Abstract
This study aimed to evaluate the clinical profiles, antibiotic susceptibility, risk factors of multi-drug resistance (MDR) and outcomes of P. aeruginosa bacteremia in children by retrospective methods at a tertiary teaching children's hospital in Seoul, Korea during 2000-2009. A total of 62 episodes were evaluated and 59 patients (95.2%) had underlying diseases. Multivariate analysis demonstrated that an intensive care unit (ICU) stay within the previous one month was the only independent risk factor for MDR P. aeruginosa bacteremia (odds ratio [OR], 6.8; 95% confidence interval [CI], 1.3-35.8, P = 0.023). The overall fatality rate associated with P. aeruginosa bacteremia was 14.5% (9 of 62). The fatality rate in patients with MDR P. aeruginosa was 57.1%, compared with 9.1% in non-MDR patients (OR 13.3; 95% CI 2.3-77.2, P = 0.006). However, the presence of respiratory difficulty was the only independent risk factor for overall fatality associated with P. aeruginosa bacteremia according to multivariate analysis (OR 51.0; 95% CI 7.0-369.0, P < 0.001). A previous ICU stay and presentation with respiratory difficulty were associated with acquisition of MDR P. aeruginosa and a higher fatality rate, respectively. Future efforts should focus on the prevention and treatment of P. aeruginosa bacteremia in high-risk children.
Keywords: Pseudomonas aeruginosa; Bacteremia; Drug Resistance, Multiple, Clinical Outcome
INTRODUCTION
Pseudomonas aeruginosa, an aerobic Gram-negative organism that is commonly discovered in soil, water, and plants, rarely causes illness in healthy people (1). However, P. aeruginosa sepsis often occurs in patients with burns, malignancies or immunodeficiency or in preterm infants (1). Most of these infections are nosocomially acquired (1). P. aeruginosa is a virulent organism that is susceptible to a limited number of antibiotic agents including antipseudomonal penicillins and cephalosporins, carbapenems, fluoroquinolones and ciprofloxacin (2-4). Despite recent improvements in therapy, P. aeruginosa bacteremia remains fatal in more than 20% of cases (5). In a recent large multicenter study of all age groups, P. aeruginosa bloodstream infection (BSI) was associated with crude mortality rates of 39% in all patients and 48% in intensive care unit patients (6).
The outcome of P. aeruginosa bacteremia has been shown to be related to microbial (7, 8) and host factors (9-11) and treatment (10-12). Understanding these factors may provide an improvement in treatment outcome.
Despite an abundance of studies on the risk factors for multi-drug resistance (MDR) and mortality of P. aeruginosa bacteremia, relatively few studies on P. aeruginosa bacteremia in children have been reported in recent years (13-16). We conducted an analysis of 62 pediatric patients with P. aeruginosa bacteremia at a tertiary care children's hospital during a recent ten-year period. We investigated the risk factors for acquisition of MDR P. aeruginosa and the mortality rate associated with P. aeruginosa bacteremia as well as its clinical characteristics and antibiotic susceptibility.
MATERIALS AND METHODS
This was a retrospective analysis conducted at the Seoul National University Children's Hospital (SNUCH), a 300-bed tertiary care university hospital and pediatric referral center located in Seoul, Korea.
Study population
From January 2000 through December 2009, we studied pediatric patients younger than 18 yr of age who had at least one positive blood culture for P. aeruginosa during hospitalization. The collected data included age, gender, type of acquisition, underlying disease, previous antimicrobial therapy, neutropenia, use of immunosuppressive treatment, prior surgery, use of vascular or urinary catheters, invasive procedures during the prior 72 hr, use of a mechanical ventilator within the previous month, presence of initial septic shock, respiratory difficulty, renal insufficiency, hepatic dysfunction, antibiotic susceptibility, MDR, and treatment outcome.
Definitions
The presence of P. aeruginosa bacteremia was defined as the isolation of P. aeruginosa in a blood culture (12). Only the first P. aeruginosa isolate, during a single clinical event, was included in the analysis. However, it was considered as a non-related, independent episode, if more than one episode was occurred with more than a two-week interval in a same patient who had been properly treated and clinically cured (17).
