Abstract
The inner ear structure of Antimora rostrata and its coupling to the swim bladder were analyzed and compared with the inner ears of several shallow-water species that also have similar coupling. The inner ear of Antimora has a long saccular otolith and sensory epithelium as compared to many other fishes. Some parts of the membranous labyrinth are thick and rigid, while other parts are thinner but attached tightly to the bony capsule. The partially rigid membranous labyrinth, along with its intimate connection to the swim bladder, may help the inner ear follow the sound oscillations from the swim bladder with better precision than would occur in a less rigid inner ear. In addition, the saccular sensory epithelium has an elaborate structure and an anterior enlargement that may be correlated with increased hearing sensitivity. Some of the features in the inner ear of Antimora may reflect the functional specialization of deep-water living and support the hypothesis that there is enhanced inner ear sensitivity in some deep-sea fishes.
Keywords: Antimora rostrata, Inner ear, Swim bladder, Deep-sea fish, Saccule, Lagena, Utricle
1. Introduction
In most oceans, sunlight is totally depleted below 1000m and temperatures are generally below 4°C at these depths. Bottom-dwelling deep-sea fishes living in these environments must overcome darkness, high pressure, low temperature, and scarce food supplies, as well as finding mates despite low population densities (Herring, 2000, 2002). Studies have demonstrated that some deep-sea fishes have evolved highly adapted and sensitive sensory organs to compensate for the lack of light. These adaptations include increased sensitivity in the visual (Munk, 1964, 1966; Locket, 1977; Douglas et al., 1998; Wagner et al., 1998; Warrant and Locket, 2004), olfactory (Herring, 2002), and lateral line systems (Marshall, 1996). However, very little is known about the auditory systems of deep-sea fishes except for some gross morphology (Bierbaum, 1914; Marshall, 1971, 1980) and the ultrastructure of portions of the inner ears in ten species (Popper, 1977, 1980).
The auditory system is likely to be especially useful in the darkness where sound travels a good deal further, and with higher directionality, than light or olfactory cues. Indeed, some deep-sea fish produce sound (Marshall, 1966) (though only a few species have been studied with regard to acoustic communication) and so it is reasonable to suggest that auditory system may be as important for deep-sea fish as other senses, particularly for relatively long distance communication. Thus, sound communication may be as important (if not more so) than other senses for deep-sea fish for survival and reproduction. Accordingly, it is as important to understand the mechanism in auditory adaptations in the deep-sea as other senses.
The acoustic environment in the deep ocean is different than the surface water because it is far away from the noise generated by wind and surface breaking waves. Although low frequency sound can propagate long distances in the sea, the “deep sound channel” below 1000 meters usually causes the propagation path of sound from the surface to bend upwards. Thus, acoustic signals from some shallower sources may never reach to the bottom (Kuperman and Roux, 2007). Thus there may be a higher signal to noise ratio for biologically relevant sounds in the deep-sea since surface sounds do not mask the detectability of sounds from deeper waters. Sound in the deep-sea may include signals from many difference sources including from the interaction between currents and bottom structures; biogenic sounds from fishes, mammals, and invertebrates; and by human activities that may still reach the deep. The mixture of acoustic signals forms a complicated “auditory scene” which must be interpreted by the animals to achieve an understanding of the acoustic environment. The process is call “auditory scene analysis” (Bregman, 1990, 2008). This auditory scene may provide fishes with a very wide acoustic “view” of the world around them (Tavolga, 1971; Fay, 1988; Popper et al., 2003).
We have begun a set of studies on the anatomy of the inner ear in a number of deep-sea fish species to fill the major gaps in our knowledge about their auditory systems (cf. Deng et al., 2002; Buran et al., 2005). We hypothesize that some deep-sea fishes may have evolved specialized inner ear structures to enhance hearing sensitivity as compared to fishes living in shallower water where there is more light. While the ideal test of this hypothesis would be to do physiological investigations, this is impossible since deep-sea fishes rarely can be taken alive. Thus, our studies focus on anatomy and ultrastructure of the auditory system, and extrapolation of function from what is known about hearing in other species for which behavioral and physiological data exist.
This study focuses on Antimora rostrata, commonly referred to as the blue antimora, blue hake, or flatnose codling. It belongs to the deep-sea cod family Moridae. Antimora lives close to the bottom at depths ranging from 300 to 3000 meters and has a broad distribution in the Atlantic, Pacific, and Indian Oceans, as well as in Antarctic waters (Cohen et al., 1990). Antimora may be the dominant scavenging species in some areas (Iwamoto, 1975; Wenner and Musick, 1977). As a sample species among deep-sea fish surveys, its distribution and biology has been documented in many areas (Gordon and Duncan, 1985; Kulka et al., 2003; Fossen and Bergstad, 2006). It is also well studied for its biochemical and physiological adaptations to many aspects of deep-sea life (e.g., Small, 1981; Siebenaller and Murray, 1990; Collins et al., 1999; Bailey et al., 2003).
As an adaptation to the low-light environment in the deep sea, Antimora has a multibank rod retina in its eye with four layers of rods stacked together rather than having a single layer of rod cells as in most other vertebrates (Fröhlich and Wagner, 1996, 1998). This structure in deep-sea fishes may increase the sensitivity of the retina to light, or may serve as spectral filters for wavelength discriminations (Denton and Locket, 1989). Antimora’s swim bladder is also adapted to overcome high pressure in the deep-sea. The swim bladder has a substantial oxygen pumping ability under great hydrostatic pressure through its gas glands that are supplied by elaborated arterial and venous capillaries, the rete mirabile (Scholander, 1954).
The auditory capsule in morid fishes is connected to the swim bladder (Paulin, 1988). This inner ear to swim bladder connection has been described in some morid fishes such as the North Atlantic codling (Lepidion eques) and tadpole codling (Salilota australis) (Marshall, 1966). In Antimora, the close proximity between the anterior chamber of the swim bladder and the skull has been demonstrated by Iwamoto (1975), but the anatomical connection with the inner ear has yet to be shown in detail.
This paper analyzes the morphology of the inner ear and its relationship to the swim bladder in Antimora in order to expand our understanding of the auditory system in deep-sea fishes. The ultrastructure of the sensory epithelia of the inner ear is analyzed with scanning electron microscopy (SEM) and compared with inner ears of shallow-water species that have swim bladder-inner ear connections. The comparison to shallow water fishes for which there are functional data may provide insight into the significance of a swim bladder-inner connection in Antimora. Some features in the inner ear of Antimora appear unique among vertebrates, so how such structures may reflect the fish’s adaptation to deep water living is also considered.
2. Materials and Methods
2.1 Station locations and sampling
The specimens of Antimora rostrata (Günther, 1878) used in this study were collected using semi-balloon otter trawls from the Porcupine Seabight in the Northeast Atlantic Ocean near Ireland during Discovery cruises D252 in April 2001, D255 in August 2001, and D260 in March 2002. The area of the stations during these cruises covered 48–52°N, 11–16°W, trawling at bottom depths of 1000–4200m with an average water temperature of 4°C.
All fish were dead when they came to the surface as a result of the two to three hours’ retrieval of the trawl from the ocean bottom. The hauling cable was approximately three times the target depth and the hauling speed was slow, thus the vertical elevation of the net was very slow during retrieval so as to reduce the damage to the fish from rapid change in pressure. No obvious expansion was observed in the fish’s swim bladder. The inner ears are incased in the bony capsule with fluid with no perceivable damage from the pressure change.
As soon as the fish came to the surface they were put on ice. Identifying (using Whitehead et al., 1984) and measuring the catch usually took one to seven hours, during which time the fish were distributed to the labs and dissected. As soon as possible, Antimora heads were fixed in cold 4% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer with 0.05% CaCl2. Several unfixed specimens were dissected on board to analyze the otoliths and the relationship between the inner ears and the swim bladder. All tissues were stored in the 4°C cold room on board the ship.
After transferring specimens to the lab at the University of Maryland (about one to three months after capture), the fixative was replaced with 0.1 M cacodylate buffer (pH 7.4) and specimens stored in a 4°C refrigerator until further analysis. The long-term fixation did not noticeably degrade the inner ear tissues, and it hardened the brain tissue very well. Most otoliths did not seem to have noticeably changed between fixed and fresh specimens, even after three months of fixation.
