Abstract
An essential step in the life cycle of human immunodeficiency virus type 1 (HIV-1) is integration of the double-stranded retroviral DNA into the genome of the host cell. HIV-1 integrase, the enzyme that inserts the vital DNA into the host chromosome, is an attractive and rational target for anti-AIDS drug design because it is essential for HIV replication and there are no known counterparts in the host cell. Inhibitors of this enzyme have the great potential to complement the therapeutic use of HIV protease and reverse transcriptase inhibitors. Natural products have provided a source of new drug candidates for anti-AIDS therapy. Baicalein and baicalin, identified components of a Chinese herbal medicine Scutellaria baicalensis Georgi, have been shown to inhibit infectivity and replication of HIV. They are therefore promising lead compounds for developing new anti-AIDS drugs. To understand how the inhibitors work and therefore design more potent and specific inhibitors, we have used molecular modeling techniques to investigate the binding modes of these inhibitors. The three-dimensional structures of these inhibitors were first built. Then, computational binding studies of these inhibitors, based on the crystal structure of the HIV-1 integrase catalytic domain, were performed to study the complex structure. The preliminary results of our computational modeling study demonstrated that Baicalein binds to the active site region of the HIV-1 integrase. Our study will be of help to identify the pharmacophores of inhibitors binding to HIV-1 integrase and design new pharmaceuticals for the treatment of AIDS.
Keywords: HIV-1 integrase, anti-AIDS drug, molecular modeling, baicalein, docking, ligand-binding interface
1 Introduction
Acquired immunodeficiency syndrome (AIDS), a set of symptoms and infections resulting from the damage to the human immune system caused by the human immunodeficiency virus (HIV) (Weiss, 1993), has been a serious, life-threatening health problem since it was first identified in 1981. It is the fourth biggest killer and the most quickly spreading disease of the century (Kallings, 2008). According to recent report of WHO and UNAIDS, in 2007, an estimated 33.2 million people globally lived with the disease, and it killed an estimated 2.1 million people, including 330 000 children (UNAIDS, WHO, 2007).
Two major types of HIV have been identified, HIV-1 and HIV-2. HIV-1 encodes three enzymes: reverse transcriptase, protease, and integrase. Combination antiviral therapy with reverse transcriptase and protease inhibitors has shown the potential therapeutic efficacy of antiviral therapy for treatment of AIDS. However, the ability of HIV to rapidly evolve drug resistance, together with toxicity problems, requires the discovery and development of new classes of anti-AIDS drugs (Matsushita, 2000; Eron, 2009; Nakanjako et al., 2009; Moszynski, 2009).
Recently, increasing evidence has demonstrated that, in treating AIDS, treatment regimens containing multiple drugs can usually amplify the therapeutic efficacies of each agent, leading to maximal therapeutic efficacy with minimal adverse effects. The pharmaceutical industry therefore has seen a shift from the search for “magic bullets” that specifically target a single disease-causing molecule to the pursuit of combination therapies that comprise more than one active ingredient. Interestingly, Traditional Chinese Medicine (TCM) has advocated combinatory therapeutic strategies for more than 2500 years (Wang et al., 2008; Kiefer et al., 2009; Cheng et al., 2009). The number of compounds exhibiting anti-HIV activity and isolated from TCM and other natural sources has increase steadily. Based on the symptoms and characteristics of patients and guided by the theories of TCM, formulae are also designed to contain a combination of different kinds of plants or minerals to improve clinical efficacy. Three-Huang Powder (THP) is one example of these formulae, whose efficacies in treating AIDS have been well established in China through pre-clinical studies and multicenter clinical trials. Designed by one of authors of this report entirely based on TCM theories, THP consists of 16 types of Chinese medicinal herbs, in which Amur cork-tree bark (Huang Bai), Scutellaria baicalensis Georgi (Huang Qin), and Astragalus membranaceus (Huang Qi) are the principal elements.
THP inhibited both integrase, reverse transcriptase, having IC50s (concentration that exhibits 50% inhibition) of 57.221 µg/mL and 155.519 µg/mL, respectively. THP are relatively nontoxic and has on LD50 (dose that cause 50% tested population death) value.