Septic shock was defined as sepsis and hypotension (18) (systolic blood pressure [BP] < 70 mmHg in infant; < 70 + [2 × age in year] after one year of age) or need for a vasopressor to maintain blood pressure (12). The primary focus of infection was defined as a culture-positive site and/or a clinically evident site of infection concomitant with bacteremia (19). Diagnosis of catheter-related bacteremia was defined as P. aeruginosa bacteremia in a patient who had an intravascular device and more than one positive blood culture result from the peripheral vein, clinical manifestations of infection (e.g., fever, chills, and/or hypotension), and no apparent source for BSI (with the exception of the catheter). One of the following should be present: a positive result of semiquantitative (> 15 colony-forming unit per catheter segment) or differential time to positivity (growth in a culture of blood obtained through a catheter hub detected via an automated blood culture system at least 2 hr earlier than a culture of simultaneously drawn peripheral blood of equal volume) (20). Prior steroid use was defined as at least 2 mg/kg/day or ≥ 20 mg of prednisone daily for at least ten of the 30 days before the diagnosis of bacteremia. Patients who had malignant disease, premature babies, and patients receiving steroid therapy were classified as immunocompromised (19).
The empirical antimicrobial therapy was considered appropriate if the causative organism was susceptible to at least one of the prescribed antimicrobials according to an in vitro test within 24 hr after blood culture sampling (12, 19).
Microbiology and antibiotic susceptibility
P. aeruginosa isolates obtained from the blood of patients at the SNUCH during the study period from January 2000 through December 2009 were collected and stored at -70℃. Species identification was carried out using VITEK-GNI cards (BioMerieux, Hazelwood, MO, USA) from 2000 to 2006 and was performed using the VITEK II system (BioMerieux) or Microscan system (MicroScan® WalkAway 96 plus system, Siemens Healthcare Diagnostics Inc., West Sacramento, CA, USA) after 2006.
Antibiotic susceptibility testing was performed using the disk diffusion method recommended by the CLSI guidelines (21) or the automatically calculated minimal inhibitory concentration (MIC) method of VITEK or a Microscan system. The tested antibiotics included amikacin, aztreonam, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, piperacillin, piperacillin/tazobactam, and tobramycin. Intermediate susceptibility to an antibiotic was considered as resistance, in correspondence with clinical practice. MDR was defined as a lack of susceptibility to three or more of the following antibiotics: 1) ciprofloxacin; 2) imipenem; 3) gentamicin, tobramycin, or amikacin; 4) ceftazidime or cefepime; and 5) piperacillin or piperacillin-tazobactam (22).
Statistical analysis
Potential risk factors for MDR P. aeruginosa bacteremia and mortality were identified using univariate analysis, and Fisher's exact test was used for categorical variables. Variables for which the P value was < 0.05 in univariate analysis were included in a logistic regression model for multivariable analysis using a backward-Wald selection process. A P value < 0.05 was considered statistically significant. A statistical package (SPSS, version 17.0, SPSS Inc, Chicago, IL, USA) was used for all analyses.
Ethics statement
This study was approved by the institutional review board of the Seoul National University Hospital (IRB registration number H-1007-151-324). Informed consent was exempted by the board.
RESULTS
Epidemiology and demographic characteristics
During the period from January 2000 to December 2009, a total of 75 patients with P. aeruginosa bacteremia were identified at the SNUCH. On average, 7.5 episodes of P. aeruginosa bacteremia occurred in one year with a range of 1-15 cases per year. The mean incidence rate (± standard deviation; SD) was 0.09 (± 0.05) episodes/1,000 patient-admission days per year. Among the 75 cases of P. aeruginosa bacteremia, medical records were unavailable for 13 (17.3%) patients, which were excluded from clinical analysis and as a result, a total of 62 episodes was analyzed in this study.
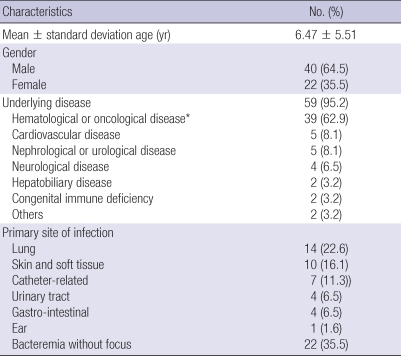
The mean (± SD) age of the patients was 6.5 (± 5.5) yr old (range; 0-17 yr old), and 40 (64.5%) patients were male (Table 1). P. aeruginosa bacteremia occurred in a mean of 29.5 hospital-days.
Table 1.
The characteristics of 62 patients with P. aeruginosa bacteremia
*These included leukemia (25.8%), solid tumor (24.2%), lymphoma (4.8%), hemophagocytic lymphoproliferative histiocytosis (4.8%), and aplastic anemia (3.2%).