2.2 Animals used in this study
In the study of swim bladder-inner ear relationships, one fixed specimen was used and two others were dissected freshly on board. In the study of inner ear structure, five fresh specimens were dissected on board for observation and preservation of the otoliths, while three additional fixed specimens were used for the gross morphology and SEM of the ultrastructure.
Four inner ears from the three fish were used for SEM study. One was a left inner ear from a 602 g female with a standard length of 402 mm; one was a right inner ear from an 1100 g female with a standard length of 484 mm. Two inner ears were from a 370 g male with a standard length of 354 mm. The fixed specimen (un-sexed) used for anatomical drawing of the swim bladder was 375 mm in standard length and weighed 390 g. All body measurements were made before fixation.
2.3 Scanning electron microscopy (SEM) procedures
Inner ears were dissected in 0.1 M cacodylate buffer under a binocular microscope. Photographs were taken during the dissection. The inner ears were then post-fixed in 1% OsO4 with 0.1 M cacodylate buffer or PIPES buffer at room temperature for 30 to 60 minutes. After three buffer rinses followed by three double distilled water rinses, the inner ears were dehydrated in 30%, 50%, and 75% ethanol at 10 minute intervals. The whole inner ears were then further dissected into individual end organs during the 75% ethanol dehydration step, and the otolith and all otolith membrane was removed. The dehydration continued at 85%, 95% and three ×100% ethanol at 10-minute intervals immediately before critical point drying.
Critical point drying was done using CO2 as the intermediary fluid (maximum pressure 2000 psi). Tissues were then mounted on aluminum stubs using silver paste to preserve the natural curve of the lagenar and utricular epithelia. The stubs were coated with about 4–6 nm thickness of Au-Pd from a filament/vacuum using a Denton Vacuum DV 503 and viewed with an AMRAY 1820D scanning electron microscope.
Mapping of the hair bundle orientation was done by scanning up and down at high magnification across the entire sensory epithelium. Orientations of sensory hair bundles were marked on photos of the maculae. Neither the shapes of the sensory epithelia, nor the hair bundle orientation patterns, differed between the four inner ears.
3. Results
3.1 Gross morphology of the inner ear
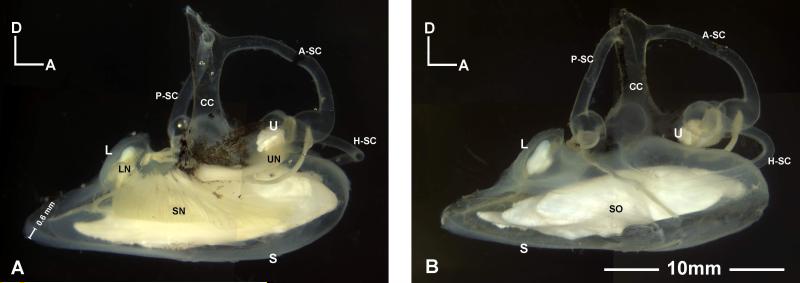
The inner ear of Antimora rostrata, like that of other bony fishes, is a membranous labyrinth that can be divided into upper and lower compartments (Fig. 1). The upper labyrinth (often called the pars superior in older literature) includes the utricle and three semicircular canals: the anterior, posterior, and horizontal (or lateral) semicircular canals. Each canal has an enlarged region, the ampulla, at one end. The lower labyrinth (pars inferior) includes two otolithic end organs, the saccule and lagena. The upper and lower parts of the labyrinth are joined together at base of the common crux (Fig. 1). No macula neglecta was found.
Figure 1.
Left and right inner ears of Antimora rostrata. The total length and standard length of the specimen are 440mm and 402mm, respectively. A: Medial view of a left inner ear showing the three end organs and their innervating eighth nerve branches. A measurement bar indicates the thickness of the posterior wall of the saccule. B: Lateral view of a right inner ear with all three otoliths clearly present in their pouches. A-SC, P-SC, H-SC, anterior, posterior, and horizontal semicircular canals; CC, common crux; L, lagena; LN, nerves to lagena; S, saccule; SN, nerves to saccule; SO, saccular otolith (sagitta). U, utricle; UN, nerves to utricle.
Each otolithic end organ is a membranous pouch enclosing a solid calcareous otolith (Figs. 1, 2A, 3A). The saccule is long and extends beyond the longitude length of upper labyrinth (from anterior ampulla to posterior ampulla) in specimens of all sizes. The specimens used in this study are within the average size range (38-47cm in total length) of mature Antimora rostrata (Kulka, et al., 2003). Previous studies have shown that in Antimora rostrata the weight of saccular otolith is closely correlated with fish length (Fossen and Bergstad, 2006), thus specific measurements of the inner ear are given with the information of fish length. In a specimen of 402mm standard length (440mm total length), the length of saccule is 18.5mm while the length of the upper labyrinth is only 10.6mm (Fig. 1). Inside each otolithic end organ is a sensory epithelium (or macula) which contains numerous sensory hair cells and which is overlain by the otolith. A gelatinous otolith membrane (Fig. 2B, C) connects the otolith with the sensory epithelium. The sensory hair cells are innervated by branches of the eighth cranial nerve that leave each macula and enter the brain stem (Figs. 1A, 4B).
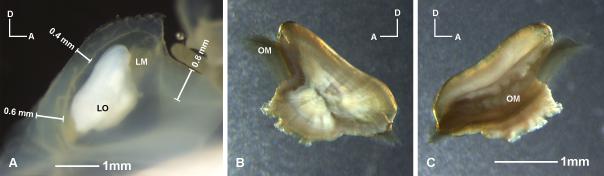
Figure 2.
Lagena of Antimora rostrata. A: Lateral view of a right lagena. The walls of the sac are from 0.4 to 0.8mm thick. B: Left lagenar otolith and otolithic membrane taken out from the lagenar sac after osmium fixation. C: Medial view of the same otolith. The brown veil-like substance is the otolithic membrane (OM) stained by osmium. Note that the otolithic membrane extends out from two ends of the lagenar otolith. Dissections show that the otolithic membrane covers the regions of the sensory epithelium that are not covered by the otolith itself. LM, lagenar macula; OM, otolithic membrane.
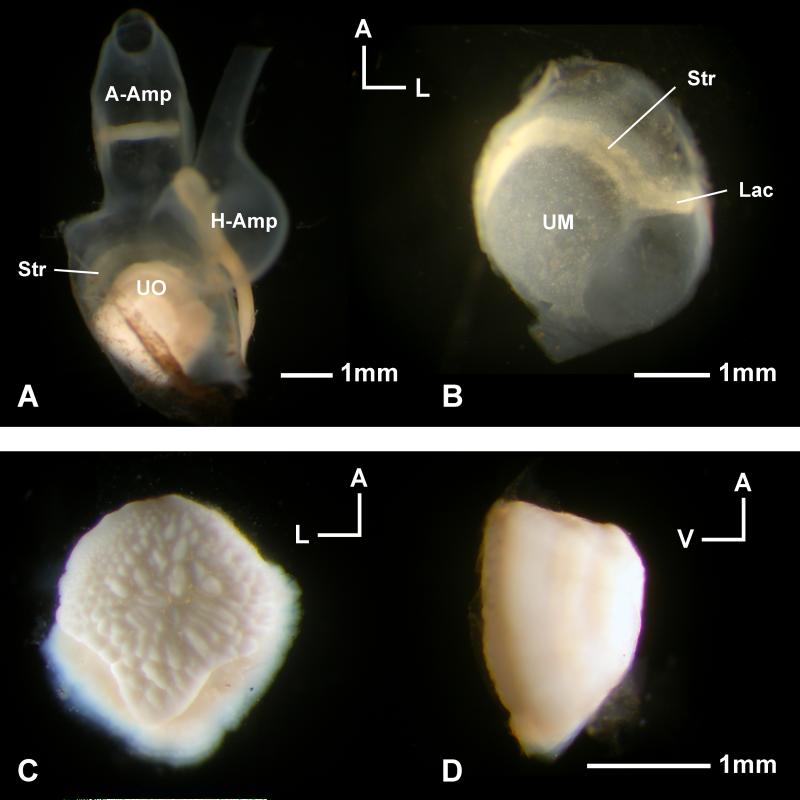
Figure 3.
