
Cervical spinal injections of vascular endothelial growth factor (VEGFA), which also signals via receptor tyrosine kinases (Robinson et al. 2000), elicits robust PMF that is also sensitive to PI3K/Akt inhibition (LY249002; Dale-Nagle & Mitchell, 2008). Collectively, common points of AIH-induced pLTF (Baker-Herman et al. 2004), A2A (Golder et al. 2008) and 5-HT7 (this study) agonist-induced PMF, and VEGF-induced PMF (Dale-Nagle & Mitchell, 2008) suggest that PMF arises from a convergence of signalling cascades downstream from TrkB. Specifically, it appears that many forms of PMF require some combination of Akt and/or ERK signalling. The ubiquity of this ‘pathway to PMF’ must be tested more comprehensively. Furthermore, studies concerning interactions between these pathways are necessary before we will gain an adequate picture of what role (if any) these pathways play in different circumstances that lead to PMF.
Potential cellular/synaptic signalling mechanisms
A2A receptor activation stimulates new synthesis and phosphorylation of an intracellular low molecular mass (80 kDa, hypoglycosylated) TrkB receptor isoform, which is essential for Akt phosphorylation (Rajagopal et al. 2004; Golder et al. 2008). Increasing intracellular TrkB protein can induce constitutive Trk signalling (Watson et al. 1999) without ligand, possibly because increased Trk monomer concentrations enable auto-dimerization and phosphorylation. In our working cellular/synaptic model of PMF, we postulate that activation of relevant spinal GsPCRs (i.e. 5-HT7 and/or A2A, among others) stimulates new synthesis of an immature TrkB isoform which auto-dimerizes and auto-phosphorylates, thereby activating the PI3K/Akt pathway. Activated Akt and/or ERK1/2 may elicit synaptic plasticity by controlling protein synthesis via translational regulation (Kelleher et al. 2004; Horwood et al. 2006), modulating membrane excitability (e.g. potassium channels; Yuan et al. 2002) and/or increasing membrane expression of glutamate receptors (Sheng & Hyoung Lee, 2003).
Although yet untested, we believe that activation of spinal GqPCRs (e.g. 5-HT2 during intermittent hypoxia) and GsPCRs (e.g. 5-HT7, A2A) induces PMF via distinctive mechanisms that converge downstream of TrkB receptor activation, differentiated by new synthesis of BDNF and/or TrkB protein as well as details in their reliance on the downstream serine/threonine kinases (i.e. Akt versus ERK).
Significance
Acute intermittent hypoxia strengthens synaptic pathways to phrenic motor neurons and improves spontaneous phrenic activity by increasing endogenous BDNF synthesis and release (Baker-Herman et al. 2004; Golder & Mitchell, 2005). Thus, intermittent hypoxia may have beneficial effects in restoring respiratory motor function after cervical spinal injury (Golder & Mitchell, 2005; Mitchell, 2007). However, intermittent hypoxia is a non-specific stimulus and may affect multiple physiological systems and, in excess, may induce severe morbidity such as systemic hypertension, hippocampal apoptosis, and learning deficits (Gozal et al. 2001; Bass et al. 2004). While exogenous BDNF delivery to the cervical spinal cord is sufficient to elicit phrenic LTF (Baker-Herman et al. 2004), protein delivery to the CNS is often complicated by the need for invasive delivery (due to the blood–brain barrier), poor penetration, immune reactions and complications attendant to receptor downregulation (Poduslo & Curran, 1996; Mitchell, 2007; Golder, 2008). Small molecules that cross the blood–brain barrier have advantages because they can mimic the activities of BDNF while minimizing negative reactions.
Since systemic adenosine receptor activation has both beneficial and deleterious effects on the cardiovascular and nervous systems, other molecules should be considered for therapeutic development (Abbracchio & Cattabeni, 1999; Pedata et al. 2001). Thus small, highly permeant molecules that activate 5-HT7 receptors and mimic BDNF effects on respiratory motor output could be a useful alternative and provide multiple potential targets for ‘harnessing’ respiratory plasticity for treatment of ventilatory control disorders (Mitchell, 2007).
