Abstract
This article reviews existing methods for the isolation, fractionation, or capture of rare cells in microfluidic devices. Rare cell capture devices face the challenge of maintaining the efficiency standard of traditional bulk separation methods such as flow cytometers and immunomagnetic separators while requiring very high purity of the target cell population, which is typically already at very low starting concentrations. Two major classifications of rare cell capture approaches are covered: (1) non-electrokinetic methods (e.g., immobilization via antibody or aptamer chemistry, size-based sorting, and sheath flow and streamline sorting) are discussed for applications using blood cells, cancer cells, and other mammalian cells, and (2) electrokinetic (primarily dielectrophoretic) methods using both electrode-based and insulative geometries are presented with a view towards pathogen detection, blood fractionation, and cancer cell isolation. The included methods were evaluated based on performance criteria including cell type modeled and used, number of steps/stages, cell viability, and enrichment, efficiency, and/or purity. Major areas for improvement are increasing viability and capture efficiency/purity of directly processed biological samples, as a majority of current studies only process spiked cell lines or pre-diluted/lysed samples. Despite these current challenges, multiple advances have been made in the development of devices for rare cell capture and the subsequent elucidation of new biological phenomena; this article serves to highlight this progress as well as the electrokinetic and non-electrokinetic methods that can potentially be combined to improve performance in future studies.
Keywords: Biomedical engineering, Electrophoresis, Separations, Hydrodynamics, Microfluidics, Rare cell capture
1. Introduction
The isolation, fractionation, and capture of cells from suspensions has a wide range of applications, from the detection of bacteria (Liu et al., 2007a; Wu et al., 2009) to the enumeration of cancer cells (Gascoyne et al., 2009; Chen and Du, 2006; Nagrath et al., 2007). The benefits and limitations of flow cytometers, immunomagnetic separators, and other macro-sized sorting equipment have been studied extensively in experimentation and in review (Pappas and Wang, 2007; Chen et al., 2008; Kulrattanarak et al., 2008) when compared to the abilities of microdevices. This article focuses on devices and techniques with potential to analyze cells that are typically found at low concentrations in suspension; such devices are currently used, or have the potential to be used, for applications in environmental pathogen detection (Lapizco-Encinas et al., 2005; Yang et al., 2006) and cancer cell isolation from patient blood samples (Gleghorn et al., 2010). The discussion is divided into sections that detail two major classifications of microfluidic approaches, non-electrokinetic and electrokinetic, followed by a summary of performance criteria by which these methods are evaluated; studies that focused on quantifying these performance specifications are highlighted in tables at the end of the article. While rare cell capture is the ultimate motivation of this paper, many of the described methods exist only as proof-of-concept studies. Thus, this article serves to highlight both the progress made in using microfluidic devices for rare cell capture and the techniques that may contribute to rare cell capture in the near future.
2. Non-electrokinetic Methods
This section focuses upon non-electrokinetic methods of cell isolation, capture, or fractionation from a suspension. As such, it lends itself naturally to organization by sorting technique: antibody capture, size-selective sorting, streamline focusing, and sheath flow. Each sorting methodology is further subdivided into cell separations of interest: blood cell fractionation, cancer cells, other mammalian cells, and prokaryotes and viruses.
Blood cell fractionation, as defined here, focuses on isolation of cell types native to circulation. Most of the studies described here revolve around the capture or elimination of white blood cells (WBCs). WBCs are of value in many diagnostic assays and studies of disease progression, but they must first be separated from the bulk blood suspension. However, WBC concentrations are low as compared to red blood cells (RBCs), roughly 1 to 1000 (Murthy et al., 2004; VanDelinder and Groisman, 2007). Conversely, for the purpose of leukemia treatments, blood transfusions, etc. it is vital to eliminate WBCs as a source of contaminantion (Sethu et al., 2005).
Studies for the isolation of cancer cells capturing circulating tumor cells (CTCs) or approximating them with model cell lines. CTCs can be found in the circulation of cancer patients (Nagrath et al., 2007; Gleghorn et al., 2010; Stott et al., 2010) and have been used as prognostic indicators of patient survival (Danila et al., 2007) as well as representative tissue for genetic analyses (Stott et al., 2010). CTCs are 106 rarer than WBCs, making their capture particuarly challenging (Nagrath et al., 2007; Adams et al., 2008; Gleghorn et al., 2010).
Non-electokinetic microfluidic techniques have also been applied to study other mammalian cells. Applications are quite disparate, ranging from sorting of cells based on stages of cell cycle (Choi et al., 2009) to isolation of fetal nucleated red blood cells (nRBCs) from maternal blood (Huang et al., 2008; Mohamed et al., 2004, 2007).
2.1. Immobilization via Antibody or Aptamer Chemistry
The microfluidic devices discussed in this section take advantage of biochemical interactions to enhance rare cell capture or fractionation. Immunocapture is a technique frequently used in the extraction of cells, viruses, and proteins from suspension. It employs anti-sera to target biological agents of interest. In rare cell isolation, immunocapture presents an opportunity to separate cells with extremely high specificity from a suspension, in a viable state. In practice, this technique is analogous to microscale affinity chromatography for cells possessing unique markers or characteristics (Plouffe et al., 2007).
2.1.1. Blood Cell Fractionation
Chang et al. studied the effect of microfludic structures on white blood cell (WBC) adhesion using different pillar geometries and orientations. They compared square and rhombic arrays with square and ellipsoidal micropillars, respectively. The micropillars were physisorbed with E-selectin to identify different leukocyte model cell lines (in cell media) via adhesive rolling speeds. Cell rolling velocities were two times as high in rhombic arrays, resulting in 130- to 160-fold enrichment, as opposed to 200-fold enrichment in square arrays. By comparing microarray geometries under identical flow and immunocapture conditions, Chang et al. demonstrated that the type of pillar geometry alone influenced cell adhesion mechanics and, by extension, isolation (Chang et al., 2002).
In contrast, Murthy et al. focused upon the effects of shear stress on leukocyte adhesion mechanics. They studied the effects of shear stress using a Hele-Shaw flow cell with a device geometry that created a linear variation in shear stress along its axis (see Figure 1C). The researchers used anti-CD5, anti-CD19, and PEG to isolate T- and B-lymphocytes from a heterogeneous PBS suspension. Non-target cells were depleted from heterogeneous mixtures, resulting in suspensions that were 97% pure (Murthy et al., 2004). Sin et al. extended this work to blood, and studied the effects of suspension density on cell binding and the time-scale of cell-antibody kinetics. Within three minutes they obtained 100% and 75% pure suspensions of T-lymphocytes and B-lymphocytes, respectively (Sin et al., 2005). Wang et al. also captured T-lymphocytes using anti-CD3-coated micropillars. They surrounded their pillars with segmented curved walls to increase the range of shear stresses experienced by the cells. Using this technique, they were able to isolate T-lymphocytes spiked in blood with 80% efficiency (Wang et al., 2010). These studies, in combination, demonstrate that the efficiency and specificity of cell immobilization can be altered by changing the flow conditions within the microfluidic device.
Figure 1.
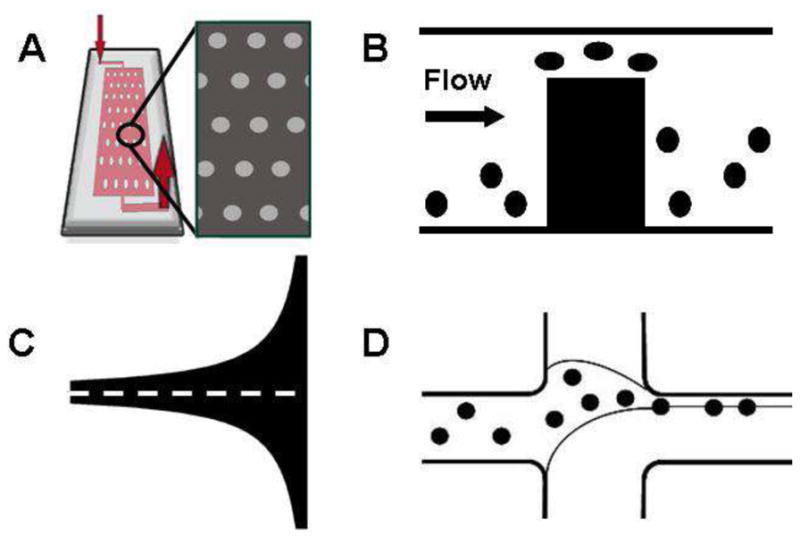
(A) Schematic of an micro-pillar device's architecture. Adapted from Gleghorn et al. (2010) (B) Schematic of a Weir microfilter's operation. Adapted from Ji et al. (2008) (C) Example of a Hele-Shaw flow cell where the dotted line is the region of linearly increasing shear (D) Schematic of a sheath-flow based separation system. Adapted from Wu et al. (2009)
2.1.2. Cancer Cells
Many microfludic devices take advantage of the 3D structure of channels to increase the surface area available to be coated with the antibody or aptamer of choice. Du et al. demonstrated the efficacy of this technique in straight microchannels by differentially capturing human mammary epithelial cells and breast cancer cells by use of epithelial membrane antigen (EMA) and epithelial growth factor receptor (EGFR) (Du et al., 2007). The sensitivity of capture to antibody dilution alone was also demonstrated using the same device geometry. Using this isolation technique, their capture rates from a PBS suspension ranged up to 30%. Xu et al. used DNA aptamers within an S-shaped microfluidic device (Xu et al., 2009) to capture cancer cells from PBS. Using aptamers targeted to various leukemia and lymphoma cell lines lines, their device efficiencies ranged from 50-83% with 88-97% purity. Recent work by Wang, et al. on silicon nanopillars (SiNPs) indicated that the topology of the microdevice itself may contribute greatly to the efficiency of rare cell capture. Comparing EpCAM functionalized SiNPs and flat surfaces, there was an approximately 6-fold increase in capture efficiency, from 4-14% to 45-65% (Wang et al., 2009).
Cancer cells have also been captured from blood-based systems. Liu et al. used nickel micro-pillars to immobilize functionalized superparamagnetic beads to create a capture zone within their microfludic devices. Using magnetic fields, they then immobilized and released an immortalized lung cancer cell line mixed with human RBCs. This method produced 133-fold enrichment with 62-74% capture efficiency (Liu et al., 2007a). Adams et al. observed cell margination along the walls of linear channels when working with whole rabbit blood. They hypothesized that this reduced the rate of cell-antibody interactions in their devices (Adams et al., 2008). This phenomenon was no longer seen when straight-walled channels were exchanged for sinusoidally varying ones. In combination with anti-epithelial growth factor receptor (EpCAM) antibodies, Adams et al. achieved immortalized breast cancer cell capture efficiences of 97%. The device was translated to the capture of model prostate cancer cells spiked in PBS, using anti-prostate specific membrane antigen (PSMA) aptamers with an efficiency of 90% (Dharmasiri et al., 2009).
While the prior studies worked with model cell lines spiked in buffer solution (Du et al., 2007; Xu et al., 2009; Dharmasiri et al., 2009; Wang et al., 2009) or blood systems (Liu et al., 2007a; Adams et al., 2008; Nagrath et al., 2007; Gleghorn et al., 2010), this method has also been used for cancer patient blood samples (Nagrath et al., 2007; Gleghorn et al., 2010). Nagrath et al. used a dense array of micro-pillars coated in EpCAM to increase the number of cell-antibody interactions for a given suspension volume. Using this approach, they isolated lung, prostate, pancreatic and other cell lines from blood samples with average effiency and purity of 65% and 50% respectively (Nagrath et al., 2007). Recently, Gleghorn et al. used computational modeling to design micro-pillar arrays such that cell-antibody interactions were size-dependent. Using microdevices functionalized with anti-PSMA antibodies, prostate cancer cells were captured at efficiences of 85-97% with purities of 68% (Gleghorn et al., 2010) (see Figure 1A).