Utricle of Antimora rostrata. A: Dorsal view of a right utricle with the otolith inside the pouch and the ampullae of the anterior (A-Amp) and horizontal semicircular canals (H-Amp) still attached. B: The utricular epithelium isolated from A, note that the striola (Str, more opaque yellowish region) and lacinia (Lac) are not covered by the otolith. C: Ventral view of the otolith taken from A, revealing the bumpy surface that is connected to the otolith membrane that lies between the otolith and the sensory epithelium. D: Side view of the same otolith to show the dome shape. UO, utricular otolith; UM, utricular macula.
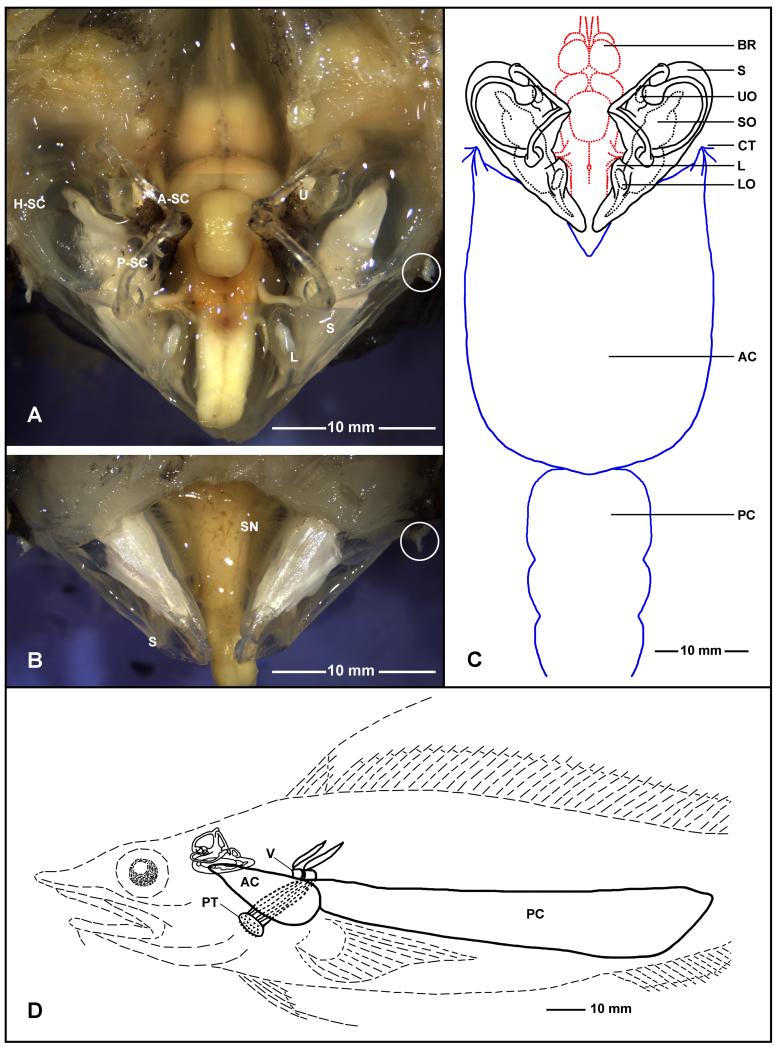
Figure 4.
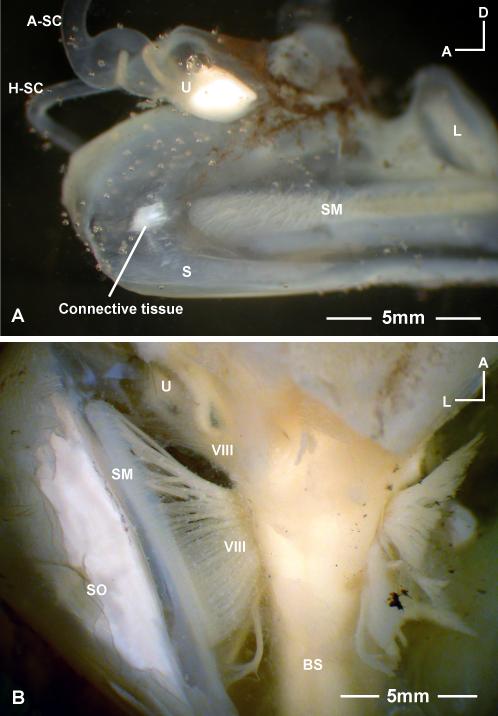
Inner ears, brain, and swim bladder of Antimora rostrata (anterior to the top for A, B, and C). A: Dorsal view of the brain and inner ears after removing part of the skull and cartilage. B: Ventral view of the inner ears and the brainstem after removing part of the bottom of the cranium, with a clear indication of the rigidity of the saccule without the support of water. After removing the two anterior chambers of the swim bladder from where they attach to the inner ear’s bony capsules, the stubs of the dense connective tissue (indicated by white circles) indicate the attachment points. C: The relationship between the brain, inner ears, and swim bladder in Antimora; note that the size of the brain is relatively small compared with the size of the inner ears. D: Lateral view of the relative position between the inner ear and the swim bladder with respect to the head and the body. Also shown in D are two vertebrae (V) and a muscle bundle to the upper pharyngeal teeth (PT). AC, PC, anterior and posterior chamber of swim bladder; A-SC, H-SC, P-SC, anterior, horizontal, and posterior semicircular canals; BR, brain; L, lagena; LO, lagenar otolith; PT, pharyngeal teeth; S, saccule; SN, nerve to saccule; SO, saccular otolith; CT, connective tissue; U, utricle; UO, utricular otolith.
The inner ears of Antimora fill a very large portion of the volume of the cranial cavity. Figures 4A and C show the relative position between the two inner ears and the brain and their relative proportions in a 402mm standard length specimen. The distance from forebrain to medulla oblongata is 15mm as compared with 18.5mm the length of saccule. The posterior and horizontal semicircular canals are completely enclosed in cartilage, while the anterior semicircular canal and the utricle are partially enclosed. The anterior and lateral walls of the saccule are tightly attached to the cranial bones that have to be peeled off carefully during dissection. The medial wall of the saccule is attached to the cranial bone via a connective tissue at a point located just anterior to the macula. The connective tissue that makes the contact with the inner side of the bony capsule is shown in Figure 5A. The outer side of the bony capsule is connected to the swim bladder via a dense connective tissue, the stub of which is shown in Figures 4A and B (highlighted with a circle).
Figure 5.
A: The bony capsule connection on the medial wall of a saccule in Antimora rostrata. This is a medial view of part of a right saccule. The contact is located just anterior to the saccular macula (SM) and is connected to the interior wall of the cranial bone via a connective tissue. B: Ventral view of the brainstem (BS) and right inner ear of Antimora, showing the array of auditory nerve fibers (VIII, eighth cranial nerve) spreading along either side of the brain stem. Refer to Figure 4 for other abbreviations.
The posterior part of the saccule, including a portion of the dorsal wall and the lagena, is not surrounded by bone, but is thick and rigid with a cartilaginous texture that can support the weight of the otolith and sustain its own shape even out of water. The rigid parts of saccular and lagenar sacs are exposed in Figure 4A and B. The anterior and posterior semicircular canals in Figure 4A are also semi-rigid and retain their natural shape and position after removing the supporting bony labyrinth. This is also observed in the fresh samples examined on board the ship. Thus, the rigidity was not a result of fixation.
The saccular otolith, the sagitta, fills up most of the space in the saccular pouch (Fig. 1). This elongated otolith has a very complex three-dimensional structure that is thicker at the rostral end and pointed at the caudal end, with bumps and concavities all over (Fig. 1B). Photographs of saccular otoliths from different sizes of Antimora specimens have been presented by Campana (2004).
