Abstract
Background: Rituximab induction together with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab maintenance (RCHOP-R) resulted to significant progression-free survival (PFS) benefit in comparison to RCHOP in the EORTC20981 trial of relapsed/refractory follicular non-Hodgkin's lymphoma (FL). However, the overall survival (OS) difference between RCHOP-R and RCHOP was insignificant. This study evaluated the cost-effectiveness of RCHOP, RCHOP-R, and CHOP in the treatment of relapsed/refractory FL.
Design: A lifetime Markov modeling based on the 5-year EORTC20981 survivals (Weibull regressions) was carried out from the public health care payer perspective. Finnish costs (drug, routine, adverse event, and relapse management) were employed. The main outcomes were incremental cost (€2008) per quality-adjusted life-year (QALY), progression-free year (PFY), and life-years gained (LYG). Analyses included cost-effectiveness acceptability frontier and multinomial expected value of perfect information (mEVPI).
Results: RCHOP-R resulted to OS (PFS) benefit compared with RCHOP and CHOP: 6 (10) and 17 (25) months, respectively. The incremental costs per QALY gained/LYG/PFY gained were €18 147/€16 380/€10 416 for RCHOP-R versus RCHOP (mEVPI €5196); €14 360/€13 041/€8976 for RCHOP-R versus CHOP (mEVPI €1986); and €12 123/€11 049/€8004 for RCHOP versus CHOP (mEVPI €1,240). RCHOP-R was the optimal option when the willingness to pay per QALY gained exceeded €18 399.
Conclusion: RCHOP-R is a potentially cost-effective treatment option for the FL.
Keywords: cancer, chemotherapy, economic evaluation, lymphoma, maintenance, opportunity cost, review, rituximab
key messages
Rituximab induction together with rituximab maintenance led to significant overall survival (OS) difference in comparison to rituximab induction alone during previously published 2-year follow-up based on the EORTC20981 trial of follicular non-Hodgkin's lymphoma (FL). In a 5-year follow-up based on the EORTC20981 trial, OS difference was insignificant probably due to rituximab salvage therapy.
Yet, rituximab induction together with rituximab maintenance was potentially cost-effective compared simultaneously with chemotherapy alone (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab induction based on the EORTC20981 5-year follow-up results of FL patients. Rituximab induction together with rituximab maintenance were the optimal option when the willingness to pay (WTP) per quality-adjusted life-year (QALY) gained exceeded €18 399.
The cost-effectiveness of rituximab induction and maintenance based on the 5-year follow-up results was robust to changes in the key model parameters and was confirmed with one-way sensitivity analysis, cost-effectiveness acceptability frontier (CEAF), and multinomial expected value of perfect information (mEVPI) methods. Also the value of additional cost-effectiveness information for the model parameters is likely to be low. Consequently, rituximab induction together with the maintenance could be positively endorsed in the current care of FL.
introduction
Lymphomas are a group of versatile cancers of the lymphatic system. FL is the most common histological type of non-Hodgkin's lymphoma (NHL) making up ∼22% of all lymphoid malignancies [1]. The economic burden of FL is showed to be significant [2].
In Finland, ∼1000 new cases of NHLs are diagnosed every year and some 250 of them are FLs—numbers that have nearly doubled during the last three decades. Only ∼20% of patients with FL present with limited stage disease (stage I–II) at diagnosis and ∼40% of patients with stage I–II disease may be cured with radiation therapy and/or chemotherapy [3]. In stages III–IV, the disease is incurable and although treatment can achieve complete or partial remissions, the clinical course of FL is characterized by a continuous pattern of relapses requiring repeated treatment. Earlier maintenance treatments have attempted to improve outcomes but have been associated with significant toxicity [4, 5].
Rituximab (R), a chimeric anti-CD20 monoclonal antibody, is well tolerated with limited toxic effects and is effective alone or in combination with chemotherapy in the treatment of indolent and aggressive forms of NHLs, including FL [6–8]. R addition to combination chemotherapy has made significant improvements in the response rate and progression-free survival (PFS) of patients with FL [6, 9–13].
Current Finnish treatment practice for relapsed or refractory indolent FL recommends the use of chemotherapy regimen consisting of R, cyclophosphamide (C), doxorubicin (H), vincristine (O), and prednisone (P) (RCHOP) [3]. In this study, the cost-effectiveness of all feasible CHOP-based treatments for patients with relapsed/refractory FL were assessed using the results obtained from EORTC20981 study [6] together with the 5-year maintenance follow-up results [12, 13].
patients and methods
patients and treatments
The patient population consisted of patients with relapsed/refractory FL. The treatment comparators were selected consistently with the current Finnish recommendation [3] and clinical practice. Accordingly, R can be added into the CHOP therapy in one of three ways:
as an induction only (RCHOP)
as both an induction and a maintenance therapy (RCHOP-R) or
not at all (CHOP).
model structure, time horizon, and perspective
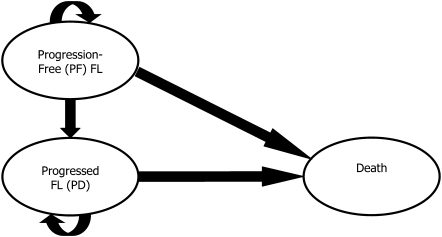
A Markov state-transition model with three health states [progression free (PF), progressive disease (PD), and death] was developed to analyze the cost-effectiveness of treatments for patients with relapsed/refractory FL. The model structure is presented in Figure 1. All patients entered the model in the PF state. PF and PD states were defined separately to account for the higher quality of life (QoL) and lower treatment costs for PFS.