Fifty-nine (95.2%) of 62 patients had underlying disease. The most common underlying diseases were hematological and oncological disease; 58.1% (36/62) of the patients had neutropenia with an absolute neutrophil count < 500 cells/µL at the onset of bacteremia. Three patients (4.8%) had no underlying disease, and all of these patients were younger than one year of age. The initial diagnoses in these three were pneumonia with pleural effusion, severe skin infection, and bacteremia without focus.
At the onset of bacteremia, 40 patients (64.5%) had infection foci. The most common infection site was the lung (22.6%; 14/62), followed by skin and soft tissue (16.1%; 10/62) and central catheter-related infection (11.3%; 7/62). Primary sites of infection could not be elucidated in 35.5% (22/62) cases.
Antimicrobial susceptibility
Of the 62 P. aeruginosa isolates, the resistance rates for the antibiotics were as follows; 24.2% for aztreonam, 16.1% for imipenem, 14.5% for gentamicin, 12.9% for piperacillin and piperacillin/tazobactam, and 11.3% for cefepime and ceftazidime, respectively.
Seven isolates (11.3%) showed MDR phenotypes, five of which were identified in 2003; remaining two isolates were identified in each year of 2005 and 2008, separately. For the MDR phenotypes, there was no overlapping patient. P. aeruginosa isolates with cefepime, piperacillin and piperacillin/tazobactam resistance showed MDR phenotypes substantially. Pan-drug resistance for P. aeruginosa was not yet identified in this study. Only one (1.6%) isolate was resistant against amikacin. All of the isolates tested were susceptible to ciprofloxacin.
Risk factors for MDR P. aeruginosa bacteremia
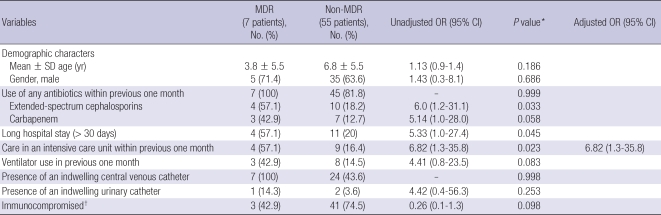
The variables considered as possible risk factors for acquisition of MDR-P. aeruginosa are shown in Table 2. In the univariate analysis, MDR-P. aeruginosa cases were more frequently associated with previous extended-spectrum cephalosporin use within one month (OR, 6.0; 95% CI, 1.2-31.1), an intensive care unit (ICU) stay (OR, 6.8; 95% CI, 1.3-35.8), and a long hospital stay (> 30 days) (OR, 5.3; 95% CI, 1.0-27.4).
Table 2.
Risk factors for MDR P. aeruginosa bacteremia in 62 patients from SNUCH over a ten-year period
*Variables with P < 0.05 on univariate analysis were included in the multivariate analysis; †Patients who had malignant disease, premature birth, or who had received steroid therapy (Reference No. 19).
An ICU stay within the previous one month was the only statistically-significant risk factor for MDR acquisition in the multivariate analysis, adjusted for age and gender in addition to the variables with P < 0.05 in the univariate analysis (adjusted OR, 6.8; 95% CI, 1.3-35.8, P = 0.023)
Clinical outcomes of P. aeruginosa bacteremia
The overall case fatality associated with P. aeruginosa bacteremia was 14.5% (9 of 62). The fatality rate of the MDR-P. aeruginosa group was 57.1% (4/7) compared with 9.1% (5/55) in the non-MDR group (OR, 13.3; 95% CI, 2.3-77.2, P = 0.006).
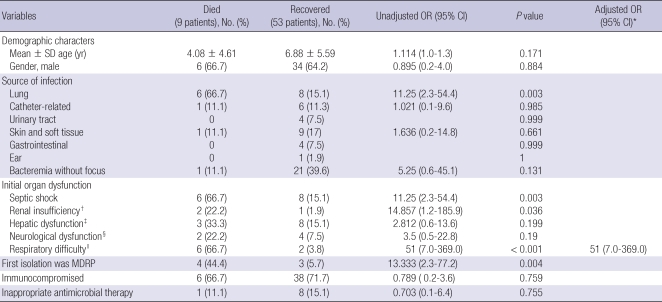
A higher fatality rate was observed in the cases with the following factors by the univariate analysis; pulmonary infection, presentation with septic shock, respiratory difficulty, renal insufficiency, and antibiogram of MDR phenotype (Table 3). Presence of respiratory difficulty was revealed to be an independent risk factor for higher all-cause fatality according to the multivariate logistic regression analysis adjusted for age, gender and the variables with P < 0.05 in the univariate analysis (OR, 51.0; 95% CI, 7.0-369.0, P < 0.001).
