Abstract
Activation of innate immunity via Toll-like receptors (TLRs) is associated with neurodegenerative diseases, and some effectors, like tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6), directly contribute to neurodegeneration. We tested the hypothesis that prostaglandin (PG) E2 receptor subtype 1 (EP1) was necessary for induction of microglial cytokines following activation of innate immunity. Primary murine microglia had cytokine secretion by activators of TLR3 > TLR9 >TLR4 > TLR2. TLR3 activation induced early expression of cyclooxygenase 2 (COX2) and delayed expression of membranous PGE synthase and secretion of PGE2. Non-selective and COX2-selective inhibitors blocked TLR3 induction of TNFα and IL-6. Moreover, of the eight out of twenty cytokines and chemokines induced by TLR3 activation, only TNFα and IL-6 were significantly dependent on EP1 signaling as determined using microglia from mice homozygous deficient for EP1 gene or wild type (WT) microglia co-incubated with an EP1 antagonist. These results were confirmed by blocking intracellular Ca2+ release with 2-aminoethoxy-diphenyl borate (2-APB) or Xestospongin C (XC), inhibitors of IP3 receptors. Our results show that suppression of microglial EP1 signaling achieves much of the desired effect of COX inhibitors by selectively blocking TLR3-induced microglial secretion of two major effectors of paracrine neuron damage. In combination with the ability of EP1 suppression to ameliorate excitotoxicity, these data point to blockade of EP1 as an attractive candidate therapeutic for neurodegenerative diseases.
Keywords: E Prostanoid Receptor 1 (EP1), TLR3, microglia, innate immunity, cytokine, calcium
INTRODUCTION
Toll-like receptors (TLRs) include a family of membrane glycoproteins that recognize exogenous structures in microorganisms and endogenous structures produced from tissue injury or disease (Takeuchi and Akira 2010). Activated TLRs coordinate gene transcription to initiate innate immune responses that, depending on the complement of receptors, intensity, duration, and repetition, may have beneficial or deleterious effects (reviewed in (Rivest 2009). Microglia express functional TLR2, TLR3, TLR4, and TLR9. Models of brain inflammation, ischemia, or Alzheimer’s disease (AD) demonstrate neuron damage following activation of microglial TLR2, TLR3, and TLR4 (Abe et al. 2010; Babcock et al. 2006; Farez et al. 2009; Field et al. 2010; Jana et al. 2008; Jin et al. 2007; Jin et al. 2008; Koedel et al. 2007; Qin et al. 2007; Reed-Geaghan et al. 2009; Sloane et al. 2010; Stewart et al. 2010), while some data support a protective role for microglial TLR9 activation (Scholtzova et al. 2009). Intriguingly, since mRNA is an endogenous ligand for TLR3 (Kariko et al. 2004), ischemia, traumatic injury, and neurodegenerative diseases including AD, Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) that accumulate RNA in pathologic lesions (Ginsberg et al. 1998), have the potential to activate this receptor.
Modulation of TLR-initiated innate immune responses is a potential therapeutic target for several neurologic diseases. Signaling following TLR 2-, 3-, or 4-initiated innate immune responses involves the prostaglandin (PG) pathway (Chang et al. 2004; Kalvegren et al. 2010; Pindado et al. 2007). Cyclooxygenase (COX) isozymes catalyze the first step in production of PGs and thromboxane A2. Large epidemiologic studies have repeatedly observed that non-steroidal anti-inflammatory drugs (NSAIDs), which act primarily in vivo by blocking activity of COX isozymes, may be effective in preventing AD [reviewed in (Szekely et al. 2007) and more recently (Vlad et al. 2008) where ibuprofen was most effective] or PD (Chen et al. 2003). Moreover, non-selective COX inhibitors, COX2-selective inhibitors, or genetic ablation of COX2 are fully or partially neuroprotective in animal models of AD (Lim et al. 2000; Lim et al. 2001; Morihara et al. 2005), PD (Aubin et al. 1998; Feng et al. 2002; Kurkowska-Jastrzebska et al. 2002; Reksidler et al. 2007; Teismann and Ferger 2001; Teismann et al. 2003), or ALS (Drachman et al. 2002; Drachman and Rothstein 2000). Disappointingly, clinical trials aimed at treating patients with AD or ALS with NSAIDs have largely failed (Aisen et al. 2003; Cudkowicz et al. 2006; Thal et al. 2005); we are unaware of a trial for PD. In contrast, a trial aimed at preventing AD in older volunteers was prematurely suspended because of unexpected increase in “thrombotic” events in treatment groups (ADAPT Research Group 2007), likely because of altered balance of PGI2 and TxA2 production (Montine et al. 2010).
Despite this setback, NSAID toxicity does not negate experimental, clinical, and epidemiologic data that underscore suppression of the PG pathway as a potential means to prevent common neurodegenerative diseases. Indeed, several groups are focused on specific PG receptors in the hope of maintaining therapeutic effect while averting toxicity. Since PGE2 levels are increased in AD, PD, ALS, and their animal models (Combrinck et al. 2006; Hoshino et al. 2007; Liang et al. 2005; Mattammal et al. 1995; Montine et al. 1999; Teismann et al. 2003), we and others have focused on PGE2 receptor subtypes, called E prostanoid (EP) receptors 1 through 4, which are linked to functionally antagonistic second messenger systems (Hata and Breyer 2004). EP1, EP2, and EP3 are expressed by microglia and most neurons (Cimino et al. 2008). Recently, genetic ablation of EP1 (EP1−/−) rescued mouse brain in a model of transient focal ischemia, at least in part from amelioration of excitotoxicity (Kawano et al. 2006). We have observed that EP1−/− microglia have altered response to LPS activation (Keene et al. 2009). Here we tested the hypothesis that microglial EP1 may be a target for modulating TLR-induced innate immune response in brain.