The part of the eighth nerve innervating the saccule fans out as an array of nerve fibers from the hindbrain and projects along the length of the saccular macula (Fig. 5B). Some parts of the sac walls are as thick as 0.6mm and the eighth nerve goes through this cartilage-like wall to reach the macula (Fig. 1A, 5B).
The lagena of Antimora has a thick wall around the macula through which the nerves penetrate to the medial side of the epithelium (Fig. 1A). Using a stereomicroscope with a stage micrometer, the thickness of this wall was found to vary from 0.4 to 0.8 mm in a 402 mm standard length fish (Fig. 2A). The lagenar otolith, the asteriscus, is convex medially and only covers two-thirds of the sensory epithelium (Fig. 2A). Most of the anterior one-third, and a small portion of the caudal end, of the lagenar macula are solely covered by the otolithic membrane that extends beyond the otolith (Fig. 2B, C).
The utricle of Antimora is not very different from that of most other teleost species (Fig. 3A). The sensory side of the utricular sac is shaped like a bowl with the utricular otolith, the lapillus, sitting on top of it. The epithelium has a striolar region with a denser population of bundles than found on the rest of the macula. The utricular striola is visible even under a light microscope and is continued laterally into an elongated tail-like region, the lacinia (Fig. 3B). It is evident that this striola-lacinia region is not covered by the utricular otolith (Fig.3A). The utricular otolith of Antimora is dome-shaped with a bumpy ventral surface attached to the epithelium via otolithic membrane and a smooth surface on the domed dorsal side (Fig. 3C, D).
3.2 Coupling between inner ear and swim bladder
The swim bladder of Antimora has anterior and posterior chambers (Fig. 4C, D). The interior of the gas chamber in a fresh specimen is filled with a foamy substance, which is a mixture of gas and lipid bi-layer membranes (Patton and Thomas, 1971; Josephson et al., 1975).
Figure 4D shows the relative position of inner ear and swim bladder with respect to the fish’s head and body in a lateral view while Figure 4C provides a dorsal view. The anterior chamber of the swim bladder has two rostral horns, which attach to the lateral part of the bony capsule of each saccule via a dense connective tissue (Fig. 4A-C). Another connective tissue links the saccule to the medial side of the bony capsule at a location just before the anterior tip of the sensory epithelium (Fig. 5A). Thus there is a physical connection between the anterior chamber of the swim bladder and the saccule. The rostral end of the anterior chamber is also attached to the fourth vertebra, which is also the root of the two muscle bundles that move the upper pharyngeal teeth (Fig. 4D).
3.3 Sensory Epithelia
3.3.1 Hair cell bundles
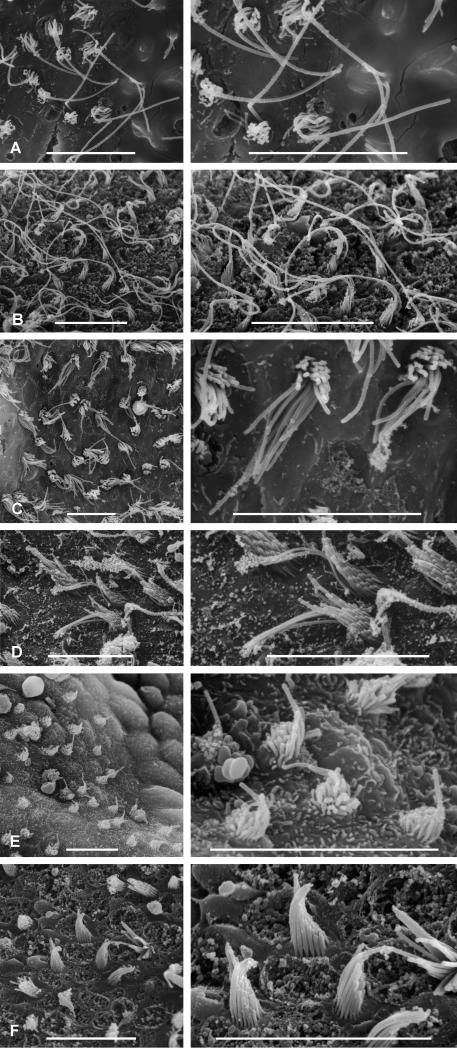
Sensory hair cells cover the maculae of each end organ of the inner ear. The apical surface of the hair cell contains a graded bundle of stereovilli and a single kinocilium that is generally longer than the tallest stereovilli. The kinocilium and all the stereovilli on top of one hair cell are referred to as a hair bundle. Figure 6 shows samples of hair bundles from the three end organs of Antimora. The orientation of a hair bundle is defined by the direction of sensitivity, which is from the shortest stereovillus to the kinocilium (Wersäll and Flock, 1963; Hudspeth and Corey, 1977). The bundle orientation patterns are shown in diagrams of the sensory epithelia with arrows representing groups of hair bundles that are oriented in the same direction (Figs. 7, 8). Each arrow points from the shortest stereovillus toward the kinocilium.
Figure 6.
Examples of hair bundles from the end organs of Antimora rostrata. A: Bundles from the ventral edge of the lagena (Fig. 8B). B: Bundles from the tip of lacinia on the utricle (Fig. 8D). C: Bundles from the striola of the lagena (Fig. 8B). D: Bundles from the caudal segment of the saccule (Fig. 7B). E: Bundles from the ventral tip of the utricle (Fig. 8D). F: Bundles from the center of the striola in the utricle (Fig. 8D). Scale bars = 10 μm.
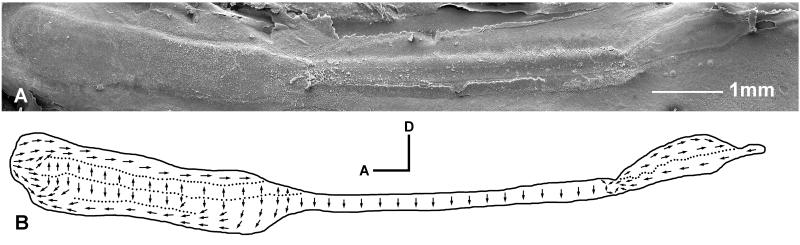
Figure 7.
Ultrastructure of the saccular sensory epithelium in Antimora rostrata. A: SEM photo of a left saccular macula. B: Hair bundle orientation pattern of the saccule. Note that the macula has three distinct segments with eight different bundle orientation groups.
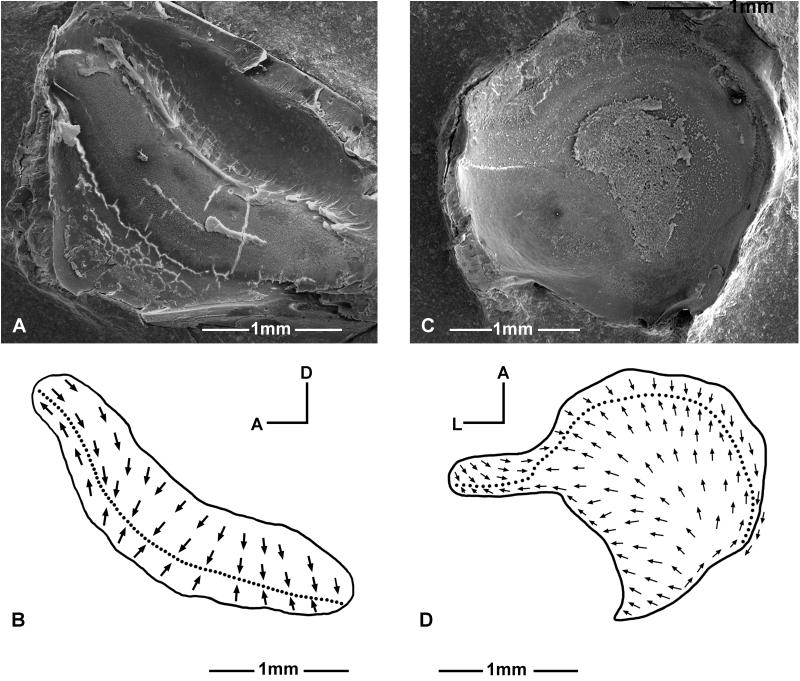
Figure 8.