2.1.3. Other Mammalian Cells
Plouffe et al. used previously discussed microfluidic devices (Murthy et al., 2004; Sin et al., 2005) to selectively isolate endothelial cells (ECs) and smooth muscle cells (SMCs) from suspension. They coated their devices with peptides (REDV and VAPG) targeted to ECs and SMCs, and investigated binding to target cells as a function of shear stress. Using these peptide sequences, they differentially isolated ECs and SMCs from homogenous and heterogenous suspensions with purities of 86% and 83%, respectively (Plouffe et al., 2007). Plouffe et al. further demonstrated the feasibility of peptide-based capture systems by using a 3-stage isolation system to deplete heterogenous suspensions of ECs, SMCs and fibroblasts (Plouffe et al., 2008). Using this system, they were able to achieve 96% to 99% depletion of the three different cell types with over 97% viability of non-immobilized cells. Their work agreed with results on shear-dependent cell capture discussed previously (Murthy et al., 2004; Sin et al., 2005), showing this relationship to be true regardless of cell type.
2.2. Size-based Sorting
Size-based sorting affords the ability to capture target cells without knowledge of the target cell's biochemical characteristics. This is an attractive option if the target cell's size is extreme in relation to its medium and also if the cell's properties are not well understood, as opposed to immunocapture, which can be employed regardless of cell size but requires knowledge of a unique cell trait that can be used as a marker. Both methods have been demonstrated to work successfully on non-pretreated biological samples(Sethu et al., 2005; VanDelinder and Groisman, 2007; Nagrath et al., 2007; Zheng et al., 2007; Adams et al., 2008; Gleghorn et al., 2010). Many approaches have been used to attempt size-sensitive isolation, ranging from size-dependent transport through small geometries to size-dependent particle pathlines in open obstacle arrays (Inglis et al., 2008; VanDelinder and Groisman, 2006, 2007; Sethu et al., 2005; Sin et al., 2005; Davis et al., 2006; Ji et al., 2008).
2.2.1. Blood Cell Fractionation
Much research has been done to develop microfludic platforms to fractionate blood components, particuarly WBCs, based on size. Sethu et al. developed a microfluidic diffusive filter for WBC depletion from whole human blood. The system allowed biconcave red blood cells (RBCs) egress from the main device while larger WBCs were retained. The filtration elements were placed on the sides of the main channel to minimize clogging by distributing RBC egress points along the length of the channel rather than focusing it in one area. To maintain equivalent volumetric flow rates in each segment, they used a flared geometry designed using Hele-Shaw analysis. Using this diffusive filter technique, over 97% WBC depletion was achieved (Sethu et al., 2005).
Ji et al. reviewed various other microfludic filtration techniques for the application of WBC depletion. They found that pillar filters and cross-flow filters had high WBC depletion rates and could be used to process large sample volumes (Ji et al., 2008). VanDelinder et al. also investigated cross-flow filters for WBC depletion, but also observed that RBC clogging hindered performance. They subsequently attempted WBC isolation using repeated microfluidic array geometries, achieving 98% WBC retention from human blood with no RBC lysis (VanDelinder and Groisman, 2006, 2007).
Davis et al. and Inglis et al. used microfludic devices featuring pillars. Rather than using the pillars to create microfludic slits to obstruct larger cell flow, they used the micropillars to create particle-size-dependent pathlines such that target cells were sorted into predetermined outlet ports based on size alone (Davis et al., 2006; Inglis et al., 2008). Using this technique, Davis et al. depleted lymphocytes and monocytes from blood with 100% efficiency and Inglis et al. were able to separate lymphocytes from diluted blood suspensions with 73% effieciency.
2.2.2. Cancer Cells
Zheng et al. developed parylene microfilters for the isolation of immortalized prostate cancer cell lines. Using pressure-driven flow to force cell suspensions through a micro-filter, their cell recoveries ranged from 87% to 89% (Zheng et al., 2007). Cells retained on the microfilters were lysed for genomic analysis. More recently, Hosokawa et al. developed microcavity arrays to select for immortalized lung carcinoma cells based size and deformability. Using negative-pressure from a peristaltic pump to drive cell suspensions through the arrays, they achieved 97% capture efficiency and 98% viability. Hosokawa et al. extended this work to breast, colon, and gastric tumor lines with greater than 80% efficiency. Chen et al. used a combination of experimental results and physical modeling to develop a weir filter to selectively isolate cancer cells based upon their deformability (Chen and Du, 2006)(see Figure 1B). Using a filter fabricated specifically for their model lung adenocarcinoma cells, they were able to achieve over 99.9% capture efficiency from diluted human blood samples.
2.2.3. Other Mammalian Cells
Mohamed et al. also used pillar filters for the goal of isolating fetal nucleated red blood cells (fNRBCs) from maternal blood (Mohamed et al., 2007). The pillars were placed to create successively narrower channels in the device such that cell capture between pillars was a function of size and deformability. RBCs and fNRBCs were isolated from goose blood and cord blood samples, respectively. Mohamed et al. reported no significant clogging using this staged pillar technique; however, blood samples were diluted pre-isolation. Huang et al. separated NRBCs based on size-dependent pathlines as described previously (Davis et al., 2006; Inglis et al., 2008). Their device successfully eliminated over 99% of RBCs; NRBCs were further purified from contaminating WBCs by use of magnetic separation. Huang et al. sucessfully enriched NRBCs by a factor of 10- to 20 more than previously reported techniques (Huang et al., 2008).
2.3. Sheath Flow & Streamline Sorting
These devices take advantage of low reynolds number fluid flow associated with the imposition of certain geometries or parallel fluid flows of different flow rates to passively sort or segregate target cells(see Figure 1D). This is another label-free and chemistry-free method of cell isolation that is most commonly used when size differences between cells is significant and, unlike size-based sorting techniques, only exerts fluid stresses on the cell rather than physical compression through filter elements. However, dense biological suspensions must be diluted to achieve maximum device performance.
2.3.1. Blood Cell Fractionation
SooHoo et al. used a microfluidics-based aqueous two-phase system (ATPS) to enrich leukocytes from blood suspension. Using one stream of polyethylene glycol (PEG) and one of dextran (DEX), with Zap-oglobin as the lysing agent, they achieved 100% RBC depletion from human blood samples (SooHoo and Walker, 2007). Zheng et al. developed devices based on T-shaped bifurcated channels to separate WBCs from RBCs. By adjusting the length of the T-channel, and the vertical distance between upstream and downstream side walls, cells were directed to different stream lines based on size alone. They were able to separate WBCs from diluted blood with 97% efficiency. However, they found that RBC orientation heavily influenced the segregation of small WBCs from RBCs (Zheng et al., 2008).
2.3.2. Other Mammalian Cells
Kuntaegowdanahalli et al. used spiral microchannels to segregate cells based on size across the width of their devices. Using a five-loop system, they sorted neuroblastoma cells from glioma cells with 80% efficiency (Kuntaegowdanahalli et al., 2009). The cells were then placed in culture and exhibited 90% viability after sorting. Lin et al. used multiple sheath flows in parallel to sort yeast cells from suspension. They used two streams of unequal flow rate to achieve a focusing effect and were able to separate yeast cells with 87.7% efficiency and 94.1% purity (Lin et al., 2009).
In contrast, Choi et al. used a series of slanted microfluidic channels of periodically varying heights to sort cells by cell-cycle phase. The slanted obstacles generated streamlines that diverted cells transverse to the flow, towards the wall of the device. There, the cell-obstacle interactions diverted larger cells out of the transverse streamlines, keeping them near the wall, while smaller cells diverged from the wall (Choi et al., 2009). They achieved lateral separation of G0/G1 phase and G2/M phase monocyte model cells with over 4-fold G2/M cell enrichment.
2.3.3. Prokaryotes & Viruses
Wu et al. used sheath flows to sort E. coli from blood. High concentrations (greater than 108 cells/ml) of E. coli cells were spiked into diluted human RBCs and were enriched 300-fold over the course of separation. They demonstrated a sorting efficiency of 62% and purity of 99.87%. The bacteria were expanded in culture and exhibited over 95% viability (Wu et al., 2009).
In summary, the devices described above all use varying non-electrokinetic techniques to succesfully isolate a broad range of cell types. However, despite a variety of isolation mechanisms and microfluidic designs, there is no single microfluidic device that can produce pure cell populations with high effciency. For these devices to be used for rigorous biochemical and genetic assays, it is essential that a method of high purity, high efficiency capture method to be found. An additional challenge is that many rare cells of interest (e.g., leukocytes, CTCs, yeast, bacteria) are found in the blood, a dense suspension that often hinders characterization of device performance. For microfludiic devices to reach their full potential as rare cell capture platforms, it is essential that these elements be addressed and improved upon.
3. Electrokinetic Methods
Electrokinetic methods comprise those methods that use electric fields to actuate cells. In microfluidic devices, the two most widespread electrokinetic techniques for manipulating cells are electrophoresis and dielectrophoresis. Electrophoresis refers to net migration due to the action of an electric field on the net free charge of a particle. This technique has been used to study cells at the membrane level (Mehrishi and Bauer, 2002), and methods such as capillary electrophoresis and microfluidic free-flow electrophoresis have been developed to separate different populations of biomolecules, viruses, bacteria, and eukaryotic cells (Kremser et al., 2004; Turgeon and Bowser, 2009). However, as the net charge of a cell's electrical phenotype is often not specific enough to distinguish between a mixture of different cells, electrophoresis has been used minimally as a cell separation technique and is not suited for applications in rare cell capture. Thus, this review will focus primarily on dielectrophoretic techniques.
Dielectrophoresis (DEP) refers to the net migration of polarized particles owing to interactions with an electric field gradient, and depends on cell wall, membrane, and cytoplasmic electrical properties (Jones, 1995; Kirby, 2010). The DEP force is a direct function of these electrical properties as well as cell size, the electrical properties of the fluid medium, and the magnitude and frequency of the applied electric field; the dependence on this wealth of parameters makes DEP an attractive tool for distinguishing between different cell types (Voldman, 2006; Hawkins et al., 2009). DEP response is classified into two regimes: when particles are more polarizable than the medium, positive DEP results and the particles are attracted to stronger field regions; conversely, when particles are less polarizable than the medium, negative DEP results and the particles are repelled from stronger field regions; the frequency at which the DEP force switches from one regime to the other (i.e. when the force is zero) is termed the “crossover frequency” (Jones, 1995; Morgan and Green, 2002). The sign and magnitude of the DEP force provides the basis for DEP cell separation techniques, and this review will cover the most common device geometries used for these techniques. The scope of this review on DEP methods will be limited to those used for capture, separation, or concentration of bulk cell populations; DEP methods for single cell capture or manipulation are covered in other reviews (Voldman, 2006; Hawkins et al., 2009; Bao et al., 2008; Kang and Li, 2009; Zhang et al., 2010). The DEP methods are organized by the type of device geometry used; each section includes a brief description of the physics associated with the technique and a summary of how it is applied to separate different cell types with a view towards pathogen detection, blood fractionation, or cancer cell isolation. Many DEP experiments have used model systems to characterize geometric performance, or as mockups of rare cell capture experiments. Thus, this section includes many devices that do not capture rare cells, but whose performance informs the potential for rare cell capture with DEP.
3.1. Electrode-based DEP
Microfabricated electrodes are the most common and practical method for creating the non-uniform electric fields necessary for DEP. While potential limitations to the use of electrode-based DEP include fouling and electrolysis at low electric field frequencies as well as increased fabrication time and cost required for more complex electrode configurations, a majority of DEP techniques use microfabricated electrodes owing to their simplicity and flexibility in implementation. The following sections will cover the most common and simple device geometries used for cell separation.