METHODS
Reagents and materials
DMEM/F12 medium and heat-inactivated fetal bovine serum (FBS) were purchased from Hyclone Laboratories (Logan, UT). G5 supplement was from Invitrogen (Carlsbad, CA). Ibuprofen, SC-51089 and NS-398 were from Cayman Chemical Company (Ann Arbor, MI). 2-aminoethoxy-diphenyl borate (2-APB) was from Tocris Bioscience (Ellisville, MO). Xestospongin C (XC) was from Tocris Bioscience (Ellisville, MO). Lipopolysaccharide (LPS) was from Calbiochem (La Jolla, CA). Double-stranded polyinosinic-polycytidylic acid (PIC) was from Sigma-Aldrich (St. Louis, MO). Pam3 CSK4 (Pam3) and CpG were from Invivogen (San Diego, CA). Papain and DNase I were from Worthington Biochemical (Lakewood, NJ).
Animals
C57BL/6 mice were from Jackson Laboratories (Bar Harbor, ME). EP1−/− mice on the C57BL/6 background were generated as described previously (Guan et al. 2007). The University of Washington IACUC approved all procedures. The animals were maintained in a specific pathogen-free environment.
Cell culture
Primary microglia were prepared as described previously (Keene et al. 2009; Shie et al. 2005) and used at a density of 78,125 cells/cm2 (25,000 cells/well of 96-well plate). Cerebral cortex was obtained from postnatal(P1–3) C57BL/6 mice and remaining meninges were removed in ice-cold Dulbecco’s Phosphate Buffered Saline. Cortex was incubated for 30 minutes at 37°C in DMEM/F12 medium containing 15U/ml papain, 0.5 mmol/L EDTA, 0.2 mg/ml L-cysteine, and 200 μg/ml DNase I, sedimented at 1500 rpm for 5 min, and the pellet was triturated with warm culture medium(DMEM/F12, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin). Cell suspension was plated on poly-ornithine coated flasks in culture medium. At 11–15 days, microglia were harvested from the underlying astrocytic monolayer by gentle agitated. Purity of microglia was determined by CD11b staining and was greater than 98%.
Reverse transcription PCR (RT-PCR) and quantitative real-time PCR
Total RNA was isolated from cells using RNeasy kit from Qiagen (Chatsworth, CA). Contaminating genomic DNA was digested by RNase-free DNase I (Qiagen). 1 μg of total RNA was used for cDNA synthesis by using Omniscript Reverse Transcriptase from Qiagen. Expression levels of COX1, COX2, membranous PGE synthase (mPGEs) and cytosolic PGE synthase (cPGEs) were determined by RT-PCR with thirty cycles. GAPDH expression levels were used as loading controls. Primer sequences for RT-PCR are listed in Table 1. Expression levels of IL-6 and TNFα were determined by quantitative real-time PCR using ABI 7900 HT with TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, CA). Primers and probes (Mm00446190_m1 for IL-6 and Mm00443258_m1 for TNFα) were from Applied Biosystems. Quantification of gene expression was calculated by the standard curve method and normalized to 18S rRNA.
Table 1.
Sequences of primers used for RT-PCR.
| Gene | Sense primer (5′-3′) | Antisense primer (5′-3′) |
|---|---|---|
| COX1 | CCTCACCAGTCAATCCCTGT | CCCAGAGCCAGTATCCATGT |
| COX2 | GCTGTACAAGCAGTGGCAAA | CCCCAAAGATAGCATCTGGA |
| mPGEs | GGCCTTTCTGCTCTGCAGCA | GGAGAACTGGGCCAGGACAT |
| cPGEs | TTGGAAAGACTGGGAGGATG | AAAATCCAGGCGATGACAAC |
| GAPDH | GACAAAATGGTGAAGGTCGGTG | TGATGTTAGTGGGGTCTCGCTC |
Cytokine assays
Microglia were seeded in 96-well plates. One day later, cells were exposed to reagents in serum-free medium with G5 supplement. In some experiments, microglia were co-incubated with SC 51089 (60 μM), 2-APB (20 μM), or XC (20 μM) for 18 hs. There was no toxicity to primary microglia under any of these conditions as determined by cell count or protein levels. Conditioned media were collected and stored at −80°C for cytokine measurements. Protein concentrations of IL-6 and TNFα were determined by ELISA kits (R & D Systems, Minneapolis, MN). Protein concentrations of 20 mice cytokines/chemokines were measured by using Mouse Cytokine 20-Plex Panel kit (Invitrogen). All assays were performed according to the manufacturers’ instructions.
PGE2 assay
One day following initial seeding, microglia were treated with 20 μg/ml PIC in the presence or absence of 500 μM Ibuprofen for various times. Conditioned media were collected for PGE2 measurement by using ELISA kit provided by Cayman Chemical Company.
Statistical analyses were performed using GraphPad Prism 5 (San Diego, CA).
