Abstract
To date, most clinical data on pro-gastrin-releasing peptide (proGRP) have been based on serum concentrations. This study evaluated the agreement between proGRP levels in fresh serum and plasma in patients with various lung diseases. Pairs of serum and EDTA plasma were collected from 49 healthy individuals. At the same time, EDTA plasma of 118 lung cancer patients and 23 patients with benign pulmonary diseases were prospectively collected. Compared to serum, plasma proGRP concentrations were higher by an average of 103.3%. Plasma proGRP was higher in malignancy (336.4 ± 925.4 pg/mL) than in benign conditions (40.1 ± 11.5 pg/mL). Small cell lung cancer (SCLC) patients showed higher levels of proGRP (1,256.3 ± 1,605.6 pg/mL) compared to other types of lung cancer. Based on the ROC curve analyses at a specificity of 95%, the diagnostic sensitivity of plasma proGRP was estimated to be 83.8% in distinguishing SCLC from all the other conditions, and 86.5% for discriminating SCLC from the nonmalignant cases. Among the SCLC cases, limited stage disease had lower levels of plasma proGRP than extensive disease. When measuring circulating levels of proGRP, the use of plasma is preferred over serum. Plasma proGRP has a potential marker for discriminating SCLC from nonmalignant conditions or non-small cell lung cancer.
Keywords: pro-gastrin-releasing peptide (31-98), Serum, Plasma, Small Cell Lung Carcinoma
INTRODUCTION
Lung cancer is the most common fatal cancer worldwide and has become the leading cause of cancer deaths in Korea. The number of new cases will continue to rise. The risk of human cancer can be associated with environmental, occupational, and recreational exposures to carcinogens (1). Pro-gastrin-releasing peptide (proGRP, residues 31-98) is a precursor of a neuropeptide hormone named gastrin-releasing peptide (GRP), and is frequently produced by small cell lung cancer (SCLC) cells (2, 3). Circulating proGRP levels have been shown to serve as a reliable marker in SCLC patients (4-6). ProGRP has been reported to be the most sensitive marker for discriminating SCLC from benign diseases of the lung (7). If measured with serum neuron-specific enolase at the same time, proGRP is known to provide additive information on pathological characteristics of lung cancer (5, 8-10). Moreover, proGRP was found to be useful in the monitoring of response to therapy and for the detection of recurrent SCLC (8, 10, 11).
ProGRP is significantly more stable in blood than GRP. However, the stability of ProGRP in serum is worse than the stability of other widely used serum cancer markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) (12, 13). The relative instability of ProGRP in serum is believed to be caused by thrombin, which is generated during the clotting process (14). By using EDTA-anticoagulated plasma specimens, the stability of proGRP can be improved especially at room temperature. However, there are different views whether proGRP concentrations of fresh serum differ from that of fresh EDTA plasma, and which matrix is preferred (14, 15). Moreover, there is little clinical information currently available about the use of plasma proGRP in patient populations, since major clinical investigations on proGRP have used almost exclusively serum specimens (2, 6, 9, 16).
The objectives of this study were to evaluate the difference of proGRP concentrations in serum and plasma using fresh specimens, and to investigate plasma proGRP concentrations in benign and malignant conditions including SCLC.
MATERIALS AND METHODS
Samples
Pairs of serum and EDTA plasma specimens from each of the healthy 27 males and 22 females were collected. The ages of the healthy individuals ranged from 45 to 83 yr with a mean of 62.1 yr. The analyses of the specimens were completed within 4 hr from the time the samples were collected.
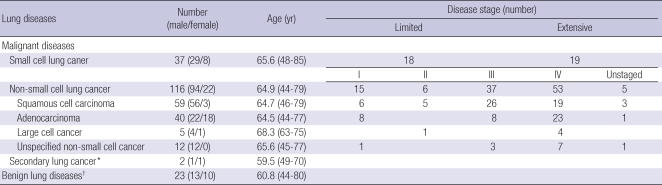
During a 9 month period starting from January 2009, EDTA plasma specimens were obtained in a consecutive manner from patients who were newly diagnosed with lung diseases during their first visit to a pulmonology clinic at a tertiary teaching hospital (n = 178). Ages ranged from 39 to 97 yr with a mean of 65.2 yr. Among the patients, 137 (77.0%) were male. From each patient, an EDTA plasma specimen was separated by immediate centrifugation and was stored at -20℃ until the proGRP assay was performed. Within the patient population, l55 were later diagnosed with lung cancer and 23 were diagnosed with benign pulmonary diseases.