3.1.1. Interdigitated array (IDA) electrodes
Interdigitated arrays consist of spatially alternating sets of grounded and energized electrodes that create non-uniform electric field regions and trap particles against a flow via positive DEP (Figure 2A). IDA electrodes are one of the most commonly used electrode configurations because their use entails minimal design parameters (electrode length and width, inter-electrode distance, and channel depth) and experimental parameters (flow rate, electric field magnitude and frequency), and yields analytical solutions for electric fields and particle motion (Sun et al., 2007). IDA electrodes are typically used for “binary” cell separation; an electric field is applied to capture the target cells from a mixture of two or more cell types via positive DEP, the non-target cells are minimally affected by the field or repelled via negative DEP and are flushed out of the device, and finally the field is turned off to release the target cells for separate collection. Through DEP characterization, a frequency regime can be selected in which one cell type is attracted to the electrodes (positive DEP) while another cell type is repelled into the regions separating the electrodes (negative DEP). Rare cell capture requires that all non-target cells be repelled, which can be demanding if the suspension is complex.
Figure 2.
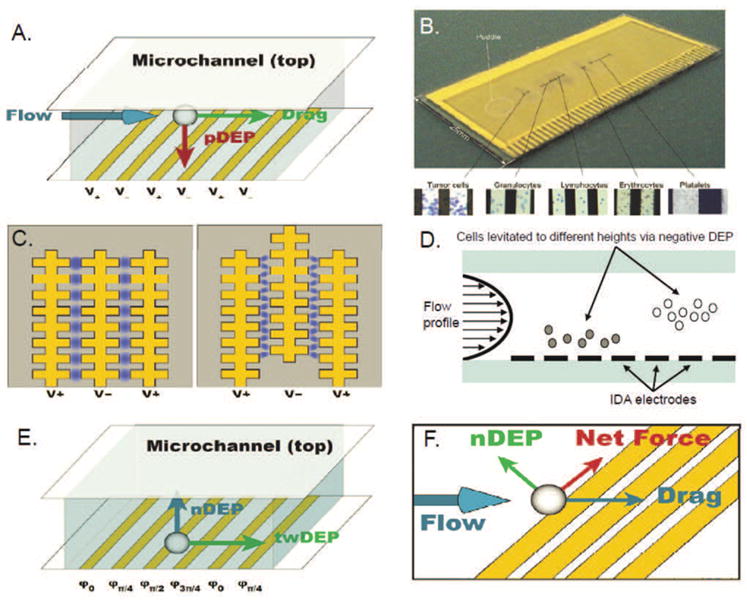
(A) Interdigitated array (IDA) electrodes. (B) Electrosmear slide showing fractionation tumor cells and blood components. Reproduced from Cristofanilli et al. (2008). (C) Castellated IDA electrodes. (D) DEP field-flow fractionation operates by levitating cells against gravity to different heights in the channel via negative DEP, allowing separation to be achieved based on their differing flow velocities. (E) Configuration and forces in a twDEP electrode array. (F) Summation of forces near an angled electrode.
IDA electrodes have been used to separate or concentrate bacteria for potential applications in pathogen sensing. Typical cell concentrations used for these studies lie in the range of 105 to 109 cells/mL. Efforts to detect foodborne pathogens such as those in the genus Literia include separation of live and heat-treated L. innocua with 90% efficiency; as the cell membrane becomes permeable upon death, large changes in conductivity can result in differences in the DEP response of live and dead cells (Li and Bashir, 2002). Researchers have also used positive DEP to attract a mixture of Listeria and Escherichia species to antibody-coated electrodes and selectively capture only L. monocytogenes (i.e. immunocapture) with 87-92% efficiency (Yang et al., 2006; Koo et al., 2009). To aid efforts in detecting environmental pathogens, researchers have demonstrated concentration of Bacillus subtilis spores (a surrogate bacteria used for research on Bacillus anthracis, i.e., anthrax) from airborne environmental samples containing diesel particulate matter with up to 60% purity; appropriate frequency ranges for separation were selected based on crossover frequency measurements (Fatoyinbo et al., 2007). Additionally, Gadish et al. concentrated B. subtilis by integration of a chaotic mixer to bring the spores into closer proximity with the IDA electrodes and enrich the sample ninefold (Gadish and Voldman, 2006), and Liu et al. captured B. anthracis with 90% efficiency for impedance measurements in order to detect viable spores electrically by their germination (Liu et al., 2007b).
IDA electrodes have also been used for blood fractionation. Cristofanilli et al. used an “electrosmear” slide that was coated to promote cell adhesion and patterned with IDA electrodes to which different electric field frequencies were applied along the length of the device (Cristofanilli et al., 2008). Near the inlet port, a low frequency was applied to levitate all cells via negative DEP to avoid adhesion to the slide, and as the blood sample obtained from a murine aspiration biopsy was flowed further along the device, different constituents of blood as well as biopsied tumor cells from a cancer line grown in nude mice were pulled toward and adhered to the electrodes via positive DEP in different regions of the slide, based on their previously characterized dielectric properties (Figure 2B) (Cristofanilli et al., 2008).
3.1.2. Castellated IDA electrodes
Castellated electrode arrays consist of interdigitated electrodes with width variation along their length, which create alternating regions of high and low electric field magnitude at the tips of the electrodes and the regions separating each electrode, respectively (Figure 2C). The advantage of castellated electrodes is the localization of high electric field regions, which can be used to trap or concentrate flowing cells in the device effectively. The procedure for cell separation using castellated electrodes is the same as that used with straight IDA electrodes; this procedure has been used for binary separation of a mixture of two bacteria types, including yeast, E. coli, and Micrococcus lysodeikticus (Marks et al., 1994), as well as for separation of viable and non-viable yeast cells (Markx and Pethig, 1995). Optical absorbance of DEP trapping was measured to calculate the effective conductivity of the cells and predict their DEP response.
Castellated IDA electrodes have been used for cell separation between bacteria and blood cells for applications in pathogen detection, with typical cell concentrations of 106 to 107 cells/mL; researchers have demonstrated separation of M. lysodeikticus from erythrocytes based on their differing dielectric properties (Wang et al., 1993). Isolation of erythrocytes infected with malaria pathogen from healthy erythrocytes was also achieved with 90% efficiency owing to the sharp increase in membrane conductivity of erythrocytes hosting malarial parasites (Gascoyne et al., 2002). In addition, Huang et al. demonstrated simultaneous separation of multiple bacteria (Bacillus cereus, E. coli, L. monocytogenes) from diluted blood with up to 97% efficiency using size-based DEP separation and post-separation PCR analysis (Huang et al., 2003).
Castellated IDA electrodes have also been used for applications in cancer cell isolation. Becker et al. characterized the dielectric parameters of cultured breast cancer cells, lymphocytes, and erythrocytes using particle electrorotation techniques, and subsequently trapped the breast cancer cells from a suspension of diluted blood, demonstrating up to 95% purity in captured cancer cells (Becker et al., 1995). More recently, Tai et al. developed an automatic platform for separation of viable and non-viable cultured human lung cancer cells based on differing dielectric properties with 81-84% efficiency and nucleus collection for nuclear protein extraction (Tai et al., 2007). While castellated IDA electrodes are similar in function and application (e.g., binary sorting) to straight IDA electrodes, their ability to create alternating regions of high and low electric field magnitude makes them better suited for concentrating samples or patterning particles at a specific location than straight IDA electrodes. As is the case for straight IDA electrodes, the challenge associated with castellated IDA electrodes is in finding a frequency or set of frequencies such that only the rare cells are attracted to the electrodes.
3.1.3. IDA electrodes for flow-field fractionation
In DEP flow-field fractionation (DEP-FFF), IDA electrodes are fabricated on the bottom of a device channel, and flowing particles of differing dielectric properties are levitated against gravity via negative DEP. The levitated particles equilibrate to different heights in the channel owing to the distinct DEP force on different types of particles, and these differing heights allow separation to be achieved by sequential collection based on different flow velocities due to the parabolic velocity distribution of low-Reynolds-number Poiseuille flow (Figure 2D). The velocities of different cells can be characterized by measuring cell elution fractograms as a function of frequency (Huang et al., 1999). The main advantage of DEP-FFF is its ability to achieve continuous-flow separation of bioparticles with size and/or dielectric differences under a constantly applied electric field, therefore avoiding the need for activation and deactivation of the field as required by binary sorting devices.
DEP-FFF has been used often as a technique to separate different cell types in blood, with cell concentrations ranging from 105 to 107 cells/mL. Researchers have demonstrated separation of erythrocytes from latex beads and characterization of their different levitation heights (Rousselet et al., 1998), as well as binary separation of human leukocyte subpopulations (T-, B-lymphocytes, monocytes, and granulocytes) based on differing membrane dielectric properties with 87-98% purity, which can be used for clinical applications in differential analysis of leukocytes (Yang et al., 2000). More recently, Hashimoto et al. performed selective capture of neutrophils and eosinophils from a mixed leukocyte suspension with 80% efficiency by deflecting the target cells away from the IDA electrodes and toward an antibody-coated layer on the opposite wall (Hashimoto et al., 2009). DEP-FFF also has been used extensively for the separation and isolation of cancer cells. In particular, the Gascoyne research group has demonstrated separation of cultured human leukemia cells from diluted blood after characterizing the cells by DEP levitation experiments (Huang et al., 1997), separation of cultured human breast cancer cells from whole blood based on measured differences in cell size and membrane capacitance (Yang et al., 1999; Gascoyne et al., 2009), and separation of cultured human breast cancer cells from normal T-lymphocytes and hematopoietic CD34+ stem cells (Huang et al., 1999; Wang et al., 2000), all with efficiencies and/or purities over 90%.
In more recent years, DEP-FFF has been used for a larger variety of applications as well as in different device geometries. These applications include separation of cells with high and low embryogenic potential in suspension cultures of carrot based on their differences in size and cytoplasm density (Falokun et al., 2003), toxicity testing by dielectric characterization of cultured human leukemia cells with membrane dissimilarities due to exposure to various toxic agents (Pui-ock et al., 2008), and enrichment of a progenitor cell population in a mixture of cell debris and erythrocytes from freshly harvested adipose tissue (Vykoukal et al., 2008). Finally, vertical IDA electrodes have been fabricated on the sidewalls of the device channel (as opposed to horizontal electrodes on the bottom of the channel) to achieve lateral separation through separate outlets. This device geometry has been used to separate mammalian cells of different sizes (Wang et al., 2009) and viable from non-viable yeast cells (Braschler et al., 2008), as well as to enrich Babesia bovis-infected erythrocytes sevenfold (Braschler et al., 2008). Unlike trapping on straight or castellated IDA electrodes, DEP-FFF allows cells to be separated based on the magnitude of the DEP response rather than just the sign of the response, and rare cell capture can be achieved in theory if the DEP response of a cell can be distinguished within the sensitivity of the device.
3.1.4. IDA electrodes for traveling-wave DEP
IDA electrodes have been used for a technique called traveling-wave DEP (twDEP) to fractionate bioparticles. The electrodes are independently driven with different electric field phases, and particles are levitated against gravity owing to negative DEP (Figure 2E). Fractionation is achieved by varying the electric field phases to drive the particles transverse to the direction of flow at different velocities. Cui and Morgan detailed the design and fabrication of a twDEP device and demonstrated particle motion using polystyrene latex particles (Cui and Morgan, 2000). The main advantage of twDEP is that fractionation may be achieved based on the particles' differing velocities alone; there is no need to drive fluid flow or to trap or concentrate particles via positive DEP.
