Abstract
Monocyte chemoattractant protein-1 (MCP-1, Ccl2) expression is increased in livers of patients with non-alcoholic steatohepatitis and in murine models of steatohepatitis. Several studies in rodents indicate that MCP-1 contributes to liver steatosis induced by feeding a high fat diet. However, the extent of MCP-1 involvement in the widely utilized methionine-choline-deficient (MCD) diet model of steatohepatitis has not been determined. We tested the hypothesis that MCP-1 contributes to steatohepatitis in mice fed the MCD diet. MCP-1-deficient mice on a C57Bl/6J background and age-matched C57Bl/6J mice were fed either MCD diet or control diet for 4 weeks. MCP-1-deficiency did not affect steatohepatitis as indicated by liver histopathology, nor did it affect serum alanine aminotransferase activity, hepatic triglyceride levels, hepatic inflammatory gene induction, or macrophage accumulation in mice fed the MCD diet. MCP-1-deficiency reduced the expression of the profibrogenic genes pro-collagen 1a1, connective tissue growth factor, and transforming growth factor-β in mice fed the MCD diet. MCP-1-deficiency significantly reduced collagen deposition and alpha-smooth muscle actin protein levels in the livers of mice fed the MCD diet. The results indicate that MCP-1 does not contribute to liver steatosis or inflammation in the MCD diet model of steatohepatitis. Rather, the data suggest that MCP-1 contributes to fibrosis in mice fed the MCD diet independent of effects on steatosis and inflammation.
Keywords: Fibrosis, inflammation, liver, MCP-1, steatohepatitis
Accumulation of fat within the liver (i.e., steatosis) is commonly associated with diseases such as non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of metabolic syndrome. Although the mechanism is not entirely clear, steatosis can lead to non-alcoholic steatohepatitis (NASH), with evidence of both hepatocellular injury and chronic inflammation; this inflammation is generally considered a critical mediator of liver fibrosis in a subset of patients. Steatosis can be induced experimentally in rodents by altering the diet or by genetic approaches. The mechanisms whereby different experimental models induce steatosis vary, and this has been reviewed elsewhere1. In contrast to models utilizing diets high in saturated fat (i.e., >20% of Calories from fat), so-called Western diets2–4, the widely utilized lipogenic methionine-choline-deficient (MCD) diet is not high in fat. Rather, the MCD diet causes defects in the handling of triglycerides, both at the level of transport and storage, as well as in de novo synthesis; these defects, combined with a high level of sucrose, lead to a rapid onset of steatohepatitis5. Diets high in fat cause obesity and insulin resistance whereas mice fed the MCD diet do not exhibit these characteristics6. Given sufficient time, both diets cause steatohepatitis, although the mechanisms whereby fat accumulates in the liver in these two models are quite different.
The progression of fatty liver disease is associated with the expression of proinflammatory factors, including the cytokine monocyte chemoattractant protein-1 (MCP-1, Ccl2). MCP-1 mRNA is markedly increased in the livers of patients with steatosis and NASH7–10. Serum levels of MCP-1 are elevated in patients with NASH compared to healthy individuals7. Similarly, hepatic MCP-1 mRNA and plasma protein levels are increased in mice fed a high fat diet2–4, and hepatic MCP-1 mRNA and MCP-1 protein levels in the plasma are increased in mice fed the MCD diet11.
MCP-1 could contribute to the pathogenesis of NASH through multiple mechanisms, and this could depend on the context in which steatosis occurs. Indeed, several recent studies indicate that MCP-1 contributes directly to hepatic lipid accumulation and insulin resistance in mice fed a high fat diet. For example, MCP-1 deficiency reduced hepatic triglyceride levels in mice fed a high fat diet2. Moreover, a pharmacologic antagonist of chemokine (C-C motif) receptor 2 (CCR2), the primary receptor for MCP-1, reduced hepatic steatosis in genetically obese diabetic db/db mice12. These studies suggest that MCP-1 contributes to steatosis induced by a high fat diet.