All cases of lung cancer were diagnosed histologically and staged with the TNM system. Of the non-small cell lung cancer (NSCLC) patients, 16.4% were in stage I or II, 32.7% were in stage III, and 50.9% were in stage IV. Of the SCLC patients, 48.4% were in limited stage and 51.6% were in extensive stage (Table 1).
Table 1.
Clinical characteristics of the patients with lung diseases
*One case was metastasized from rectal cancer and the other was from esophageal cancer; †Specific diagnoses were pulmonary tuberculosis (n = 7), pneumonia (n = 5), mycobacterial infection other than tuberculosis (n = 2), a solitary benign nodule (n = 2), nontuberculous granuloma (n = 2), a case each of parasitic infection, paraganglioma, hamartoma, eosinophilic infiltration and idiopathic pulmonary fibrosis.
Measurement of the tumor marker concentrations
All blood collection tubes were from BD Vacutainer (BD Vacutainer Systems, Plymouth, UK). For all the serum and plasma specimens, proGRP concentrations were measured using a fully automated immunoassay, the ARCHITECT ProGRP (Abbott Laboratories, Wiesbaden, Germany) assay. The ARCHITECT ProGRP assay was a two-step chemiluminescent immunoassay. In the first step, sample, assay diluent, and anti-proGRP coated paramagnetic microparticles were combined. ProGRP present in the sample binded to the anti-proGRP coated microparticles. After washing, anti-proGRP acridinium-labeled conjugate was added to create a reaction mixture in the second step. Following another wash, pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units. A direct relationship existed between the amount of proGRP in the sample and the relative light units detected.
Concentrations of squamous cell carcinoma antigen (SCC) and neuron-specific enolase (NSE) were compared between serum and plasma obtained from the healthy individuals. The ARCHITECT SCC reagent and the Modular NSE reagent (Roche Diagnostics GmbH, Mannheim, Germany) were employed for measuring the respective analyte. The ARCHITECT SCC assay was a two-step chemiluminescent immunoassay. In the first step, sample and anti-SCC coated paramagnetic microparticles were combined. SCC present in the sample binded to the anti-SCC coated microparticles. After washing, anti-SCC acridinium-labeled conjugate was added to create a reaction mixture in the second step. The later part of the procedure was identical to that of the ARCHITECT ProGRP assay. The Modular NSE assay was a chemiluminescent immunoassay. At first, NSE present in the sample, biotinylated anti-NSE and anti-NSE labeled with ruthenium complex form a sandwich complex. After addition of streptavidin coated microparticles, the complex became bound to the solid phase via interaction of biotin and streptavidin. In the measuring cell, microparticles were magnetically onto the surface of the electrode. Application of a voltage to the electrode then induced chemiluminescent emission which was measured by a photomultiplier. Results were determined via a calibration curve.
According to the manufacturers, both serum and plasma could be used for measuring SCC concentrations, whereas plasma specimens were not allowed to be used with the Modular NSE reagent.
Statistical analysis
Statistical analyses were conducted using MedCalc 9.5.2 (Med-Calc Software, Mariakerke, Belgium) with the significance level of 0.05. The median and range of proGRP, SCC and NSE were calculated for the healthy individual's serum and plasma. The median and range of plasma proGRP were calculated separately for the healthy individuals, benign lung diseases and lung cancer. These values were also calculated by histology and stage of the lung cancer cases. The Wilcoxon-rank sum test or Kruskal-Wallis test was used to determine if there was a statistically significant difference in the median values of the markers among groups. The diagnostic accuracy of proGRP was assessed by plotting receiver operating characteristic (ROC) curves and estimating the area under the curve (AUC) in discriminating SCLC from other conditions. As no definitive diagnostic threshold for proGRP has been reported in the Korean population so far, this was calculated by picking the point of the best diagnostic performance on the ROC curve. Test for difference between AUCs was done using DeLong's test for two correlated ROC curve.