Table 3.
Risk factors for fatal outcomes associated with P. aeruginosa bacteremia
*Variables with P < 0.05 on univariate analysis were included in the multivariable analysis; †A serum creatinine level of > 2.0 mg/dL or a requirement for dialysis (Reference No. 19); ‡A serum bilirubin concentration of > 2.5 mg/dL, increased aspartate aminotransferase level or alanine aminotransferase level more than twice the normal level (Reference No. 19); §Change in consciousness level (Reference No. 19); ∥A partial arterial O2 pressure of < 60 mmHg, a partial arterial CO2 pressure of > 50 mmHg, or a need for ventilator assistance (Reference No. 19).
Nine (14.5%) patients were treated with inappropriate initial antimicrobial regimens for P. aeruginosa bacteremia; six (66.6%) of these patients did not receive any antibiotics directed against P. aeruginosa (e.g. anti-pseudomonal beta-lactams) as an initial empirical treatment. Three (33.3%) of the nine patients were initially treated with antibiotics that have known anti-pseudomonal activity, for which the isolates were actually resistant based on the in vitro susceptibility test. The fatality rate of the patients who were treated with appropriate initial antimicrobial regimens for P. aeruginosa bacteremia was 15% (8/53) and the fatality rate among the patients with inappropriate initial antimicrobial regimens was 11.1% (1/9); a statistically significant difference among these two groups was not observed (P = 0.755 by Fisher's exact test).
DISCUSSION
The objective of the present retrospective study was to evaluate the clinical patterns, antibiotic sensitivity, independent risk factors for MDR and treatment outcomes of P. aeruginosa bacteremia in children. We found that fifty-nine (95.2%) of 62 patients had underlying disease, seven isolates (11.3%) showed MDR phenotypes, and overall case fatality was 14.5% (9 of 62). We also found that ICU stay within the previous one month was independently associated with the development of MDR P. aeruginosa bacteremia, and presentation with respiratory difficulty was an independent risk factor for all-cause fatality due to P. aeruginosa bacteremia. Previous investigations have already described risk factors of an MDR phenotype in P. aeruginosa infection. Cao et al. (4) demonstrated that the use of imipenem or meropenem and mechanical ventilation were independent risk factors for MDR P. aeruginosa infections. Johnson et al. (22) documented that previous transplantation, hospital-acquired BSI and ICU admission in the year before MDR P. aeruginosa BSI were independent risk factors. Gulay et al. (23) showed that the major risk factors for infection or colonization with MDR P. aeruginosa were prolonged stay in the ICU, previous and lengthy imipenem usage, and mechanical ventilation. To our knowledge, our study provides the first data revealing the risk factors of an MDR P. aeruginosa infections among pediatric groups based on the 10 yr observation, in which previous ICU stay was found to be a risk factor of MDR P. aeruginosa infection. It may be hypothesized that colonization of P. aeruginosa in the ICU can cause infection and may be a risk factor for MDR P. aeruginosa infection.
Recently, resistance to antimicrobial agents of P. aeruginosa has become a more serious clinical problem (3, 24, 25). Moreover, prevalence of MDR phenotype is increasing among P. aeruginosa in adult patients (26, 27). However, we were unable to demonstrate an increased imipenem-resistance or MDR phenotypes of P. aeruginosa bacteremia during the study period. This phenomenon might be associated with the small number of cases in this study. Furthermore, all isolates were susceptible to ciprofloxacin, which differs from results from another study (28). Recently the resistance rate for quinolone among E. coli and K. pneumoniae blood isolates obtained from children in our institute was increasing accompanying with an increment of quinolone usuage (unpublished data). As a result, continuous monitoring should be manditory for increasing trends of MDR phenotypes or quinolone resistance among P. aeruginosa isolates in pediatric hospitals.
Infections caused by MDR P. aeruginosa are difficult to treat (4). Our results show the case fatality for MDR group to be as high as 50% and higher than that in the non-MDR group, even though some studies have suggested that MDR phenotype is not a predictor for fatality with P. aeruginosa (4, 22). This fatality rate was consistent with the result of an earlier study which reported that the odds of death in patients with MDR P. aeruginosa bacteremia was 3.9 (95% CI, 1.42-10.78, P = 0.002) times higher than that for patients with non-MDR P. aeruginosa (16).