Another mechanism whereby MCP-1 could contribute to the pathogenesis of NAFLD and NASH is by enhancing the inflammatory response. MCP-1 coordinates leukocyte recruitment to the liver by activation of the CCR2 receptor on inflammatory cells including monocytes/macrophages13. Indeed, deficiency in either MCP-1 or CCR2 reduced hepatic infiltration of inflammatory cells in various models of liver disease14–17, suggesting that MCP-1 could contribute not only to steatosis, but also to inflammation. In addition, several recent studies indicate that MCP-1 is an important mediator of liver fibrosis15,18,19. Together, these studies indicate that the effects of MCP-1 in NASH could be multifaceted and model dependent.
Although MCP-1 is induced in both high-fat and MCD diet models of NAFLD/NASH, fundamental differences in the mechanism of steatosis in each model exist. We sought to characterize the contribution of MCP-1 to steatosis in mice fed the MCD diet, as this model is widely utilized to study mechanisms of NASH. Moreover, we systematically characterized the effect of MCP-1-deficiency on hepatocellular injury, hepatic inflammation, and fibrosis in mice fed the MCD diet.
Materials and Methods
Mice
All mice were used between 8–12 weeks of age. Male MCP-1−/− mice20 on a C57Bl/6J background and age-matched male C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in an AAALAC-accredited facility at the University of Kansas Medical Center. Mice were housed at an ambient temperature of 22°C with alternating 12-h light/dark cycles and allowed water and rodent chow (Teklad 8604; Harlan, Indianapolis, IN) ad libitum prior to feeding custom diets. All animal procedures were performed according to the guidelines of the American Association for Laboratory Animal Science and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Dietary model of non-alcoholic steatohepatitis
The MCD rodent chow (MCD diet, 518810) and an otherwise identical diet sufficient in methionine and choline (control diet, 518754) were purchased from Dyets, Inc. (Bethlehem, PA). Insofar as baseline differences in the purified diet, not just the methionine/choline-deficiency, could affect baseline gene expression and other parameters, the use of this methionine/choline-sufficient diet is the most appropriate control. Mice were fed either control diet or MCD diet ad libitum for 4 weeks. The mice were not fasted prior to sample collection. Under isoflurane anesthesia, blood was then collected from the caudal vena cava into sodium citrate (final concentration, 0.38%) for the collection of plasma and into an empty syringe for the collection of serum. Sections of liver from the left lateral lobe were fixed in 10% neutral-buffered formalin for 48 hours, and then embedded in paraffin. The right medial lobe was affixed to a cork with OCT (Fisher Scientific, Pittsburgh, PA) and immersed for 3 minutes in liquid nitrogen-chilled isopentane. The remaining liver was snap frozen in liquid nitrogen.
Histopathology, sirius red staining, clinical chemistry, and triglyceride determination
Formalin-fixed livers were sectioned at 5 microns, stained with hematoxylin and eosin (H & E), and evaluated by a pathologist (O.T.). Steatosis was scored from 0–4 in a masked fashion. Sirius red staining and fast-green counterstaining of formalin-fixed, paraffin-embedded liver sections were performed as described previously21. The serum activity of alanine aminotransferase (ALT) was determined using a commercially available reagent (Thermo Fisher, Rockford, IL). Hepatic triglyceride content was determined as described previously22 using commercially available reagents (Wako, Richmond, VA). Serum triglyceride levels were determined using commercially available reagents (Pointe Scientific, Inc, Canton, MI). Plasma glucose levels were determined using a commercial glucometer.