Building on the successful implementation of twDEP on polystyrene beads, a number of biological separations have been achieved. Bacteria separation has been demonstrated by use of viable and non-viable yeast cells (Talary et al., 1996; Kua et al., 2007); also, blood fractionation has been demonstrated by separating T-lymphocytes and erythrocytes by applying multiple frequencies to direct the cells to move in opposite directions such that they were collected separately through different outlets (Loire and Mezic, 2003). twDEP has also been used for applications in pathogen detection; a spiral electrode array was characterized and used for a 1000-fold enrichment of malaria-infected erythrocytes from normal erythrocytes with 90% purity (Wang et al., 1997; Gascoyne et al., 2002). Application of the traveling field caused normal erythrocytes to be trapped at the electrode edges via positive DEP, while infected cells were levitated via negative DEP and carried to the center of the spiral (Gascoyne et al., 2002). More recently, Cheng et al. developed a high-throughput 3D twDEP device used for focusing and sorting particles, and demonstrated its ability to separate bacteria and blood cells based on DEP mobility magnitude as well as direction (Cheng et al., 2009). Other recent studies used twDEP for characterization of cultured lymphoma and myeloma cells for potential applications in rare cell capture (Cen et al., 2004) and the development of a DEP pump for blood delivery in microfluidic devices (Lei et al., 2009).
3.1.5. Angled electrodes
Angled electrodes are most often used for binary separation of bioparticles or to create localized particle pathlines due to the particles' negative DEP mobilities. As the particles approach an electrode, the negative DEP force that acts on them can exceed drag forces, resulting in a net force parallel to the electrodes. Particles then travel along the length of the electrode until drag forces exceed the DEP force, at which point the particles can flow past the electrodes (Figure 2F). Displacing particles transverse to the direction of flow allows angled electrodes to preferentially direct particles to different outlets or focus them into concentrated streams.
Angled electrodes have been used to sort and concentrate various bacterial samples. Cheng et al. designed a device with 3D electrode gates to focus and separate yeast and E. coli into different outlets, after which surface-enhanced Raman scattering was used to detect bacteria concentration and evaluate efficiency (Cheng et al., 2007). Kim et al. tagged E. coli with different sized microspheres and used angled electrodes to separate the two target cell types into different outlets, after which capture efficiency and purity was evaluated using flow cytometry (Kim et al., 2008). More recently, a magnetic separation module was incorporated into the device to capture magnetically-tagged cells and separate them from unlabeled non-target cells, which improved the device's ability to separate multiple cell types (Kim and Soh, 2009). Vahey and Voldman developed a separation method termed “isodielectric separation,” which uses a diffusive mixer to establish an electrical conductivity gradient across the width of a channel containing angled electrodes (Vahey and Voldman, 2008). DEP forces vary along the length of the electrodes, which direct and separate viable and non-viable yeast cells across the device in the direction of decreasing conductivity until they reach their respective isodielectric points, where there is no net force (Vahey and Voldman, 2008).
Angled electrodes have also been used for binary sorting of mammalian and blood cells. To address the need for a noninvasive method for sorting cell populations according to their cell-cycle phase, Kim et al. separated cultured human breast cancer cells based on their differing sizes due to their cell cycle phase (Kim et al., 2007). Angled electrodes were also used to demonstrate a low-stress platelet size-based DEP separation technique, separating platelets from diluted whole blood with 95% purity (Pommer et al., 2008).
3.2. Insulative DEP
Insulative DEP techniques rely on constrictions or expansions in channel geometry to generate electric field non-uniformities and deflect or trap bioparticles via negative DEP. While this approach places limits on the frequencies and geometries used, the main advantage of insulative DEP is that no internal electrodes are used. This leads to simpler device fabrication, reduced propensity for fouling, and the possibility of using a DC field for electrokinetic particle transport as well as trapping via DEP (Lapizco-Encinas et al., 2004a).
3.2.1. Angled and curved constrictions
The simplest geometry in an insulative DEP device is a perpendicular insulative constriction in the device channel. Kang et al. demonstrated size-based separation of live cells by using rectangular constrictions to deflect larger cells (white blood cells and cultured mammalian breast cancer cells) via negative DEP to a different trajectory than smaller blood components (red blood cells, platelets) (Kang et al., 2008). Binary sorting is achieved by fabricating two outlet channels for the separate trajectories.
Extending the basic principles of rectangular constrictions, angled constrictions have also been used to separate and concentrate bioparticles. The DEP force acting perpendicular to the constriction depends on the angle that the constriction forms with the channel. If this DEP force is smaller than the drag force, then particles will flow past the constriction unaffected; if, however, the DEP force exceeds the drag force, then the particles are stopped at and deflected parallel to the constriction. Angled constrictions have been used to demonstrate size-based separation of B. subtilis from polystyrene particles (Barrett et al., 2005). Curved constrictions, in which the angle of constriction varies continuously across the channel, have also been used to separate different sized particles (Figure 3A) (Hawkins et al., 2007).
Figure 3.

(A) Schematic of curved constriction in channel depth. Inset: top view of device fabricated in Zeonor 1020R polymer substrate. Reproduced from Hawkins et al. (2007). (B) Trapping of live (green) and dead (red) E. coli with separation of populations using insulative post array. Reproduced from Lapizco-Encinas et al. (2004a).
3.2.2. Post arrays
DEP trapping using an array of insulating posts was reported by Cummings and Singh, who investigated various geometric variables that affect the electric field, including post shape, distance between posts, and array angle to the applied field (Cummings and Singh, 2003). Using an array of circular posts etched in a glass substrate, researchers at Sandia National Laboratories have demonstrated trapping of polystyrene beads (Mela et al., 2005) and separation of live and dead E. coli based on their differing magnitudes in negative DEP response (Figure 3B) (Lapizco-Encinas et al., 2004a). The group later demonstrated separation and concentration of any two pairs of E. coli, B. subtilis, B. cereus, and Bacillus megaterium (Lapizco-Encinas et al., 2004b), as well as tobacco mosaic virus (Lapizco-Encinas et al., 2005). A direct application of this technique is for the detection of microbes in drinking water, which is hindered by current analytical instruments that require significant concentration of microbes in order to detect them (Lapizco-Encinas et al., 2005).
3.2.3. Other geometries
A variety of other device geometries have been designed for bioparticle separation and isolation using insulative DEP. Chou et al. used an array of constrictions to trap and concentrate single- and double-stranded DNA (Chou et al., 2002). Pysher et al. designed channel walls with a sawtooth pattern to produce spatially resolved separation of live and dead E. coli and B. subtilis (Pysher and Hayes, 2007). More recently, Church et al. fabricated a serpentine channel to filter E. coli from yeast cells (Church et al., 2009), Cho et al. positioned plastic membranes with honeycomb-shaped pores between electrodes to trap and release E. coli in the flow channel (Cho et al., 2009), and Shafiee et al. developed a “contactless” DEP technique to isolate live/dead cultured human leukemia cells by using thin insulating barriers to separate the electrodes used to apply the electric field from the sample channel, thus preventing potential issues such as contamination and bubble formation (Shafiee et al., 2010).
3.3. Prospects for DEP rare cell capture
The preceding sections on electrode-based and insulative DEP techniques introduced the most common device geometries that researchers have used to separate different populations of cells. Those studies that focused on quantifying experimental performance criteria such as efficiency, enrichment, and/or purity are summarized in Table 2. Overall, DEP methods are advantageous because they do not require a biochemical labeling step to achieve continuous-flow separation. Additionally, it is possible to achieve DEP cell separation without a priori knowledge of the different cells' properties. For binary separation using IDA electrodes, only the frequency range in which the cells experience DEP forces opposite in sign needs to be known; for methods that use angled electrodes or insulative constrictions and techniques such as DEP-FFF and twDEP, only the cells' relative DEP response magnitudes are required to achieve separation of several cell types. As such, DEP offers the ability to isolate single cells (because of its sensitivity to cellular dielectric properties) as well as the possibility for separation of cell populations in which not all cell types have been characterized. In the latter case, DEP potentially can be used to screen for cells with unknown membrane phenotypes, which can facilitate research on bacterial species such as Mycobacterium tuberculosis, whose pathogenicity is closely tied to membrane composition (Rhoades et al., 2005).
Table 2. Electrokinetic cell fractionation/isolation.
| Application | Cell Type Modeled | Cell Type Used | Carrier Media | Off-line Processing | Experimental Parameters | Efficiency | Enrichment | Purity | DEP Technique | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| L. monocytogenes | L. innocua | DI water, 2 μS/cm; 105 cells/mL | Cell counting | 1 Vpp, 50 kHz | 90% | – | – | IDA | Li and Bashir (2002) | |
| Pathogen detection | L. monocytogenes | L. monocytogenes | DI water, 1-15 μS/cm; 102-103 cells/mL | Cell counting | 0.2 μL/min, 800 μm/s; 20 Vpp, 1 MHz | 87-92% | – | – | IDA + IC | Yang et al. (2006) |
| B. anthracis | B. subtilis | DI water, 5×10−4S/m; 3.8×106 cells/mL | Measure absorbance | 100 μL/min; 40 Vpp, 100 kHz | – | 9× | – | IDA | Gadish and Voldman (2006) | |
| B. anthracis | B. subtilis | DI water, 7.6 mS/m; 9.9×107 spores/mL, 2.1×107 diesel particles/mL | Hemacytometer | 0.5-4 mL/hr, 94 μm/s; 10 Vpp, 1 MHz | na | na | ≤ 60% | IDA | Fatoyinbo et al. (2007) | |
| B. anthracis | B. anthracis | DI water, 2-3 μS/cm; 107-109 spores/mL | Cell counting | 0.2 μL/min, 40 cm/min; 20 Vpp, 100 kHz | 90% | na | na | IDA | Liu et al. (2007b) | |
| Plasmodium falciparum | Malaria-infected erythrocytes | Sucrose buffer, 22-55 mS/m; 107 cells/mL | Cell counting | 5Vpp, 200 kHz | 90% | 50-200× | na | Castellated | Gascoyne et al. (2002) | |
| na | B. cereus, E. coli, L. monocytogenes | Mannitol + PBS, 180 μS/cm; 4 μL blood + 1 μL of 106 B. cereus or 7×105 E. coli or 106 L. monocytogenes | PCR amplification | 10 Vpp, 10 kHz | ≤97% | na | na | Castellated | Huang et al. (2003) | |
| na | E. coli | DI water, 10-20 μS/cm; 105 cells/mL | Cell counting | 100 Pa; 2000 V/cm | 90+% | 3000× | na | iDEP | Lapizco-Encinas et al. (2005) | |