Baicalein and baicalin, isolated from a Chinese herbal medicine Scutellaria baicalensis Georgi, have been shown to inhibit infectivity and replication of HIV. They are therefore promising lead compounds for developing new anti-AIDS drugs (Wu et al., 2001). While baicalin inhibits HIV-1 infection at the level of viral entry (Li et al., 2000), baicalein is a novel class of integrase inhibitors (Ahn et al., 2001). Both of them represent promising lead compounds for developing new anti-AIDS drugs and offer a significant advance in the search for new HIV enzyme targets as they are both specific for HIV-1 integrase and relatively nontoxic.
HIV-1 integrase, the enzyme responsible for integrating the viral DNA into the host genome, provides a fertile area of investigation using rational drug discovery approaches (Bhattacharya and Osman, 2009; Tanaka et al., 2009; Schafer and Squires, 2010; Garrido et al., 2010; McColl and Chen, 2010; Marchand et al., 2009). It can be divided into three discrete domains, N-terminus, core, and C-terminus. The structure of a complex of its core domain with a novel inhibitor has been determined (Goldgur et al., 1999) which provide us a platform for the investigation and discovery of anti-AIDS agents using computational methodology (Gupta et al., 2009).
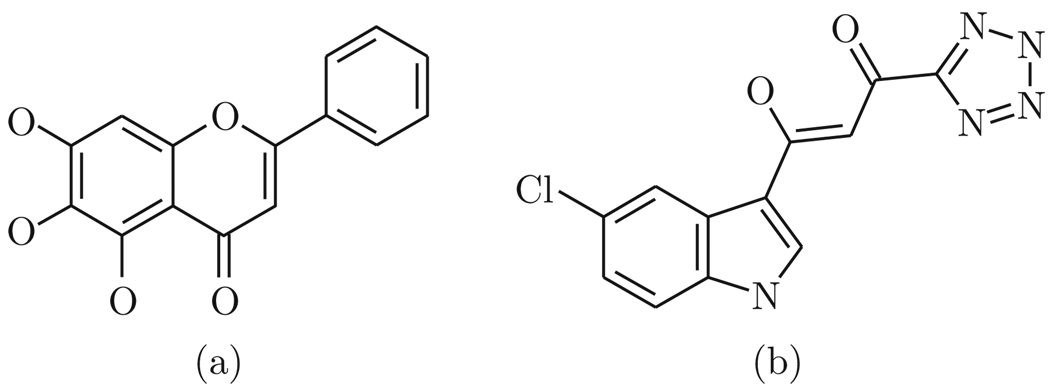
In the present study, we use molecular modeling techniques to examine the binding mode of baicalein (Fig. 1) in order to understand how and where the inhibitor binds to HIV-1 integrase.
Figure 1.
Chemical structures of baicalein (a) and the inhibitor 5CITEP (b)
2 Methodology
All calculations in this study were performed on a Windows XP based DELL Optiplex GX620 computer with Intel Pentium 4 CPU and 2 GB of RAM
2.1 Protein and ligand structures
The coordinates of the core domain of HIV-1 integrase were taken from the Protein Data Bank (PDB code 1QS4). The PDB file was modified to include only chain A of the core domain dimer, which was then converted from pdb format to the mol 2 format using Windock’s PMOL2Q module (Hu and Southerland, 2007). Hydrogen atoms and chargers were added to the entire protein. The structure of baicalein was first modeled using ISIS Draw program (MDL ISIS Draw 2.5, MDL Information System Inc, 2002), then converted into three-dimensional structure by ViewerLite softwere (ViewerLite 4.2, Accelrys Inc, 2001). Hydrogens were added and Gaisteiger charges were calculated. Energy minimization was performed with the MM force field using ArgusLab program (ArgusLab 4.0.1, http://www.arguslab.com). The integrase inhibitor included in the original PDB file, 5CITEP, was used as the comparison structure (Fig. 1).