The fatality rate associated with P. aeruginosa bacteremia is higher than those of all other bacteremia in the hospital settings. Thus, appropriate initial antimicrobial therapy is of particular importance in these cases. There are several studies emphasizing the importance of appropriate initial antimicrobial treatment (11, 12, 29). Kang et al. (12) emphasized that delayed effective antimicrobial therapy for P. aeruginosa bacteremia, presentation with septic shock, pneumonia and increasing APACHE II score tended to be related to higher mortality. However, in another study, inactive empiric antibiotic therapy was not an independent predictor of mortality with MDR P. aeruginosa infection, but the authors postulated a small number of patients who received inappropriate empiric therapy (4.6%; 23/503) as the cause (22). In the current study, the initial appropriateness of antibiotics was not a predictor of fatality like a previous study (22). This result was probably due to the small number (14.5%) of the patients who had initially received inappropriate empirical therapy and who then later received the appropriate antibiotics.
In a pediatric study, Grisaru-Soen et al. (14) demonstrated that underlying disease was the only factor correlated with mortality according to multiple regression analysis. In our study, most of the P. aueruginosa bacteremia cases occurred to the patients with underlying disease, but underlying disease was not an independent risk factor (data was not shown). In our study, the presence of respiratory difficulty was the only independent risk factor related to overall fatality.
This study has several limitations. First, it was retrospective and performed at a single health-care center. Therefore, the results are not representative of all pediatric patients in Korea. Second, we did not conduct an analysis of the genotypes of the P. aeruginosa isolates and did not elucidate clustered occurrence of MDR phenotypes in 2003. Third, for the outcome analysis, the formal severity of the illness scores could not be calculated due to the retrospective nature of this study and to the absence of certain data in the medical records.
However, this study has some advantages. Our hospital is a referral institution and a university-affiliated tertiary hospital with relatively many pediatric patients diagnosed with P. aeruginosa bacteremia. In addition, this study included for invasive P. aeruginosa isolates obtained over a consecutive 10 yr.
In conclusion, our study revealed that a previous ICU stay was associated with acquisition of MDR P. aeruginosa and presentation with respiratory difficulty was independent predictors of fatal outcomes among pediatric patients with P. aeruginosa bacteremia. P. aeruginosa bacteremia has become a major concern not only for adults but also for children with underlying disease who are at high risk for health care-associated infection. Continuous monitoring is required for early detection of increment of MDR or PDR-phenotypes among P. aeruginosa isolates and for timely management of patients with probably higher fatality rate. Additionally, future efforts should be concentrated on the prevention and treatment of P. aeruginosa bacteremia in high-risk children.
AUTHOR SUMMARY
Pseudomonas aeruginosa Bacteremia in Children Over Ten Consecutive Years: Analysis of Clinical Characteristics, Risk Factors of Multi-drug Resistance and Clinical Outcomes
Mi Ae Yang, Jina Lee, Eun Hwa Choi, and Hoan Jong Lee
This study aimed to evaluate the clinical profiles, antibiotic susceptibility, risk factors of multi-drug resistance (MDR) and outcomes of P. aeruginosa bacteremia in children. We retrospectively reviewed medical records of pediatric patients with P. aeruginosa bacteremia at a tertiary children's hospital in Seoul, Korea from January 2000 to December 2009. A total of 62 episodes were evaluated. An intensive care unit (ICU) stay within the previous one month was the only independent risk factor for MDR P. aeruginosa bacteremia. The overall fatality rate associated with P. aeruginosa bacteremia was 14.5%. The fatality rate in patients with MDR P. aeruginosa was 57.1%, compared with 9.1% in non-MDR patients. However, the presence of respiratory difficulty was the only independent risk factor for overall fatality associated with P. aeruginosa bacteremia. A previous ICU stay and presentation with respiratory difficulty were associated with acquisition of MDR P. aeruginosa and a higher fatality rate, respectively.