| na | E. coli | PBS, 0.5 mS/m; 9.3×103 cells/mL | Cell counting | 100 μL/min; 128 V/mm, 300 kHz | 66% | na | na | iDEP | Cho et al. (2009) | |
| Cancer cell isolation | Lung cancer | A549-luc-C8 | DMEM buffer, 72 μS/cm | Flow cytometry | 3 μL/min; 15 Vpp, 16 MHz | 81-84% | na | na | Castellated | Tai et al. (2007) |
| Breast cancer | MDA231 | Sucrose buffer; 107 malignant, 3×107 normal cells/mL | Cell counting | 5 Vpp, 200 kHz | na | na | 95% | Castellated | Becker et al. (1995) | |
| Breast cancer | MDA-435 | Sucrose buffer, 56 mS/m; 5×106 cells/mL, 2:3 ratio of MDA-435:RBCs | Cell counting | 0.5 mL/min, 780 μm/s; 1.4 Vpp, 5 kHz | na | na | 98% | FFF | Yang et al. (1999) | |
| Breast cancer | MDA-435, -468, -231 cells | Sucrose buffer, 30 mS/m; 105-106 cells/mL, 1:1000 ratio of tumor cell to PBMNs | Cell counting | 1.5 mL/min; 2.8 Vpp, 60 kHz | ≤92% | na | na | FFF | Gascoyne et al. (2009) | |
| Breast cancer | MDA-435, CD34+ stem cells | Sucrose buffer, 10 mS/m; 106 cells/mL, 3:2 ratio of CD34+ to MDA-435 | Flow cytometry | 2 mL/min; 4 Vpp, 40 kHz | na | na | 96-99% | FFF | Huang et al. (1999) | |
| Breast cancer | MDA-MB-231 | PBS, 100-200 mS/m; 106 cells/mL | Flow cytometry | 200-400 μL/hr; 20 Vpp, 500 kHz | na | 4.4× | 96% | Angled electrodes | Kim et al. (2007) | |
| Leukemia | THP-1 | Sucrose buffer, 110-115 μS/cm; 106 cells/mL | Cell counting | 0.02 mL/hr, 222 μm/s; 20-50 Vrms, 200-500 kHz | 90+% | na | na | Contactless DEP | Shafiee et al. (2010) | |
| Blood fractionation or enrichment | Leukocytes | Leukocytes | Sucrose buffer, 10 mS/m; 2×106 cells/mL, 1:1 ratio | Flow cytometry | 2 mL/min; 4Vpp, 20-50 kHz | na | na | 87-98% | FFF | Yang et al. (2000) |
| Leukocytes | Leukocytes | GIT medium, 13 mS/cm; 5×106 cells/mL | Cell counting | 1.5 μL/min; 20 Vpp, 1 MHz | 80% | na | na | FFF + IC | Hashimoto et al. (2009) | |
| Leukocytes | MDA-435, CD34+ stem cells | Sucrose buffer, 10 mS/m; Separation: 1.2×106 cells/mL, Leukocyte enrichment: 5×106 cells/mL | Flow cytometry | Separation: 2 mL/min, Leukocyte enrichment: 0.5 mL/min; 4 Vpp, 40 kHz | 55-75% | na | 92-99% | FFF | Wang et al. (2000) | |
| Malaria | Erythrocytes infected with B. bovis | PBS, 60 mS/m | Cell counting | 500 μm/s; 4.7-9 Vrms, 90 kHz-4 MHz | na | 7× | na | FFF | Braschler et al. (2008) | |
| Malaria | Erythrocytes infected with P. falciparum | Sucrose buffer, 0.055 S/m; 2000 cells with 5% parasitized cells | Cell counting | 3 Vpp, 2 MHz | na | 1000× | 90% | twDEP | Gascoyne et al. (2002) | |
| Platelets | Concentrated platelets + whole blood | Sucrose buffer, 50 mS/m; 107 cells/mL | Flow cytometry | 150 μL/hr; 6.6 mm/s; 100 Vpp, 1 MHz | na | 5.3× | 95% | Angled electrodes | Pommer et al. (2008) | |
Using only DEP techniques for rare cell capture in pathogen detection or tumor cell isolation, however, is challenging; studies have reported significant decreases in cell capture efficiency or purity as target cell concentrations became more dilute (Gascoyne et al., 2009; Fatoyinbo et al., 2007; Gascoyne et al., 2002; Huang et al., 2003). While numerous DEP methods for cell separation of artificial samples have been reviewed in this article, we are not aware of a study that demonstrates strictly dielectrophoretic capture of pathogens from environmental (air or water) samples or capture of viable tumor cells from whole blood of cancer patients. In the future development of rare cell capture microfluidic devices, it may be beneficial to merge DEP methods with techniques such as magnetic-activated cell sorting (Kim et al., 2008; Kim and Soh, 2009) or immunocapture (Yang et al., 2006; Hashimoto et al., 2009). Such hybrid techniques combine the actuation of DEP with the chemical-specificity of immunocapture techniques; a system could be developed in which the applied electric field is tuned low enough to cause no physiological harm to target cells while inducing a strong enough DEP force to cause or prevent interactions with immunocapture surfaces. These synergistic effects have the potential to minimize problems associated with immunocapture techniques (e.g., nonspecific binding) and yield higher performance in rare cell capture efficiency and purity compared to using DEP techniques alone.
4. Performance Criteria
In the previous sections, we have described a variety of different methods to isolate a multitude of rare cell types. In this section, we quantitatively compare these disparate studies with a unified set of performance criteria. Comparing the literature systematically identifies the strengths and weaknesses of the field as a whole and provides insights into future research directions. In the following paragraphs, we define performance metrics by which the literature will be evaluated (see Tables 1 & 2) and draw conclusions upon analyzing these criteria. Italics are used to highlight headings in these tables.
Table 1. Non-electrokinetic cell fractionation/isolation.
| Application | Cell Type Modeled |
Cell Type Used |
Carrier Media |
# Steps/ Stages |
Off-line Processing |
Volumetric/Linear Flowrate |
Efficiency | Enrichment | Purity | Viability | Analysis Technique |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-lymphocytes/ T-lymphocytes |
Raji/ Molt-3 | PBS | 1 | staining | ; na | 97% | na | na | na | anti-CD5, anti-CD19, PEG IC | Murthy et al. (2004) | |
| Blood cell fractionation |
BC/PC/CC/Lymphoblast | MCF7/PC3/HeLa/Daudi | DMEM, Blood | 1 | staining, enumeration | 0; 0 | 80% | na | na | na | EpCAM IC | Wang et al. (2010) |
| B-lymphocytes/ T-lymphocytes |
Raji/ Molt-3 | PBS | 1 | staining, enumeration |
; na | 75%-100% | na | na | na | anti-CD5, anti-CD19 IC | Sin et al. (2005) | |
| Myeloid cells | HL-60/U-937 | RPMI-1600 | 1 | labeling, enumeration | na; 700 to | na | 130× to 200× | na | na | E-selectin IC | Chang et al. (2002) | |
| Leukocytes | Leukocytes | Whole Human Blood | 2 | enumeration, lysing | ; na | na | na | 97% | na | SBS | Sethu et al. (2005) | |
| Leukocytes | Leukocytes | Whole Human Blood | 1 | labeling, enumeration |
; na | 98% | na | na | na | SBS | VanDelinder and Groisman (2007) | |
| Leukocytes | Leukocytes | Diluted Human Blood | 1 | enumeration | 10 to ; na | 70% | na | na | na | SBS | Ji et al. (2008) | |
| Leukocytes | Leukocytes | Diluted Human Blood | 1 | enumeration | ; na | 72 to 85% | na | na | na | SBS | Ji et al. (2008) | |
| Leukocytes | Leukocytes | Diluted Human Blood | 1 | enumeration | ; na | 70 to 95% | na | na | na | SBS | Ji et al. (2008) | |
| Leukocytes | Leukocytes | Diluted Human blood | 1 | flow cytometry, staining, lysing, enumeration | ; na | 99.6% | na | na | na | SBS | Davis et al. (2006) | |
| Lymphocytes/Monocytes | CD4+ cells/CD14+ cells/ J45 lymphocytes | Diluted Human blood | 1 | labeling, enumeration | na; | 73% | na | na | na | SBS | Inglis et al. (2008) | |
| Leukocytes | Leukocytes | Diluted Human blood | 1 | lysing, enumeration | ; na | na | 100× | na | na | ShF | SooHoo and Walker (2007) | |
| Leukocytes | Leukocytes | Diluted Human Blood | 1 | Dilution, enumeration | ; na | 97% | na | na | na | StF | Zheng et al. (2008) | |
| Normal breast cell/ BC | HME/TTU-1 | PBS | 1 | enumeration, staining | ; na | 30% | na | na | na | EMA/ EGFR IC | Du et al. (2007) | |
| Leukemia/lymphoma | CCRF-CEM/Ramos/Toledo | Modified PBS | 2 | cytometry, staining, enumeration | ; na | 50-83% | 135× | 88-97% | na | Scg8/ TD05/ Sgd5 IC | Xu et al. (2009) | |
| Cancer Cells | BC | MCF7 | Whole Rabbit blood | 3 | check | na; | 1-97% | na | na | na | EpCAM IC | Adams et al. (2008) |
| PC | LNCaP | PBS | 3 | enumeration, staining | na; | 90% | na | na | na | PSMA, EpCAM IC | Dharmasiri et al. (2009) | |
| BC | MCF7 | DMEM | 1 | staining, enumeration, SEM | 0; 0 | 45-60% | na | na | na | EpCAM IC | Wang et al. (2009) | |
| LC | SPC-A-1 | Diluted Human blood | 1 | enumeration | ; | 99.9% | na | na | na | SBS | Chen and Du (2006) | |
| LC | A549 | Human RBCs | 2 | staining, enumeration | 0; 0 | 62-74% | 133× | na | na | WGA IC | Liu et al. (2007a) | |
| LC/PC/ Pancreatic Cancer/ Colon Cancer | LC/PC/ Pancreatic CTC/ Colon CTC | Whole Human Blood | 1 | staining, enumeration | ; na | na | na | 42%-67% | na | EpCAM IC | Nagrath et al. (2007) | |
| LC/PC/BC, Bladder Cancer | NCI-H1650/ PC3-9/ SKBr-3/ T-24 | PBS | 1 | staining, enumeration | ; na | >65% | na | na | na | EpCAM IC | Nagrath et al. (2007) | |
| LC | NCI-H1650 | Whole Human Blood | 1 | staining, enumeration | ; na | >60% | na | na | na | EpCAM IC | Nagrath et al. (2007) | |
| PC | LNCaP | PBS | 1 | labeling, enumeration | ; na | 97% | na | na | na | PSMA IC | Gleghorn et al. (2010) | |
| PC | LNCaP | Whole Human Blood | 1 | labeling, enumeration | ; na | 85% | na | 68% | na | PSMA IC | Gleghorn et al. (2010) | |
| PC | PC CTCs | Whole Human Blood | 1 | labeling, enumeration | ; na | na | na | 62% | na | PSMA IC | Gleghorn et al. (2010) | |
| LC/GC/Colon Cancer/ BC | NCI-H358/ AGS/ SNU-1/ SW620/MCF-7 / Hs578T | Whole Human Blood | 1 | labeling, enumeration, SEM, staining | ; na | >80% | na | na | 98% | SBS | Hosokawa et al. (2010) | |
| PC | LNCaP | PBS | 1 | labeling, enumeration, electrolysis, PCR | Manual; na | 87-89% | na | na | na | SBS | Zheng et al. (2007) | |
| PC | LNCaP | Whole Human Blood | 1 | labeling, enumeration, electrolysis, PCR | Manual; na | 89% | na | na | na | SBS | Zheng et al. (2007) | |
| Other Mammalian Cells | Endothelial Cells/ Smooth Muscle Cells | H5V/ A7r5 | PBS | 1 | labeling, enumeration, staining | ; na | na | na | 86%; 83% | na | REDV/VAPG Peptide IC | Plouffe et al. (2007) |
| Endothelial Cells/ Smooth Muscle Cells/ Fibroblasts | H5V/ A7r5/ 3T3-6 | PBS | 2 | labeling, enumeration, staining | ; na | na | na | 96-99%* | 97% | REDV/VAPG/ RGDS Peptide IC | Plouffe et al. (2008) | |
| Neural Stem Cells | SH-SY5Y/ C6 | PBS | 1 | staining, enumeration, flow cytometry | TBD | 89% | na | na | 90% | SBS | Kuntaegowdanahalli et al. (2009) | |
| G2/M myeloid cells | U937 | 10mM sodium borate | 1 | flow cytometry | ; na | na | 4× | na | na | StF | Choi et al. (2009) | |
| Nucleated RBC | Nucleated RBC | Diluted Human Blood | 2 | filtration, dilution, staining | ; na | na | 10× to 20× | na | na | StF | Huang et al. (2008) | |
| Prokaryotes & Viruses | E. coli | E. Coli | Diluted Human RBCs | 1 | staining, enumeration, SDS page | ; na | 62% | 300× | 99.87% | 95% | ShF | Wu et al. (2009) |
0IC = Immunocapture, SBS = Size-based Sorting, ShF = Sheath Flow, StF = Streamline Focusing
0BC = Breast Cancer, CC = Cervical Cancer, GC = Gastric Cancer, LC = Lung Cancer, PC = Prostate Cancer
When comparing different rare cell capture devices, it is important to distinguish between the cell type modeled (e.g., cells obtained from biological samples) and the cell type used (often an immortalized cell line). This is imperative when the target cell's biological characteristics are not well understood, e.g. circulating tumor cells. While the use of well-understood model cell lines eases the characterization of device performance, their relations to clinical samples are not always well defined. Likewise, the carrier media used for experimentation is often chosen to simplify device characterization. Many rare cells that are targeted for isolation exist in dense biological suspension when in vivo, e.g. blood. However, many such fluids present other cellular material that confound quantification of performance e.g., changing viscous or conductivity properties. For this reason, rare cells are often captured from diluted biological solutions or even buffer solutions. For devices that use DEP methods, the conductivity of the media and the cell concentrations used are also important as these parameters affect the DEP force and capture efficiency or purity, respectively.
