Abstract
NK cells become functionally competent to be triggered by their activation receptors through the interaction of NK cell inhibitory receptors with their cognate self-MHC ligands, an MHC-dependent educational process termed “licensing.” For example, Ly49A+ NK cells become licensed by the interaction of the Ly49A inhibitory receptor with its MHC class I ligand, H2Dd while Ly49C+ NK cells are licensed by H2Kb. Structural studies indicate that the Ly49A inhibitory receptor may interact with two sites, termed site 1 and site 2, on its H2Dd ligand. Site 2 encompasses the α1/α2/α3 domains of the H2Dd heavy chain and β2-microglobulin (β2m), and is the functional binding site for the Ly49A in effector inhibition. Ly49C functionally interacts with a similar site in H2Kb. However, it is currently unknown whether this same site is involved in Ly49A or Ly49C-dependent licensing. Herein, we produced transgenic C57BL/6 mice expressing wild type or site 2 mutant H2Dd molecules and studied whether or not Ly49A+ NK cells are licensed. We also investigated Ly49A and Ly49C-dependent NK licensing in murine β2m-deficient mice which are transgenic for human β2m which has species-specific amino acid substitutions in β2m. Our data from these transgenic mice indicate that site 2 on self-MHC is critical for Ly49A and Ly49C-dependent NK cell licensing. Thus, NK cell licensing through Ly49 involves specific interactions with its MHC ligand that are similar to those involved in effector inhibition.
Keywords: Innate immunity, NK cell, Ly49, MHC, Licensing
Introduction
NK cells play a major role in innate immunity against tumors and virally infected cells (1). When NK cells engage cellular targets, they can be activated to kill and secrete immunoregulatory cytokines such as IFNγ (2). NK cells express two functional types of cell surface receptors that modulate these effects: activation and inhibitory receptors (3). Engagement of activation NK cell receptors with induced endogenous and virally-encoded ligands results in stimulation of NK cell responses. By contrast, inhibitory NK cell receptors recognize MHC class I molecules and block NK cell stimulation. The recognition of self-MHC class I molecules by inhibitory receptors in part explains the “missing-self” hypothesis and prevents dysregulation of NK cells in the normal host.
According to the “missing-self” hypothesis, NK cells survey the cell surfaces of tissues for the presence of MHC class I molecules (“self”) that are normally ubiquitously expressed and prevent NK cell attack (4). Down-regulation of MHC class I, such as in virus-infected or tumor cells, permits NK cell stimulation via activation receptors. However, one prediction of the missing-self hypothesis is that NK cells should be overactive or autoreactive in MHC class I-deficient hosts. Yet, NK cells from MHC class I-deficient humans and mice have generally poor cytotoxic capacity (5, 6), indicating that another tolerance mechanism must also be operative.
Studies from several laboratories on mouse and human NK cells indicate that the inhibitory receptors for MHC class I have a second function in “licensing” or education of NK cells (7-11). The licensing model predicts that engagement of inhibitory receptors by self-MHC class I confers NK cells with the capacity to be subsequently stimulated through their activation receptors, i.e., NK cells require licensing to attain functional competence. NK cells that do not express the appropriate inhibitory receptors for self-MHC class I, do not attain full functional competence and are thus self-tolerant. For example, in vitro stimulation of murine NK cells via antibody cross-linking of the Nkrp1c (NK1.1, Klrb1) receptor resulted in IFNγ production primarily from NK cells that expressed an inhibitory receptor for self-MHC, such as Ly49A in a mouse expressing H2Dd, the cognate MHC class I ligand for Ly49A (7). Similarly, Ly49C+ NK cells are licensed by the cognate ligand (H2Kb) for Ly49C. Thus, Ly49A-H2Dd and Ly49CH2Kb interactions have provided support for a licensing or an MHC-dependent education effect.
The interactions between Ly49 receptors and their MHC ligands have been analyzed at the crystallographic level and in assays of effector inhibition. For example, the structure of Ly49A in complex with H2Dd revealed potential two interaction sites on H2Dd (12). Site 1 consists of the “left” side of the peptide binding cleft, as viewed from above with the α1 helix at the top, whereas site 2 consists of all three domains of H2Dd and β2-microglobulin (β2m) underneath the peptide-binding cleft. In vitro mutagenesis studies of H2Dd showed that site 2 is the key binding site for Ly49A receptors in trans interactions, i.e., when Ly49A engages H2Dd on a target cell and inhibits natural killing of the target (13, 14). For example, a point mutation (Arg to Ala) at residue 6 (R6A) in site 2 of H2Dd completely prevented Ly49A-dependent inhibition of natural killing of the T cell tumor C1498 (13). Moreover, Ly49A-dependent interactions with H2Dd is dependent on species-specific residues in β2m, such that H2Dd associated with human β2m does not interact with Ly49A (13-16). A similar site on H2Kb involving β2m is involved in interaction with Ly49C (17). In addition, Ly49A can also use site 2 to interact in cis with H2Dd expressed on the NK cell itself (18). Cis interactions can be detected by decreased binding of an anti-Ly49A monoclonal antibody, such as diminished mean fluorescence intensity (MFI) during flow cytometry (19). Thus, although a role for binding at site 1 has not yet been described, Ly49 receptors can interact with site 2 on their MHC ligands in both trans and cis.