Figure 1.
Diagram of health states and transitions in the model. In the model, patients are assigned across a series of health states reflecting their disease status and treatment received.
Patients were followed through the health states in monthly cycles over a period of 30 years. A lifetime horizon was used in order to capture the all treatment-related economic and health outcomes [e.g. full life-expectancy (LE) and QALYs] of the patients.
The health care provider perspective was applied in the analysis (i.e. the analysis was based on direct health care costs excluding, for example, possible productivity losses, income transfers, and value added tax). All outcomes were discounted at the rate of 3% per annum as recommended by the Finnish authorities.
outcomes
The primary outcome of the study was incremental cost (€2008) per incremental quality-adjusted survival measured as QALYs. In addition, incremental cost per OS measured as life-years gained (LYG) and incremental cost per PFS measured as progression-free years (PFYs) gained constituted the secondary outcomes.
clinical data
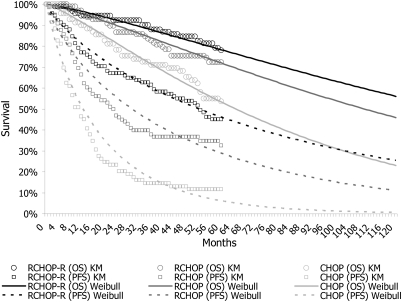
PFS and OS of relapsed/refractory FL patients receiving CHOP-based therapies were estimated based on the EORTC20981 trial's [6] 5-year follow-up update [12, 13]. In the current analysis, the 5-year results of EORTC20981 were used as a primary source for efficacy data since the design of the trial is consistent with the current Finnish recommendations [3] and practice. Parametric survival models together with covariance matrices were used to extrapolate the survival estimates with Stata 10 statistical software. The survival models are presented in Appendix 1.The original survival data [12] and estimated Weibull survival curves are also presented in Figure 2. After 5 years, the transition probabilities were equalized in every treatment group because although PFS difference was significant (Weibull treatment effect P = 0.013) between RCHOP-R and RCHOP, OS result was not (P = 0.316) (compare Appendix 1). The impact of survival functions was tested in sensitivity analyses.
Figure 2.
Original 5-year Kaplan–Meier (KM) survival data and curves based on Weibull regression estimation. In the model, transition probabilities were equalized after 5 years.
quality of life
Since Finnish utility data were not available, applied utility weights were based on the British study with 222 FL patients [14]. The EQ-5D utility weights were 0.805 [standard error (SE) 0.018] for the PF, 0.618 (SE 0.056) for the PD, and 0 for the dead state. In the case of serious drug-related adverse event (SAE), a QoL loss of 0.19 was assumed to take place (this is equal with the change in QoL between PF and PD).
resource use and unit costs
Drug use data for different CHOP-based regimens were obtained from EORTC20981 since the study regimens were found to correspond the practice in the Finnish setting. Current Finnish market prices for pharmaceuticals [15] were used. Cost of medication was estimated as euro per milligram using the most economical generic product prices. This assumption illustrates the most efficient use of clinical medications in hard-to-cure conditions like FL that can be typically achieved in relatively large clinics treating the FL patients in Finland. The unit costs are given in Table 1.
Table 1.
Unit costs (€2008) used in the model
| Resource unit | Cost | Source |
| Drug administration visit (including traveling €30.28) | 247.34 | [16] |
| Drug costs | Milligram | |
| Rituximab (Mabthera 10 mg/ml infusion), per mg | 3.06 | [15] |
| CHOP | 13.65 | [15] |
| Routine treatment (including traveling €30.28) | Month | |
| Progressive disease/progression-free disease | 247.34/82.45 | [15] |
| Adverse events (including traveling €30.28) | Event | |
| AE/SAE | 247.34/2247.82 | [15] |
| Relapse costs (including traveling €30.28) | Relapse | |
| Chemotherapy | 12 138.76 | [15, 16] |
| Rituximab single agent | 15 456.00 | [15, 16] |
| Rituximab chemotherapy combination | 27 594.76 | [15, 16] |
| Radiotherapy | 7849.92 | [16] |
| Allogeneic stem cell transplantation | 43 714.91 | [16, 17] |
| Autologous stem cell transplantation | 15 047.25 | [17] |
| Rituximab and stem cell transplantation | 44 397.28 | [16, 17] |
| Chemoradiotherapy | 19 988.68 | [15, 16] |
| Rituximab chemotherapy combination | 24 703.68 | [15, 16] |
| Other | 23 432.36 | [15, 16] |
CHOP = Syklofosfamid 1 g, Doxorubicin Meda 2 mg/ml, Oncovin 1 mg/ml, and Prednison 40 mg; SAE, serious adverse event.