Ethics statement
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (approved number 2008-75). Informed consent was acquired from all subjects.
RESULTS
Comparison between serum and plasma level
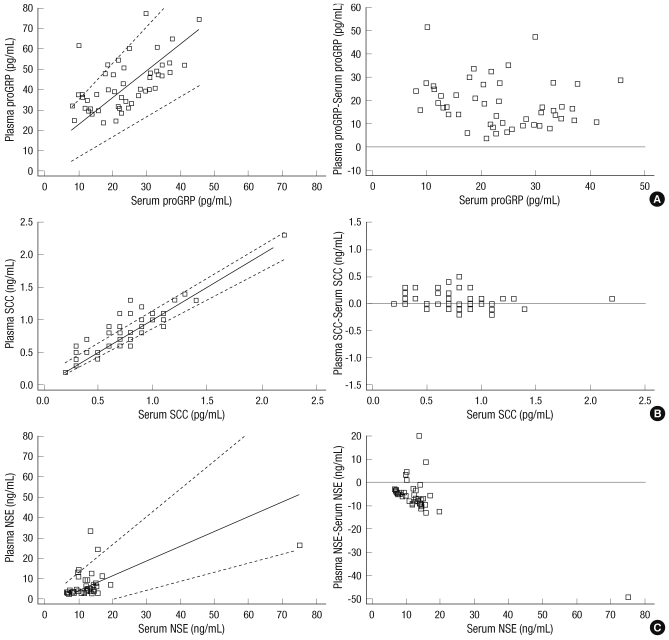
Plasma proGRP was only moderately correlated with serum proGRP (r = 0.583, P < 0.01). On average, compared to the serum proGRP levels, the plasma proGRP levels were higher by 103.3%. In the same samples, plasma SCC was higher than the serum SCC level by 15.3%. The serum SCC concentrations were significantly better correlated with the plasma SCC (r = 0.905, P < 0.001) than the serum proGRP was with the plasma proGRP. One healthy individual was excluded from the measurement of NSE due to insufficient sample volume. The correlation coefficient was calculated to be 0.533 between serum and plasma NSE. The correlation coefficients between serum and plasma NSE was not significantly different from that of serum and plasma proGRP (P = 0.729). Contrary to the other 2 markers, NSE was lower in plasma than in serum, and the difference was 46.2%. Results of the comparison of the tumor markers between serum and plasma are depicted in Fig. 1.
Fig. 1.
Comparison of concentrations of the 3 tumor markers between fresh serum and plasma using Passing-Bablok regression and difference plots in healthy individuals. (A) pro-gastrin-releasing peptide (proGRP): slope, 1.32; intercept, 9.62; mean difference, + 18.8. (B) squamous cell cancer antigen (SCC): slope, 1.00; intercept, 0.00; mean difference, + 0.1. (C) neuron- specific enolase (NSE): slope, 0.72; intercept, -2.99; mean difference, -5.9.
Plasma proGRP in various clinical conditions
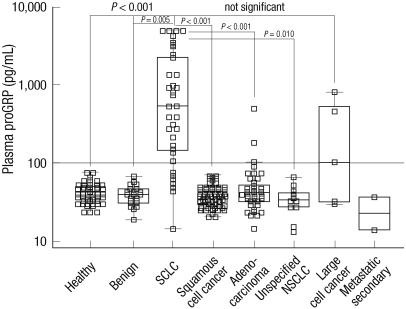
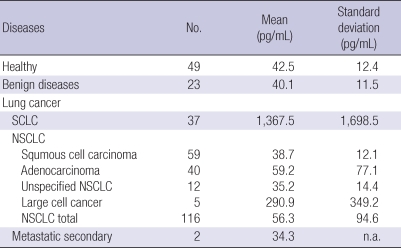
At the time of diagnosis, the average plasma proGRP concentration of the cancer patients was 336.4 pg/mL and the standard deviation was 925.4 pg/mL. Compared to the benign lung diseases (mean, 40.1 pg/mL; standard deviation, 11.5 pg/mL), the plasma proGRP level was significantly higher in the lung cancer patients. Once the cancer patients were grouped by their histological type, the SCLC patients showed the highest level of proGRP with a mean of 1,357.5 pg/mL and a standard deviation of 1,698.5 pg/mL. Large cell lung cancer cases, which along with SCLC is thought to originate from neuroendocrine cells, followed next showing mean plasma proGRP of 290.9 pg/mL and standard deviation of 349.2 pg/mL. Compared to the cases of SCLC and large cell, patients diagnosed with adenocarcinoma of the lung, squamous cell carcinoma or unspecified NSCLC showed significantly lower levels of proGRP (P < 0.001, Fig. 2, Table 2).