Structure of the sensory epithelia of the lagena (A-B) and utricle (C-D) in Antimora rostrata. A and C: SEM photo of the left lagenar macula and left utricular macula. B and D: Hair bundle orientation pattern of the lagena and utricle.
3.3.2 Saccule
The saccular macula of Antimora is long and narrow and may be divided into three segments (Fig. 7A). Hair bundle orientation maps, derived from SEM images, are shown in Figure 7B. The three distinct segments on the saccule have different hair bundle orientation patterns, and these can be further divided into eight regions based upon bundle orientation (Fig. 7B).
The rostral segment of the saccular epithelium is much wider than the other two regions and can be horizontally divided into four hair bundle orientation groups. From dorsal to ventral they are a horizontal-posterior oriented group, a vertical-dorsal oriented group, a vertical-ventral oriented group, and a horizontal-anterior oriented group (Fig. 7B). The very tip of the rostral segment does not have clear boundaries between bundles that are oriented in different directions, but instead has bundles that gradually change in direction.
The middle segment of the macula has all hair bundles oriented vertical-ventrally, while the caudal segment is horizontally bi-directional. At the junction of the middle and caudal segments, a small group of bundles form a swirl with counterclockwise (for a left inner ear) orientations (Fig. 7B).
Hair bundle densities on the maculae were calculated using SEM photographs of different regions of the maculae with sample areas from 2000 to 10000 μm2. The average bundle density varies between specimens of different sizes. As an example, in a specimen of 402 mm standard length, the average bundle density of the entire macula is 227/104μm2 (SE=11, n=23). The bundle density is slightly lower in the anterior segment and slightly higher in the posterior segment. When calculated separately, the anterior, middle, and posterior segment has an average bundle density of 190/104μm2(SE=13, n=8), 232/104μm2 (SE=24, n=10), and 259/104μm2 (SE=28, n=7), respectively.
3.3.3 Lagena and utricle
The lagenar macula is concave and shaped like a “boomerang” (Fig. 8A, B). The curvature of the “boomerang” shape bends upward and backward against the saccule.
The dorsal one-third of the lagenar macula has two opposing vertically oriented hair bundle groups; the directions of the bundle orientation axes are parallel to the length of the macula. The ventral one-quarter of the macula also has two opposing vertically oriented bundle groups, though their axes are almost perpendicular to the length of the macula. The middle part of the macula has two oppositely oriented bundle groups giving about a 45° direction relative to vertical, and they are perpendicular to the length of the macula (Fig. 8B). The very posterior tip of the macula is only covered by the otolithic membrane and not by the otolith.
The hair bundle orientation pattern of the utricular macula in Antimora (Fig. 8D) is similar to other fishes that have been mapped (Lewis et al., 1985). The axes of posterior to anterior oriented bundles spread out radially from the narrow posterior region towards the broader anterior border.
The average hair bundle density in the lagena of a 402 mm standard length specimen is 521/104μm2 (SE=35, n=6). In the utricle of the same specimen, the striola region has a hair bundle density of 276/104μm2 (SE=15, n=6).
4. Discussion
Anatomical and SEM studies show that Antimora rostrata has some unique inner ear structural features that have not been described in other fishes. These include a rigid and cartilaginous-like end-organ wall in the lagena and part of the saccule, a complex hair bundle orientation pattern in the saccular macula with more orientation groups than previously described for any vertebrate, and a direct saccule-swim bladder connection. While a tight direct connection between the anterior projections of the swim bladder and the saccule is seen in some shallow-water fishes, in Antimora, there are some unique features of this connection. Some of these characters may reflect adaptation to living in deep water.
4.1 Bigger and thicker inner ears
The inner ears of Antimora, especially its saccule, are very large as compared with its brain (Fig. 4A-C). For comparison, in the shallow water gadiform, the Atlantic cod (Gadus morhua), the inner ear is only half the length of the brain (from forebrain to medulla oblongata) (Dale, 1976). In contrast, in Antimora rostrata, the length of inner ear is slightly longer than the same brain region. Similarly, Fine et al. (1987) reported that a deep-sea fish, the bony-eared assfish (Acanthonus armatus), has two inner ears with a mass that is several times greater than that of the brain. Large inner ears were also found in a deep-sea gadiform family Macrouridae (Deng et al., 2002), and in other deep-sea macrourids (Hymenocephalus) (Bierbaum, 1914).
Large inner ears in fish may not necessarily be related to deep-water living. Flying fish, for example, have huge semicircular canals as compared to brain size. However, the lower labyrinth in the flying fish (Exochoetus volitans) is small compared to the size of the brain (Retzius, 1881). The large inner ears observed in deep-sea gadiform fishes may simply exist because many gadiform fishes have large saccules in general. For example, tadpole fish (Raniceps raninus) and Atlantic cod both have large lower labyrinths (Retzius, 1881). Nevertheless, none of these inner ears are comparable to Antimora with a saccule that extends 175% the length of the upper labyrinth. Other members in the Moridae family, such as the beardless codling (Brosmichlus (Gadella) imberbis) and the shortbeard codling (Laemonema barbatula), also have large saccular otoliths with similar sculpted features to those found in Antimora (Campana, 2004). It would be worthwhile to see if the inner ear structures are similar throughout this deep-water family.
The rigidity and thickness of the end organ walls, which are seen in Antimora rostrata, have rarely been reported in fish species except in large sized specimens of bluefin tuna (Thunnus thynnus). The bluefin tuna specimens were close to three meters long and weighed about 230 to 380 kg (Song et al., 2006), which is about five to six times longer and several hundred times heavier than the Antimora specimens studied here. Interestingly, the length of saccule in a tuna of 2.55 meters long is about the same as that of the saccule in an Antimora only one-fifth of its size. One possibility is that the cartilaginous wall of bluefin tuna inner ear may be an adaptation to protect the inner ear during fast swimming and deep diving, or it may be simply a feature of very large fish inner ears (Song et al., 2006). Since the size and life style of bluefin tuna are very different from Antimora, the implication of the cartilaginous labyrinth wall may be very different in the two species. In the bluefin tuna, the whole membrane surrounding the labyrinth, including the semicircular canals, are all cartilaginous, whereas in Antimora only the lagenar sac and the posterior part of the saccule is extra thick and rigid.
Considering the fact that the thin portion of the Antimora saccular sac is tightly attached to the bony capsule, and the other parts of the inner ear sacs are rigid, the whole membranous structure of the inner ear may be able to move in synchrony with the oscillation of the swim bladder via the dense connective tissue. This may provide direct stimulation from the swim bladder to the saccular epithelium and can be considered as a mechanism that may improve inner ear sensitivity in Antimora.
On the other hand, although for many fishes, the saccule may be primarily hearing, the utricle may be primarily vestibular, and the lagena may have dual functions (Platt and Popper, 1981; Platt, 1983), it is nevertheless possible that the unique structure in Antimora’s saccule may result from adaptations for vestibular function. However, since virtually all adaptations to the saccule can be associated with improved hearing bandwidth and sensitivity in shallow-water fish (Plat and Popper, 1981), it is likely that the same relationship holds for deep-sea fish.
4.2 Lagena and utricle
The curvature of the lagenar macula bends upward and backward against the saccule, which is opposite to the commonly described lagena shapes in many other fishes, including some deep-sea fishes (Popper, 1977, 1980; Buran et al., 2005) in which the macula bends downward and forward to embrace the posterior end of saccule. It is also different from the lagena of another gadiform fish, the European hake (Merluccius merluccius), which is bent towards the saccule (Lombarte and Popper, 1994, 2004). The lagenar macula of Atlantic cod reported by Dale (1976) is shaped like a narrow tongue with an anterior-ventral process.
The utricle shape is similar to that in most bony fishes (Lewis et al., 1985). The hair bundle orientation pattern is similar to that in Atlantic cod (Dale, 1976) and European hake (Lombarte and Popper, 1994, 2004). It is also similar to those seen in many other fishes studied to date (Platt and Popper, 1981; Platt, 1977, 1993; Lu and Popper 1998; Buran et al., 2005).