It is not known, however, if site 2 is critical for Ly49-dependent NK cell licensing, the topic of the current study. Herein, we examined licensing of Ly49A+ NK cells with novel transgenic animal models expressing either wild type (WT) H2Dd or site 2 mutant H2Dd molecules. We also investigated whether substitution of mouse with human β2m influences natural killer licensing with respect to Ly49A and Ly49C.
Materials and Methods
Mice
C57BL/6 (B6) mice were purchased from the National Cancer Institute (NCI). H2Kb-/- Db-/- (KODO) mice were described previously (20) and were purchased from National Institute of Allergy and Infectious Diseases (NIAID) repository through Taconic (Hudson, NY). The D8 mouse is a H2Dd-transgenic line produced with an H2Dd genomic construct on B6 background and were described previously (21). Human β2m transgenic mice (22) were kindly provided by Dr. Chella David (Mayo Clinic, Rochester, MN) and bred to D8 KODO mice deficient in mouse β2m such that H2Dd is expressed with human β2m. Triple knockout (TKO) mice which lack H2KbDb and murine β2m were obtained from Dr. Ted Hansen (Washington University in St. Louis, MO). All mice were used in accordance with institutional guidelines for animal experimentation.
Development of H2Dd transgenic mice
H2Dd wild type (WT Dd) and site 2 mutant (R6A) constructs were described previously (13). These H2Dd transgene inserts were subcloned into the expression vector pHSE3’ which contains the H2Kb promoter and immunoglobulin enhancer sequences for transgene expression (23). For the R6A-1 mouse, we subcloned the R6A transgene insert into the expression vector pHβ-Apr-1-neo, which contains a human β-globin promoter sequence for transgene expression (24). Linearized transgene constructs were purified by electoelution, resuspended in microinjection buffer, and microinjected into C57BL/6 (B6) mouse embryos at the Transgenic Knockout Microinjection Core in the Department of Pathology and Immunology, supported in part by the Rheumatic Diseases Core Center (Washington University in St. Louis, MO). Transgenic founder mice were screened by Southern blot and PCR. Candidate founder mice were bred to B6 mice to generate H2Dd transgenic mice on B6 background. Each H2Dd transgenic line was then crossed to KODO mice to yield H2Dd transgenic mice on KODO background (R6A-1 KODO, R6A-2 KODO, and WT Dd KODO respectively). The genetic backgrounds of all mice were double checked for genotype by the Speed Congenics Core Facility at Washington University, supported by the Rheumatic Diseases Core Center. The characteristics of each H2Dd transgenic line are summarized in Table I.
Table I.
H2Dd transgenic mice
| Mouse | H2Dd insert | Expression vector | H2Dd(%) | Reference |
| D8 | WT Dd gene | Genomic construct | 99% | Bieberich et al (21) |
| WT Dd | WT Dd cDNA | pHSE3' | 50-60% | This paper |
| R6A-1 | R6A (site 2 mutant) cDNA | pHβ-Apr-neo | 50-60% | This paper |
| R6A-2 | R6A (site 2 mutant) cDNA | pHSE3' | 99% | This paper |
Monoclonal antibodies
145-2C11 (anti-CD3), 1D3 (anti-CD19), PK136 (anti-NK1.1), A1 (anti-Ly49A), 34-2-12 (anti-H2Dd α3), 34-5-8 (anti-H2Dd α1/α2), 28-8-6 (anti-H2Kb/Db), XMG1.2 (anti-IFNγ) antibodies were purchased from BD Biosciences (San Jose, CA). Culture supernatants were produced from hybridoma 2.4G2 (anti-FcγRII/III) (American Type Culture Collection, Manassas, VA). JR9 (anti-Ly49A) (25) was purified from culture supernatants derived from hybridomas kindly provided by Jacques Roland (Pasteur Institute, Paris, France), and then conjugated to FITC. 4LO (anti-Ly49C) was purified from culture supernatants derived from the hybridoma kindly provided by Dr. Suzanne Lemieux (Institut Armand-Frappier, Montreal, Canada), and then biotinylated in the lab.
Staining and flow cytometry
Splenocytes were suspended in RPMI-1640 media supplemented with 10% FBS, L-glutamine, penicillin, streptomycin, and 2-mercaptoethanol (R10 media). After RBC lysis, all cells were adjusted to the concentration of 107 cells per mL of R10 media. For most staining, 106 cells were used for staining. After monoclonal antibody staining for 30 minutes, cells were washed with and resuspended in ice-cold sorter buffer (PBS containing 2% FCS and 0.1% sodium azide). 2.4G2 supernatant (anti-FcγRII/III) was added to all staining to prevent non-specific Fc receptor effects. All flow cytometry data were collected using FACS Canto/Calibur/Scan flow cytometers (BD Biosciences) and then analyzed using FlowJo software (Tree Star, Ashland, OR).
Immobilized anti-NK1.1 stimulation assay
Wells in six-well culture plates were coated with 1mL of purified PK136 (anti-NK1.1) antibody diluted in PBS at concentrations of 2μg/mL or 5μg/mL for 90 minutes, then washed three times with PBS. 107 splenocytes in one mL R10 media were placed in each well and incubated for one hour at 37°C and 5% CO2. Subsequently, Golgi-Plug (BD Biosciences) containing brefeldin A was added to each well. Three mL of ice-cold sorter buffer was added to each well after 6 hours of additional incubation, and cells were then harvested. Cells were stained for 30 minutes with monoclonal antibodies for cell surface antigens, fixed with the Cytofix (BD Biosciences) and permeablized by Perm/Wash buffer (BD Biosciences) containing saponin. The cells were washed twice with Perm/Wash buffer and stained with XMG1.2 (anti-IFNγ) antibody.
