Abstract
Morbidity and mortality due to seasonal and pandemic influenza could be reduced by simpler vaccination methods that enable improved vaccination coverage. In this study, solid metal microneedles coated with influenza virus-like particle (VLP) vaccine were inserted into skin for intradermal immunization. Microneedles were applied to the skin by hand and designed for simple administration with little or no training. Inclusion of trehalose in the coating formulation significantly increased vaccine stability during coating by maintaining hemagglutination activity. Mice vaccinated with stabilized microneedles developed strong antibody responses comparable to conventional intramuscular vaccination and were fully protected against subsequent viral challenge. Whereas, coating microneedles with a coating solution lacking trehalose led to only partial protection against lethal viral challenge. Therefore, our results show that microneedles coated with trehalose-stabilized VLP vaccine can be a promising tool for improving influenza vaccination.
1. Introduction
The influenza virus is in the family of Orthomyxoviridae and RNA-containing viruses with immunogenic surface proteins such as hemagglutinin (HA) and neuraminidase (NA) (1). Influenza viruses have been a critical cause of death and hospitalization, and their global impact on public health has received increased attention due to the recent emergence of the new swine H1N1 virus (2).
The spread of influenza viruses can be prevented effectively by vaccination (3). In contrast to conventional vaccination by injection with a hypodermic needle by a trained medical expert, vaccination using microneedles has recently been investigated as a simple method of vaccine administration that targets vaccine antigen delivery to the skin (4). Microneedles have been designed in various ways, including formulation as a patch coated with vaccine that can be applied to the skin by personnel with minimal training or possibly by patients themselves. In addition, immunization via the skin is attractive in part because the epidermis and dermis have abundant antigen-presenting cells such as Langerhans and dermal dendritic cells (5). Previously, microneedles have been studied for influenza vaccination using inactivated virus antigens using coated microneedle patches (6–11), as well as hollow microneedles for injection (12, 13). These studies showed that influenza vaccination in this way induced strong humoral and cellular immune responses.
Virus-like particle (VLP) vaccines have been a major advance in subunit immunogen research and derived attention due to their expected improvements in safety and cellular immunogenicity compared to inactivated virus vaccines (14). Influenza VLPs have been widely used for influenza vaccination and shown promise for effective vaccination (15).
In this study, we evaluate the use of microneedle patches coated with VLPs as a simple method of influenza vaccination. We further examine the role of trehalose to stabilize the VLPs during microneedle coating and thereby retain immunogenicity.
2. Materials and methods
2.1. Preparation of influenza VLPs and coated microneedles
VLP vaccine containing influenza M1 and HA (A/PR/8/34) was prepared as described in our previous study (15). Stainless steel microneedles were fabricated using laser cutting and electro-polishing, as described before (6). To apply a vaccine coating, microneedles were dipped six times at room temperature into coating solution using a dip-coating device and air dried. The standard coating solution was composed of 1% (w/v) carboxymethylcellulose sodium salt (Carbo-Mer), 0.5% (w/v) Lutrol F-68 NF (BASF), and 2 mg/ml VLP vaccine in phosphate buffered saline (PBS). In some cases, 15% (w/v) trehalose (Sigma Aldrich) was added.
2.2. Antigenicity of influenza VLPs according to drying time
A 1 μL droplet of coating solution either with or without trehalose was mixed with 1 μL of VLP vaccine on a 3 mm × 3 mm piece of the same stainless steel used to make microneedles. After drying in air at room temperature for different drying times, the coating was dissolved from the metal piece in 100 μL of PBS for 12 h. Coating on these metal pieces produced similar antigen stability to coating stainless steel microneedles (data not shown), which suggests that using these metal pieces is a suitable model system. To determine hemagglutination (HA) titers as a measurement of VLP antigenicity after coating, VLP vaccine dissolved from the metal pieces was serially diluted in 100 μL of PBS deficient in Mg2+ and Ca2+, mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire), and incubated for 1 h at 25°C. The titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells (16).
2.3. Immunization using microneedles
BALB/c mice (Charles River) were anesthetized intramuscularly with 110 mg/kg ketamine (Abbott Laboratories) mixed with 11 mg/kg xylaxine (Phoenix Scientific). The skin on the back of the mouse was exposed by removing hair with depilatory cream (Nair), washed with 70% ethanol, and dried with a hair dryer. An in-plane, five-needle array of microneedles coated with 1 μg of influenza VLP vaccine formulated either with or without trehalose was manually inserted into the skin and left for 10 min to dissolve the vaccine coating in the skin. The vaccine dose coated on microneedles was measured by a BCA protein assay kit (Pierce). As a positive control, 1 μg of influenza VLP vaccine formulated either with or without trehalose was dissolved from a microneedle array in 100 μl PBS and injected intramuscularly in the upper quadriceps muscles of mice. Naïve (i.e., negative control) mice received no treatment.