4.3 Swim bladder to inner ear connection
Swim bladders, or other gas chambers, can help to enhance the sound signal that reaches the fish inner ears by radiating the pressure component of the sound as a displacement that can be detected by the inner ear, thereby expanding the fish’s hearing range in sensitivity and bandwidth (Fay, 1988; Popper and Fay, 1999; review see Popper et al., 2003). This is best known in some otophysan fishes that have Weberian ossicles as the linkage between the inner ear and the swim bladder (von Frisch, 1938; van Bergeijk, 1967; Popper, 1983, Popper and Tavolga, 1981).
The swim bladder of Antimora and its connection to the skull or ear capsule has been described by Iwamoto (1975) and Paulin (1988). However, these investigators did not show the relative position between the swim bladder and the inner ears. In the diagrams of the lateral view of the swim bladder, the authors depicted a relatively ventrally pointed anterior chamber, which is the opposite of the current observation (Fig. 4D). The anterior chambers are actually pointed slightly dorsally, thus they reach upwards to connect with the lateral wall of the inner ear’s bony capsules.
The left and right projection of the anterior chamber of the swim bladder each make an intimate connection to an anterior lateral spot at the bony capsule around the respective saccule (Fig. 4A-C). Inside the interior medial wall of the bony capsule, another piece of connective tissue located just anterior to the macula affixes the saccule to the bone (Fig. 5A). The inner ear may thus receive direct stimulation from sound pressure since the swim bladder has a direct mechanical connection with the anterior macula of the saccule. Although the connection seen in Antimora is different from the Weberian ossicle connection in the Ostariophysan fishes (Weber, 1820; Poggendorf, 1952), the structure in Antimora may serve as a direct link connecting the motions of the swim bladder to the cranial bone, and then in turn to the saccule since the wall of the sac is tightly attached to the bony capsule.
Otophysic (inner ear to swim bladder) connections without a Weberian apparatus are found in many fish families (Nelson, 1955; Schellart and Popper, 1992). Different species of the squirrelfish family Holocentridae have different configurations between the swim bladder and the ear capsule; from no direct contact to intimate contact. Hearing studies in the squirrelfish family (Coombs and Popper, 1979) showed that a species with a direct inner ear to swim bladder connection (shoulderbar soldierfish, Myripristis kuntee) has a broader hearing range (100–3000 Hz) than a species that has no such connection (Hawaiian squirrelfish, Adioryx xantherythrus, synonym of Sargocentron xantherythrus, 100–800 Hz), as well as much better hearing sensitivity, with a 30 dB difference in thresholds at the best frequency.
With an otophysic connection similar to shoulderbar soldierfish, it is possible that Antimora has enhanced hearing sensitivity or a broader frequency range via this acoustic coupling. However, such enhancements may be limited because the wall of Antimora’s deep-sea adapted swim bladder is less elastic than that of the goldfish and other fishes with good hearing, and the Antimora swim bladder is filled not entirely by gas, but by a mixture of gas and lipid bi-layer membrane foams (Josephson et al., 1975). These characteristics may reduce the amplitude of the swim bladder’s vibration. This limitation may be overcome, however, by the rigid connection between Antimora’s inner ear and swim bladder. The rigid wall of the ear capsule may help the inner ear to synchronize with the vibration from the swim bladder more precisely than a softer inner ear, thus making the inner ear better able to pick up most of the reduced radiated signal from the swim bladder.
4.4 Complex saccular hair bundle orientation patterns and a convergent implication of non-Weberian otophysic connection
The highly complicated saccular hair bundle orientation pattern in Antimora suggests specialization for hearing. One characteristic of some fishes that hear well is an elaboration in the hair bundle orientation pattern on the saccular epithelium (e.g., Popper and Coombs, 1982; Popper and Fay, 1999), and/or an enlarged rostral segment in the saccular macula (Ramcharitar et al., 2001, 2006). The pattern in Antimora (Fig. 7B), with eight orientation groups, has not been seen in other fishes studied to date and adds a new category to the saccular hair bundle orientation patterns summarized by Popper and Coombs (1982). Such a complicated pattern may imply differentiated signal processing of sound stimuli at the peripheral level.
The swim bladder’s anterior projection overlaps with two thirds of the length of the saccular pouch, almost embracing the entire length of the saccular macula. This feature may provide signal enhancement to the whole length of the macula. Although the anterior gas chamber is closer to the posterior portion of the saccule (Fig. 4C), where there are only two horizontally oriented bundle groups, the direct connection to the swim bladder is at the anterior part of the macula (Fig. 4A-C, Fig. 5) which has all four orientation directions in that area. Thus, it is not easy to determine which part of the macula is under stronger stimulation from the swim bladder and how the different parts work.
Although a complex saccular structure is correlated with better hearing in some fishes (Popper and Coombs, 1982; Ramcharitar et al., 2001, 2006), it is not necessarily the case for all fishes. The highly specialized inner ears of ostariophysines with the Weberian apparatus have a relatively small saccule and a large lagena. The hair bundle orientation pattern in the saccule has only two vertically orientated groups (Platt 1977, 1993). Ostariophysines, such as goldfish and catfish, form a unique group of fish with the innovation of Weberian apparatus to improve hearing (Fay, 1988). They may not be comparable with fishes that have non-Weberian otophysic connections and the mechanism for enhance hearing may be very different for different group of fishes. In this study, Antimora rostrata is compared only with fishes that possess non-Weberian otophysic connections.
Complex saccular hair bundle orientation patterns have been noted in two groups of fishes that are not related to morids, but which have similar coupling between the swim bladder and the inner ear. For example, in clown knifefish (Notopterus chitala), the swim bladder projections cover two-thirds of the saccule with a sandwich attachment of the swim-bladder/bony-capsule/saccular-sac (Coombs and Popper, 1982). Intriguingly similar to Antimora, the saccular macula of clown knifefish is also elongated and can be separated into three parts by narrow bridges (Coombs and Popper, 1982). The orientation pattern on the saccular macula is also elaborated in the anterior portion, which is also seen in the anterior saccule of Antimora that couples with the swim bladder via bony capsule. The hearing range of clown knifefish is slightly wider than that of non-otophysic fishes (from 100 to 1000 Hz in behavioral tests), but the sensitivity is not as great as in other fishes with swim bladder-inner ear connections (Coombs and Popper, 1982).
The saccular macula of the pinecone soldierfish (Myripristis murdjans), another species from the squirrelfish family that has an otophysic connection, also has separate parts with narrow linkages between them (Popper, 1977), as seen in Antimora. The shape and length of the saccular macula in the pinecone soldierfish is very different from the Hawaiian squirrelfish, a non-otophysic member in the same family, and the hair bundle orientation pattern in the anterior portion of the macula in pinecone soldierfish is more complicated than in Hawaiian squirrelfish (Popper, 1977). As mentioned in the previous section, the hearing sensitivity and frequency range in shoulderbar soldierfish (Myripristis kuntee) are much better than in Hawaiian squirrelfish (Coombs and Popper, 1979).
On the other hand, the swim bladder of a gadiform fish from shallow water, the Atlantic cod, has very thin ducts that extended from the swim bladder to about 6 to 7mm from the saccule (Dale, 1976). The structure and hair bundle orientation pattern of Atlantic cod’s saccular macula is not as complex as in Antimora. There is no enlargement in the anterior part of the saccule and there are only six hair bundle orientation groups (Dale, 1976). A similar orientation pattern is found in another gadiform, the European hake (Lombarte and Popper, 1994, 2004). Interestingly, the saccules in the Atlantic cod and European hake are very similar to those in a non-otophysic deep-sea gadiform family Macrouridae, which ecologically coexists with Antimora on the deep-sea slope (Deng et al., 2002 and personal observations). Based on these data, it seems that at least in the order Gadifomes, the enlarged and elaborate saccular macula is not necessarily related to deep-water living, but to the presence of an otophysic connection.
It is difficult to imply any physiological characteristics of Antimora’s saccule based on data from the clown knifefish and soldierfish, but the similar “eccentric” organization in bundle orientation in the saccules of these three species may give a clue for such non-Weberian swim bladder-saccule relationships. Cross-taxon comparisons suggest that saccular complexity associated with an otophysic connection may be a convergent evolutionary feature in some groups of fishes.