References
- 1.Pier GB, Ramphal R. Pseudomonas aeruginosa. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005. pp. 2587–2615. [Google Scholar]
- 2.Harris A, Torres-Viera C, Venkataraman L, DeGirolami P, Samore M, Carmeli Y. Epidemiology and clinical outcomes of patients with multiresistant Pseudomonas aeruginosa. Clin Infect Dis. 1999;28:1128–1133. doi: 10.1086/514760. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43:1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang H, Sun H, Zhu Y, Chen M. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. J Hosp Infect. 2004;57:112–118. doi: 10.1016/j.jhin.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Kuikka A, Valtonen VV. Factors associated with improved outcome of Pseudomonas aeruginosa bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis. 1998;17:701–708. doi: 10.1007/s100960050164. [DOI] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 7.Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 9.Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med. 2000;160:501–509. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- 10.Lodise TP, Jr, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;51:3510–3515. doi: 10.1128/AAC.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, Kim EC, Choe KW. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37:745–751. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- 13.Fergie JE, Shema SJ, Lott L, Crawford R, Patrick CC. Pseudomonas aeruginosa bacteremia in immunocompromised children: analysis of factors associated with a poor outcome. Clin Infect Dis. 1994;18:390–394. doi: 10.1093/clinids/18.3.390. [DOI] [PubMed] [Google Scholar]
- 14.Grisaru-Soen G, Lerner-Geva L, Keller N, Berger H, Passwell JH, Barzilai A. Pseudomonas aeruginosa bacteremia in children: analysis of trends in prevalence, antibiotic resistance and prognostic factors. Pediatr Infect Dis J. 2000;19:959–963. doi: 10.1097/00006454-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Lin TY, Wang CH. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J. 2002;21:1049–1052. doi: 10.1097/00006454-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Caselli D, Cesaro S, Ziino O, Zanazzo G, Manicone R, Livadiotti S, Cellini M, Frenos S, Milano GM, Cappelli B, Licciardello M, Beretta C, Aricò M, Castagnola E Infection Study Group of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) Multidrug resistant Pseudomonas aeruginosa infection in children undergoing chemotherapy and hematopoietic stem cell transplantation. Haematologica. 2010;95:1612–1615. doi: 10.3324/haematol.2009.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suárez C, Peña C, Tubau F, Gavaldà L, Manzur A, Dominguez MA, Pujol M, Gudiol F, Ariza J. Clinical impact of imipenem-resistant Pseudomonas aeruginosa bloodstream infections. J Infect. 2009;58:285–290. doi: 10.1016/j.jinf.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Valoor HT, Singhi S, Jayashree M. Low-dose hydrocortisone in pediatric septic shock: an exploratory study in a third world setting. Pediatr Crit Care Med. 2009;10:121–125. doi: 10.1097/PCC.0b013e3181936ab3. [DOI] [PubMed] [Google Scholar]
- 19.Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, Kim JH, Kim EC. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002;46:1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. Document M100-S19. [Google Scholar]
- 22.Johnson LE, D'Agata EM, Paterson DL, Clarke L, Qureshi ZA, Potoski BA, Peleg AY. Pseudomonas aeruginosa bacteremia over a 10-year period: multidrug resistance and outcomes in transplant recipients. Transpl Infect Dis. 2009;11:227–234. doi: 10.1111/j.1399-3062.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 23.Gülay Z, Atay T, Amyes SG. Clonal spread of imipenem-resistant Pseudomonas aeruginosa in the intensive care unit of a Turkish hospital. J Chemother. 2001;13:546–554. doi: 10.1179/joc.2001.13.5.546. [DOI] [PubMed] [Google Scholar]
- 24.Aris RM, Gilligan PH, Neuringer IP, Gott KK, Rea J, Yankaskas JR. The effects of panresistant bacteria in cystic fibrosis patients on lung transplant outcome. Am J Respir Crit Care Med. 1997;155:1699–1704. doi: 10.1164/ajrccm.155.5.9154879. [DOI] [PubMed] [Google Scholar]
- 25.Burwen DR, Banerjee SN, Gaynes RP. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. National Nosocomial Infections Surveillance System. J Infect Dis. 1994;170:1622–1625. doi: 10.1093/infdis/170.6.1622. [DOI] [PubMed] [Google Scholar]
- 26.Tacconelli E, Tumbarello M, Bertagnolio S, Citton R, Spanu T, Fadda G, Cauda R. Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: analysis of trends in prevalence and epidemiology. Emerg Infect Dis. 2002;8:220–221. doi: 10.3201/eid0802.010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HY, Yuan M, Ibrahim-Elmagboul IB, Livermore DM. National survey of susceptibility to antimicrobials amongst clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother. 1995;35:521–534. doi: 10.1093/jac/35.4.521. [DOI] [PubMed] [Google Scholar]
- 28.Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47:1681–1688. doi: 10.1128/AAC.47.5.1681-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal F, Mensa J, Almela M, Martinez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156:2121–2126. [PubMed] [Google Scholar]