Fig. 2.
Distribution of plasma proGRP concentrations in healthy individuals, benign lung diseases and lung cancer. The average proGRP level in small cell lung cancer was higher than those of other conditions except large cell lung cancer which is thought to be originated from the neuroendocrine cells. SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
Table 2.
Plasma proGRP in healthy individuals, benign lung diseases and lung cancer patients
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; n.a., not available.
Cancer patients with a distant metastasis showed higher levels of plasma proGRP (mean, 5,159.0 pg/mL; standard deviation, 1,247.1 pg/mL) than the patients without metastasis (mean, 159.6 pg/mL; standard deviation, 404.0 pg/mL, P < 0.05). The SCLC patients with an extensive disease had higher proGRP (mean, 1,940.1 pg/mL; standard deviation, 1,947.7 pg/mL) than the cases with limited disease (mean, 533.0 pg/mL; standard deviation, 759.6 pg/mL, P < 0.05).
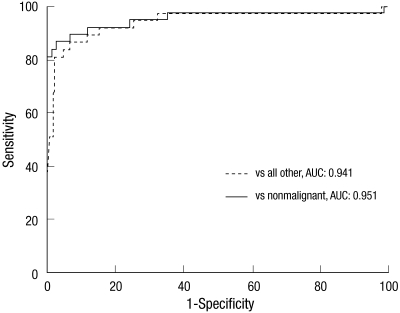
Based on the ROC curve analyses, at specificity of 95%, the diagnostic sensitivity of plasma proGRP was estimated to be 83.8% in distinguishing SCLC from all the other conditions, and 86.5% for discriminating SCLC from the nonmalignant cases (Fig. 3).
Fig. 3.
Accuracy of plasma proGRP for detecting small cell lung cancer at the time of diagnosis using ROC curve analysis. The AUCs of the 2 curves were not significantly different. ROC, Receiver Operating Characteristics; AUC, area under curve.
DISCUSSION
We investigated the difference of proGRP concentrations in fresh serum and plasma. In addition, plasma proGRP levels in various benign and malignant lung diseases were compared. Until recently, serum was the single recommended sample type of proGRP assays. However, poor stability of serum proGRP has been a challenging issue (12, 13). According to Yoshimura et al. (14), average plasma proGRP concentration was higher than serum by 26% even when tested at a fresh state (14). However, another investigation showed there was no difference observed between serum and plasma from SCLC patients when assayed immediately after collection (15). In this study, we found each pair of plasma and serum from a same healthy individual showed different proGRP concentration by 103.3% in average, although the slope of serum versus plasma was 1.32, Considering there still has to be preanalytical steps requiring various period of time, the exceptional vulnerability of serum proGRP resulted in the seemingly conflicting outcomes from different study settings. Also, the low levels of proGRP in healthy individuals seemed to contribute to the more pronounced proportional difference between serum and plasma in our study.
A recently introduced automated chemiluminescent immunoassay, ARCHITECT ProGRP, recommends plasma as a preferred specimen. On the contrary, Nordlund et al. (15), preferred fresh serum to EDTA plasma, partly because the plasma proGRP can be increased by the release of proGRP from thrombocytes or leukocytes (15). However, if plasma is separated from the cellular component by simple centrifugation in a timely manner, which is quite familiar to most clinical laboratories, spurious increase of proGRP in stored plasma specimens may be avoided. Therefore, plasma is deemed to be the specimen of choice for measuring circulating proGRP concentration, given the undesirably lowered serum proGRP observed in this study.
Interestingly, serum and plasma proGRP showed a correlation (r = 0.583) significantly lower than that of SCC but closer to that of NSE, in spite of the manufacturer's option to use either type of specimen. Based on the correlation result, the fact that some commercially available proGRP assay kits only accepts serum proves that additional revision in them is required.