2.4. Antibody responses and viral challenge infection after immunization
Influenza virus-specific antibody (IgG) was measured by enzyme-linked immunosorbent assay (ELISA)(15). Data are presented as optical densities read at 450 nm. For virus challenge, isoflurane-anesthetized mice were intranasally infected with the mouse-adapted A/PR8 virus (20 LD50) five weeks after vaccination. Mice were observed daily to monitor changes in body weight and to record mortality. All animal studies were approved by the Emory University Institutional Animal Care and Use Committee.
2.5. Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated. A two-tailed Student’s t-test (α=0.05) was performed when comparing two different conditions. In all cases, a value p<0.05 was considered statistically significant.
3. Results and discussion
3.1. Effect of trehalose on the kinetics of HA activity loss by VLPs coated onto microneedles
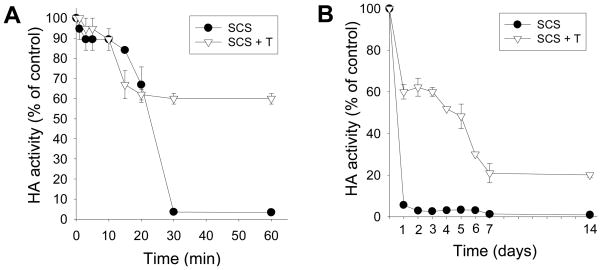
Previous studies have shown that coating of other influenza vaccines onto microneedles can damage their antigenicity (6). Because VLPs contain hemagglutinin as a major antigenic component, we measured HA activity to assess vaccine antigenicity during the drying process of influenza VLP vaccine. Because previous studies showed that the addition of trehalose to the coating formulation can help stabilize other influenza vaccines (6), we also studied the effect of trehalose on the kinetics of VLP vaccine activity during a 1-h short-term study and 2-week long-term study (Fig. 1)
Figure 1.
Effect of trehalose on the kinetics of HA activity loss by VLPs coated onto microneedles. VLPs were coated (●) without and (▽) with 15% trehalose present in the coating formulation. HA activity was measured at different times to assess antigenicity. (A) Short-term and (B) long-term kinetics of HA activity loss (SCS: standard coating solution, T: trehalose).
During the short-term study, HA activity of the VLP vaccine dropped to approximately 60% within 20 min independent of the presence of trehalose in the coating formulation (Fig. 1A). Without trehalose, HA activity continued to drop, such that almost all HA activity was lost by the 30 min time point. However, with trehalose in the formulation, HA activity remained stable from the 20 min time point until the 1 h time point. This indicates that there were two phases of HA activity loss during the drying process. While trehalose did not protect the vaccine during the first phase, it did provide significant protection during the second phase and thereby retained HA activity at 60%.
During the long-term study, almost all HA activity was lost before the first measurement at 1 day for VLP vaccine lacking trehalose stabilization (Fig.1B). In contrast, the presence of trehalose helped the VLP vaccine to retain 60% HA activity until 3 days. There was then a gradual decrease in HA activity to 25% during the period up to 7 days. After day 7, there was no significant drop in HA activity through day 14. This suggests that there is a third phase of antigencity loss over a time scale of days, for which trehalose provided partial stabilization.
Overall, we found that microneedle coating of VLPs caused a rapid loss in vaccine antigenicity as determined by HA activity. The addition of trehalose disaccharide sugar to the coating formulation significantly stabilized VLPs during microneedle coating and initial storage. However, the VLPs slowly lost HA activity within 1 week. Therefore, additional studies are required to further stabilize the VLPs and thereby maintain HA activity during extended storage.
3.2. Induction of antibody response and protection against viral challenge after vaccination
To validate in vitro findings of antigenicity, we next vaccinated mice to evaluate immunogenicity after immunization using microneedles with and without trehalose. Groups of mice were immunized with a single 1 μg dose of influenza VLP vaccine in the skin after coating the vaccine in the absence (MN, n=6) or presence of trehalose (MN+T, n=6) in the coating formulation. In order to compare with the conventional intramuscular route of immunization, mice were immunized with influenza VLP vaccine that was dissolved from coated microneedles in the absence (IM, n=6) or presence of trehalose (IM+T, n=6) using intramuscular injection. A naive control group was not treated with any immunization (n= 6).