5. Conclusion
A number of unique features in the inner ear of Antimora rostrata support the hypothesis of enhanced sensitivity in the inner ear of some deep-sea fishes as compared with shallow-water fishes. In Antimora rostrata, the large inner ears and elongated saccular otolith and macula, and especially the enlarged anterior part of the saccular macula, may imply increased hearing sensitivity. The complicated eight-way bundle orientation pattern may facilitate enhanced directional coding at the peripheral level. The suggestion of enhanced sensitivity is also supported by a close connection between the saccule and the swim bladder. The rigidity and thickness of end organ walls may be an adaptation for deep-water living. The membrane wall of the sensory epithelium is several times thicker than in other fishes and is partially sclerotic, which may help the whole inner ear move together with the membrane attached to the bony capsule; and the attachment between the end organ walls and the surrounding bones implies that the chambers may oscillate exactly with the swim bladder’s movements while the otoliths are fully suspended via the otolith membrane and hair bundles. Some of these specialized structures in the inner ear of Antimora may be resulted form adaptations to deep-water living.
Acknowledgements
Thanks to Professor Imants Priede for inviting Deng and Wagner to the RRS Discovery cruises D252, 255 and 260. These cruises were funded by a NERC grant awarded to Priede, Collins, and Bagley from the University of Aberdeen (GR3/12789). Also thanks to the Masters and crews of RRS Discovery for expert nautical work. Thanks to T. Maugel for his training and expert advice on SEM work. We thank Drs. W. Hodos, C. Platt, A. Coffin, B. Casper for reading and commenting on earlier versions of this paper. Portions of the microscopy work were supported by P-30 grant 2 P30 DC004664 from the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey DM, Bagley PM, Jamieson AJ, Collins MA, Priede IG. In situ investigation of burst swimming and muscle performance in the deep-sea fish Antimora rostrata. J. Exp. Mar. Biol. Ecol. 2003;286:295–311. [Google Scholar]
- Bierbaum G. Untersuchungen über den Bau der Gehörorgane von Tiefseefischen. Z. Wiss. Zool. 1914;III:281–380. [Google Scholar]
- Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Bregman AS, Peter Dallos, Donata Oertel. Auditory scene analysis. In: Basbaum AI, Kanenko A, Shepherd GM, Westheimer G, editors. The Senses: A Comprehensive Reference. Vol 3, Audition. Academic Press; San Diego: 2008. pp. 861–870. [Google Scholar]
- Buran BN, Deng X, Popper AN. Structural variation in the inner ears of four deep-sea Elopomorph fishes. J. Morph. 2005;265:215–225. doi: 10.1002/jmor.10355. [DOI] [PubMed] [Google Scholar]
- Campana SE. Photographic atlas of fish otoliths of the Northwest Atlantic Ocean. National Research Council Canada; Ottawa: 2004. [Google Scholar]
- Cohen DM, Inada T, Iwamoto T, Scialabba N. FAO Fisheries Synopsis. No. 125. Vol. 10. FAO; Rome: 1990. FAO species catalogue. Vol. 10. Gadiform fishes of the world (Order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date; pp. 352–354. [Google Scholar]
- Collins MA, Priede IG, Bagley PN. In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. Biol. Sci. 1999;266:2011–2016. [Google Scholar]
- Coombs S, Popper AN. Hearing differences among Hawaiian squirrelfish (Family Holocentridae) related to difference in the peripheral auditory system. J. Comp. Physiol. A. 1979;132:203–207. [Google Scholar]
- Coombs S, Popper AN. Structure and function of the auditory system in the clown knife fish, Notopterus chitala. J. Exp. Biol. 1982;97:225–239. [Google Scholar]
- Dale T. The labyrinthine mechanoreceptor organs of the cod Gadus morhua L. (Teleostei: Gadidae) Norwegian J. Zool. 1976;24:85–128. [Google Scholar]
- Deng X, Wagner H-J, Popper AN. Messages from the bottom of the Atlantic Ocean: Comparative studies of anatomy and ultrastructure of the inner ears of several gadiform deep-sea fishes. Abstr. Midwinter Res. Meet. Assoc. Res. Otolaryngol. 2002;25:383. http://www.aro.org/archives/2002/2002383.html. [Google Scholar]
- Denton EJ, Locket NA. Possible wavelength discrimination by multibank retinae in deep-sea fishes. J. Mar. Biolog. Assoc. U.K. 1989;46:685–722. [Google Scholar]
- Douglas RH, Partridge JC, Marshall NJ. The eyes of deep-sea fish. I: Lens pigmentation, tapeta and visual pigments. Prog. Retin. Eye. Res. 1998;17:597–636. doi: 10.1016/s1350-9462(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in Vertebrates: A Psychophysics Databook. Hill-Fay Associates; Winnetka, IL: 1988. [Google Scholar]
- Fine ML, Horn MH, Cox B. Acanthonus armatus, a deep-sea teleost fish with a minute brain and large ears. Proc. R. Soc. Lond., B, Biol. Sci. 1987;230:257–265. doi: 10.1098/rspb.1987.0018. [DOI] [PubMed] [Google Scholar]
- Fossen I, Bergstad OA. Distribution and biology of blue hake, Antimora rostrata (Pisces: Moridae), along the mid-Atlantic Ridge and off Greenland. Fisheries Res. 2006;82:19–29. [Google Scholar]
- Fröhlich E, Wagner HJ. Rod outer segment renewal in the retinae of deep-sea fish. Vision Res. 1996;36:3183–3194. doi: 10.1016/0042-6989(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Fröhlich E, Wagner HJ. Development of multibank rod retinae in deep-sea fishes. Vis. Neurosci. 1998;15:477–483. doi: 10.1017/s095252389815304x. [DOI] [PubMed] [Google Scholar]
- Gordon JDM, Duncan JAR. The biology of fish of the family Moridae in the deep-water of the Rockall Trough. J. Mar. Biol. Assoc. U.K. 1985;65:475–485. [Google Scholar]
- Günther A. Preliminary notices of deep-sea fishes collected during the voyage of H. M. S. “Challenger.”. (Ser. 5).Ann. Mag. Nat. Hist. 1878:17–28. [Google Scholar]
- Herring PJ. Species abundance, sexual encounter and bioluminescent signallin in the deep sea. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2000;355:1273–1276. doi: 10.1098/rstb.2000.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring PJ. 7, Chemical messages. Oxford University Press; New York: 2002. The biology of the deep ocean; pp. 148–160. [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. U.S.A. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T. The abyssal fish Antimora rostrata (Günther) Comp. Biochem. Physiol., B. 1975;52:7–11. doi: 10.1016/0305-0491(75)90108-x. [DOI] [PubMed] [Google Scholar]
- Josephson RV, Holtz RB, Misock JP, Phleger CF. Composition and partial protein characterization of swim bladder foam from deep-sea fish Coryphaenoides acrolepis and Antimora rostrata. Comp. Biochem. Physiol., B. 1975;52:91–95. doi: 10.1016/0305-0491(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Kulka DW, Simpson MR, Inkpen TD. Distribution and Biology of Blue Hake (Antimora rostrata Günther 1878) in the Northwest Atlantic with Comparison to Adjacent Areas. J. Northwest Atlantic Fisheries Sci. 2003;31:299–318. [Google Scholar]
- Kuperman WA, Roux P. Underwater acoustics. In: Rossing TD, editor. Springer Handbook of Acoustics. Springer; New York: 2007. pp. 149–201. [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. Comparative inner ear anatomy. In: Edwin RL, Ellen LL, William B, editors. The Vertebrate Inner Ear. CRC press; Boca Raton, FL: 1985. pp. 13–94. [Google Scholar]