Patients in the PD health state were assumed to require greater healthcare resources compared with those in the PF health state. For the sake of simplicity and to avoid the double counting of costs, the routine treatment costs were assumed to consist of outpatient visits only: patient was assumed to visit physician once every 3 months in PF health state and once monthly in PD health state. The case-mix-adjusted Finnish national unit costs from year 2006 [16] (converted to 2008 real value using the official health care price index published by the Association of Finnish Local and Regional Authorities) were used as a proxy for the costs of health care resources as recommended by the Finnish authorities.
Treatment costs of SAEs and relapses were assigned based on the Diagnosis-Related Group (Nord-DRG) classification system [16] (the cost of SAE in the Table 1 is simple average) and confirmed by clinical specialists. The incidence of adverse events (AE) and relapses was defined based on EORTC20981 [6]. When a non-serious AE took place, a visit to the physician was assumed.
sensitivity analysis
The impact of discounting to the results was also assessed using 0% discount rate. In addition, the effect of selected survival curve type, AE and relapse costs, QoL, and body surface area on the results was assessed. This was done by using log-logistic survival curves (compare Appendix 1) and Kaplan–Meier data instead of Weibull, by varying the applied AE and relapse costs, by using average QoL of Finnish cancer patients (EQ-5D 0.741 [18]), and by using the more common 1.7 m2 as the body surface area (1.9 m2 was the mean in EORTC20981).
The parameter uncertainty was handled as probability distributions for individual input parameters [19]. The utilities were assumed to follow normal distributions trimmed between 0 and 1 (no utilities worse than death or >1 were accepted). The number of induction and R doses was trimmed from 0 to infinity and was assumed to follow normal distribution. The probabilities of AEs and relapse treatments were characterized with beta distributions. The unit costs, such as the price of drug treatments, were handled as fixed tariffs. These distributions were sampled using the second-order Monte Carlo simulation, and the model outputs (i.e. expected costs and effects) were recorded for each set of samples. The sampling and recording were repeated 2000 times to ensure that uncertainty associated with the model inputs was taken into account.
A net monetary benefit (NMB = expected health outcome × WTP per health outcome − expected costs) method was applied in the representation of parameter uncertainty [20]. Since the optimal treatment option (i.e. the option with the highest NMB) may not always have the highest probability of being cost-effective for a given value of WTP per health benefit gained, CEAF [21, 22] was applied to represent the parameter uncertainty. The CEAF takes into account the potential skewness of NMB distribution and plots the probability of the most cost-effective (i.e. optimal) treatment being cost-effective.
Since CEAF does not consider the consequences of wrong decision, mEVPI was also estimated. Expected value of perfect information (EVPI) combines both the probability of wrong decision and the consequences of that wrong decision (‘opportunity costs’) in terms of NMB forgone. The EVPI estimate (for a single patient) represents the value of parameter uncertainty that can be resolved by acquiring additional evidence for the model parameters.
results
efficacy
The treatment regimen RCHOP-R was associated on average with 5.21 QALYs. The expected QALYs for RCHOP and CHOP alone were 4.72 and 3.90, respectively. RCHOP-R resulted to 0.49 and 1.31 QALYs gained when compared with RCHOP and CHOP alone, respectively. The detailed efficacy results are given in Table 2.
Table 2.
Base-case cost-effectiveness and one-way sensitivity analyses
| Outcome | Treatment |
ICER |
||||
| RCHOP-R | RCHOP | CHOP | RCHOP-R versus RCHOP | RCHOP-R versus CHOP | RCHOP versus CHOP | |
| Base case, 3% discounting | ||||||
| Costs (€2008) | 68 331 | 59 521 | 49 562 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 18 147 | 14 360 | 12 123 |
| LYs | 7.25 | 6.72 | 5.81 | 16 380 | 13 041 | 11 049 |
| PFYs | 3.86 | 3.01 | 1.76 | 10 416 | 8976 | 8004 |
| 0% discounting | ||||||
| Costs (€2008) | 79 079 | 69 918 | 59 500 | |||
| QALYs | 6.10 | 5.50 | 4.51 | 15 264 | 12 358 | 10 586 |
| Log logistic survivals | ||||||
| Costs (€2008) | 72 842 | 66 852 | 61 871 | |||
| QALYs | 6.68 | 5.95 | 4.89 | 8271 | 6123 | 4666 |
| Kaplan–Meier survivals | ||||||
| Costs (€2008) | 68 008 | 59 121 | 49 756 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 18 307 | 13 965 | 11 400 |
| BSA 1.7 m2 | ||||||
| Costs (€2008) | 66 449 | 58 662 | 49 562 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 16 039 | 12 920 | 11 077 |
| Routine management (€0) | ||||||
| Costs (€2008) | 54 609 | 45 708 | 36 091 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 18 335 | 14 169 | 11 706 |
| Relapse costs halved | ||||||
| Costs (€2008) | 56 226 | 45 441 | 33 507 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 22 214 | 17 383 | 14 527 |
| AE and SAE costs doubled | ||||||
| Costs (€2008) | 68 506 | 59 606 | 49 712 | |||
| QALYs | 5.21 | 4.72 | 3.90 | 18 332 | 14 380 | 12 044 |
| QoL 0.741 in PF and PD | ||||||
| Costs (€2008) | 68 331 | 59 521 | 49 562 | |||
| QALYs | 5.38 | 4.99 | 4.29 | 22 524 | 17 167 | 14 183 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; LY, life-year; PFY, progression-free year; BSA, body surface area; SAE, serious adverse event; QoL, quality of life.