2.2 WinDock docking study
The docking study was performed with the program WinDock developed in our laboratory (Hu and Southerland, 2007), which uses the widely distributed DOCK searching engine (Ewing et al., 2001; Moustakas et al., 2006; Lang et al., 2009) to generate a set of spheres as the “negative image” of the protein binding site, and use an incremental construction and random conformation search method to dock flexible small molecules to macromolecular sites, and a Coulombic and Lennard-Jones grid-based scoring function to evaluate the binding affinity of ligands. WinDock’s SPHBOC module was used to determine the binding site and produce a set of spheres for binding site characterization. Contact scores and energy scores were calculated using an energy cutoff distance of 6.0 Å and a van der Waals repulsive exponent of 8.0 Å. Ligands were oriented to the spheres with a distance tolerance of 0.5 Å and distance minimum of 2.0 Å. A minimum anchor size of 50 was used with an internal energy repulsive exponent of 8.0 Å and clash overlap of 0.25 Å. All other parameters were left as their defaults.
2.3 MVD docking study
In order to investigate if there are other particular regions of the protein that are preferred by the ligand (blind docking), docking calculation was performed using Molegro Virtual Docker (MVD) program (Thomsen and Christensen, 2006). The docking module of MVD is based on an evolution algorithm variant called differential evolution. Both structures of protein and ligand were uploaded into MVD. Bond orders, hybridizations, and hydrogen atoms were added, chargers were assigned, and flexible torsions of ligand were detected. A grid volume that is big enough to cover the entire surface of the protein was used for docking calculation, while other parameters were default.
3 Results
3.1 Prediction of baicalein binding to the core domain of HIV integrase
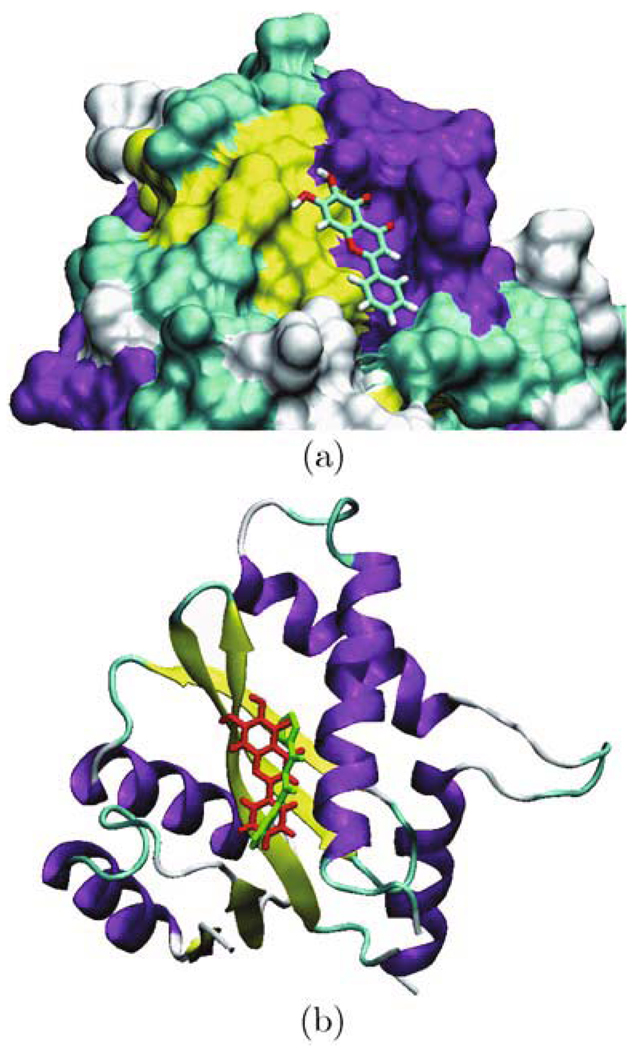
The Windock docking study revealed that the baicalein was bound in the middle of the active site of subunit A of the enzyme. A schematic view of baicalein bound at the active site of the enzyme is shown in Fig. 2(a). This is consistent with the previous findings that the inhibition by baicalein on integrase is directed toward conserved amino acids in the central core domain of integrase during catalysis (Goldgur et al., 1999).
Figure 2.