The licensing ratio for a given Ly49 receptor was determined by assessing the percentage of IFNγ-producing cells in receptor-positive and receptor-negative NK cell populations. For example, Ly49A-dependent licensing ratio=(% IFNγ-producing Ly49A+ cells)/(% IFNγ-producing Ly49A- cells) whereas Ly49C-dependent licensing ratio=(% IFNγ-producing Ly49C+ cells)/(% IFNγ-producing Ly49C– cells). These ratios were calculated from the dot plots where Ly49 is on the x-axis and IFNγ staining is on the y-axis, using the following formula: [(UR)/(UR+LR)]/[(UL)/(UL+LL)].
Chromium release assay
The general protocol for chromium release assay by Ly49A+ IL-2-activated NK cells was described previously (26). In brief, splenocytes from D8 KODO (H2Kb-/- Db-/-) mice were harvested and cultured in 800 IU/mL concentration of IL-2 for 9 days. Panning by JR9 (anti-Ly49A) monoclonal antibody was performed on Day 7 to isolate Ly49A+ and Ly49A– cells. The percentage of Ly49A+ IL-2-activated NK cells in effector cell was 90-95% by FACS verification. For target cells, splenocytes were harvested from each mouse (KODO, R6A-2 KODO, WT Dd KODO, D8 KODO) and cultured with Con A (6μg/ml) for 2 days then washed and labeled with 51Cr. YAC-1 tumor cells were used as control target cells. %Specific lysis was calculated by standard formula (26).
Results
Transgenic expression of WT and mutant H2Dd
To specifically assess the role of site 2 on H2Dd in Ly49A-mediated licensing, we produced transgenic mice expressing WT and site 2 mutant H2Dd (R6A) molecules (13) directly in C57BL/6 (B6) mice. Initial screening of founder mice was performed by PCR and Southern blot. Candidate founder mice showing high levels of H2Dd transgene expression were bred to C57BL/6 mice and then crossed to H2Kb-/-Db-/- (KODO) mice for further analysis. In this manner, we obtained one new wild-type (WT) H2Dd and two new R6A H2Dd mutant (R6A-1 and R6A-2) transgenic lines on the KODO background (Table I). Similar H2Dd mRNA expression by RT-PCR and H2Dd alloreactivity by mixed lymphocyte reaction were obtained with all WT and mutant H2Dd mice (data not shown), indicating that all of the lines carry functionally expressed transgenes. Moreover, H2Dd transgenic protein expression on the cell surface of splenocytes was assessed by FACS staining with monoclonal antibodies that detect different portions of the H2Dd molecule. When stained with monoclonal antibody 34-5-8 that binds the α1/α2 domain of H2Dd, splenocytes from R6A-1 mice express mutant H2Dd at intermediate levels in a monophasic manner (Fig. 1A). These levels were somewhat lower than H2Dd in D8 mice (21), a previously produced WT H2Dd transgenic line that expresses H2Dd at levels comparable to non-transgenic H2d mice (data not shown). By contrast, R6A-2 splenocytes have biphasic expression of mutant H2Dd, with both populations expressing mutant H2Dd at levels equal to or higher than splenocytes in D8 mice. Finally, splenocytes from our new WT Dd transgenic mice also have biphasic expression of H2Dd at levels equal to or lower than R6A-2 mice. Similar results were obtained with monoclonal antibody 34-2-12 that binds the α3 domain of H2Dd (Fig. 1B). Thus, expression of R6A mutant and WT H2Dd was readily detectable with monoclonal antibodies specific for all three extracellular domains in the transgenic mice.
FIGURE 1.
Surface expression of transgenic H2Dd. Two monoclonal antibodies that detect different portions of H2Dd were used to assess transgene expression on splenocytes. A, Histograms of splenocytes from the indicated H2Dd transgenic mice (solid lines) stained with PE-conjugated monoclonal antibody 34-5-8, which detects the α1/α2 domain of H2Dd. B, Histograms of splenocytes from the indicated H2Dd transgenic mice (solid lines) stained with FITC-conjugated monoclonal antibody 34-2-12, which detects α3 domain of H2Dd. These histograms are representative of six independent experiments. In all histograms, the profiles of splenocytes from KODO (dotted line) and D8 KODO mice (dashed line) are shown. The percentage of splenocytes expressing transgenic H2Dd is shown in each histogram.
R6A mutant H2Dd expression on primary cells does not inhibit natural killing by Ly49A+ IL-2-activated NK cells
Previous studies indicated that transfected WT H2Dd expressed on C1498 tumor cells inhibited killing by Ly49A+ IL-2-activated NK cells, whereas tumor cells expressing transfected R6A mutants were killed (13, 26). Here we performed chromium release cytotoxicity assays using Con A blast targets derived from splenocytes of the H2Dd transgenic mice on KODO (H2Kb-/- Db-/-) background. Con A blasts retained similar level of H2Dd expression on cell surface as in primary cells (data not shown). As expected, similar to Con A blasts from D8 KODO mice, WT Dd KODO Con A blasts inhibited natural killing by Ly49A+ IL-2 activated NK cells (Fig. 2). However, Con A blasts from R6A-2 KODO did not inhibit killing despite expression of H2Dd on R6A-2 at levels equal to or higher than on WT Dd cells. Of note, specific lysis of Con A blasts from R6A-2 KODO mice was similar to that of KODO targets, indicating that WT but not R6A mutant H2Dd molecules on primary cell targets were recognized in effector responses by the inhibitory Ly49A receptor on NK cells in trans.