The average LE using RCHOP-R was 7.25 years and using RCHOP and CHOP alone was 6.72 and 5.81 years, respectively. Thus, the use of RCHOP-R provided 0.54 and 1.44 additional life-years when compared with RCHOP and CHOP alone, respectively.
The use of RCHOP-R regimen provided 3.9 PFYs. The expected PFYs for RCHOP and CHOP alone were 3.0 and 1.8, respectively. RCHOP-R resulted to additional 0.9 and 2.1 PFYs when compared with RCHOP and CHOP alone, respectively.
costs
Expected lifetime costs using CHOP alone were €49 562 followed by RCHOP €59 521 and RCHOP-R €68 331. The use of RCHOP-R results to the additional costs of €8810 and €18 769 when compared with RCHOP and CHOP alone, respectively. These cost differences can be largely attributed to maintenance drug costs in the RCHOP-R group. However, this cost is partly offset by the lower cost of treatment upon relapse when using RCHOP-R due to a longer estimated time in the PF health state.
cost-effectiveness
RCHOP-R resulted to an incremental cost-effectiveness ratio (ICER) of €14 360 per QALY gained followed by RCHOP (€12 123 per QALY gained) when compared with CHOP alone. In the worst base case comparison for RCHOP-R, which was RCHOP-R versus RCHOP, an ICER of €18 147 per QALY gained was established. In the base case, the incremental costs per LYG ranged from €11 049 to €16 380 and the respective incremental costs per PFY gained ranged from €8004 to €10 416.
In one-way sensitivity analyses, the cost-effectiveness estimates were found to be relatively insensitive to the changes in the key parameters.
probabilistic sensitivity analysis
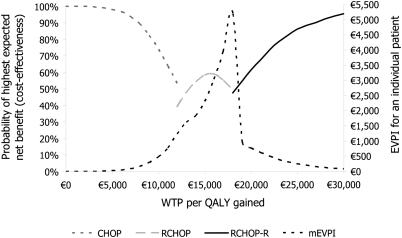
CEAF and mEVPI are depicted in the Figure 3. They reveal that in the decision situation, where all feasible CHOP-based options were assessed simultaneously, the optimal options were presented and the probability of cost-effectiveness was >50% (potential cost-effectiveness), RCHOP-R was the optimal treatment with the WTP level exceeding €18 399 per QALY gained. When WTP per QALY gained was between €13 252 and €17 894, RCHOP was the optimal treatment with the probability of cost-effectiveness >50%. With the WTP per QALY gained values below €12 103, CHOP was the optimal treatment with the probability of cost-effectiveness >50%.
Figure 3.
Cost-effectiveness acceptability frontier depicts the efficient choices as the function of WTP per quality-adjusted life-year (QALY) gained. The multinomial expected value of perfect information (mEVPI) analysis reveals the expected consequences of the wrong decision in monetary terms.
With the WTP of €12 104–13 251 and €17 895–18 398 per QALY gained, none of the treatments were potentially cost-effective. The probability of RCHOP-R being cost-effective with the threshold values of €20 000, €30 000, €40 000, and €50 000 per QALY were acceptable: 61.6%, 95.3%, 99.7%, and 100.0%, respectively. Since the probability of cost-effectiveness for a single treatment option is likely to be low when the number of comparators is more than two [23], RCHOP-R treatment seems to be very potential for FL based on the 5-year follow-up.
The patient-level mEVPI was €1240 at a WTP level of €12 123 and €5196 at a WTP level of €18 147 per QALY gained—the mEVPI was highest with the ICER values. These values can be interpreted as the upper limit of the value of further research per patient that would eliminate the uncertainty around the parameters in the model, given WTP. According to the EVPI analysis, the decision between RCHOP-R and RCHOP is more uncertain than the decision between RCHOP and CHOP. However, with the WTP values exceeding some €19 000 per QALY gained, the need for additional cost-effectiveness information is low or, saying it with other words, the consequences of that wrong decision would be minimal if RCHOP-R is chosen.
discussion
In the present study, the use of RCHOP-R regimen was found to be cost-effective when it is compared with all feasible CHOP-derived treatment options in the Finnish setting using the EORTC20981 5-year follow-up data. When the WTP for QALY gained exceeds €18 399, RCHOP-R is potentially cost-effective option offering the highest value for money invested in the treatment of relapsed/refractory FL. The secondary outcomes (i.e. incremental cost per PFY gained and LYG) also show that RCHOP-R is a potentially cost-effective intervention.