As indicated in the previous investigations, circulating proGRP levels were significantly higher in the SCLC patients compared to the nonmalignant cases or the other types of cancer. However, plasma proGRP of the SCLC patients was not statistically significantly different compared to large cell lung cancer patients. This is partly because only a small number of large cell lung cancer patients were included in this study. However, it is also possible that such a lack of difference was caused by the common neuroendocrine origin of both types of cancer. Among NSCLC, large cell lung cancer has been known to be closer to SCLC rather than to other types of NSCLC in terms of pathological characteristics.
In discriminating SCLC from NSCLC, plasma proGRP showed a sensitivity of 51.4% at a specificity of 99%. Against all the benign lung diseases, the diagnostic sensitivity of plasma proGRP for detecting SCLC was estimated to be 86.5% at specificity of 99%. Compared to a previous study (sensitivity 46% and specificity 96% in the diagnosis of SCLC), the diagnostic performance of proGRP revealed in this study was significantly enhanced (5, 17). We attributed the improved performance of proGRP to the use of plasma instead of serum, contrary to the previous clinical trials mainly incorporating serum specimens. In addition, the diagnostic performance of plasma proGRP for discriminating SCLC looked better than other commonly available tumor markers (18).
In conclusions, plasma is preferred to serum for measuring a circulating level of proGRP. Plasma proGRP concentration measured by the two-step chemiluminescent immunoassay was sensitive and specific for discriminating SCLC from nonmalignant conditions or NSCLC.
ACKNOWLEDGMENTS
The authors thank Abbott Diagnostics for providing the reagent kits used in this study.
Footnotes
This work (No. R01-2008-000-10620-0) was supported by Midcareer Researcher Program through NRF grant funded by the MEST, Korea. All authors have no conflict of interest to declare for this study.
AUTHOR SUMMARY
Plasma proGRP Concentration is Sensitive and Specific for Discriminating Small Cell Lung Cancer from Nonmalignant Conditions or Non-small Cell Lung Cancer
Hye-Ran Kim, In-Jae Oh, Myung-Geun Shin, Joon-Seok Park, Hyun-Jung Choi, Hee-Jung Ban, Kyu-Sik Kim, Young-Chul Kim, Jong-Hee Shin, Dong-Wook Ryang, and Soon-Pal Suh
We compared serum and plasma concentrations of proGRP using fresh specimens, and evaluated the plasma proGRP concentrations in benign and malignant conditions including small cell lung carcinoma (SCLC). The plasma concentration is superior to serum level for measuring the circulating proGRP. Plasma proGRP concentration measured by the two-step chemiluminescent immunoassay was revealed to be a sensitive and specific parameter for discriminating SCLC from nonmalignant conditions or NSCLC.
References
- 1.Paik KH, Park YH, Ryoo BY, Yang SH, Lee JC, Kim CH, Ki SS, Kim JM, Park MJ, Ahn HJ, Choi W, Chung JH. Prognostic value of immunohistochemical staining of p53, bcl-2, and Ki-67 in small cell lung cancer. J Korean Med Sci. 2006;21:35–39. doi: 10.3346/jkms.2006.21.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide (31-98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res. 1994;54:2136–2140. [PubMed] [Google Scholar]
- 3.Yamaguchi K, Abe K, Kameya T, Adachi I, Taguchi S, Otsubo K, Yanaihara N. Production and molecular size heterogeneity of immunoreactive gastrin-releasing peptide in fetal and adult lungs and primary lung tumors. Cancer Res. 1983;43:3932–3939. [PubMed] [Google Scholar]