Immunohistochemistry and immunofluorescence
Immunohistochemical staining of macrophages in frozen liver sections was performed as described11 utilizing a 1:1 mix of rat anti-mouse CD68/rat anti-mouse F4/80 antibodies (AbD Serotec, Raleigh, NC), and Vector NovaRed substrate (Vector Laboratories, Burlingame, CA). The extent of macrophage accumulation in liver sections stained immunohistochemically for macrophages was determined in a masked fashion and each liver sample assigned a score from 1–10. Oil Red O staining was performed as previously described22. Immunofluorescent staining for Type 1 collagen was performed as described previously23 utilizing a rabbit anti-mouse Type 1 collagen antibody (Millipore, Billerica, MA) and Alexa 594-conjugated secondary antibody (Invitrogen, Carlsbad, CA). The slides were visualized using an Olympus BX41 microscope (Olympus, Lake Success, NY). Images were captured using an Olympus DP70. Quantification of Type 1 collagen antibody utilizing Scion Image software was performed as described previously23. The evaluator was masked to both treatment and genotype.
Liver homogenate preparation and α-smooth muscle actin (α-SMA) Western blotting
Whole liver homogenates were prepared by homogenizing 100 mg of snap frozen liver in 1 ml of RIPA buffer containing protease inhibitor cocktail and PhosStop phosphatase inhibitor (Roche Applied Science). The homogenate was transferred to 1.7 ml tubes, rotated end-over-end for 15 minutes at 4°C then subjected to centrifugation at 14,000 × g for 10 minutes. The supernatant was collected and protein concentration determined using the Bio-rad DC protein assay (Bio-rad, Hercules, CA). Five μg of protein for each sample was diluted in 1X LDS buffer (Invitrogen, San Diego, CA) and heated at 95°C in the presence of 2-mercaptoethanol for 10 minutes. Samples were separated by electrophoresis utilizing precast 4–20% Bis-tris Criterion gels (Bio-rad) and transferred to Immobilon PVDF membrane (Millipore, Billerica, MA) by semi-dry transfer. Membranes were blocked with 3% BSA in 1X TBST for 60 minutes at room temperature and then incubated overnight at 4°C with a 1:1000 dilution of a monoclonal mouse anti-α-SMA antibody or 1:5000 dilution of a monoclonal mouse anti-glyceraldehyde 3 phosphate dehydrogenase (GAPDH) antibody (Millipore), followed by incubation for 1 h at room temperature with a secondary anti-mouse IgG-HRP conjugated antibody diluted at 1:5000 (Cell Signaling Technology, Danvers, MA). All antibodies were diluted in 1% BSA in 1X TBST. Membranes were then washed and incubated with Supersignal West Pico substrate (ThermoScientific) solution and exposed to Blue lite autoradiography film (ISC Bioexpress, Kaysville, UT).
RNA isolation, cDNA synthesis, and real-time PCR
Total RNA was isolated from approximately 100 mg of snap-frozen liver using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH). One microgram of RNA was utilized for the synthesis of cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) and MyCycler thermal cycler (Bio-Rad, Hercules, CA). Levels of tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2), MCP-1, macrophage inflammatory protein (MIP-2, Cxcl2), keratinocyte chemoattractant/growth related gene (KC/Gro, Cxcl1), intercellular adhesion molecule-1 (ICAM-1), tissue inhibitor of metalloproteinase-1 (TIMP-1), connective tissue growth factor (CTGF), pro-collagen 1a1 (Col1a1), transforming growth factor-β (TGF-β), cell surface glycoprotein F4/80, macrosialin (CD68), interleukin (IL)-1β, IL-6, and GAPDH mRNA were determined using the TaqMan gene expression assay from Applied Biosystems and TaqMan gene expression master mix (Applied Biosystems) on an ABI Prism 7300 sequence detection system (Applied Biosystems). The expression of each gene was adjusted relative to GAPDH expression levels, and relative expression level determined using the comparative Ct method.