In order to elaborate the results of this study, a systematic search in PubMed/Medline was done (18 July 2010) by one author (EJOS) to find published Finnish cancer studies reporting full economic evaluations. In the search, following words were used: cancer or oncology or malignancy and cost-effectiveness or cost-utility or cost–benefit and Finland or Finnish. Based on abstracts, six publications [24–29] presented full incremental economic evaluations of cancer treatments (studies published before year 2000, screening studies, and reviews were excluded) in Finland and were included in further review. These studies are presented in Table 3. For comparison, the results of present study are also presented in the Table 3.
Table 3.
Incremental cost-effectiveness, methods, and emergent concerns based on Finnish cancer studies: results of systematic search and review
| Reference | Disease | Costs | Time frame | Base | Comparators (new first) | New drug | ICUR | Outcomes | Methodsa | New drug | Concerns |
| Current study | FL | Payer | Lifetime | M | RCHOP-R, RCHOP, CHOP | i.v. | 18 400 (highest) | QALY, LYG, PFY | Probabilistic (Monte Carlo), Weibull regressions, log-logistic, CEAF, mEVPI | Hospital use | Foreign QoL |
| [24] | mSTS | Payer | Lifetime | M | Trabectedin, multiple options | i.v. | 42 600; 38 000b | QALY, LYG | Probabilistic (Monte Carlo), linear survival, CEAF, EVPI, mapping | Reimbursed | Same QoL for stable and progessive state |
| [25] | mRCC | Payer | Lifetime | M | Sunitinib malate, BSC | p.o. | 42 900 | QALY, LYG, PFY | Probabilistic (Gibbs sampler), Weibull regressions | Reimbursed | Comparability of effectiveness data between groups, foreign QoL |
| [26] | GM | Payer | Lifetime | M | Temozolomide, PCV | p.o. | 32 500 | QALY, LYG, PFY | Probabilistic (Monte Carlo), pertinency scores, EVPI | Reimbursed | Proxy QoL data |
| [27] | CC | Payer | Varying | M | HPV16 and HPV18 vaccination, no vaccination | Vaccination | 18 400 | CCs, QALY, deaths avoided | Probabilistic (@Risk), regressions | na | Very high utilities, low discounting rate for QALYs (1.5%), foreign QoL |
| [28] | BC | Societal | 3/5 years | P | de FEC, FEC+ HDCT + BMS | i.v. | na | LYG | Probabilistic (bootstrap) | Hospital use | Comparability, 3-year direct costs, 5-year efficacy, no QoL or discounting |
| [29] | CC | Payer | Lifetime | M | HPV16 and HPV18 vaccination, no vaccination | Vaccination | 17 300 | CCs, QALY, LYG, deaths avoided | Probabilistic (@Risk) | na | Very high utilities, foreign QoL |
In addition to the conventional incremental analysis.
Assuming hospital price for trabectedin.
ICUR, incremental cost-utility ratio (incremental cost per QALY gained); FL, follicular lymphoma; M, modeling; QALY, quality-adjusted life-year; LYG, life-years gained; PFY, progression-free year; CEAF, cost-effectiveness acceptability frontier; mEVPI, multinomial expected value of perfect information; QoL, quality of life; mSTS, metastatic soft-tissue sarcoma; EVPI, expected value of perfect information; mRCC, metastatic renal cell carcinoma; BSC, best supportive care; p.o., by mouth; GM, glioblastoma multiforme; PCV, procarbazine, lomustine, and vincristine; HPV, human papillomavirus; CC, cervical cancer; BC, breast cancer; P, piggy-back; de, dose escalated; FEC, 5-fluorouracil, epirubicin and cyclophosphamide; HDCT, high-dose chemotherapy; BMS, bone marrow supported; na, not applicable.
In Finnish cancer studies generally, higher magnitudes of cost-effectiveness thresholds for cost per QALY gained were found when compared with the results presented in this study. Also only one of the previous studies [24] compared multiple options and none of them evaluated the impact of different survival functions. When interpreting the results of various Finnish cancer studies, it should be noted that among others malignancies, the sources of information (e.g. cost, QoL and treatment practice) and base years (costs) vary between studies, which diminishes the comparability of the different results markedly. Thus, the results should be cautiously observed.
In addition to the search and selection, the studies were reviewed by one author (EJOS) based on the findings of a previous publication [30]. Most emergent concerns in the Finnish economic evaluations of cancer treatments were related to the inconsistent time frame of costs and effectiveness, utilities, and comparability of the patient populations.
Comparison of these FL results to previous cost-effectiveness studies of FL is hard due to different settings, data, and treatments. However, these cost-effectiveness results are in concordance with the large B-cell [31] assessment. Previous studies comparing the cost-effectiveness of RCHOP-R and RCHOP based on a 2-year follow-up data from EORTC20981 [6] and relying on the significant OS difference between RCHOP-R and RCHOP in a 2-year follow-up have resulted to €7600 per LYG and €8700 per QALY gained in France [32] and €11 200 per LYG and €12 600 per QALY gained in Sweden [33]. These studies did not include comparisons to CHOP alone. A study based on the 2-year follow-up and comparison between RCHOP and CHOP demonstrated £10 700 per QALY gained and £9300 per LYG in the UK [34] but did not assess RCHOP-R. The current study demonstrated somewhat higher ICERs. This was no surprise since only the current study used the EORTC20981 5-year follow-up data as the basis for economic evaluation for the first time, compared all feasible CHOP-based treatment options simultaneously and included, for example, disutility due to SAEs. The disutility due to SAE is not likely to benefit RCHOP-R or RCHOP since R-addition and observation have recently demonstrated comparable QoL results [35].