FIGURE 2.
Primary cells from WT and mutant H2Dd transgenic mice as targets for natural killing. Effector cells were Ly49A+ IL-2-activated NK cells from D8 KODO (H2Kb-/-Db-/-) mice and target cells were Con A blasts from the indicated H2Dd transgenic mice on KODO background. YAC-1 tumor cells were used as a positive control. Effector to target ratios (E:T ratios) ranging from 1:1 to 20:1 are shown. Results are representative of three independent experiments.
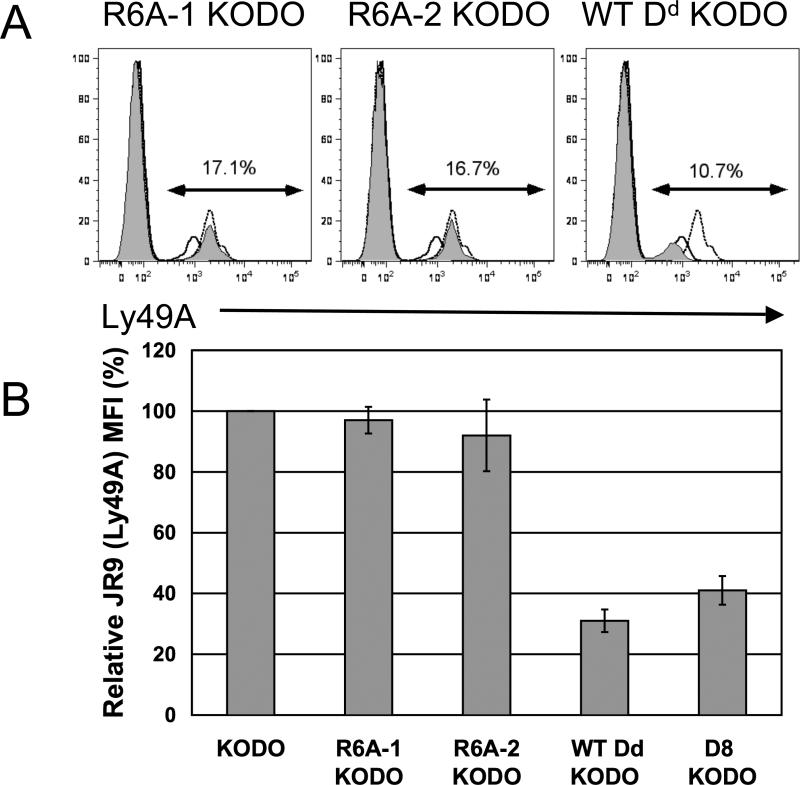
No down-modulation of Ly49A mean fluorescence intensity in R6A mutant transgenic mice
Ly49A molecules can also bind site 2 on H2Dd molecules when expressed on the NK cell itself, a cis interaction, as indicated by decreased mean fluorescent intensity (MFI) of anti-Ly49A staining by flow cytometry (19). Here we compared the MFI of FITC-conjugated JR9 (anti-Ly49A) on Ly49A+ NK cells from the various H2Dd transgenic mice on KODO (H2Kb-/- Db-/-) background to the MFI of FITC-JR9 in KODO mice, an environment lacking H2Dd (Fig. 3A). The Ly49A MFI in KODO mice was set as 100% for ease of comparison (Fig. 3B). NK cells from D8 KODO and WT Dd KODO mice had relative Ly49A MFI values of 41% and 31%, respectively, whereas NK cells from R6A-1 KODO and R6A-2 KODO mice had relative Ly49A MFI values of 97% and 92% respectively. Similar results were obtained when monoclonal antibody A1 (anti-Ly49A) was used to stain Ly49A+ NK cells from H2Dd transgenic mice on the B6 background (Supplemental Fig. 1), indicating that Ly49A down-modulation was not observed in R6A-1 and R6A-2 KODO mice. Thus, the site 2 mutation on H2Dd also affected cis recognition of transgenic H2Dd by Ly49A receptors.
FIGURE 3.
JR9 (anti-Ly49A) MFI in WT and mutant H2Dd transgenic mice. A, Representative histograms of natural killer cells from individual spleens of indicated H2Dd transgenic mice stained with FITC-conjugated JR9 (anti-Ly49A) monoclonal antibody. In all histograms, the dotted line is the histogram of a KODO (H2Kb-/-Db-/-) mouse, the solid line is that of a D8 KODO mouse, and the shaded histogram is from a representative mouse of the indicated H2Dd transgenic line. In each histogram, the percentage of NK cells expressing Ly49A is shown. B, For ease of comparison, MFIs of Ly49A from the indicated H2Dd transgenic mice are compared to the MFI of Ly49A in KODO mice, which is set as 100%. MFIs of Ly49A from KODO, R6A-1 KODO, R6A-2 KODO, D8 KODO, and Dd WT KODO mice were 2346, 2182, 2158, 727, and 962 respectively. These results are pooled from data from six independent experiments and are shown as mean + SD.