Statistics
Comparison of two groups was performed using Student’s t-test. Comparison of 3 or more groups was performed using one-way analysis of variance followed by the Student-Newman-Keuls post-hoc test. The criterion for statistical significance was P<0.05
Results
Effect of MCP-1-deficiency on MCD diet-induced hepatocellular injury
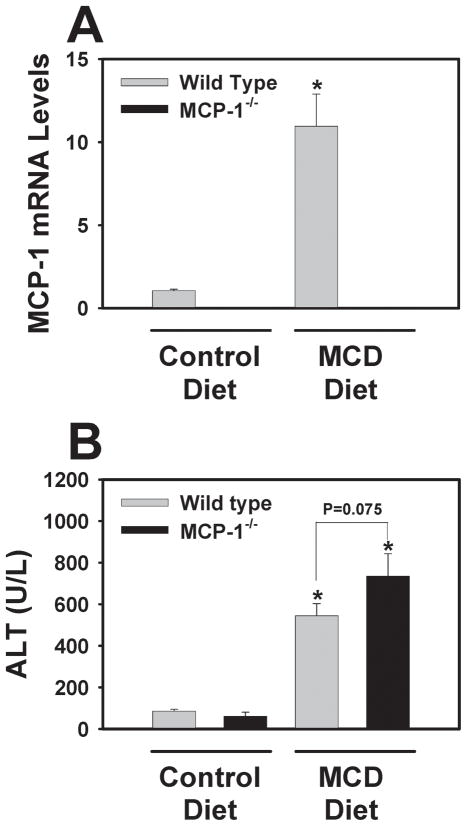
In agreement with previous studies11,24, hepatic MCP-1 mRNA levels increased in wild type mice fed the MCD diet (Fig. 1A). Serum ALT activity increased in mice fed the MCD diet compared to mice fed the control diet. Surprisingly, MCP-1-deficiency did not significantly affect serum ALT activity, although a trend towards higher serum ALT activity was observed in MCP-1-deficient mice fed the MCD diet (P = 0.075 for comparison between WT and MCP-1−/− mice fed the MCD diet). (Fig. 1B). This result indicates that MCP-1-deficiency does not reduce hepatocellular injury in mice fed the MCD diet.
Figure 1. Effect of MCP-1-deficiency on MCP-1 mRNA levels and serum ALT activity in mice fed the MCD diet.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days and (A) hepatic levels of MCP-1 mRNA and (B) serum ALT activity were determined. Data are expressed as mean ± SEM. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet.
Effect of MCP-1-deficiency on MCD diet-induced steatosis
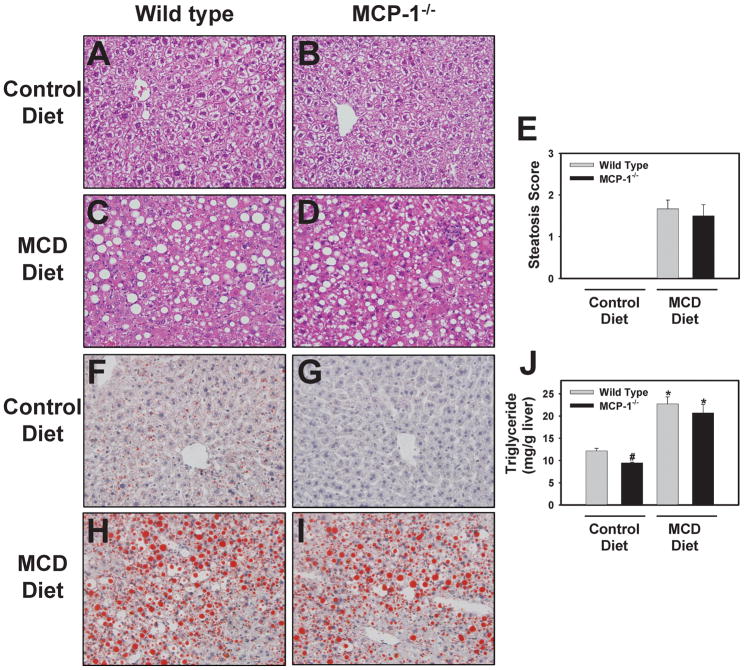
H&E-stained slides were examined by a pathologist and no substantial differences in liver histology were observed between wild-type mice and MCP-1-deficient mice fed control diet (Fig. 2A–2B). Both wild type and MCP-1-deficient mice fed the MCD diet developed panacinar macrovesicular hepatic steatosis of similar severity (Fig. 2C–E and Fig. 2H–I). Hepatic triglyceride levels increased significantly in both wild type and MCP-1-deficient mice fed the MCD diet (Fig. 2J). These results are in contrast to mice fed a high fat diet, in which both steatosis and hepatic triglyceride levels were reduced by MCP-1-deficiency2,3. Although the mice were not fasted, changes in circulating triglyceride and glucose levels mirrored previous studies25,26. Serum triglyceride levels were significantly reduced in mice fed the MCD diet (Control vs. MCD diet, mean ± SEM: Wild type mice, 57 ± 6 vs. 24 ± 1 mg/dL; MCP-1−/− mice, 57 ± 13 vs. 28 ± 2 mg/dL). Similarly, plasma glucose levels were significantly reduced in mice fed the MCD diet (Control vs. MCD diet, mean ± SEM: Wild type mice, 289 ± 43 vs. 160 ± 11 mg/dL; MCP-1−/− mice, 348 ± 34 vs. 146 ± 23 mg/dL). Interestingly, glucose levels were higher in MCP-1−/− mice fed control diet compared to wild type mice fed control diet.