The cost-effectiveness of a health technology should reflect the balance of all relevant aspects of a treatment, including both treatment- and decision-based (i.e. opportunity) costs, clinical benefits, safety, and quantity and the QoL gained. The presentation of costs and clinical benefits can provide the overall value of multiple interventions through valid approaches like CEAF and EVPI. Since it has been demonstrated that the optimal option may not always have the highest probability of being cost-effective for given WTP [21, 22], CEAF was employed. The EVPI analysis demonstrated that the expected consequences of a wrong decision would be minimal if RCHOP-R is chosen with WTP values exceeding €19 000 per QALY.
This study was based on the survivals from a single trial with a 5-year follow-up after the randomization to R-maintenance and observation. In this intention-to-treat (ITT) setting, however, the randomization was not broken and assumptions between 2- and 5-year survivals were not needed. Yet, confounding by treatment took place: high rates of R-salvage therapy was used in RCHOP (34%) and CHOP (59%) groups meanwhile the rate of R-salvage therapy in RCHOP-R group was relatively low (26%). Conservatively for RCHOP-R, the excess cost of R-salvage therapy after the initial relapse treatment was not included in the modeling. Despite this conservative approach, RCHOP-R was both efficacious and cost-effective.
EORTC20981 [6, 12, 13] had among the lowest overall response rates compared with the other trial of relapsed/refractory FL [36]. However, it included a large sample size compared with most other trials and, in addition, CHOP-based therapies are recommended for FL treatment in the Finnish setting [3] and in Europe [37]. Thus, estimates based on EORTC20981 5-year data can be regarded as the most conservative, reliable, and clinically relevant estimates currently available for the purposes of this study in Finland and in other European settings. Also, we may say that among CHOP-based therapies, RCHOP-R should be positively endorsed in the care recommendations due to value it provides for money.
The drug use data for different CHOP-based regimens were obtained from EORTC20981 and hospital price for R was used. This was done to maintain the comparability of results and the association between drug dose and efficacy. Also, the drug use data were found to be in concordance with the practice in the Finnish setting. The Finnish drug use is likely to be consistent with the European way of treating relapsed/refractory FL patients. In addition to the trial-based modeling approach, the use of hospital price is likely to improve the comparability of results to other European countries (i.e. two-tier financing system is used in Finland [24, 38–41] and R is a hospital drug). The pharmacy retail premium is high in Finland.
Since there were no Finnish data obtainable to estimate the rate of hospitalizations related to routine care of FL, the routine treatment costs were assumed to consist of outpatient visits only. This is a rather conservative assumption from RCHOP-R's perspective since majority of these hospitalizations are likely to occur early when, for example, CHOP is used and later when, for example, RCHOP-R is used, and therefore, discounting would have impact on these. The incidence of AEs and relapses was based on EORTC20981, and the resource use and costs related to these were estimated using Finnish sources.
The LYGs, PFYs gained, and QALYs gained through RCHOP-R appeal to be clinically significantly higher than the corresponding values obtained using RCHOP or CHOP even based on the modeling of a 5-year data although there was no difference in OS at 5 years of follow-up. However, no FL-specific Finnish QoL data were obtainable and therefore UK values were used. The Finnish age- and sex-matched population reports an EQ-5D-based utility of 0.835 on average and the respective average value for cancer patients is 0.741 [18]—thus, the UK-based values seem to be consistent with the Finnish values. This was explored in sensitivity analysis.
Using modeling approach, the time horizons were expanded beyond the trial period and lifetime costs and benefits of different strategies were estimated. A limitation of this approach is the requirement to make extrapolations and assumptions regarding some unknown variables. Based on the 5-year data [12, 13], no continued survival benefits for any treatments were assumed beyond 5 years. In addition, the impacts of discounting, QoL, the dosing of R, routine management, relapses, AEs, and survival curve type on the cost-effectiveness results were assessed. The results of base-case analysis were found to be robust.
One limitation of the study was that patients could not return to the PF state from the PD state. Although clinically FL patients may return to the PF state after treatment, there were insufficient data from EORTC20981 to reflect this transition within the model structure. However, accounting for this transition is not likely to clinically discriminate between the groups over the lifetime course of FL. In addition, EORTC20981 was not sufficiently powered to detect difference in outcomes between the three strategies. Probabilistic sensitivity analysis, however, accounted for the lack of statistical power and depicted the uncertainty in this economic evaluation through CEAF and mEVPI.
conclusion
Based on the available evidence, RCHOP-R is a potentially cost-effective strategy for the Finnish patients with relapsed/refractory FL. The cost-effectiveness ratio of RCHOP-R obtained in this study was less than previously published values for novel treatments in oncology in Finland.
funding
Roche Oy, Finland.
disclosure
EJOS and JAM are consultants and shareholders of ESiOR Oy that was commissioned by Roche Oy to perform this study; ESiOR also carries out commissioned studies and health-economic analysis for several other pharmaceutical companies, food industry companies, and hospitals. The other author has declared no conflicts of interest. All authorship decisions were made on the basis of scientific consideration with no editorial role of the sponsor.