(a) baicalein is located in the active site of the protein; (b) baicalein (in red) and the inhibitor 5CITEP (in green) in the active site of the protein
3.2 Comparison of baicalein binding with the inhibitor in the complex
The comparison of baicalein binding with the inhibitor 5CITEP is shown in Fig. 2(b). They are all located in the active site between the three catalytic acidic residues, Asp-64, Asp-116, and Glu-152. This suggests that baicalein has the similar binding modes as the inhibitor 5CITEP. Both of these compounds interacted with the central core domain of integrase.
3.3 Comparison of interface residues between baicalein and 5CITEP
The contacting residues (interface residues) for both baicalein and the inhibitor 5CITEP are presented in Table 1. The baicalein binding looks quite similar to the known inhibitor binding. Several residues that are known to be important for catalysis or DNA binding are involved in binding both ligands. They are all hydrogen-bonded to Asn-155, Lys-159, and Lys-156. The difference is that baicalein does not form a hydrogen bond to Gln-148 as the inhibitor 5CITEP, but is close to the His-67. Since experiments have shown that several residues near the active site, including Thr-143, Gln-148, lys-156, and lys-159, are critical for binding viral DNA (Farnet et al., 1996; Jenkins et al., 1997), and that lys-156 and lys-159 are also involved in baicalein binding, it is tempting to speculate that the interactions between baicalein and integrase at least partially mimic the DNA substrate/integrase interaction.
Table 1.
Comparison of interface residues between baicalein and the inhibitor 5CITEP
| Baicalein | 5CITEP | ||||||
|---|---|---|---|---|---|---|---|
| Protein atom | Ligand atom | Distance | Protein atom | Ligand atom | Distance | ||
| ASP64 | OD2 | C1 | 3.25 | ASP64 | OD2 | C12 | 3.35 |
| CYS65 | O | C10 | 3.77 | CYS65 | O | N4 | 4.73 |
| THR66 | OG1 | O3 | 3.46 | THR66 | OG1 | N2 | 2.75 |
| HIS67 | CD2 | O2 | 3.01 | ||||
| ASP116 | OD1 | C5 | 3.43 | ASP116 | CB | C6 | 4.23 |
| GLN148 | NE2 | C4 | 3.06 | GLN148 | NE2 | N9 | 3.63 |
| ILE151 | CG2 | C3 | 3.41 | ILE151 | CG2 | C12 | 3.61 |
| GLU152 | O | O5 | 3.51 | GLU152 | OE2 | O2 | 2.58 |
| ASN155 | ND2 | C13 | 3.49 | ASN155 | ND2 | N4 | 3.49 |
| LYS156 | CG | O5 | 4.02 | LYS156 | NZ | O1 | 3.27 |
| LYS159 | NZ | O3 | 2.89 | LYS159 | NZ | N1 | 2.82 |
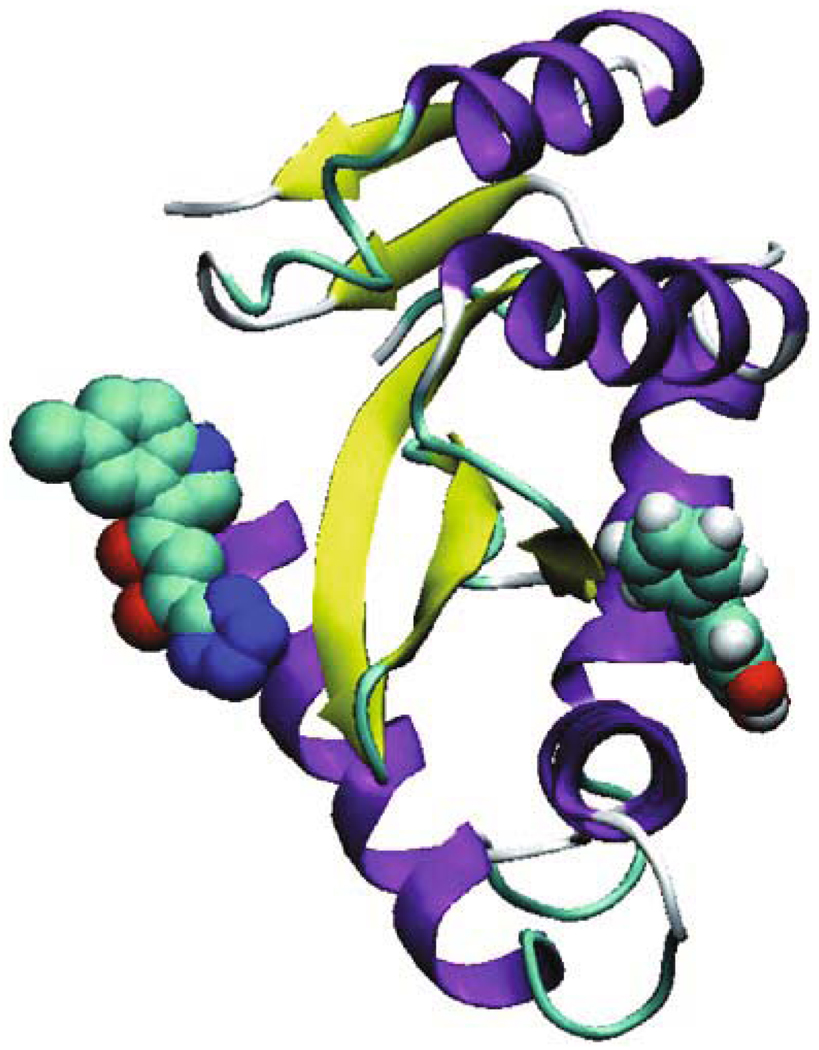
3.4 Another possible binding mode predicted by blind docking
The blind docking study using MVD predicted another possible binding mode of baicalein in which the compound docked into another binding pocket of HIV-1 integrase. The location of baicalein is in close proximity to the active site of integrase, but is quite distant from the site to which the inhibitor 5CITEP binds (Fig. 3). Baicalein is located on the other side of the flexible loop from the catalytic residues. It seems that rather than preventing DNA binding to integrase directly, baicalein might interacts with the flexible loop, alters loop conformation, and affects the conformations of active site residues. Therefore, baicalein’s inhibition of HIV-1 integrase is to likely proceed by an equivalent mechanism, which is similar to another HIV integrase inhibitor Y3 (Lubkowski et al., 1998).