RESULTS
TLR-specific activation in wt murine primary microglia
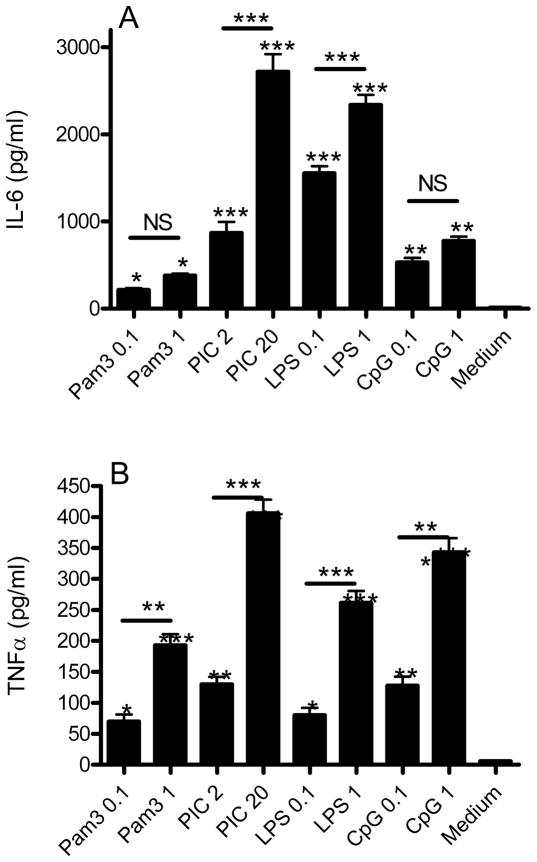
Our first series of experiments explored the activation of the TLRs known to be expressed functionally by microglia (Block et al. 2007) by ligands that have relative specificity at the concentrations used (Iliev et al. 2004; Jack et al. 2005; Olson and Miller 2004; Park et al. 2006; Ribes et al. 2009; Shah et al. 2009). These included Pam3 for TLR2 (0.1 and 1 μg/ml) PIC for TLR3 (2 and 20 μg/ml), LPS for TLR4 (0.1 and 1 μg/ml), and CpG for TLR9 (0.1 and 1 μM). We assessed microglial activation by quantifying secreted IL-6 (Figure 1A) and TNFα (Figure 1B) after 18 hr incubation. All TLR activators significantly increased IL-6 and TNFα medium concentrations, and all showed a significant concentration-response relationship for TNFα while only PIC and LPS had a significant concentration-response for IL-6 secretion. Activation of TLR3 by PIC yielded the greatest increase in both analytes, followed by LPS, and then Pam3 and CpG. It is important not to over-interpret these data since we are using usual but limited concentrations of TLR activators; however, higher levels might compromise specificity of TLR activation. Thus, under these conditions of commonly used concentrations of relatively specific activators of TLR2, TLR3, TLR4, or TLR9, activation of TLR3 by PIC yielded the greatest increase in IL-6 and TNFα secretion by murine primary microglia.
Figure 1.
WT murine primary microglia were incubated with medium or with two different concentrations of activators specific for TLR2 (Pam3), TLR3 (PIC), TLR4 (LPS), or TLR9 (CpG) for 18 hr and then medium concentrations (pg/ml) of IL-6 (A) and TNFα (B) quantified. Data are average concentration ± SEM (n=3). ANOVA for both analytes had P < 0.0001 and Bonferroni-corrected paired comparisons for each condition with medium only noted above the column. Horizontal lines indicate Bonferroni-corrected paired comparisons between the two concentrations of each TLR activator (*P < 0.05, **P < 0.01, ***P<0.001)
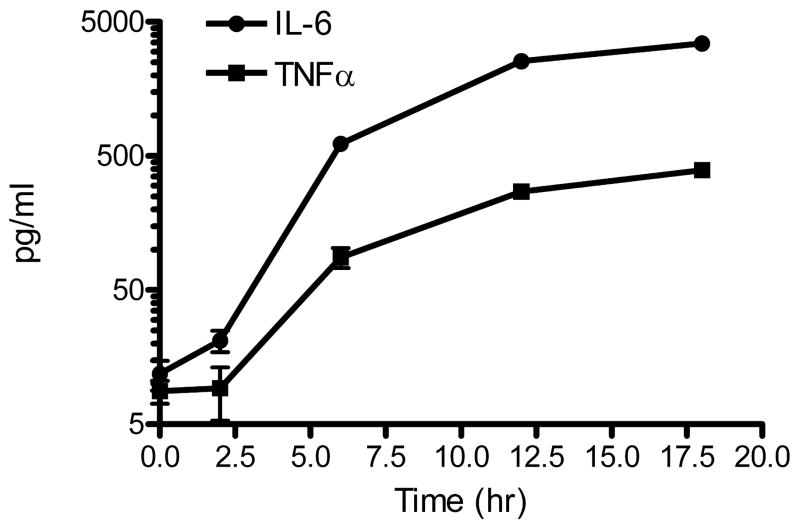
We focused further study on TLR3 activation by 20 μg/ml PIC. Figure 2 presents the time courses for IL-6 and TNFα secretion by wt murine primary microglia and demonstrates their logarithmic rise from 2 to 18 hr in the presence of PIC. We next sought to determine the extent of wt primary microglial activation by PIC by assaying medium using a cytokine/chemokine multiplex assay. We analyzed medium from wt microglia cultures using a mouse 20-plex Luminex array (FGF-basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, TNFα and VEGF) and determined that eight cytokines or chemokines were significantly induced by PIC at 6 hr or 18 hr (Table 2). In aggregate, these results demonstrated concentration- and time-dependent effects of TLR3 activation on a subset of cytokines and chemokines in wt murine primary microglia and confirmed our time course for IL-6 and TNFα secretion following incubation with PIC with a different set of reagents.