Acknowledgments
The authors wish to thank Mrs Taru Hallinen for comments during the manuscript phase of the study and David Laaksonen for the language revision.
The results based on shorter term (2-year) data have been presented as a poster at the 11th International Society for Pharmacoeconomics and Outcomes Research European Congress, 8–11 November 2008, Athens, Greece.
appendix 1: survival models
The Weibull survival regressions were estimated based on the 5-year trial data [12] in order to extrapolate the results and to achieve probabilistic analysis. The Weibull regressions were established as follows: S(t) = exp(−λtγ), where S(t) = survival in time t, λ = exp(−μ/σ), γ = 1/σ, and μ = intercept + treatment effect. Following parameter values were estimated:
RCHOP-R/RCHOP PFS [model P = 0.012, Akaike Information Criteria (AIC) = 574.880, Bayesian Information Criteria (BIC) = 584.605]: γ = 1.216520, intercept = 7.229883, and treatment effect = 0.577092 (P = 0.013).
CHOP PFS (model P < 0.001, AIC = 452.897, BIC = 461.786): γ = 1.117781, intercept = 6.346413, and treatment effect = 0.000000.
RCHOP-R/RCHOP OS (model P = 0.310, AIC = 297.497, BIC = 307.239): γ = 0.803794, intercept = 8.391771, and treatment effect = 0.239529 (P = 0.316).
CHOP OS (model P = 0.043, AIC = 294.864, BIC = 303.794): γ = 0.816271, intercept = 7.877270, and treatment effect = 0.000000.
In a sensitivity analysis scenario, log-logistic survival regressions were also used: S(t) = 1/(1 + λtγ); where λ = exp(−(intercept + treatment effect)/scale) and γ = 1/scale. For log-logistic survival, following parameter values were established:
RCHOP-R/RCHOP PFS (model P = 0.056, AIC = 564.918, BIC = 574.643): γ = 0.947780, intercept = 6.627432, and treatment effect = 0.699412 (P = 0.005).
CHOP PFS (model P < 0.001, AIC = 442.396, BIC = 451.284): γ = 0.783611, intercept = 5.757276, and treatment effect = 0.000000.
RCHOP-R/RCHOP OS (model P = 0.280, AIC = 296.959, BIC = 306.700): γ = 0.747126, intercept = 8.194182, and treatment effect = 0.269684 (P = 0.284).
CHOP OS (model P = 0.041, AIC = 294.535, BIC =303.466): γ = 0.726554, intercept = 7.606067, and treatment effect = 0.000000.
Although the log-logistic survival curves fitted marginally better to the data when compared with the Weibull survival curves, the Weibull models were used. The Weibull-based approach was chosen as the base case because it produced conservative results and neither of the approaches fitted optimally to the OS data.
References
- 1.Ansell SM, Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2005;80:1087–1097. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 2.Foster T, Miller JD, Boye ME, Russell MW. Economic burden of follicular non-Hodgkin's lymphoma. Pharmacoeconomics. 2009;27:657–679. doi: 10.2165/11314820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Jyrkkiö S, Jantunen E, Keskinen L, et al. Follikulaarisen lymfooman hoito. Finnish Med J. 2007;(17):1729–1733. [Google Scholar]
- 4.Rohatiner AZ, Gregory WM, Peterson B, et al. Meta-analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol. 2005;23:2215–2223. doi: 10.1200/JCO.2005.06.146. [DOI] [PubMed] [Google Scholar]
- 5.Steward WP, Crowther D, McWilliam LJ, et al. Maintenance chlorambucil after CVP in the management of advanced-stage, low-grade histologic type non-Hodgkin's lymphoma. A randomized prospective study with an assessment of prognostic factors. Cancer. 1998;61:441–447. doi: 10.1002/1097-0142(19880201)61:3<441::aid-cncr2820610306>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 7.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 8.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse humanmonoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 9.Salles G, Mounier R, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 10.Herold M, Haas A, Scrock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 11.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 12.van Oers MH, Van Glabbeke M, Baila L, et al. EORTC 20981 Intergroup Study. Rituximab in remission induction and maintenance treatment of relapsed follicular NHL: a phase III randomized clinical trial. Updated analysis of maintenance. 2008. American Society of Hematology 2009 Annual Meeting (Abstr 838) [Google Scholar]
- 13.van Oers MJH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008;19:570–576. doi: 10.1093/annonc/mdm543. [DOI] [PubMed] [Google Scholar]
- 15.FMT. Finnish Medicine Tariff. Helsinki, Finland: SII & NAM; 2010. [Google Scholar]
- 16.Hujanen T, Kapiainen S, Tuominen U, Pekurinen M. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Helsinki, Finland: Stakes Työpapereita; 2008. [Google Scholar]
- 17.Helsinki University Hospital. HYKS-sairaanhoitoalue: Hinnasto. Helsinki, Finland: HUS; 2009. [Google Scholar]