Figure 3.
Baicalein (space filling representation) docked into another binding pocket of HIV-1 integrase, compared with the inhibitor at the left (space filling representation)
4 Conclusions
Development of inhibitors that are specifically directed against additional targets, such as integrase, is a useful strategy for expanding the current combination therapy involving reverse transcriptase and protease inhibitors.
Our goal in working with this class of inhibitors was to provide leads to potential anti-AIDS drugs targeting HIV-1 integrase and develop a better understanding of the role of crucial target residues in integrase binding site. In the present study, molecular modeling techniques have been applied in prediction of the binding mode of baicalein with HIV-1 integrase.
Our Windock docking study shows the baicalein bound in the middle of the active site of the integrase and makes similar close contacts with the protein as the inhibitor 5CITEP in the complex. This is consistent with the previous findings that the inhibition by baicalein on integrase is directed toward conserved amino acids in the central core domain of integrase during catalysis. Analysis of the crystal structure of HIV-1 integrase reveals a cluster of lysine residues near the active site. Site-directed mutagenesis and photo-crosslinking studies have found that Lys156 and Lys159 are critical for the functional interaction of integrase with viral DNA. These two residues are also involved in baicalein binding. The interactions between baicalein and integrase might at least partially mimic the DNA substrate/integrase interaction.
An alternative binding mode (from our blind docking study) indicated that the compound docked into another binding pocket of HIV-1 integrase, which is in close proximity to the active site of integrase, but is located on the other side of the flexible loop from the catalytic residues, quite distant from the site to which the inhibitor 5CITEP binds. It is possible for the compound to interact with the flexible loop, alter loop conformation, and therefore affect the conformations of active site residues.
Many compounds identified as integrase inhibitors, though structurally belong to different chemical classes, contain one or more hydroxyl substituents. The potency of these chemically diverse compounds is often associated with the presence of a bis-catechol structure. However, it has shown that the action of baicalein is different from other bis-catechols (Ahn et al., 2001; Robinson et al., 1996).