Figure 2.
WT murine primary microglia were incubated with 20 μg/ml PIC and medium concentrations of IL-6 and TNFα were determined at the times indicated. Data are the average concentration ± SEM (n=3, error bars are smaller than most symbols). ANOVA for each analyte vs. time had P < 0.0001 with Bonferroni-corrected paired comparisons had P < 0.01 for each time point with all others for that analyte except for 0 vs. 2 hr for IL-6 and TNFα.
Table 2.
WT murine primary microglia were incubated with 20 μg/ml PIC and medium analyzed at 6 and 18 hr using a 20-plex array for FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, IP-10, KC, MCP-1, MIG, MIP-1α, TNFα and VEGF. Of these, only the eight listed were significantly (P < 0.05) induced by PIC. Data are average ± SEM pg/ml for three duplicate determinations. Average coefficient of variance (CV) for these eight analytes were FGF basic (12.1%), IL-1α (15.7%), IL-5 (14.8%), IL-6 (17.9%), IL-12 (10.1%), IP-10 (13.9%), MCP-1 (11.3%), MIP-1α (8.4%), TNFα (14.2%).
| pg/ml at 6 hr | pg/ml at 18 hr | |||
|---|---|---|---|---|
| Medium | 20 mg/ml PIC | Medium | 20 mg/ml PIC | |
| FGF basic | 323 ± 50 | 428 ± 15 | 261 ± 49 | 250 ± 7 |
| IL-1α | 19 ± 5 | 56 ± 5 | 23 ± 3 | 58 ± 7 |
| IL-5 | 17 ± 6 | 81 ± 13 | 38 ± 9 | 207 ± 6 |
| IL-6 | 73 ± 13 | 387 ± 61 | 48 ± 3 | 1242 ± 168 |
| IL-12 | 15 ± 3 | 46 ± 7 | 17 ± 2 | 134 ± 16 |
| IP-10 | 26 ± 9 | 1634 ± 153 | 33 ± 15 | 1624 ± 195 |
| MCP-1 | 25 ± 8 | 497 ± 88 | 92 ± 24 | 1670 ± 126 |
| MIP-1α | 295 ± 16 | 5567 ± 1027 | 285 ± 21 | 27246 ± 1214 |
| TNFα | 49 ± 12 | 1599 ± 256 | 67 ± 12 | 2463 ± 409 |
Induction of the prostaglandin pathway by PIC
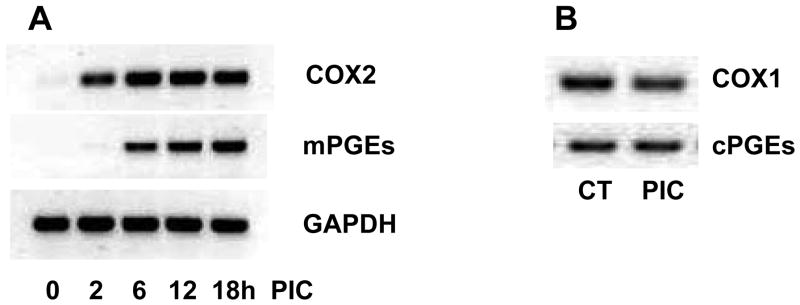
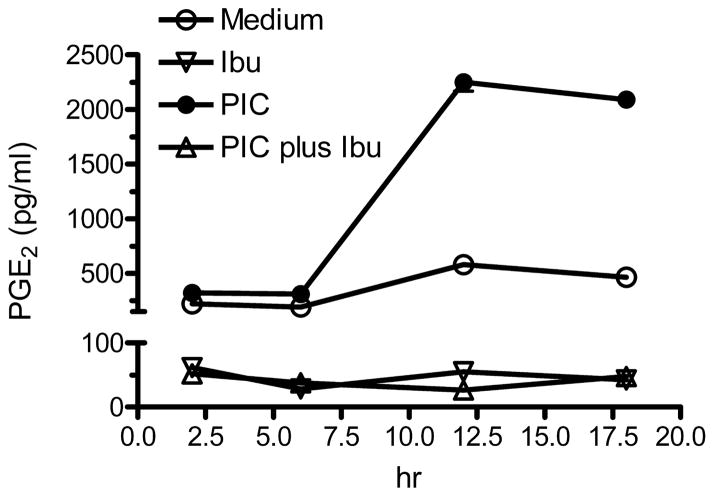
We are aware of only one report that describes PG signaling following TLR3 activation and this was in an immortalized cell line (Pindado et al. 2007). Therefore, we next tested the hypothesis that PIC activation of wt murine primary microglia required participation of the PG pathway, specifically PGE2. We first examined transcription of COX2 and mPGEs by PCR. As shown in Figure 3A, COX2 mRNA was detected at very low levels and mPGEs mRNA was undetectable in primary microglia cultures in the absence of PIC. COX2 mRNA increased dramatically following 2 hr incubation with PIC and reached its maximum by 6 hr. In contrast, mPGEs mRNA expression was delayed with detectable induction at 6 hr that continued to rise to 18 hr incubation with PIC. As expected, the levels of mRNA of COX1 and cPGEs were not increased by 18 hr incubation with PIC (Figure 3B). We also measured the concentration of PGE2 in medium under similar conditions (Figure 4). Incubation with PIC yielded a delayed, significant increase in medium PGE2 at 6 (P < 0.05), 12 (P < 0.01), and 18 (P < 0.01) hr.
Figure 3.