Ly49A+ NK cells from R6A mutant mice are not licensed
To assess licensing of NK cells from mutant mice, NK cells were stimulated with immobilized PK136 (anti-NK1.1) which has previously been shown to more robustly stimulate IFNγ production by licensed as compared to unlicensed NK cells (7). The Ly49A-dependent licensing ratio is calculated by dividing the percentage of IFNγ-producing Ly49A+ NK cells by the corresponding percentage among Ly49A- NK cells, and it indicates whether the Ly49A+ NK cells are more reactive or licensed than the counterpart Ly49A- population from the same mouse (Fig. 4A). The KODO (H2Kb-/- Db-/-) mice and D8 KODO mice were used as a negative and a positive control, respectively. At a low concentration (2μg/mL) of immobilized PK136 (anti-NK1.1), the licensing ratio in WT Dd KODO mice was greater than 1, indicating a positive licensing effect of transgenic H2Dd on Ly49A+ NK cells. However, there was a significantly lower effect on NK cells from R6A-1 and R6A-2 mice, which exhibited a Ly49A-dependent licensing ratio similar to KODO mice. Similar results were obtained when we used a higher concentration (5μg/mL) of PK136 to stimulate the NK cells (Fig. 4 B, 4C). These results indicate that R6A mutants do not have the capacity to license NK cells via Ly49A and that preservation of site 2 in H2Dd is critical for licensing of NK cells via Ly49A and H2Dd interactions.
FIGURE 4.
NK cell licensing in WT and mutant H2Dd transgenic mice. A, A representative experiment is shown when cells from an individual mouse of indicated genotype were stimulated by plate-bound PK136 (anti-NK1.1) antibody. In this experiment, PK136 antibody at the concentration of 2μg/mL was used for coating the culture well. The Ly49A licensing ratio (R) is shown in each plot. See Methods for calculation of licensing ratio. Cells shown are gated on CD3-, CD19-, NK1.1+ splenocytes. The licensing ratios (mean + SD) are shown for pooled results from six independent experiments with PK136 coating concentration of 2μg/mL (Fig. 4B) and 5μg/mL (Fig. 4C). The p value for Student's t-test is shown.
Ly49A+ NK cells from human β2m transgenic mice are not licensed
To corroborate the studies of mutant H2Dd mice, we next sought to assess the role of β2m subunit in Ly49-dependent licensing because Ly49 interactions with MHC class I ligands is dependent on species-specific residues in mouse β2m (13-16). For this analysis, we used human β2m transgenic mice that had been well studied previously for T cell responses (22, 27). These mice were then backcrossed to the D8 TKO (triple knockout, murine β2m-/- H2Kb-/-Db-/-) mice (Table II). When stained with monoclonal antibody 34-5-8 which detects α1/α2 domain of H2Dd molecule, huβ2m D8 TKO mice showed levels of H2Dd transgene expression comparable to that of D8 KODO mice (Fig. 5A). Similar results were obtained with monoclonal antibody 34-2-12 which detects the α3 domain of H2Dd. Thus, H2Dd Tg protein complexed with human β2m was normally expressed on splenocytes from these mice as assessed by FACS staining with monoclonal antibodies that detect different portions of the H2Dd molecule.
Table II.
H2Dd and human β2m double transgenic mice.
| Mice | H2Dd Tg | human β2m Tg | KbDb | murine β2m |
| D8 KODO | + | - | -/- | +/+ |
| huβ2m D8 TKO | + | + | -/- | -/- |
| TKO | - | - | -/- | -/- |
FIGURE 5.
Ly49A-dependent NK cell licensing in human β2m transgenic mouse. A, Expression of H2Dd is shown. Splenocytes from each transgenic mice were stained with monoclonal antibody 34-5-8 which detects α1/α2 domain of H2Dd and 34-2-12 which detects α3 domain of H2Dd. Dotted line, dashed line and solid line are the histograms of TKO (murine β2m-/- H2Kb-/-Db-/-), D8 KODO (H2Kb-/-Db-/-), and huβ2m D8 TKO mice, respectively. huβ2m D8 TKO mice showed comparable level of H2Dd expression on cell surface when compared with D8 KODO mice, as indicated by two different monoclonal antibodies which detect different domains of H2Dd. B, Ly49A MFI is shown. For ease of comparison, MFIs of Ly49A from the indicated transgenic mice were compared to the MFI of Ly49A in TKO mice, which was set as 100%. huβ2m D8 TKO mouse showed comparable level of Ly49A MFI (97%) while D8 KODO mouse showed much lower level (43%). MFIs of Ly49A from TKO, huβ2m D8 TKO, and D8 KODO mice were 92, 89, and 40 respectively. C and D, Ly49A-dependent licensing ratios at PK136 (anti-NK1.1) concentration of 2μg/mL and 5μg/mL are shown in Fig. 5C and Fig. 5D respectively. These results are shown in mean ± SD. Mice compared in Fig. 5 are listed in Table II.
Then we compared the MFI (mean fluorescence intensity) of FITC-conjugated JR9 (anti-Ly49A) on Ly49A+ NK cells from TKO, huβ2m D8 TKO, and D8 KODO mice. The Ly49A MFI in TKO mice was set as 100% for ease of comparison (Fig. 5B). Huβ2m D8 TKO and D8 KODO mice had relative Ly49A MFI values of 97% and 43% respectively. Thus, Ly49A down-modulation was not observed in huβ2m D8 TKO mouse where mouse β2m was replaced by human β2m.