AIDS is now a pandemic. In sub-Saharan Africa, it is now the leading cause of death (Lawn, 2004; Schneider et al., 2005; Morgan et al., 2002). There are urgent needs to develop novel class of anti-AIDS drugs. Molecular modeling of baicalein with the core catalytic domain of HIV-1 integrase would provide useful information about how the compound works and may lead to the development of more potent and specific pharmaceuticals for the treatment of AIDS.
Acknowledgments
We wish to thank Mr. Guy M. Lingani for laboratory assistance and Dr. Rene Thomsen and Molegro ApS, Denmark, for giving us the possibility of using the trial version of MVD. This work is supported by grant 2 G12 RR003048 from the RCMI Program, Division of Research Infrastructure, National Center for Research Resources, NIH.
References
- 1.Ahn HC, Lee SY, Kim JW, Son WS, Shin CG, Lee BJ. Binding aspects of baicalein to HIV-1 integrase. Mol Cells. 2001;12:127–130. [PubMed] [Google Scholar]
- 2.Bhattacharya S, Osman H. Novel targets for anti-retroviral therapy. J Infect. 2009;59:377–386. doi: 10.1016/j.jinf.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CW, Bian ZX, Wu TX. Systematic review of Chinese herbal medicine for functional constipation. World J Gastroenterol. 2009;15:4886–4895. doi: 10.3748/wjg.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eron JJJ. Antiretroviral therapy: new drugs, formulations, ideas, and strategies. Top HIV Med. 2009;17:146–150. [PubMed] [Google Scholar]
- 5.Ewing TJ, Makino S, Skillman AG, Kuntz ID. DOCK 4.0: search strategies for automated molecular docking of flexible molecule databases. J Comput Aided Mol Des. 2001;15:411–428. doi: 10.1023/a:1011115820450. [DOI] [PubMed] [Google Scholar]
- 6.Farnet CM, Wang B, Lipford JR, Bushman FD. Differential inhibition of HIV-1 preintegration complexes and purified integrase protein by small molecules. Proc Natl Acad Sci USA. 1996;93:9742–9747. doi: 10.1073/pnas.93.18.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrido C, Geretti AM, Zahonero N, Booth C, Strang A, Soriano V, De Mendoza C. Integrase variability and susceptibility to HIV integrase inhibitors: Impact of subtypes, antiretroviral experience and duration of HIV infection. J Antimicrob Chemother. 2010;65:320–326. doi: 10.1093/jac/dkp423. [DOI] [PubMed] [Google Scholar]
- 8.Goldgur Y, Craigie R, Cohen GH, Fujiwara T, Yoshinaga T, Fujishita T, Sugimoto H, Endo T, Murai H, Davies DR. Structure of the HIV- 1 integrase catalytic domain complexed with an inhibitor: A platform for antiviral drug design. Proc Natl Acad Sci USA. 1999;96:13040–13043. doi: 10.1073/pnas.96.23.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta P, Roy N, Garg P. Docking-based 3DQSAR study of HIV-1 integrase inhibitors. Eur J Med Chem. 2009;44:4276–4287. doi: 10.1016/j.ejmech.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z, Southerland W. WinDock: Structure-based drug discovery on Windows-based PCs. J Comp Chem. 2007;28:2347–2351. doi: 10.1002/jcc.20756. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins TM, Esposito D, Engelman A, Craigie R. Critical contacts between HIV-1 integrase and viral DNA identified by structure-based analysis and photo-crosslinking. EMBO J. 1997;16:6849–6859. doi: 10.1093/emboj/16.22.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallings LO. The first postmodern pandemic: 25 years of HIV/AIDS. J Intern Med. 2008;263:218–243. doi: 10.1111/j.1365-2796.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer D, Pitluk J, Klunk K. An overview of CAM: components and clinical uses. Nutr Clin Pract. 2009;24:549–559. doi: 10.1177/0884533609342437. [DOI] [PubMed] [Google Scholar]