Figure 2. Effect of MCP-1-deficiency on liver histology and triglyceride levels in mice fed the MCD diet.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days. Representative photomicrographs showing H&E-stained liver sections (A–D) and Oil Red O-stained liver sections (F–I) from wild type mice fed control diet (A,F) or MCD diet (C,H) and from MCP-1-deficient mice fed control diet (B, G) or MCD diet (D, I). (E) H&E-stained slides were evaluated by a pathologist and assigned a macrovesicular steatosis severity score. (J) Hepatic triglyceride levels were determined as described in Methods. Data are expressed as mean ± SEM. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet. #Significantly different from wild type mice fed the control diet.
Of importance, MCP-1-deficiency significantly reduced baseline triglyceride levels in mice fed control diet (Fig. 2J). Similarly, Oil Red O staining indicated a decrease in lipid content of livers from MCP-1-deficient mice fed control diet (Fig. 2F–G).
Effect of MCP-1-deficiency on MCD diet-induced hepatic inflammatory gene induction and macrophage accumulation
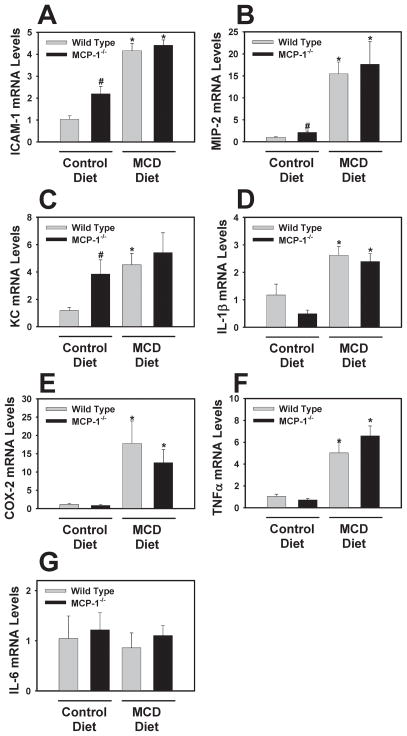
Compared to wild type mice fed control diet, levels of ICAM-1, MIP-2 and KC/Groα mRNA were increased in livers of MCP-1-deficient mice fed control diet (Fig. 3A–C). Previous studies have identified a similar, and likely compensatory, effect of MCP-1-deficiency on the expression of other chemokines 27. Interestingly, IL-1β mRNA levels were lower in MCP-1−/− mice fed the MCD diet, although this did not achieve statistical significance (Fig. 3D). Induction of ICAM-1, MIP-2, KC/Gro, IL-1β, COX-2, and TNFα mRNA in livers of mice fed the MCD diet was unaffected by MCP-1-deficiency (Fig. 3A–F). Hepatic IL-6 mRNA levels were not significantly affected by either the MCD diet or MCP-1-deficiency (Fig. 3G).