(A) WT murine primary microglia were incubated with 20 μg/ml PIC for indicated times. RNA was isolated and PCR was performed for COX2 and mPGEs expression. GAPDH was shown as loading control. (B) RNA was harvested from WT murine primary microglia treated with 20 μg/ml PIC for 18 hrs and analyzed for COX1 and cPGEs expression by PCR.
Figure 4.
WT murine primary microglia were incubated with 20 μg/ml PIC starting at time 0 and medium concentrations of PGE2 were determined at the times indicated. Exposure to ibuprofen (Ibu; 500 μM) began at time = −0.5 hr and continued throughout the experiment. Data are the average concentration ± SEM (n=3, error bars are smaller than symbols). Two-way ANOVA for the four exposure groups vs. time had P < 0.0001 for exposure group, time, and interaction. Bonferroni corrected post tests had P < 0.01 for all times points for Medium vs. Ibu, Medium vs. PIC plus Ibu, Ibu vs. PIC, and PIC vs. PIC plus Ibu; for Medium vs. PIC had P > 0.05 at 2 hr, P < 0.05 at 6 hr, and P < 0.001 at 12 and 18 hr; and P > 0.05 at all time points for Ibu vs. PIC plus Ibu.
Incubation of wt murine primary microglia with ibuprofen reduced medium concentrations to near background levels and reduced medium concentrations in cultures co-incubated with PIC to similar levels from 2 to 18 hr (Figure 4); the former likely through inhibition of COX1 and in the latter likely through inhibition of COX1 and COX2. These results show that PIC induced early expression of COX2 and delayed expression of mPGEs and production of PGE2 over a time course that raises the possibility that products of COX2, and perhaps PGE2, might be necessary for PIC-induced secretion of IL-6 and TNFα by murine primary microglia.
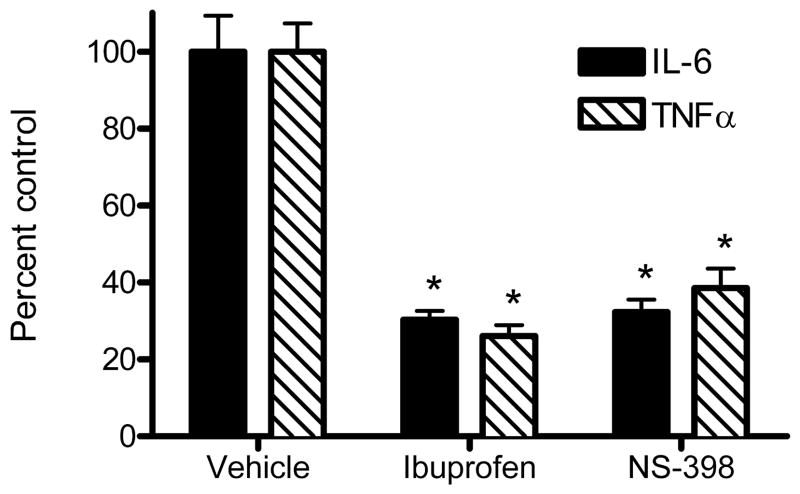
We tested this possibility by determining whether ibuprofen (non-selective COX inhibitor) or NS-398 (COX2-selective inhibitor) suppressed PIC-induced secretion of IL-6 or TNFα. Figure 5 presents the results of this experiment. We present data as % control to plot results for both analytes on the same axis; control levels were similar to our other experiments (average + SEM): IL-6 = 2847 ± 270 pg/ml, TNFα = 373 ± 28 pg/ml and statistical analyses were performed on concentration data. Results of these experiments are consistent with the PG pathway being necessary for two-thirds to three-quarters of PIC-induced IL-6 and TNFα secretion by murine primary microglia. Moreover, there was no further significant suppression by ibuprofen over NS-398, suggesting a central role for COX2 in PIC-induced secretion of IL-6 and TNFα. However, although we have used PGE2 as a marker for PG biosynthesis, these results do not prove that PGE2, as opposed to some other eicosanoid product of COX, is necessary for PIC-induced microglial secretion of IL-6 or TNFα.
Figure 5.
WT murine primary microglia were incubated with 20 μg/ml PIC starting at time 0 and medium concentrations of IL-6 and TNFα were determined at 18 hr. Exposure to ibuprofen (500 μM), NS-398 (50 μM), or DMSO vehicle began at time = −0.5 hr and continued throughout the experiment. Shown are average percent ± SEM (n=3) of vehicle treated controls. One-way ANOVA was performed on concentration data for each analyte. ANOVA for IL-6 had P < 0.0005 and TNFα had P < 0.001 for the three groups. Bonferroni-corrected post tests had *P < 0.001 compared to vehicle. Results for each analyte were not significantly different (P > 0.05) between Ibuprofen and NS-398 groups.