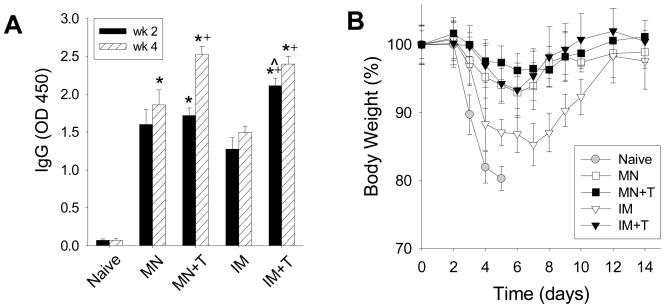
Serum samples taken 2 weeks and 4 week after immunization were used to determine A/PR8 virus-specific total IgG. As shown in Fig. 2A, microneedle immunizations (MN, MN+T) induced lower levels of IgG antibodies than those by the stabilized intramuscular injection (IM+T) but higher than those by the unstabilized intramuscular injection (IM) immunizations at week 2. However, levels of IgG in the stabilized microneedle group (MN+T) caught up those from the corresponding intramuscular group (IM+T) at week 4, which was significantly higher than those induced by unstabilized influenza VLP vaccination by either route (MN, IM).
Figure 2.
Induction of antibody response and protection against viral challenge after vaccination. (A) Total serum antibody responses specific to influenza virus (IgG) measured 2 and 4 weeks after immunization with either microneedles coated with VLP vaccine or intramuscular injection of the same VLP vaccine (+: p<0.05 compared to MN, ^: p<0.05 compared to MN+T, *: p<0.05 compared to IM). (B) Body weight change of immunized mice after lethal challenge infection. (MN: microneedles without trehalose, MN+T: microneedles with trehalose, IM: intramuscular injection of reconstituted vaccine without trehalose, IM+T: intramuscular injection of reconstituted vaccine with trehalose, Naive: no treatment.)
Five weeks after immunization, groups of mice including the naïve mice were challenged with a lethal dose of influenza virus infection. All of the naïve mice lost over 20% of body weight by day 5 and died (survival rate=0%). In contrast, both of the microneedles groups (MN, MN+T) and the stabilized intramuscular group of mice (IM+T) were completely protected from lethal virus challenge (survival rate=100%). The stabilized microneedle group (MN+T) lost slightly less body weight than the other two groups (MN, IM+T) and these groups did not show any serious sickness (Fig. 2B). However, only 67% of mice in the unstabilized intramuscular group (IM) survived lethal challenge infection and lost body weight up to 15%, which indicates serious illness (Fig. 2B).
From these results, we found that vaccination using trehalose-stabilized VLP vaccine (MN+T) induced a robust antibody response that conferred complete protection from challenge similar to intramuscular vaccination using the same formulation (IM+T). Microneedle vaccination without trehalose stabilization (MN) yielded an inferior antibody response compared to the trehalose-stabilized formulation (MN+T). However, intramuscular injection of VLPs lacking trehalose stabilization (IM) were even less protective.
4. Conclusion
Microneedles used in this study offer the opportunity to simplify vaccination through the use of a patch that could be applied by minimally trained personnel or possibly through self administration. In vitro antigenicity and in vivo immunogenicity studies showed that microneedle vaccination using influenza VLPs stabilized with trehalose induced similar protective immunity compared to intramuscular injection. We also found that VLPs coated without trehalose in the formulation were more immunogenic when administered using microneedles compared to intramuscular injection, indicating that the microneedle route of administration in the skin was more immunogenic in this situation. Our results thus demonstrate that microneedles coated with trehalose-stabilized VLP vaccine can be a promising tool for improving influenza vaccination.
Acknowledgments
This work was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. It was supported in part by NIH grants R01-EB006369 (M.R.P.), U01-AI0680003 (R.W.C.), SERCEB (R.W.C) and the Georgia Research Alliance (S.M.K). We thank Dr. Vladimir Zarnitsyn for microneedle fabrication and Dr. Mark Allen for use of his laser microfabrication facilities.
References
- 1.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26:D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiris JSM, Poon LLM, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–173. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monto AS, Ohmit SE. Seasonal influenza vaccines: evolutions and future trends. Expert Rev Vaccines. 2009;8:383–389. doi: 10.1586/erv.09.9. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partidos CD, Beignon AS, Mawas F, Belliard G, Briand JP, Muller S. Immunity under the skin: potential application for topical delivery of vaccines. Vaccine. 2003;21:776–780. doi: 10.1016/s0264-410x(02)00597-2. [DOI] [PubMed] [Google Scholar]
- 6.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010 doi: 10.1016/j.jconrel.2009.10.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang CL, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salam C, Leroux-Roels G. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–6619. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Damme PV, Oosterhuis-Kafeja F, Wielen MVD, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 14.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 15.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath S. Estimation of Hemagglutination-Inhibition Titers. Arch Virol. 1975;49:199–205. doi: 10.1007/BF01317538. [DOI] [PubMed] [Google Scholar]