A number of quantitative metrics can be used to describe device performance. Efficiency is the most commonly used measure of performance in rare cell isolation literature. Efficiency is defined as the fraction of successfully isolated/fractionated cells with respect to the total number of target cells introduced into the device. High-efficiency microfludic cell isolation devices are often operated at higher volumetric flowrates than high-purity ones, resulting in increased throughput (Gleghorn et al., 2010; Sethu et al., 2005). Another common metric is enrichment, the multiplicative factor by which the number of rare cells per unit volume is increased. Depletion, in contrast, operates by capturing non-target cells within the device, leaving a more pure subpopulation at the outlet (Plouffe et al., 2008, 2007). Purity is the number of target cells captured divided by the total captured cell population. Purity is an important metric for measuring the selectivity of a device, but its optimization usually results in lower efficiencies and throughputs. However, high purity samples are desirable for a variety of biomolecular assays and tools. Equally important is the viability of cells post-capture. Some devices define viability as the percentage of cells left in a functional state post-capture and others post-culture ex-vitro. When comparing results from different methods, it is also important to compare the number of steps/stages involved. The possibility of increased performance with multi-stage processing versus the simplicity of device operation are major concerns for devices designed for clinical applications. However, the number of steps/stages was not included for devices that employ DEP methods, as a majority of those listed in Table 1 had similar procedures that include dielectric characterization, cell staining, on-chip capture or fractionation, and post-process cell counting. Given the data in Tables 1 & 2 organized under the headings described in previous paragraphs, we can make a number of observations about rare cell capture in microdevices.
5. Discussion & Conclusion
Multiple strides have been made in the enrichment, fractionation, and capture of rare cells. The devices outlined in this review have been successfully used for the enrichment of bacteria to the genetic analyses of cancer cells (Wu et al., 2009; Stott et al., 2010). Microfluidic devices for rare cell capture have elucidated new biological phenomena and afforded multiple avenues of further scientific investigation. Current devices have been successfully implemented in the enumeration of rare cells ranging from NRBCs to CTCs (Huang et al., 2008; Nagrath et al., 2007; Gleghorn et al., 2010); however the lack of a single microfluidic device that can isolate pure cell populations with high efficiency limits the number of molecular and genetic tools that can be used on these populations.
Additionally, few cell capture studies directly process biological samples (Nagrath et al., 2007; Gleghorn et al., 2010; VanDelinder and Groisman, 2006, 2007). In contrast, most devices spike cell lines into buffer solution (Chang et al., 2002; Murthy et al., 2004; Sin et al., 2005; Plouffe et al., 2007; Zheng et al., 2007; Kuntaegowdanahalli et al., 2009; Choi et al., 2009; Dharmasiri et al., 2009; Xu et al., 2009; Plouffe et al., 2008), or pre-diluted/lysed blood samples (Zheng et al., 2008; Davis et al., 2006; Huang et al., 2008; Wu et al., 2009). Importantly, in devices that employ DEP methods, efficiency and purity performance is low when target cell concentrations are dilute (Fatoyinbo et al., 2007; Gascoyne et al., 2002; Huang et al., 2003; Gascoyne et al., 2009), thus making rare cell capture using DEP techniques alone extremely difficult. In addition, many more cell capture devices approximate the ex vivo target with a model equivalent (Xu et al., 2009; Dharmasiri et al., 2009; Li and Bashir, 2002; Gadish and Voldman, 2006; Fatoyinbo et al., 2007; Becker et al., 1995; Yang et al., 1999; Huang et al., 1999) rather than capture of the actual in vivo target (VanDelinder and Groisman, 2007; Sethu et al., 2005; Yang et al., 2006; Liu et al., 2007b). WBC fractionation is the only technique where undiluted samples are commonly used, there are a few exceptions for other rare cell types (Nagrath et al., 2007; Huang et al., 2008; Gleghorn et al., 2010).
Similarly, the viability of cells after the capture process is not a well-quantified area, but one that is a crucial performance evaluation metric for rare cell capture devices. Mechanical stresses from shear, either from electric- (e.g., DEP forces), contact- (e.g., from pillar filters) or fluid-induced forces (e.g., obstacle-based arrays) can lead to gene upregulation or even induce an apoptotic response (Wernig et al., 2003; Okahara et al., 1998). Directly tied to cell viability is cell release and culture post-capture. Some attempts have been made to elute rare cells from devices (Xu et al., 2009; Adams et al., 2008; Dharmasiri et al., 2009; Liu et al., 2007a; Zhang et al., 2008; Zheng et al., 2007; Wu et al., 2009), especially those using affinity-based methods (i.e. immunocapture) (Adams et al., 2008; Dharmasiri et al., 2009). Although a majority of devices that employ DEP methods do not quantify post-process viability, other researchers have established that exposure to electric fields from microfabricated electrodes used for DEP techniques often does not alter cell viability (Wang et al., 1999; Ho et al., 2006). Electric field magnitudes and frequencies used for these devices are listed in the Experimental Parameters column of Table 2. Ultimately, in situations where the target cell can be as few as 1 per billion non-target cells (e.g., bacteria, viruses, CTCs), cell expansion in culture will be a critical step in obtaining enough material for further experimentation.
For future studies and biological applications, the major areas for improvement are ability to elute cells in an undamaged state, increased cell survivability, and systems capable of delivering both high capture efficiency and purity. The development of such a platform could be facilitated by incorporating both electrokinetic and non-electrokinetic methods to create hybrid systems, as in recent efforts (Yang et al., 2006; Hashimoto et al., 2009; Kim et al., 2008; Kim and Soh, 2009). Combining the sensitivity of DEP cell manipulation with the robustness of immunocapture has the potential to improve rare cell capture efficiency and purity, and such hybrid systems have scientific value and applicability across a variety of biological fields.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Gottert J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. Journal of the American Chemical Society. 2008;130:10. doi: 10.1021/ja8015022. [2, 4, 5, 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao N, Wang J, Lu C. Recent advances in electric analysis of cells in microfluidic systems. Analytical and bioanalytical chemistry. 2008;391:933–42. doi: 10.1007/s00216-008-1899-x. [9] [DOI] [PubMed] [Google Scholar]
- Barrett LM, Skulan AJ, Singh AK, Cummings EB, Fiechtner GJ. Dielectrophoretic manipulation of particles and cells using insulating ridges in faceted prism microchannels. Analytical chemistry. 2005;77:6798–804. doi: 10.1021/ac0507791. [15] [DOI] [PubMed] [Google Scholar]
- Becker FF, Gascoyne PR, Wang XB, Huang Y, Pethig R, Vykoukal J. Separation of human breast cancer cells from blood by differential dielectric affinity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:860–4. doi: 10.1073/pnas.92.3.860. [11, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschler T, Demierre N, Nascimento E, Silva T, Oliva AG, Renaud P. Continuous separation of cells by balanced dielectrophoretic forces at multiple frequencies. Lab on a chip. 2008;8:280–6. doi: 10.1039/b710303d. [12] [DOI] [PubMed] [Google Scholar]
- Cen EG, Dalton C, Li Y, Adamia S, Pilarski LM, Kaler KVIS. A combined dielectrophoresis, traveling wave dielectrophoresis and electrorotation microchip for the manipulation and characterization of human malignant cells. Journal of microbiological methods. 2004;58:387–401. doi: 10.1016/j.mimet.2004.05.002. [13] [DOI] [PubMed] [Google Scholar]
- Chang WC, Lee LP, Liepmann D. Adhesion-based capture and separation of cells for microfluidic devices. Materials Research Society Proceedings. 2002;79 [3, 18] [Google Scholar]
- Chen DF, Du H. Simulation studies on electrothermal fluid flow induced in a dielectrophoretic microelectrode system. Journal of Micromechanics and Microengineering. 2006;16:2411–2419. [1, 6] [Google Scholar]
- Chen P, Feng X, Du W, Liu B. Microfluidic chips for cell sorting. Frontiers in Bioscience. 2008 doi: 10.2741/2859. [1] [DOI] [PubMed] [Google Scholar]
- Cheng IF, Chang HC, Hou D, Chang HC. An integrated dielectrophoretic chip for continuous bioparticle filtering, focusing, sorting, trapping, and detecting. Biomicrofluidics. 2007;1:21503. doi: 10.1063/1.2723669. [14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng IF, Froude VE, Zhu Y, Chang HC, Chang HC. A continuous high-throughput bioparticle sorter based on 3D traveling-wave dielectrophoresis. Lab on a chip. 2009;9:3193–201. doi: 10.1039/b910587e. [13] [DOI] [PubMed] [Google Scholar]
- Cho YK, Kim S, Lee K, Park C, Lee JG, Ko C. Bacteria concentration using a membrane type insulator-based dielectrophoresis in a plastic chip. Electrophoresis. 2009;30:3153–9. doi: 10.1002/elps.200900179. [15] [DOI] [PubMed] [Google Scholar]
- Choi S, Song S, Choi C, Park JK. Microfludic self-sorting of mammalian cells to achieve cell cycle synchrony by hydrophoresis. Analytical Chemistry. 2009;81:1964–1968. doi: 10.1021/ac8024575. [2, 7, 18] [DOI] [PubMed] [Google Scholar]
- Chou C, Tegenfeldt JO, Bakajin O, Chan S, Cox E, Darnton N, Duke T, Austin R. Electrodeless Dielectrophoresis of Single- and Double-Stranded DNA. Biophysical Journal. 2002;83:2170–2179. doi: 10.1016/S0006-3495(02)73977-5. [15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church C, Zhu J, Wang G, Tzeng TRJ, Xuan X. Electrokinetic focusing and filtration of cells in a serpentine microchannel. Biomicrofluidics. 2009;3:044109. doi: 10.1063/1.3267098. [15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Krishnamurthy S, Das CM, Reuben JM, Spohn W, Noshari J, Becker F, Gascoyne PR. Dielectric cell separation of fine needle aspirates from tumor xenografts. Journal of separation science. 2008;31:3732–9. doi: 10.1002/jssc.200800366. [10, 22] [DOI] [PubMed] [Google Scholar]