EP1 activation is necessary for approximately half of PIC-induced IL-6 and TNFα secretion
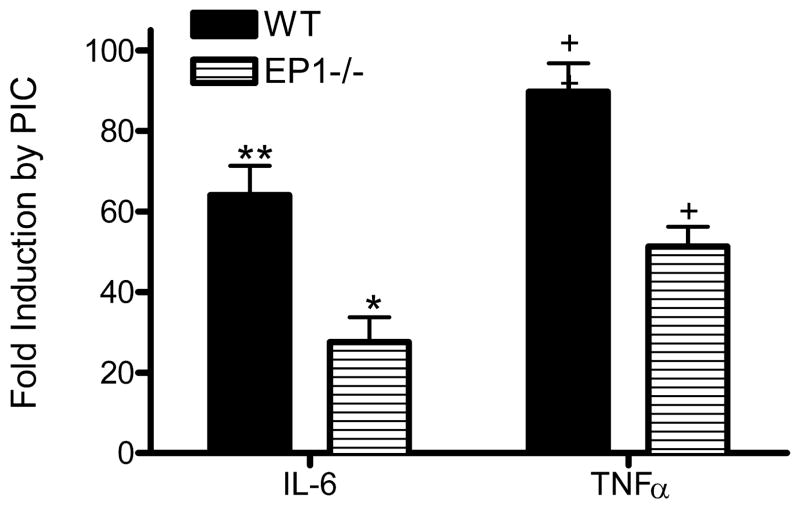
We repeated our 20-plex assay for cytokines and chemokines following 18 hr PIC incubation but now with either wt primary murine microglia co-incubated with an antagonist of EP1 (SC-51089) or with EP1−/− primary murine microglia. Our results with Luminex reagents showed that of the 8 analytes significantly induced by PIC (Table 2), only IL-6 (P < 0.01) and TNFα (P < 0.01) were significantly suppressed in wt cultures co-incubated with SC-51089 or in EP1−/− cultures (not shown). We confirmed these findings with ELISAs for IL-6 and TNFα (Figures 6 and 7). Indeed, PIC-induced secretion of IL-6 and TNFα were each reduced by about half of wt cultures when co-incubated with SC-51089 or when EP1−/− microglia were substituted for wt. Since expression of IL-6 and TNFα is transcriptionally regulated, we sought to confirm these findings with quantitative real-time PCR (qPCR). Using results from wt murine primary microglia incubated with PIC for 6 hr to define normal, identically exposed EP1−/− murine primary microglia had levels of IL-6 mRNA that averaged (± SEM) 42 ± 3% of wt (n=6; P < 0.001) and TNFα mRNA that averaged (± SEM) 73 ± 5% of wt (n=6; P < 0.001) of control. These genetic and pharmacologic approaches show that PIC-induced murine primary microglia expression and secretion of IL-6 and TNFα are significantly dependent on EP1 activation.
Figure 6.
WT or EP1−/− murine primary microglia were incubated with vehicle or 20 μg/ml PIC starting for 18 hr when medium concentrations of IL-6 and TNFα were quantified. Shown are average fold induction ± SEM (n=3) by PIC over vehicle control for both analytes and genotypes. Two-way ANOVA was performed on concentrations of each analyte: Medium vs. PIC and WT vs. EP1−/−. Results of two-way ANOVA for IL-6 concentration had *P < 0.01 for genotype, **P < 0.001 for exposure group, and P < 0.01 for interaction between these two terms. Results of two-way ANOVA for TNFα concentration had +P < 0.01 for genotype, ++P < 0.001 for exposure group, and P < 0.01 for interaction between these two terms.
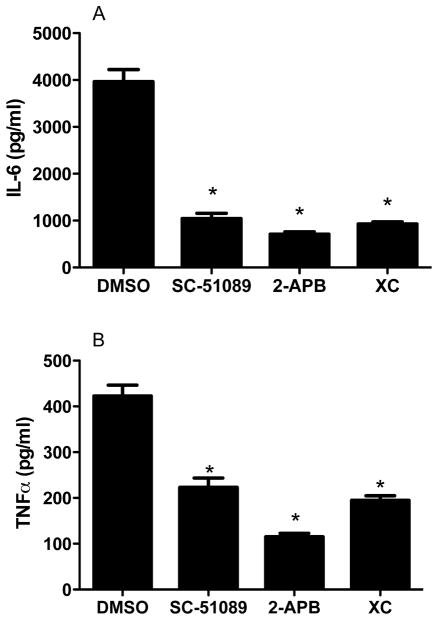
Figure 7.
WT murine primary microglia were incubated with 20 μg/ml PIC starting at time 0 and medium cytokine concentrations were determined at 18 hr. Exposure to SC-51089 (60 μg/ml), 2-APB (20 μM), XC (20 μM), or DMSO vehicle began at time = −0.5 hr and continued throughout the experiment. Data are average concentration ± SEM (n=4). (A) IL-6: ANOVA had P < 0.0001 with Bonferroni corrected paired comparisons showing *P < 0.001 for DMSO vs. SC-51089, 2-APB, or XC but P > 0.05 for SC-51089 vs. 2-APB or XC, or 2-ABP vs. XC (B) TNFα: ANOVA had P < 0.0001 with Bonferroni-corrected paired comparisons showing *P < 0.001 for DMSO vs. SC-51089, 2-APB, or XC, P < 0.05 for SC-51089 or XC vs. 2-ABP but P > 0.05 for SC-51089 or XC.
2-APB and XC replicate effects of EP1 suppression in PIC-stimulated microglia
EP1 is a G protein-coupled receptor that activates intracellular signaling by releasing Ca2+ from intracellular stores via the inositol 1,4,5-trisphosphate receptor (IP3R). We tested the hypothesis that an inhibitor of this second messenger system would replicate the effects of suppressing EP1 activation (Figure 7). 2-APB and XC, IP3R antagonists, significantly inhibited PIC-induced wt microglial secretion of IL-6 and TNFα; the extent of suppression of IL-6 secretion by 2-APB or XC was not significantly different from SC-51089; however, 2-ABP was more effective than XC in suppressing TNFα secretion, perhaps reflecting greater specificity of XC over 2-ABP. 2-ABP also significantly suppressed PIC-induced levels of COX-2 and mPGEs mRNA by 50% or more (P < 0.01 for each). These data confirm that intracellular Ca2+ signaling is necessary for most of PIC-induced microglial secretion of IL-6 and TNFα and suggest it is mediated largely by EP1 activation.