- 14.Lang PT, Brozell SR, Mukherjee S, Pettersen EF, Meng EC, Thomas V, Rizzo RC, Case DA, James TL, Kuntz ID. DOCK 6: Combining techniques to model RNA-small molecule complexes. RNA. 2009;15:1219–1230. doi: 10.1261/rna.1563609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000;276:534–538. doi: 10.1006/bbrc.2000.3485. [DOI] [PubMed] [Google Scholar]
- 17.Lubkowski J, Yang F, Alexandratos J, Wlodawer A, Zhao H, Burke TRJ, Neamati N, Pommier Y, Merkel G, Skalka AM. Structure of the catalytic domain of avian sarcoma virus integrase with a bound HIV-1 integrase-targeted inhibitor. Proc Natl Acad Sci USA. 1998;95:4831–4836. doi: 10.1073/pnas.95.9.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchand C, Maddali K, Métifiot M, Pommier Y. HIV-1 IN inhibitors: 2010 update and perspectives. Curr Top Med Chem. 2009;9:1016–1037. doi: 10.2174/156802609789630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushita S. Current status and future issues in the treatment of HIV-1 infection. Int J Hematol. 2000;72:20–27. [PubMed] [Google Scholar]
- 20.McColl DJ, Chen X. Strand transfer inhibitors of HIV-1 integrase: Bringing IN a new era of antiretroviral therapy. Antiviral Res. 2010;85:101–118. doi: 10.1016/j.antiviral.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JA. HIV-1 infection in rural Africa: Is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 22.Moszynski P. New HIV drug patent pool “offers hope to millions”. BMJ. 2009;339:b5557. doi: 10.1136/bmj.b5557. [DOI] [PubMed] [Google Scholar]
- 23.Moustakas DT, Lang PT, Pegg S, Pettersen E, Kuntz ID, Brooijmans N, Rizzo RC. Development and validation of a modular, extensible docking program: DOCK 5. J Comput Aided Mol Des. 2006;20:601–619. doi: 10.1007/s10822-006-9060-4. [DOI] [PubMed] [Google Scholar]
- 24.Nakanjako D, Colebunders R, Coutinho AG, Kamya MR. Strategies to optimize HIV treatment outcomes in resource-limited settings. AIDS Rev. 2009;11:179–189. [PubMed] [Google Scholar]
- 25.Robinson WEJ, Cordeiro M, Abdel-Malek S, Jia Q, Chow SA, Reinecke MG, Mitchell WM. Dicaffeoylquinic acid inhibitors of human immunodeficiency virus integrase: Inhibition of the core catalytic domain of human immunodeficiency virus integrase. Mol Pharmacol. 1996;50:846–855. [PubMed] [Google Scholar]
- 26.Schafer JJ, Squires KE. Integrase inhibitors: A novel class of antiretroviral agents. Ann Pharmacother. 2010;44:145–156. doi: 10.1345/aph.1M309. [DOI] [PubMed] [Google Scholar]
- 27.Schneider MF, Gange SJ, Williams CM, Anastos K, Greenblatt RM, Kingsley L, Detels R, Muñoz A. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005;19:2009–2018. doi: 10.1097/01.aids.0000189864.90053.22. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka R, Tsujii H, Yamada T, Kajimoto T, Amano F, Hasegawa J, Hamashima Y, Node M, Katoh K, Takebe Y. Novel 3alpha-methoxyserrat-14-en-21beta-ol (PJ-1) and 3beta-methoxyserrat-14-en-21beta-ol (PJ-2)-curcumin, kojic acid, quercetin, and baicalein conjugates as HIV agents. Bioorg Med Chem. 2009;17:5238–5246. doi: 10.1016/j.bmc.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen R, Christensen MH. MolDock: A new technique for high-accuracy molecular docking. J Med Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 30.UNAIDS, WHO. “2007 AIDS epidemic update” (PDF) [Retrieved on 12 February 2009];December 2007; http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 31.Wang JF, Wei DQ, Chou KC. Drug candidates from traditional Chinese medicines. Curr Top Med Chem. 2008;8:1656–1665. doi: 10.2174/156802608786786633. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 33.Wu JA, Attele AS, Zhang L, Yuan CS. Anti-HIV activity of medicinal herbs: usage and potential development. Am J Chin Med. 2001;29:69–81. doi: 10.1142/S0192415X01000083. [DOI] [PubMed] [Google Scholar]