Figure 3. Effect of MCP-1-deficiency on hepatic proinflammatory gene mRNA levels in mice fed the MCD diet.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days. Hepatic levels of (A) ICAM-1, (B) MIP-2, (C) KC/Groα, (D) IL-1β, (E) COX-2, (F) TNFα, and (G) IL-6 mRNAs were quantified. Data are expressed as mean ± SEM. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet. #Significantly different from wild type mice fed the control diet.
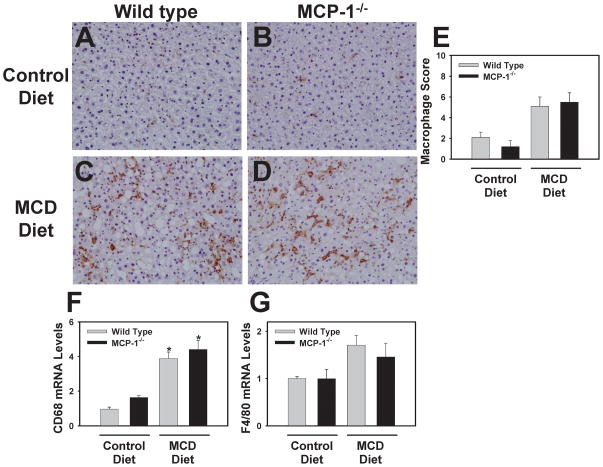
Scattered sinusoidal macrophage staining was observed in the livers of wild type and MCP-1-deficient mice fed control diet (Fig. 4A–B), representing Kupffer cells. Hepatic macrophage accumulation increased in mice fed the MCD diet and was not affected by MCP-1-deficiency (Fig. 4C–E). In agreement with this observation, liver CD68 and F4/80 mRNA levels tended to increase in mice fed the MCD diet, and were unaffected by MCP-1-deficiency (Fig. 4F–G)
Figure 4. Effect of MCP-1-deficiency on hepatic macrophage accumulation.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days. Representative photomicrographs show combined CD68- and F4/80-positive staining (i.e., macrophages) in livers of wild type mice fed control diet (A) or MCD diet (C) and from MCP-1-deficient mice fed control diet (B) or MCD diet (D). (E) The extent of macrophage accumulation was assessed in a masked fashion and each tissue assigned a score (1–10). Hepatic levels of (F) CD68 and (G) F4/80 mRNA were determined. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet.