DISCUSSION
A clear therapeutic imperative exists for AD, PD, and ALS. While there are ample experimental, epidemiologic, and clinical data to support a contribution from innate immune activation in these diseases, treatment with NSAIDs failed to show efficacy in symptomatic stages of AD or ALS (Aisen et al. 2003; Cudkowicz et al. 2006; Thal et al. 2005) and was suspended because of toxicity in a prevention trial (ADAPT Research Group 2007). Despite these setbacks, the underlying experimental and epidemiologic data still strongly support suppression of the PG pathway as a means to prevent or delay symptom onset from AD and PD and perhaps ALS. It is for this reason that several laboratories pursue therapeutic targets in the PG pathway that will retain therapeutic benefit but avert toxicity by focusing on specific PG receptors rather than suppression of the entire PG pathway as with NSAIDs.
Our experiments focused on EP1, one of four PGE2 receptor subtypes, that is expressed widely in the central nervous system on neurons and microglia, and perhaps other cells (Cimino et al. 2008). Indeed, this promiscuity represents a serious complication to the interpretation of in vivo experiments or ex vivo models that include multiple cell types. Given our focus on suppressing paracrine damage to neurons from innate immune activation, we restricted our experiments to microglia, the major immune effectors in the central nervous system. Our results showed that TLR3 activation stimulated primary microglial secretion of eight (out of twenty) cytokines and chemokines, and that among these eight secreted factors only two, TNFα and IL-6, were partially dependent on EP1 signaling as determined with either pharmacologic or genetic tools, apparently though EP1’s known second messenger system of increasing intracellular Ca2+. Indeed, PIC-induced microglial secretion of TNFα and IL-6 were reduced by approximately one-half when EP1 signaling was suppressed. Comparison with our experiments using NSAIDs indicated that suppression of EP1 signaling achieved most of the effect of these drugs on TLR3-induced microglial secretion of TNFα and IL-6 (one-third to three-quarters reduction) but also suggests the possibility of a smaller PG-independent pathway.
Most of our experiments used the TLR3 activator, PIC. This approach was based on results from our initial experiments that showed that activators of TLR2, TLR3, TLR4, and TLR9, used at concentrations that maintain receptor specificity, yielded different degrees of activation of primary microglia with PIC yielding the greatest. Microglial TLR3 is the least studied of the TLRs from the perspective of neurodegenerative disease, yet mRNA is an endogenous activator of TLR3 and others have shown already that RNA accumulates in pathologic structures of AD, PD, and ALS (Ginsberg et al. 1998). We further demonstrated that activation of TLR3 in primary murine microglia led to induction of COX2 and delayed induction mPGEs transcription, accompanied by increased production of PGE2. We are aware of only one other report that links the PG pathway with TLR3 activation, and this work used an immortalized macrophage-like cell line (Pindado et al. 2007).
The roles of TNFα and IL-6 in the CNS are complex and areas of intense investigation in neurodegenerative diseases. In broad strokes, increased TNFα, alone or sometimes in concert with other inflammatory effectors like IL-6, can lead to neuronal dysfunction or death through several mechanisms including altered ion conductance, altered synaptic plasticity, and activation of pro-apoptotic pathways (Park and Bowers 2010). Many clinical-based studies have associated increased levels of TNFα and IL-6 in diseased regions of brain or cerebrospinal fluid from patients with AD or PD (reviewed in (Nagatsu and Sawada 2005; Rosenberg 2005). Several experiments have demonstrated that suppression of TNFα or IL-6 signaling partially or fully protects from neurodegeneration in models of AD (Billings et al. 2005; Blasko et al. 1999; Garcao et al. 2006; Janelsins et al. 2008; Liao et al. 2004; McAlpine et al. 2009; Mehlhorn et al. 2000) or PD (Austin et al. 2006; Ferger et al. 2004; Gao et al. 2008; McCoy et al. 2006; Sriram et al. 2002; Sriram et al. 2006; Su et al. 2008), and pilot clinical investigations raise the possibility that specific inhibition of TNFα signaling may bring therapeutic benefit to patients with AD (Tobinick 2009). While the ultimate outcome of modulating microglial innate immune response on nearby neurons, either trophic or toxic, is a complex topic that is difficult to address accurately in cell culture, our data raise the possibility that suppression of microglial EP1 signaling may be beneficial to neurons under some circumstances of innate immune activation in brain.
Our results show that suppression of microglial EP1 signaling may achieve much of the desired effect of NSAIDs by selectively blocking the induced secretion of two major effectors of paracrine immune damage to neurons following TLR3 activation. In combination with the already demonstrated ability of EP1 suppression to ameliorate neuronal excitotoxicity (Kawano et al. 2006), these data point to blockade of EP1 as an attractive candidate therapeutic for neurodegenerative diseases.
Acknowledgments
This work was supported by NIH grants ES16754, DK37097, and GM15431; and by the Nancy and Buster Alvord Endowment. We thank Dr. Kathleen Montine for editorial assistance and Ms. Carol Arnold for managerial support.