Lastly, we compared the Ly49A-dependent licensing ratio of huβ2m D8 TKO mouse to that of TKO and D8 KODO mice. At a low concentration (2μg/mL) of immobilized PK136 (anti-NK1.1), the licensing ratio in huβ2m D8 TKO mouse was similar to that of TKO mouse which was used as a negative control, whereas the licensing ratio in D8 KODO was close to 2 (Fig. 5C). Similar results were obtained at a higher concentration (5μg/mL) of immobilized PK136 (Fig. 5D). These results indicate that H2Dd complexed with human β2m does not have the capacity to license NK cells via Ly49A. Thus, the β2m subunit provides species-specific sites crucial for licensing of NK cells via Ly49A and H2Dd interactions, consistent with a role for site 2 because mouse but not human β2m provides all critical β2m residues in site 2 (13-16).
Role of human β2m subunit in Ly49C-mediated NK licensing
The interaction of Ly49C with H2Kb is also dependent on species-specific residues in β2m (15, 17). To investigate the role of human β2m in Ly49C-dependent NK licensing, we produced human β2m Tg, murine β2m knockout mice (huβ2mTg muβ2mKO) on the C57BL/6 (H2b) background (Table III). When we compared MHC expression on cell surface of B6 versus huβ2mTg muβ2mKO splenocytes with monoclonal antibody 28-8-6 which detects H2Kb/Db, both mice showed comparable levels (Fig. 6A). Human β2m transgene protein expression was directly assessed by FACS staining with monoclonal antibody FITC-TU99 (Fig. 6A). Also, the MFI of Ly49C was compared by staining by monoclonal antibody 4LO (anti-Ly49C). The Ly49C MFI of β2mKO mouse was set as 100% for ease of comparison. The relative values of Ly49C MFI of huβ2mTg muβ2mKO mouse and B6 mouse was 56% and 25% respectively (Fig. 6B), showing that Ly49C staining was susceptible to species-specific changes in β2m but less than expected as compared with Ly49A (Fig. 5B).
Table III.
Human β2m Tg mice on H2b background.
| Mice | KbDb | murine β2m | human β2m Tg |
| B6 (H2b) | +/+ | +/+ | - |
| huβ2mTg muβ2mKO | +/+ | -/- | + |
| β2mKO | +/+ | -/- | - |
FIGURE 6.
Ly49C-dependent licensing in human β2m transgenic mice. A, H2KbDb and human β2m expression is shown. Splenocytes from indicated mice were stained with monoclonal antibody 28-8-6 (anti-H2KbDb) and with monoclonal antibody TU99 (anti-human β2m) respectively. In all histograms, dotted line is β2mKO mouse, dashed line is B6 mouse, and solid line is huβ2mTg muβ2mKO mouse. B, Ly49C MFI is shown. Relative MFIs of Ly49C of Ly49C+ NK cells from indicated mice are compared, when Ly49C MFI of β2mKO mouse is set as 100%. C, Ly49C-dependent licensing ratios are shown at a lower PK136 concentration (2μg/mL). D, Ly49C-dependent licensing ratios are shown at a higher PK136 concentration (5μg/mL) These results are shown in mean ± SD. Mice compared in Fig. 6 are listed in Table III.
Finally we compared the Ly49C-dependent licensing ratio in NK cells from β2mKO, huβ2mTg muβ2mKO, and B6 mice. At a low concentration (2μg/mL) of immobilized PK136 (anti-NK1.1), huβ2mTg muβ2mKO NK cells had a licensing ratio virtually identical to that of β2mKO cells, with both much lower than B6 cells (Fig. 6C). Similar results were obtained at a high concentration (5μg/mL) of PK136 (Fig. 6D), indicating a role for species-specific β2m substitutions in Ly49C-dependent licensing.
Discussion
We developed new transgenic mice to study the role of site 2 on the MHC molecule in Ly49-dependent natural killer cell licensing. For Ly49A, WT and site 2 mutant H2Dd molecules were well expressed as determined by FACS staining of splenocytes with two different monoclonal antibodies specific for different extracellular domains of H2Dd. As expected, site 2 mutant H2Dd molecules on primary cells did not inhibit natural killing by Ly49A+ IL-2-activated NK cells in contrast to WT H2Dd. Moreover, site 2 mutant H2Dd did not bind Ly49A in cis as measured by Ly49A MFI, while WT Tg H2Dd showed an effect on Ly49A MFI even at levels only 50-60% of normal expression levels, consistent with previous reports (19). Most importantly, site 2 mutant H2Dd did not contribute to Ly49A-dependent NK cell licensing. These data were corroborated by investigation of licensing in human β2m Tg mice which lack murine β2m. Despite otherwise normal MHC class I expression, both Ly49A and Ly49-dependent licensing were perturbed in these animals that is likely due to species-specific substitutions in β2m that influence site 2 interactions of MHC class I with Ly49 receptors in vitro (13-17). Taken together, our studies indicate that site 2 in MHC class I molecules is critical for Ly49-dependent NK cell licensing as well as for inhibition of natural killing.