- 4.Takada M, Kusunoki Y, Masuda N, Matui K, Yana T, Ushijima S, Iida K, Tamura K, Komiya T, Kawase I, Kikui N, Morino H, Fukuoka M. Pro-gastrin-releasing peptide (31-98) as a tumour marker of small-cell lung cancer: comparative evaluation with neuron-specific enolase. Br J Cancer. 1996;73:1227–1232. doi: 10.1038/bjc.1996.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina R, Filella X, Augé JM. ProGRP: a new biomarker for small cell lung cancer. Clin Biochem. 2004;37:505–511. doi: 10.1016/j.clinbiochem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Stieber P, Dienemann H, Schalhorn A, Schmitt UM, Reinmiedl J, Hofmann K, Yamaguchi K. Pro-gastrin-releasing peptide (ProGRP)--a useful marker in small cell lung carcinomas. Anticancer Res. 1999;19:2673–2678. [PubMed] [Google Scholar]
- 7.Molina R, Auge JM, Alicarte J, Filella X, Viñolas N, Ballesta AM. Pro-gastrin-releasing peptide in patients with benign and malignant diseases. Tumour Biol. 2004;25:56–61. doi: 10.1159/000077724. [DOI] [PubMed] [Google Scholar]
- 8.Niho S, Nishiwaki Y, Goto K, Ohmatsu H, Matsumoto T, Hojo F, Ohe Y, Kakinuma R, Kodama T. Significance of serum pro-gastrin-releasing peptide as a predictor of relapse of small cell lung cancer: comparative evaluation with neuron-specific enolase and carcinoembryonic antigen. Lung Cancer. 2000;27:159–167. doi: 10.1016/s0169-5002(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Aoyagi K, Urakami K, Fukutani T, Maki N, Yamamoto S, Otsubo K, Miyake Y, Kodama T. Enzyme-linked immunosorbent assay of pro-gastrin-releasing peptide for small cell lung cancer patients in comparison with neuron-specific enolase measurement. Jpn J Cancer Res. 1995;86:698–705. doi: 10.1111/j.1349-7006.1995.tb02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunaga N, Tsuchiya S, Minato K, Watanabe S, Fueki N, Hoshino H, Makimoto T, Ishihara S, Saito R, Mori M. Serum pro-gastrin-releasing peptide is a useful marker for treatment monitoring and survival in small-cell lung cancer. Oncology. 1999;57:143–148. doi: 10.1159/000012022. [DOI] [PubMed] [Google Scholar]
- 11.Okusaka T, Eguchi K, Kasai T, Kurata T, Yamamoto N, Ohe Y, Tamura T, Shinkai T, Saijo N. Serum levels of pro-gastrin-releasing peptide for follow-up of patients with small cell lung cancer. Clin Cancer Res. 1997;3:123–127. [PubMed] [Google Scholar]
- 12.Kuwabara M, Shibata N, Ariyoshi Y. Lung cancer: progress in diagnosis and treatment. I. Diagnosis and physiopathology: 4. Progress in the study of tumor markers. Nippon Naika Gakkai Zasshi. 1997;86:20–26. [PubMed] [Google Scholar]
- 13.Banfi G, Parma P, Pontillo M. Stability of tumor markers CA 19.9, CA 125, and CA 15.3 in serum obtained from plain tubes and tubes containing thixotropic gel separator. Clin Chem. 1997;43:2430–2431. [PubMed] [Google Scholar]
- 14.Yoshimura T, Fujita K, Kawakami S, Takeda K, Chan S, Beligere G, Dowell B. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol. 2008;29:224–230. doi: 10.1159/000152940. [DOI] [PubMed] [Google Scholar]
- 15.Nordlund MS, Bjerner J, Warren DJ, Nustad K, Paus E. Progastrin-releasing peptide: stability in plasma/serum and upper reference limit. Tumour Biol. 2008;29:204–210. doi: 10.1159/000148188. [DOI] [PubMed] [Google Scholar]
- 16.Aoyagi K, Miyake Y, Urakami K, Kashiwakuma T, Hasegawa A, Kodama T, Yamaguchi K. Enzyme immunoassay of immunoreactive progastrin-releasing peptide (31-98) as tumor marker for small-cell lung carcinoma: development and evaluation. Clin Chem. 1995;41:537–543. [PubMed] [Google Scholar]
- 17.Gruber C, Hatz R, Reinmiedl J, Nagel D, Stieber P. CEA, cyfra 21-1, NSE, and proGRP in the diagnosis of lung cancer: a multivariate approach. J Lab Med. 2008;32:361–371. [Google Scholar]
- 18.Nisman B, Biran H, Ramu N, Heching N, Barak V, Peretz T. The diagnostic and prognostic value of ProGRP in lung cancer. Anticancer Res. 2009;29:4827–4832. [PubMed] [Google Scholar]