References
- Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, Iadecola C. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADAPT Research Group. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998;71:1635–42. doi: 10.1046/j.1471-4159.1998.71041635.x. [DOI] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–63. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–37. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–8. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–77. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–64. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- Cimino PJ, Keene CD, Breyer RM, Montine KS, Montine TJ. Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr Med Chem. 2008;15:1863–9. doi: 10.2174/092986708785132915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck M, Williams J, De Berardinis MA, Warden D, Puopolo M, Smith AD, Minghetti L. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:85–8. doi: 10.1136/jnnp.2005.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Frank K, Dykes-Hoberg M, Teismann P, Almer G, Przedborski S, Rothstein JD. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52:771–8. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Rothstein JD. Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann Neurol. 2000;48:792–5. [PubMed] [Google Scholar]
- Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol. 2009;10:958–64. doi: 10.1038/ni.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354–8. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–33. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcao P, Oliveira CR, Agostinho P. Comparative study of microglia activation induced by amyloid-beta and prion peptides: role in neurodegeneration. J Neurosci Res. 2006;84:182–93. doi: 10.1002/jnr.20870. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Galvin JE, Chiu TS, Lee VM, Masliah E, Trojanowski JQ. RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol. 1998;96:487–94. doi: 10.1007/s004010050923. [DOI] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Nakaya T, Homan T, Tanaka K, Sugimoto Y, Araki W, Narita M, Narumiya S, Suzuki T, Mizushima T. Involvement of prostaglandin E2 in production of amyloid-beta peptides both in vitro and in vivo. J Biol Chem. 2007;282:32676–88. doi: 10.1074/jbc.M703087200. [DOI] [PubMed] [Google Scholar]
- Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9) FASEB J. 2004;18:412–4. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–30. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181:7254–62. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–82. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Shie FS, Liu J, Wang Y, Davis J, Schantz AM, Montine KS, Montine TJ, Zhang J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvegren H, Skoglund C, Helldahl C, Lerm M, Grenegard M, Bengtsson T. Toll-like receptor 2 stimulation of platelets is mediated by purinergic P2X1-dependent Ca2+ mobilisation, cyclooxygenase and purinergic P2Y1 and P2Y12 receptor activation. Thromb Haemost. 2010;103:398–407. doi: 10.1160/TH09-07-0442. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–9. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Keene CD, Chang R, Stephen C, Nivison M, Nutt SE, Look A, Breyer RM, Horner PJ, Hevner R, Montine TJ. Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol. 2009;174:2300–9. doi: 10.2353/ajpath.2009.081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Merbt UM, Schmidt C, Angele B, Popp B, Wagner H, Pfister HW, Kirschning CJ. Acute brain injury triggers MyD88-dependent, TLR2/4-independent inflammatory responses. Am J Pathol. 2007;171:200–13. doi: 10.2353/ajpath.2007.060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Babiuch M, Joniec I, Przybylkowski A, Czlonkowski A, Czlonkowska A. Indomethacin protects against neurodegeneration caused by MPTP intoxication in mice. Int Immunopharmacol. 2002;2:1213–8. doi: 10.1016/s1567-5769(02)00078-4. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–7. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–32. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–14. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22:983–91. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Mattammal MB, Strong R, Lakshmi VM, Chung HD, Stephenson AH. Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson’s disease. J Neurochem. 1995;64:1645–54. doi: 10.1046/j.1471-4159.1995.64041645.x. [DOI] [PubMed] [Google Scholar]
- McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–77. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–75. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423–31. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, 2nd, Morrow JD. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Sonnen JA, Milne G, Baker LD, Breitner JC. Elevated ratio of urinary metabolites of thromboxane and prostacyclin is associated with adverse cardiovascular events in ADAPT. PLoS One. 2010;5:e9340. doi: 10.1371/journal.pone.0009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, Frautschy SA, Cole GM. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer’s models. Neuropsychopharmacology. 2005;30:1111–20. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–56. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–83. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindado J, Balsinde J, Balboa MA. TLR3-dependent induction of nitric oxide synthase in RAW 264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. J Immunol. 2007;179:4821–8. doi: 10.4049/jimmunol.179.7.4821. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–92. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reksidler AB, Lima MM, Zanata SM, Machado HB, da Cunha C, Andreatini R, Tufik S, Vital MA. The COX-2 inhibitor parecoxib produces neuroprotective effects in MPTP-lesioned rats. Eur J Pharmacol. 2007;560:163–75. doi: 10.1016/j.ejphar.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Ribes S, Ebert S, Czesnik D, Regen T, Zeug A, Bukowski S, Mildner A, Eiffert H, Hanisch UK, Hammerschmidt S, et al. Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Escherichia coli K1 strains by murine microglial cells. Infect Immun. 2009;77:557–64. doi: 10.1128/IAI.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB. Clinical aspects of inflammation in Alzheimer’s disease. Int Rev Psychiatry. 2005;17:503–14. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease-related pathology. J Neurosci. 2009;29:1846–54. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah VB, Williams DL, Keshvara L. beta-Glucan attenuates TLR2- and TLR4-mediated cytokine production by microglia. Neurosci Lett. 2009;458:111–5. doi: 10.1016/j.neulet.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–7. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–60. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–6. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006;20:670–82. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Breitner JC, Zandi PP. Prevention of Alzheimer’s disease. Int Rev Psychiatry. 2007;19:693–706. doi: 10.1080/09540260701797944. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Teismann P, Ferger B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse. 2001;39:167–74. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–8. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–15. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- Tobinick E. Tumour necrosis factor modulation for treatment of Alzheimer’s disease: rationale and current evidence. CNS Drugs. 2009;23:713–25. doi: 10.2165/11310810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–7. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]