Our interpretations were dependent on appropriate transgenic expression of WT and mutant H2Dd molecules. Inasmuch as transgenic effects in any single transgenic mouse could be due to construct insertion and not necessarily to expression of the transgenic molecule, we studied two transgenic mutant H2Dd founder lines and analyzed mice on the KODO background in order to minimize effects of endogenous MHC class I. Our WT H2Dd transgenic mice produced licensing effects on Ly49A+ NK cells comparable to a previously produced H2Dd transgenic mice (D8) (21), although the effects on licensing in D8 mice were slightly more robust. These effects could be related to a somewhat higher level of H2Dd in D8 versus our newly described WT H2Dd transgenic mice. Regardless, these data suggest that a relatively low level of H2Dd is sufficient to generate licensing effects, consistent with our previous observations with MHC heterozygous mice and with different MHC haplotypes (28). On the other hand, we found no evidence for licensing of Ly49A+ NK cells in the two different R6A founder lines. The founder line R6A-1 expresses mutant H2Dd at levels comparable to the new WT H2Dd transgenic line, whereas the R6A-2 line expresses mutant H2Dd at levels much higher than WT H2Dd. However, neither R6A mutant demonstrated licensing effects on Ly49A+ NK cells. Moreover, the R6A-2 transgenic line expresses mutant H2Dd with a promoter construct identical to that used for our new WT H2Dd transgenic line, and R6A-2 still does not induce the licensing phenotype in Ly49A+ NK cells. These data with new mutant H2Dd Tg mice suggest that site 2 is required for Ly49A-dependent licensing.
Most importantly, these studies were corroborated by compound Tg and ko mice where we replaced mouse β2m with human β2m which lacks appropriate murine β2m residues for Ly49A-dependent interactions. In addition, human β2m Tg mice on the H2b background allowed us to extend our findings to another receptor and MHC molecule, Ly49C and H2Kb, respectively. Finally, site 2 in both H2Dd and H2Kb is specifically involved in Ly49A and Ly49C-dependent effector inhibition, respectively (13-17). Therefore, these considerations strongly support the conclusion that site 2 in cognate self-MHC molecules is required for licensing of Ly49A+ and Ly49C+ NK cells as well as in effector inhibition.
Although structural analysis suggested that there are potentially two sites on H2Dd for Ly49A binding (12), our data also extend previous studies indicating that site 2 is necessary for both trans and cis functional interactions with Ly49A (13, 14, 19). In the case of trans interactions, Ly49A on an NK cell interacts with H2Dd on a tumor target and confers inhibitory effects. Previous site-directed mutagenesis of both site 1 and site 2 residues established that site 2 is the interaction site for trans recognition of a tumor transfectant (13, 14). Here we also showed that site 2 on H2Dd on primary cells is critical for the Ly49A-dependent inhibitory effect.
In terms of cis interactions, Held and colleagues demonstrated that H2Dd on the NK cell itself can interact in cis with Ly49A on the same cell (18, 19). Prior site-directed mutagenesis have established that site 2 is involved in these cis interactions and that Ly49A reactivity with anti-Ly49A mAbs provide an indirect measure of such interactions. Herein, we provide additional evidence of the importance of site 2 for cis interactions of Ly49A with H2Dd because site 2 mutant H2Dd and H2Dd with human β2m do not alter anti-Ly49A reactivity as compared to H2b mice.
Interestingly, however, site 2 substitutions via species-specific differences in β2m affected Ly49C-dependent licensing but had less effect on Ly49C reactivity with mAb 4LO. These effects could be due to a higher number of residues in contact with β2m between Ly49A and Ly49C or the specific residues contacted (12, 13, 16)(17). Regardless, while mAb 4LO reactivity is only an indirect marker of cis interactions, these data nonetheless suggest that cis interactions may be less important for licensing interactions between Ly49C and H2Kb.
Although this interpretation will require additional studies, it may highlight the subtle differences between Ly49A and Ly49C with respect to functional interaction with its MHC ligands, such as the previously noted effect of MHC-bound peptides on Ly49C but not Ly49A interactions (29-31). Despite these differences, both receptors interact with site 2 in their cognate MHC ligand for licensing and effector inhibition.
Our conclusions come with two caveats. 1) Our current interpretation is partially based on the assumption that the R6A mutation specifically disrupts a critical interaction at site 2. It is theoretically possible that this mutation causes a more profound conformational change in the H2Dd structure that affects site 1. However, we think this is unlikely to be the case because antibody binding is preserved and the mutant MHC class I molecule can still be functionally recognized in allo-MLR. Moreover, we provide corroborating data with the species-specific β2m studies. 2) It should be noted that we have not formally excluded the possibility that site 1 may be involved in licensing because we did not test any site 1 mutant MHC class I molecules in this study. Our expectation is that site 1 mutant MHC class I molecules will still permit licensing to occur, although interpretations of such experiments may be problematic because there are no data, other than the crystallographic results, showing that disruption of putative site 1 residues perturbs interaction between Ly49 receptors and MHC class I. Such experiments may need to simultaneously mutate multiple residues putatively involved in site 1 recognition but then more profound conformational changes may occur that disturb site 2. Nonetheless, at the current time, the available data strongly support a role for site 2 in NK cell licensing by Ly49 receptors.
Therefore, the most important conclusions from this study are that both licensing and inhibition of effector function involve the same interaction site on the cognate MHC ligand for Ly49A and Ly49C. Both functions for both Ly49s are related to tolerance. That is, a licensed Ly49A+ or Ly49C+ NK cell should be inhibited by the same site on the same MHC allele that conferred licensing in the first place. Thus, the current study provides additional evidence that licensing and inhibition of effector function are related properties of NK cells and their receptors.