- 18.Saarni SI, Härkänen T, Sintonen H, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res. 2006;15:1403–1414. doi: 10.1007/s11136-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 19.Briggs AH, Sculpher MJ, Claxton K. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- 20.Stinnett A, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18:S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 21.Barton GR, Briggs AH, Fenwick EAL. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11:886–897. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 22.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10:779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 23.Hallinen TA, Soini EJ, Eklund K, Puolakka K. Cost-utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology. 2010;49:767–777. doi: 10.1093/rheumatology/kep425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soini EJ, García San Andrés B, Joensuu T. Trabectedin in the treatment of metastatic soft tissue sarcoma: cost-effectiveness, cost-utility and value of information. Ann Oncol. 2011;22:215–223. doi: 10.1093/annonc/mdq339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purmonen T, Martikainen JA, Soini EJ, et al. Economic evaluation of sunitinib malate in the second line treatment of metastatic renal cell carcinoma (mRCC)—a Bayesian approach. Clin Ther. 2008;30:382–392. doi: 10.1016/j.clinthera.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Martikainen JA, Kivioja A, Hallinen T, Vihinen P. Economic evaluation of temozolomide in the treatment of recurrent glioblastoma multiforme. Pharmacoeconomics. 2005;23:803–815. doi: 10.2165/00019053-200523080-00006. [DOI] [PubMed] [Google Scholar]
- 27.Suarés E, Smith JS, Bosch FX, et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccination. 2008;26(Suppl. 5):F29–F45. doi: 10.1016/j.vaccine.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 28.Kellokumpu-Lehtinen P, Bergh J, Salminen E, et al. Cost-effectiveness of intensive adjuvant chemotherapy for high-risk breast cancer: is tailored and dose-escalated chemotherapy with growth factor support (GFS) more costly and less effective than marrow-supported high-dose chemotherapy—results from a randomized study. Acta Oncol. 2007;46:146–152. doi: 10.1080/02841860600965012. [DOI] [PubMed] [Google Scholar]
- 29.Torvinen S, Nieminen P, Lehtinen M, et al. Cost effectiveness of prophylactic HPV 16/18 vaccination in Finland: results from a modelling exercise. J Med Econ. 2010;13:284–294. doi: 10.3111/13696998.2010.485951. [DOI] [PubMed] [Google Scholar]
- 30.Drummond M, Sculpher M. Common methodological flaws in economic evaluations. Med Care. 2005;43:5–14. doi: 10.1097/01.mlr.0000170001.10393.b7. [DOI] [PubMed] [Google Scholar]
- 31.Groot MT, Lugtenburg PJ, Hornberger J, et al. Cost-effectiveness of rituximab (MabThera) in diffuse large B-cell lymphoma in The Netherlands. Eur J Haematol. 2005;74:194–202. doi: 10.1111/j.1600-0609.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 32.Deconinck E, Hiadi-Fargier H, Pen CL, Brice P. Cost effectiveness of rituximab maintenance therapy in follicular lymphoma. Pharmacoeconomics. 2010;28:35–46. doi: 10.2165/11314070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Kasteng F, Erlanson M, Hagberg H, et al. Cost-effectiveness of maintenance rituximab treatment after second line therapy in patients with follicular lymphoma in Sweden. Acta Oncol. 2008;47:1029–1036. doi: 10.1080/02841860802120028. [DOI] [PubMed] [Google Scholar]
- 34.Ray JA, Carr E, Lewis G, Marcus R. An evaluation of the cost-effectiveness of rituximab in combination with chemotherapy for the first-line treatment of follicular non-Hodgkin's lymphoma in the UK. Value Health. 2010;13:346–357. doi: 10.1111/j.1524-4733.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 35.Witzens-Harig M, Reiz M, Heiss C, et al. Quality of life during maintenance therapy with the anti-CD20 antibody rituximab in patients with B cell non-Hodgkin's lymphoma: results of a prospective randomized controlled trial. Ann Hematol. 2009;88:51–57. doi: 10.1007/s00277-008-0560-2. [DOI] [PubMed] [Google Scholar]
- 36.Vidal L, Gafter-Gvili L, Leipovici L, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2009;101:248–255. doi: 10.1093/jnci/djn478. [DOI] [PubMed] [Google Scholar]
- 37.Dreyling M ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl. 4):119–120. doi: 10.1093/annonc/mdp148. [DOI] [PubMed] [Google Scholar]
- 38.OECD. Reviews of Health Systems. Finland: OECD; 2005. [Google Scholar]
- 39.Teperi J, Porter ME, Vuorenkoski L, Baron JF. The Finnish Health Care System. A Value-Based Perspective. Helsinki, Finland: Sitra; 2009. [Google Scholar]
- 40.Häkkinen U. The impact of changes in Finland's health care system. Health Econ. 2005;14(Suppl 1):S101–S118. doi: 10.1002/hec.1030. [DOI] [PubMed] [Google Scholar]
- 41.Hermanson T, Aro S, Bennett CL. Finland's health care system. Universal access to health care in a capitalistic democracy. J Am Med Assoc. 1994;271:1957–1962. [PubMed] [Google Scholar]