- Locket NA. Adaptations to the deep-sea environment. In: Crescitelli F, editor. Handbook of sensory physiology. Vol. VII/5. The Visual System in Vertebrates. Springer-Verlag; Berlin: 1977. pp. 67–192. [Google Scholar]
- Lombarte A, Popper AN. Quantitative analyses of postembryonic hair cell addition in the otolithic endorgans of the inner ear of the European hake, Merluccius merluccius (Gadiformes, Teleostei) J. Comp. Neurol. 1994;345:419–428. doi: 10.1002/cne.903450308. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Popper AN. Quantitative changes in the otolithic organs of the inner ear during the settlement period in European hake (Merluccius merluccius) Mar. Ecol. Prog. Ser. 2004;267:233–240. [Google Scholar]
- Lu Z, Popper AN. Morphological polarizations of sensory hair cells in the three otolithic organs of a teleost fish: fluorescent imaging of hair bundles. Hear. Res. 1998;126:47–57. doi: 10.1016/s0378-5955(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Marshall NB. Sound-producing mechanisms and the biology of deep-sea fishes. In: Tavolga WN, editor. Marine Bio-acoustics. Volume 2. Pergamon Press; Oxford: 1966. pp. 123–133. [Google Scholar]
- Marshall NB. Explorations in the Life of Fishes. Harvard University Press; Cambridge, MA: 1971. [Google Scholar]
- Marshall NB. Deep-Sea Biology: Developments and Perspectives. Garland STPM Press; New York and London: 1980. [Google Scholar]
- Marshall NJ. The lateral line system of three deep-sea fish. J. Fish. Biol. 1996;49(Suppl. A):239–258. [Google Scholar]
- Munk O. Ocular degeneration in deep-sea fishes. Galathea Rep. 1964;8:21–32. [Google Scholar]
- Munk O. Ocular anatomy of some deep-sea teleosts. Dana Rep. 1966;70:1–62. [Google Scholar]
- Nelson EM. The morphology of the swim bladder and auditory bulla in the Holocentridae. Fieldiana Zool. 1955;37:121–130. [Google Scholar]
- Patton S, Thomas AJ. Composition of lipid foams from swim bladders of two deep ocean fish species. J. Lipid Res. 1971;12:331–335. [PubMed] [Google Scholar]
- Paulin CD. Swim bladder structure in morid cods (Pisces: Gadiformes) Copeia. 1988;1988:450–454. [Google Scholar]
- Platt C. Hair cell distribution and orientation in goldfish otolith organs. J. Comp. Neurol. 1977;172:283–287. doi: 10.1002/cne.901720207. [DOI] [PubMed] [Google Scholar]
- Platt C. The peripheral vestibular system of fishes. In: Northcutt RG, Davis RE, editors. Fish Neurobiology. Vol . I, Brainstem and Sensory Organs. University of Michigan Press; Ann Arbor: 1983. pp. 89–123. [Google Scholar]
- Platt C. Zebrafish inner ear sensory surface are similar to those in goldfish. Hear .Res. 1993;65:133–140. doi: 10.1016/0378-5955(93)90208-i. [DOI] [PubMed] [Google Scholar]
- Platt C, Popper AN. Fine structure and function of the ear. In: Tavolga WN, Popper AN, Fay RR, editors. Hearing and Sound Communication in Fishes. Springer; New York: 1981. pp. 3–38. [Google Scholar]
- Poggendorf D. Die absoluten Hörschwellen des Zwergwelses (Amiurus nebulosus) und Beiträge zur physic des Weberschen Apparates der Ostariophysen. Z. Vergl. Physiol. 1952;34:222–257. [Google Scholar]
- Popper AN. A scanning electron microscopic study of the sacculus and lagena in the ears of fifteen species of teleost fishes. J. Morphol. 1977;153:397–418. doi: 10.1002/jmor.1051530306. [DOI] [PubMed] [Google Scholar]
- Popper AN. Scanning electron microscopic study of the saccules and lagena in several deep-sea fishes. Am. J. Anat. 1980;157:115–136. doi: 10.1002/aja.1001570202. [DOI] [PubMed] [Google Scholar]
- Popper AN. Organization of the inner ear and auditory processing. In: Northcutt RG, Davis RE, editors. Fish Neurobiology. University of Michigan Press; Ann Arbor: 1983. pp. 125–178. [Google Scholar]
- Popper AN, Coombs S. The morphology and evolution of the ear in Actinopterygian fishes. Am. Zool. 1982;22:311–328. [Google Scholar]
- Popper AN, Fay RR. The auditory periphery in fishes. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. Springer-Verlag; New York: 1999. pp. 43–100. [Google Scholar]
- Popper AN, Fay RR, Platt C, Sand O. Sound detection mechanisms and capabilities of teleost fishes. In: Collin SP, Marshall NJ, editors. Sensory Processing in Aquatic Environments. Springer-Verlag; New York: 2003. pp. 3–38. [Google Scholar]
- Popper AN, Tavolga WN. Structure and function of the ear in the marine catfish, Arius felis. J. Comp. Physiol. A. 1981;144:27–34. [Google Scholar]
- Ramcharitar J, Higgs DM, Popper AN. Sciaenid inner ears: a study in diversity. Brain Behav. Evol. 2001;58:152–162. doi: 10.1159/000047269. [DOI] [PubMed] [Google Scholar]
- Ramcharitar J, Higgs DM, Popper AN. Audition in sciaenid fishes with different swim bladder-inner ear configurations. J. Acoust. Soc. Am. 2006;119:439–443. doi: 10.1121/1.2139068. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehororgan der Wirbelthiere. Vol I. Das Gehororgan der Fische und Amphibien. Samson and Wallin; Stockholm: 1881. [Google Scholar]
- Schellart NAM, Popper AN. Functional aspects of the evolution of the auditory system of Actinopterygian fish. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. Springer-Verlag; New York: 1992. pp. 295–322. [Google Scholar]
- Scholander PF. Secretion of gases against high pressures in the swimbladder of deep sea fishes. II. The rete mirabile. Biol. Bull. 1954;107:260–277. [Google Scholar]
- Siebenaller JF, Murray TF. A1 adenosine receptor modulation of adenylyl cyclase of a deep-living teleost fish, Antimora rostrata. Biol. Bull. 1990;178:65–73. doi: 10.2307/1541538. [DOI] [PubMed] [Google Scholar]
- Small GJ. A review of the bathyal fish genus Antimora (Moridae: Gadiformes) Proc. Calif. Acad. Sci. 1981;42:341–348. [Google Scholar]
- Song J, Matieu A, Soper RF, Popper AN. Structure of the inner ear of bluefin tuna (Thunnus thynnus) J. Fish. Biol. 2006;68:1767–1781. [Google Scholar]
- Tavolga WN. Sound production and detection. In: Hoar WS, Randall DJ, editors. Fish physiology. vol 5. Academic Press; New York: 1971. pp. 135–205. [Google Scholar]
- van Bergeijk WA. The evolution of vertebrate hearing. In: Neff WD, editor. Contributions to Sensory Physiology. Academic Press; New York: 1967. pp. 1–49. [DOI] [PubMed] [Google Scholar]
- von Frisch K. Zur psychologie des Fisch-Schwarmes. Naturwissenschaften. 1938;26:601–606. [Google Scholar]
- Warrant EJ, Locket NA. Vision in the deep sea. Biol. Rev. 2004;79:671–712. doi: 10.1017/s1464793103006420. [DOI] [PubMed] [Google Scholar]
- Wagner H-J, Fröhlich K, Negishi K, Collin SP. The eyes of deep-sea fish. II: Functional morphology of the retina. Prog. Retin. Eye. Res. 1998;17:637–685. doi: 10.1016/s1350-9462(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Weber EH. De Aure et Auditu Hominis et Animalium. Pars I. de aure animalium aquatilium. Gerhard Fleischer; Leipzig: 1820. [Google Scholar]
- Wenner CA, Musick JA. Biology of the morid fish, Antimora rostrata, in the western North Atlantic. J. Fish. Res. Board Can. 1977;34:2362–2368. [Google Scholar]
- Wersäll J, Flock A. Physiological aspects on the structure of vestibular end organs. Acta Otolaryngol. Suppl. 1963;192:85–9. [PubMed] [Google Scholar]
- Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E. Fishes of the North-eastern Atlantic and Mediterranean. UNESCO; Paris: 1984. [Google Scholar]