Acknowledgments
This work was supported by NIH HL080209 and NRSA HL092785.
Glossary
Abbreviations
- AIH
acute intermittent hypoxia
- Akt
protein kinase B
- BDNF
brain derived neurotrophic factor
- ERK
extracellular signal-regulated kinase
- LTF
long term facilitation
- MEK
Map-erk kinase
- PACAP
pituitary adenylate cyclase activating polypeptides
- PI3K
phosphatidylinositol 3-kinase
- pLTF
phrenic long term facilitation
- PMF
phrenic motor facilitation
- ROS
reactive oxygen species
- TrkB
tropomyosin-related kinase B
Author contributions
Both authors fully contributed to the conception and design of the study, analysis and interpretation of data, and the drafting and revising of the manuscript; they both approved the version to be published. All experiments where performed at the University of Wisconsin-Madison School of Veterinary Medicine.
References
- Abbracchio MP, Cattabeni F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann N Y Acad Sci. 1999;890:79–92. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, Schonwald A, Wilker RE, Stehle S, Kinane TB. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Mitchell GS. Intrathecal administration of vascular endothelial growth factor (VEGF) elicits phrenic motor facilitation. FASEB J. 2008;22:1232.6. (abstract) [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Verge D, Conrath M. Pre- and postsynaptic localization of the 5-HT7 receptor in rat dorsal spinal cord: immunocytochemical evidence. J Comp Neurol. 2005;490:256–269. doi: 10.1002/cne.20667. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest. 1976;70(1 Suppl):154–157. doi: 10.1378/chest.70.1_supplement.154. [DOI] [PubMed] [Google Scholar]
- Errico M, Crozier RA, Plummer MR, Cowen DS. 5-HT7 receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001;102:361–367. doi: 10.1016/s0306-4522(00)00460-7. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2000–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Golder FJ. Receptor tyrosine kinases and respiratory motor plasticity. Respir Physiol Neurobiol. 2008;164:242–251. doi: 10.1016/j.resp.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus. Learn Mem. 1999;6:243–256. [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2A receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kishino A, Nakayama C. Enhancement of BDNF and activated-ERK immunoreactivity in spinal motor neurons after peripheral administration of BDNF. Brain Res. 2003;964:56–66. doi: 10.1016/s0006-8993(02)04066-0. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002a;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002b;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem. 2003;87:1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol. 2009;102:337–348. doi: 10.1152/jn.91239.2008. [DOI] [PubMed] [Google Scholar]
- Macfarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2007;102:337–348. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Potenza RL, Pezzola A, Blum D, Bantubungi K, Popoli P. Effects of the adenosine A2A receptor antagonist SCH 58621 on cyclooxygenase-2 expression, glial activation, and brain-derived neurotrophic factor availability in a rat model of striatal neurodegeneration. J Neuropathol Exp Neurol. 2007;66:363–371. doi: 10.1097/nen.0b013e3180517477. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. 1st edn. New York: Springer; 2007. pp. 291–306. [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Jeong GB, Crocker A, Arneja A, David S, Russell DS, Kalb RG. Protecting motor neurons from toxic insult by antagonism of adenosine A2a and Trk receptors. J Neurosci. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia GS, Meneses A. Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav Brain Res. 2005;163:136–140. doi: 10.1016/j.bbr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen Z-Y, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Frey JU. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Curr Opin Neurobiol. 2005;15:607–613. doi: 10.1016/j.conb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Watson FL, Porcionatto MA, Bhattacharyya A, Stiles CD, Segal RA. TrkA glycosylation regulates receptor localization and activity. J Neurobiol. 1999;39:323–336. doi: 10.1002/(sici)1097-4695(199905)39:2<323::aid-neu15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McGuire M, White DP, Ling L. Serotonin receptor subtypes involved in vagus nerve stimulation-induced phrenic long-term facilitation in rats. Neurosci Lett. 2004;363:108–111. doi: 10.1016/j.neulet.2004.03.067. [DOI] [PubMed] [Google Scholar]