- Cui L, Morgan H. Design and fabrication of travelling wave dielectrophoresis structures. Journal of Micromechanics and Microengineering. 2000;10:72–79. [13] [Google Scholar]
- Cummings EB, Singh AK. Dielectrophoresis in Microchips Containing Arrays of Insulating Posts: Theoretical and Experimental Results. Analytical Chemistry. 2003;75:4724–4731. doi: 10.1021/ac0340612. [15] [DOI] [PubMed] [Google Scholar]
- Danila D, Heller G, Gignac G, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher H. Circulating tumor cell number and prognosis in progressive castration resistant prostate cancer. Clinical Cancer Research. 2007 doi: 10.1158/1078-0432.CCR-07-1506. [2] [DOI] [PubMed] [Google Scholar]
- Davis J, Inglis D, Morton K, Lawrence D, Huang L, Chou S, Sturm J, Austin R. Deterministic hydrodynamics: Taking blood apart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14779–14784. doi: 10.1073/pnas.0605967103. [5, 6, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri U, Balamurugan S, Adams AA, Okagbare PI, Obubuafo A, Soper SA. Highly efficient capture and enumeriation of low abundance prostate cancer cells using prostate-specific membrane antigen aptamers immobilized to a polymeric microfluidic device. Electrophresis. 2009;30:3289–3300. doi: 10.1002/elps.200900141. [4, 18, 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Baune M, Thoming J. Insulator-based dielectrophoresis in viscous media - Simulation of particle and droplet velocity. Journal of Electrostatics. 2007;65:452–458. [4] [Google Scholar]
- Falokun CD, Mavituna F, Markx GH. AC electrokinetic characterisation and separation of cells with high and low embryogenic potential in suspension cultures of carrot (Daucus carota) Plant Cell Tissue and Organ Culture. 2003;75:261–272. [12] [Google Scholar]
- Fatoyinbo HO, Hughes MP, Martin SP, Pashby P, Labeed FH. Dielectrophoretic separation of Bacillus subtilis spores from environmental diesel particles. Journal of environmental monitoring : JEM. 2007;9:87–90. doi: 10.1039/b614556f. [10, 16, 18] [DOI] [PubMed] [Google Scholar]
- Gadish N, Voldman J. High-throughput positive-dielectrophoretic bioparticle microconcentrator. Analytical chemistry. 2006;78:7870–6. doi: 10.1021/ac061170i. [10, 18] [DOI] [PubMed] [Google Scholar]
- Gascoyne P, Mahidol C, Ruchirawat M, Satayavivad J, Watcharasit P, Becker FF. Microsample preparation by dielectrophoresis: isolation of malaria. Lab on a chip. 2002;2:70–5. doi: 10.1039/b110990c. [11, 13, 16, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne PRC, Noshari J, Anderson TJ, Becker FF. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30:1388–98. doi: 10.1002/elps.200800373. [1, 12, 16, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (gedi) and a prostate-specific antibody. Lab on a Chip. 2010;10:27–29. doi: 10.1039/b917959c. [1, 2, 4, 5, 17, 18, 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Kaji H, Nishizawa M. Selective capture of a specific cell type from mixed leucocytes in an electrode-integrated microfluidic device. Biosensors & bioelectronics. 2009;24:2892–7. doi: 10.1016/j.bios.2009.02.025. [12, 16, 19] [DOI] [PubMed] [Google Scholar]
- Hawkins BG, Gleghorn JP, Kirby BJ. Dielectrophoresis for Particle and Cell Manipulations. 1st. Artech House; Boston, MA: 2009. [9] [Google Scholar]
- Hawkins BG, Smith AE, Syed YA, Kirby BJ. Continuous-flow particle separation by 3D Insulative dielectrophoresis using coherently shaped, dc-biased, ac electric fields. Analytical chemistry. 2007;79:7291–300. doi: 10.1021/ac0707277. [15, 22] [DOI] [PubMed] [Google Scholar]
- Ho CT, Lin RZ, Chang WY, Chang HY, Liu CH. Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap. Lab on a Chip. 2006;6:724–734. doi: 10.1039/b602036d. [19] [DOI] [PubMed] [Google Scholar]
- Huang JT, Wang GC, Tseng KM, Fang SB. A chip for catching, separating, and transporting bio-particles with dielectrophoresis. Journal of industrial microbiology & biotechnology. 2008;35:1551–7. doi: 10.1007/s10295-008-0459-x. [2, 7, 18] [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang X, Becker F, Gascoyne P. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophysical Journal. 1997;73:1118–1129. doi: 10.1016/S0006-3495(97)78144-X. [12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang J, Wang XB, Becker FF, Gascoyne PR. The removal of human breast cancer cells from hematopoietic CD34+ stem cells by dielectrophoretic field-flow-fractionation. Journal of hematotherapy & stem cell research. 1999;8:481–90. doi: 10.1089/152581699319939. [12, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang JM, Hopkins PJ, Kassegne S, Tirado M, Forster AH, Reese H. Separation of simulants of biological warfare agents from blood by a miniaturized dielectrophoresis device. Biomedical Microdevices. 2003;5:217–225. [11, 16, 18] [Google Scholar]
- Inglis D, Davis J, Zieziulewicz T, Lawrence D, Austin R, Sturm J. Determining blood cell size using microfluidic hydrodynamics. Journal of Immunological Methods. 2008;329:151–156. doi: 10.1016/j.jim.2007.10.004. [5, 6] [DOI] [PubMed] [Google Scholar]
- Ji H, Samper V, Chen Y, Heng C, Lim T, Yobas L. Silicon-based microfilters for whole blood cell separation. Biomedical Microdevices. 2008;10:251–257. doi: 10.1007/s10544-007-9131-x. [5, 6, 22] [DOI] [PubMed] [Google Scholar]
- Jones TB. Electromechanics of Particles. Cambridge University Press; New York, NY: 1995. [8, 9] [Google Scholar]
- Kang Y, Li D. Electrokinetic motion of particles and cells in microchannels. Microfluidics and Nanofluidics. 2009;6:431–460. [9] [Google Scholar]
- Kang Y, Li D, Kalams SA, Eid JE. DC-Dielectrophoretic separation of biological cells by size. Biomedical microdevices. 2008;10:243–9. doi: 10.1007/s10544-007-9130-y. [15] [DOI] [PubMed] [Google Scholar]
- Kim U, Qian J, Kenrick SA, Daugherty PS, Soh HT. Multitarget dielectrophoresis activated cell sorter. Analytical chemistry. 2008;80:8656–61. doi: 10.1021/ac8015938. [14, 16, 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Shu CW, Dane KY, Daugherty PS, Wang JYJ, Soh HT. Selection of mammalian cells based on their cell-cycle phase using dielectrophoresis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20708–12. doi: 10.1073/pnas.0708760104. [14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Soh HT. Simultaneous sorting of multiple bacterial targets using integrated dielectrophoretic-magnetic activated cell sorter. Lab on a chip. 2009;9:2313–8. doi: 10.1039/b903950c. [14, 16, 19] [DOI] [PubMed] [Google Scholar]
- Kirby BJ. Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices. Cambridge University Press; 2010. [8] [Google Scholar]
- Koo OK, Liu Y, Shuaib S, Bhattacharya S, Ladisch MR, Bashir R, Bhunia AK. Targeted capture of pathogenic bacteria using a mammalian cell receptor coupled with dielectrophoresis on a biochip. Analytical chemistry. 2009;81:3094–101. doi: 10.1021/ac9000833. [10] [DOI] [PubMed] [Google Scholar]
- Kremser L, Blaas D, Kenndler E. Capillary electrophoresis of biological particles: viruses, bacteria, and eukaryotic cells. Electrophoresis. 2004;25:2282–91. doi: 10.1002/elps.200305868. [8] [DOI] [PubMed] [Google Scholar]
- Kua CH, Lam YC, Rodriguez I, Yang C, Youcef-Toumi K. Dynamic cell fractionation and transportation using moving dielectrophoresis. Analytical chemistry. 2007;79:6975–87. doi: 10.1021/ac070810u. [13] [DOI] [PubMed] [Google Scholar]
- Kulrattanarak T, van der Sman R, Schron C, Boom R. Classification and evaluation of microfluidic devices for continuous suspension fractionation. Advances in Colloid and Interface Science. 2008 doi: 10.1016/j.cis.2008.05.001. [1] [DOI] [PubMed] [Google Scholar]
- Kuntaegowdanahalli S, Bhagat AAS, Kumar G, Papautsky I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab on a Chip. 2009;9:2973–2980. doi: 10.1039/b908271a. [7, 18] [DOI] [PubMed] [Google Scholar]
- Lapizco-Encinas BH, Davalos RV, Simmons BA, Cummings EB, Fintschenko Y. An insulator-based (electrodeless) dielectrophoretic concentrator for microbes in water. Journal of microbiological methods. 2005;62:317–26. doi: 10.1016/j.mimet.2005.04.027. [1, 15] [DOI] [PubMed] [Google Scholar]
- Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y. Dielectrophoretic concentration and separation of live and dead bacteria in an array of insulators. Analytical chemistry. 2004a;76:1571–9. doi: 10.1021/ac034804j. [14, 15, 22] [DOI] [PubMed] [Google Scholar]
- Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y. Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis. 2004b;25:1695–704. doi: 10.1002/elps.200405899. [15] [DOI] [PubMed] [Google Scholar]
- Lei U, Huang CW, Chen J, Yang CY, Lo YJ, Wo A, Chen CF, Fung TW. A travelling wave dielectrophoretic pump for blood delivery. Lab on a chip. 2009;9:1349–56. doi: 10.1039/b822809d. [13] [DOI] [PubMed] [Google Scholar]
- Li H, Bashir R. Dielectrophoretic separation and manipulation of live and heat-treated cells of Listeria on microfabricated devices with interdigitated electrodes. Sensors and Actuators B: Chemical. 2002;86:215–221. [10, 18] [Google Scholar]
- Lin CH, Lee CY, Tsai CH, Fu LM. Novel continuous particle sorting in microfluidic chip utilizing cascaded squeeze effect. Microfludics and Nanofluidics. 2009;7:499–508. [7] [Google Scholar]
- Liu CX, Lagae L, Borghs G. Manipulation of magnetic particles on chip by magnetophoretic actuation and dielectrophoretic levitation. Applied Physics Letters. 2007a;90 [1, 4, 19] [Google Scholar]
- Liu YS, Walter TM, Chang WJ, Lim KS, Yang L, Lee SW, Aronson A, Bashir R. Electrical detection of germination of viable model Bacillus anthracis spores in microfluidic biochips. Lab on a chip. 2007b;7:603–10. doi: 10.1039/b702408h. [10, 18] [DOI] [PubMed] [Google Scholar]
- Loire S, Mezic I. Separation of bioparticles using the travelling wave dielectrophoresis with multiple frequencies. IEEE, Maui, Hawaii, USA. 2003;6 [13] [Google Scholar]
- Marks GH, Huang Y, Zhou XF, Pethig R. Dielectrophoretic characterization and separation of microorganisms. Microbiology. 1994;140:585–591. [11] [Google Scholar]
- Markx GH, Pethig R. Dielectrophoretic separation of cells: Continuous separation. Biotechnology and bioengineering. 1995;45:337–43. doi: 10.1002/bit.260450408. [11] [DOI] [PubMed] [Google Scholar]
- Mehrishi JN, Bauer J. Electrophoresis of cells and the biological relevance of surface charge. Electrophoresis. 2002;23:1984. doi: 10.1002/1522-2683(200207)23:13<1984::AID-ELPS1984>3.0.CO;2-U. [8] [DOI] [PubMed] [Google Scholar]
- Mela P, van Den Berg A, Fintschenko Y, Cummings EB, Simmons BA, Kirby BJ. The zeta potential of cyclo-olefin polymer microchannels and its effects on insulative (electrodeless) dielectrophoresis particle trapping devices. Electrophoresis. 2005;26:1792–9. doi: 10.1002/elps.200410153. [15] [DOI] [PubMed] [Google Scholar]