Supplementary Material
Acknowledgements
The authors thank Mike White, James Mohan, Kate Render, Melissa Berrien, and Darryl Higuchi for their help in the development of transgenic animals. We thank Chella David and Ted Hansen for ko and Tg mice. We also thank Megan Cooper, Julie Elliott, and Helena Jonsson for critical review of this manuscript.
Abbreviations
- B6
C57BL/6
- KODO
H2Kb-/-Db-/-
- MFI
Mean Fluorescence Intensity
- TKO
Triple Knockout, murine β2m-/- H2Kb-/-Db-/-
- WT
Wild Type
Footnotes
This work was supported by the Howard Hughes Medical Institute and grants from NIH to W.M.Y. The Microinjection and Speed Congenics Core Facilities were supported in part by the Rheumatic Diseases Core Center grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
References
- 1.Yokoyama WM. Chapter 16. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. 6th ed. Lippincott-Raven; New York: 2008. pp. 483–517. [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa H, Yabe T, Watanabe K, Miyamoto R, Miki A, Akaza T, Tadokoro K, Tohma S, Inoue T, Yamamoto K, Juji T. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I). Hum Immunol. 1999;60:32–40. doi: 10.1016/s0198-8859(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 6.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, Mohanakumar T, Hsu KC, Dupont B, Yokoyama WM. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, Levy F, Leclercq G, Hoglund P, Beermann F, Held W. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Tormo J, Natarajan K, Margulies DH, Mariuzza RA. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402:623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto N, Mitsuki M, Tajima K, Yokoyama WM, Yamamoto K. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp Med. 2001;193:147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Whitman MC, Natarajan K, Tormo J, Mariuzza RA, Margulies DH. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd and beta 2-microglobulin. Journal of Biological Chemistry. 2002;277:1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- 15.Michaelsson J, Achour A, Rolle A, Karre K. MHC Class I Recognition by NK Receptors in the Ly49 Family Is Strongly Influenced by the beta(2)-Microglobulin Subunit. J Immunol. 2001;166:7327–7334. doi: 10.4049/jimmunol.166.12.7327. [DOI] [PubMed] [Google Scholar]
- 16.Mitsuki M, Matsumoto N, Yamamoto K. A species-specific determinant on beta2-microglobulin required for Ly49A recognition of its MHC class I ligand. Int Immunol. 2004;16:197–204. doi: 10.1093/intimm/dxh017. [DOI] [PubMed] [Google Scholar]
- 17.Dam J, Guan R, Natarajan K, Dimasi N, Chlewicki LK, Kranz DM, Schuck P, Margulies DH, Mariuzza RA. Variable MHC class I engagement by Ly49 natural killer cell receptors demonstrated by the crystal structure of Ly49C bound to H-2K(b). Nat Immunol. 2003;4:1213–1222. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- 18.Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 20.Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H. Major histocompatibility complex (MHC) class I KbDb -/- deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci U S A. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bieberich C, Scangos G, Tanaka K, Jay G. Regulated expression of a murine class I gene in transgenic mice. Mol Cell Biol. 1986;6:1339–1342. doi: 10.1128/mcb.6.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khare SD, Hansen J, Luthra HS, David CS. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human beta2-microglobulin (beta2m) double transgenic mice with disrupted mouse beta2m. The Journal of clinical investigation. 1996;98:2746–2755. doi: 10.1172/JCI119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pircher H, Mak TW, Lang R, Ballhausen W, Ruedi E, Hengartner H, Zinkernagel RM, Burki K. T cell tolerance to Mlsa encoded antigens in T cell receptor V beta 8.1 chain transgenic mice. Embo J. 1989;8:719–727. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunning P, Leavitt J, Muscat G, Ng SY, Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987;84:4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roland J, Cazenave PA. Ly-49 antigen defines an αβ TCR population in i-IEL with an extrathymic maturation. Int Immunol. 1992;4:699–706. doi: 10.1093/intimm/4.6.699. [DOI] [PubMed] [Google Scholar]
- 26.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 27.Kievits F, Ivanyi P, Krimpenfort P, Berns A, Ploegh HL. HLA-restricted recognition of viral antigens in HLA transgenic mice. Nature. 1987;329:447–449. doi: 10.1038/329447a0. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson AH, Yang L, Kim S, Taffner SM, Yokoyama WM. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J Immunol. 2010;184:3424–3432. doi: 10.4049/jimmunol.0904057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franksson L, Sundback J, Achour A, Bernlind J, Glas R, Karre K. Peptide dependency and selectivity of the NK cell inhibitory receptor Ly-49C. Eur J Immunol. 1999;29:2748–2758. doi: 10.1002/(SICI)1521-4141(199909)29:09<2748::AID-IMMU2748>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Correa I, Raulet DH. Binding of diverse peptides to MHC class I molecules inhibits target cell lysis by activated natural killer cells. Immunity. 1995;2:61–71. doi: 10.1016/1074-7613(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 31.Orihuela M, Margulies DH, Yokoyama WM. The natural killer cell receptor Ly-49A recognizes a peptide-induced conformational determinant on its major histocompatibility complex class I ligand. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11792–11797. doi: 10.1073/pnas.93.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.