- Mohamed H, McCurdy LD, Szarowski DH, Duva S, Turner JN, Caggana M. Development of a rare cell fractionation device: Application for cancer detection. IEEE Transactions on Nanobioscience. 2004;3:251–256. doi: 10.1109/tnb.2004.837903. [2] [DOI] [PubMed] [Google Scholar]
- Mohamed H, Turner JN, Caggana M. Biochip for separating fetal cells from mathernal circulation. Journal of Chromatography A. 2007;1162:187–192. doi: 10.1016/j.chroma.2007.06.025. [2, 6] [DOI] [PubMed] [Google Scholar]
- Morgan H, Green N. AC Electrokinetics: Colloids and Nanoparticles. 1. Research Studies Press, Ltd.; Baldock, Hertfordshire, England: 2002. [9] [Google Scholar]
- Murthy SK, Sin A, Tompkins RG, Toner M. Effect of flow and surface conditions on human lymphocyte isolation using microfluidic chambers. Langmuir. 2004;20:8. doi: 10.1021/la048047b. [2, 3, 5, 18] [DOI] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith M, Kwak EL, Digurmarthy S, Muzikansky A, Ryan P, Balis U, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumor cells in cancer patients by microchip technology. Nature. 2007;450 doi: 10.1038/nature06385. [1, 2, 4, 5, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi Jichi. Upregulation of prostacyclin synthesisrelated gene expression by shear stress in vascular endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998 doi: 10.1161/01.atv.18.12.1922. [19] [DOI] [PubMed] [Google Scholar]
- Pappas D, Wang K. Cellular separations: A review of new challenges in analytical chemistry. Analytica Chimica Acta. 2007;601:26–35. doi: 10.1016/j.aca.2007.08.033. [1] [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Njoka DN, Harris J, Liao J, Horick NK, Radisic M, Murthy SK. Peptide-mediated selective adhesion of smooth muscle and endothelial cells in microfluidic shear flow. Langmuir. 2007;23:7. doi: 10.1021/la0700220. [3, 5, 17, 18] [DOI] [PubMed] [Google Scholar]
- Plouffe BD, Radisic M, Murthy SK. Microfluidic depletion of endothelial cells, smooth muscle cells, and fibroblasts from heterogeneous suspensions. Lab on a Chip. 2008;8:11. doi: 10.1039/b715707j. [5, 17, 18] [DOI] [PubMed] [Google Scholar]
- Pommer MS, Zhang Y, Keerthi N, Chen D, Thomson JA, Meinhart CD, Soh HT. Dielectrophoretic separation of platelets from diluted whole blood in microfluidic channels. Electrophoresis. 2008;29:1213–8. doi: 10.1002/elps.200700607. [14] [DOI] [PubMed] [Google Scholar]
- Pui-ock S, Ruchirawat M, Gascoyne P. Dielectrophoretic field-flow fractionation system for detection of aquatic toxicants. Analytical chemistry. 2008;80:7727–34. doi: 10.1021/ac801095p. [12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysher MD, Hayes MA. Electrophoretic and dielectrophoretic field gradient technique for separating bioparticles. Analytical chemistry. 2007;79:4552–7. doi: 10.1021/ac070534j. [15] [DOI] [PubMed] [Google Scholar]
- Rhoades ER, Geisel RE, Butcher BA, McDonough S, Russell DG. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: characterization of a new model of the granuloma-tous response to mycobacterial components. Tuberculosis (Edinburgh, Scotland) 2005;85:159–76. doi: 10.1016/j.tube.2004.10.001. [16] [DOI] [PubMed] [Google Scholar]
- Rousselet J, Markx G, Pethig R. Separation of erythrocytes and latex beads by dielectrophoretic levitation and hyperlayer field-flow fractionation. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1998;140:209–216. [12] [Google Scholar]
- Sethu P, Sin A, Toner M. Microfluidic diffusive filter for apheresis (leukapheresis) Lab on a Chip. 2005;6:83–89. doi: 10.1039/b512049g. [2, 5, 17, 18] [DOI] [PubMed] [Google Scholar]
- Shafiee H, Sano MB, Henslee EA, Caldwell JL, Davalos RV. Selective isolation of live/dead cells using contactless dielectrophoresis (cDEP) Lab on a chip. 2010;10:438–45. doi: 10.1039/b920590j. [16] [DOI] [PubMed] [Google Scholar]
- Sin A, Murthy SK, Revzin A, Tompkins RG, Toner M. Enrichment using antibody-coated microfluidic chambers in shear flow: model mixtures of human lymphocytes. Bioengineering and Biotechnology. 2005;91:816–826. doi: 10.1002/bit.20556. [3, 5, 18] [DOI] [PubMed] [Google Scholar]
- SooHoo J, Walker G. Microfluidic liquid filters for leukocyte isolation. Proceedings of the 29th Annual International Conference of the IEEE EMBS; 2007. [7] [DOI] [PubMed] [Google Scholar]
- Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswara S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Science and Translational Medicine. 2010 doi: 10.1126/scitranslmed.3000403. [2, 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Morgan H, Green N. Analytical solutions of ac electrokinetics in interdigitated electrode arrays: Electric field, dielectrophoretic and traveling-wave dielectrophoretic forces. Physical Review E. 2007;76 doi: 10.1103/PhysRevE.76.046610. [9] [DOI] [PubMed] [Google Scholar]
- Tai CH, Hsiung SK, Chen CY, Tsai ML, Lee GB. Automatic microfluidic platform for cell separation and nucleus collection. Biomedical microdevices. 2007;9:533–43. doi: 10.1007/s10544-007-9061-7. [11] [DOI] [PubMed] [Google Scholar]
- Talary MS, Burt JPH, Tame JA, Pethig R. Electro-manipulation and separation of cells using travelling electric fields. Journal of Physics D-Applied Physics. 1996;29:2198–2203. [13] [Google Scholar]
- Turgeon RT, Bowser MT. Micro free-flow electrophoresis: theory and applications. Analytical and bioanalytical chemistry. 2009;394:187–98. doi: 10.1007/s00216-009-2656-5. [8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey MD, Voldman J. An equilibrium method for continuous-flow cell sorting using dielectrophoresis. Analytical chemistry. 2008;80:3135–43. doi: 10.1021/ac7020568. [14] [DOI] [PubMed] [Google Scholar]
- VanDelinder V, Groisman A. Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Analytical Chemistry. 2006;78:3765–3771. doi: 10.1021/ac060042r. [5, 6, 18] [DOI] [PubMed] [Google Scholar]
- VanDelinder V, Groisman A. Perfusion in microfluidic cross-flow: Separation of white blood cells from whole blood and exchange of medium in a continuous flow. Analytical Chemistry. 2007;79:2023–2030. doi: 10.1021/ac061659b. [2, 5, 6, 18] [DOI] [PubMed] [Google Scholar]
- Voldman J. Electrical forces for microscale cell manipulation. Annual Review of Biomedical Engineering. 2006;8:425–454. doi: 10.1146/annurev.bioeng.8.061505.095739. [9] [DOI] [PubMed] [Google Scholar]
- Vykoukal J, Vykoukal DM, Freyberg S, Alt EU, Gascoyne PRC. Enrichment of putative stem cells from adipose tissue using dielectrophoretic field-flow fractionation. Lab on a chip. 2008;8:1386–93. doi: 10.1039/b717043b. [12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lu J, Marchenko SA, Monuki ES, Flanagan LA, Lee AP. Dual frequency dielectrophoresis with interdigitated sidewall electrodes for microfluidic flow-through separation of beads and cells. Electrophoresis. 2009;30:782–91. doi: 10.1002/elps.200800637. [4, 12] [DOI] [PubMed] [Google Scholar]
- Wang X, Huang Y, Wang X, Becker F, Gascoyne P. Dielectrophoretic manipulation of cells with spiral electrodes. Biophysical Journal. 1997;72:1887–1899. doi: 10.1016/S0006-3495(97)78834-9. [13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Huang Y, Burt JPH, Markx GH, Pethig R. Selective Dielectrophoretic Confinement of Bioparticles in Potential-Energy Wells. Journal of Physics D-Applied Physics. 1993;26:1278–1285. [11] [Google Scholar]
- Wang XB, Yang J, Huang Y, Vykoukal J, Becker FF, Gascoyne PRC. Cell Separation by Dielectrophoretic Field-flow-fractionation. Analytical Chemistry. 2000;72:832–839. doi: 10.1021/ac990922o. [12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Yang J, Gascoyne PRC. Role of peroxide in AC electrical field exposure effects on Friend murine erythroleukemia cells during dielectrophoretic manipulations. Biochimica Et Biophysica Acta-General Subjects. 1999;1426:53–68. doi: 10.1016/s0304-4165(98)00122-6. [19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chin SY, Chin CD, Sarik J, Harper M, Justman J, Sia SK. Microfluidic cd4+ t-cell counting device using chemiluminescence-based detection. Analytical Chemistry. 2010;82:39–40. doi: 10.1021/ac902144w. [3] [DOI] [PubMed] [Google Scholar]
- Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by 1-integrin signaling pathways. Hypertension. 2003 doi: 10.1161/01.HYP.0000062882.42265.88. [19] [DOI] [PubMed] [Google Scholar]
- Wu Z, Willing B, Bjerketorp J, Jansson JK, Hjort K. Soft inertial microfluidics for high throughput separation of bacteria from human blood cells. Lab on a Chip. 2009;9:1193–1199. doi: 10.1039/b817611f. [1, 8, 18, 19, 22] [DOI] [PubMed] [Google Scholar]
- Xu Y, Philips JA, Yan J, Li Q, Fan ZH, Tan W. Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer cells. Analytical Chemistry. 2009;81:7436–7442. doi: 10.1021/ac9012072. [4, 18, 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang Y, Wang X, Becker F, Gascoyne P. Differential Analysis of Human Leukocytes by Dielectrophoretic Field-Flow-Fractionation. Biophysical Journal. 2000;78:2680–2689. doi: 10.1016/S0006-3495(00)76812-3. [12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang Y, Wang XB, Becker FF, Gascoyne PRC. Cell Separation on Microfabricated Electrodes Using Dielectrophoretic/Gravitational Field-Flow Fractionation. Analytical Chemistry. 1999;71:911–918. doi: 10.1021/ac981250p. [12, 18] [DOI] [PubMed] [Google Scholar]
- Yang L, Banada PP, Chatni MR, Seop Lim K, Bhunia AK, Ladisch M, Bashir R. A multifunctional microfluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of Listeria monocyto-genes. Lab on a chip. 2006;6:896–905. doi: 10.1039/b607061m. [1, 10, 16, 18, 19] [DOI] [PubMed] [Google Scholar]
- Zhang C, Khoshmanesh K, Mitchell A, Kalantar-Zadeh K. Dielectrophoresis for manipulation of micro/nano particles in microfluidic systems. Analytical and bioanalytical chemistry. 2010;396:401–20. doi: 10.1007/s00216-009-2922-6. [9] [DOI] [PubMed] [Google Scholar]
- Zhang YT, Bottausci F, Rao MP, Parker ER, Mezic I, MacDonald NC. Titanium-based dielectrophoresis devices for microfluidic applications. Biomedical Microdevices. 2008;10:509–517. doi: 10.1007/s10544-007-9159-y. [19] [DOI] [PubMed] [Google Scholar]
- Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. Journal of Chromatography A. 2007;1162:8. doi: 10.1016/j.chroma.2007.05.064. [5, 6, 18, 19] [DOI] [PubMed] [Google Scholar]
- Zheng S, Liu JQ, Tai YC. Streamline-based microfluidic devices for erythrocytes and leukocytes separation. Journal of Microelectromechanical systems. 2008;17:1029–1038. [7, 18] [Google Scholar]