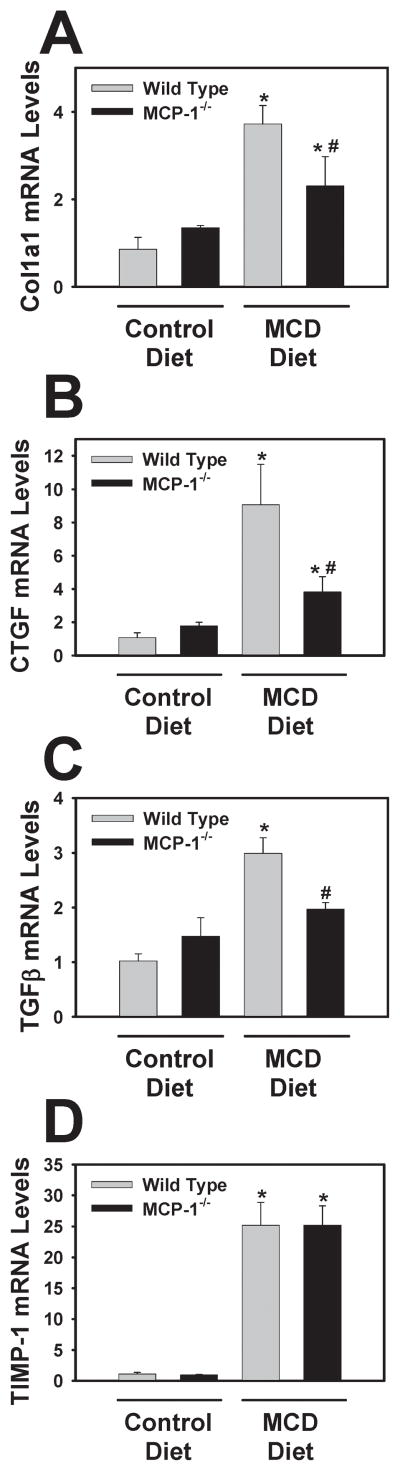
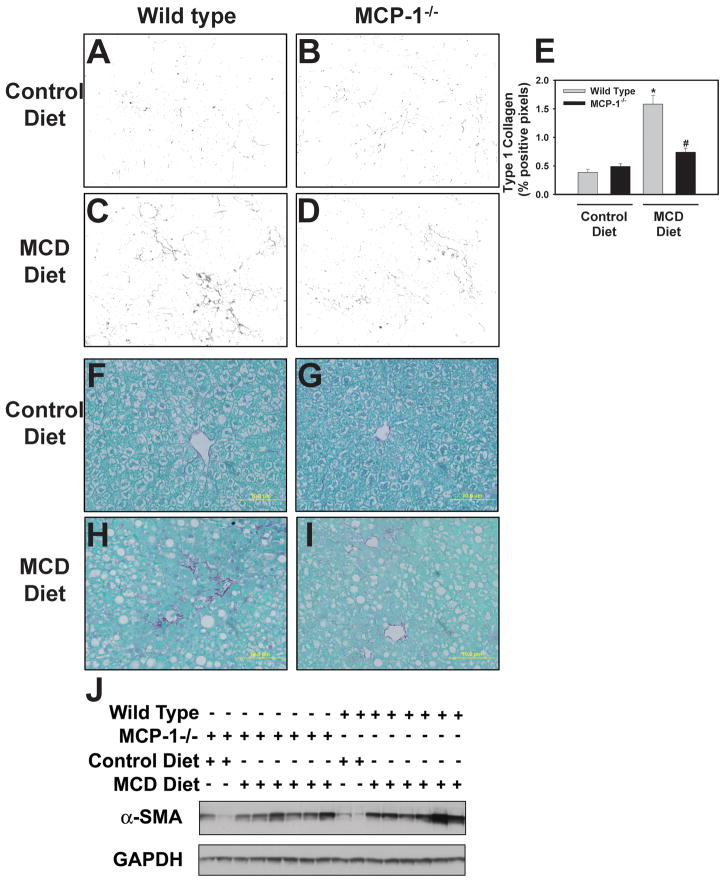
Effect of MCP-1-deficiency on profibrogenic gene expression and collagen deposition in livers of mice fed the MCD diet
MCP-1 has been implicated in hepatic fibrosis and has been shown to directly activate stellate cells15,19. This suggests that MCP-1 could affect liver fibrosis without altering steatosis or inflammation. Therefore, we also determined the effect of MCP-1-deficiency on induction of various profibrogenic genes. Induction of Col1a1, CTGF, and TGF-β mRNA, but not TIMP-1 mRNA, were significantly reduced in MCP-1-deficient mice fed the MCD diet (Fig. 5A–D). Moreover, hepatic collagen deposition, determined utilizing immunofluorescent staining for Type 1 collagen (Fig. 6A–D) and sirius red staining (6F–I), was decreased in MCP-1-deficient mice fed the MCD diet. Quantification of the Type 1 collagen staining indicated a significant reduction in collagen protein levels in the livers of MCP-1-deficient mice fed the MCD diet (Fig. 6E). The levels of α-SMA increased significantly in mice fed the MCD diet, and this was reduced in MCP-1-deficient mice (Fig. 6J). Densitometry indicated a significant reduction in α-SMA protein in MCP-1-deficient mice fed the MCD diet compared to wild type mice fed the MCD diet (mean ± SEM, n = 6, 1.5 ± 0.25 [wild type] vs 0.8 ± 0.1 [MCP-1−/−])
Figure 5. Effect of MCP-1-deficiency on levels of profibrogenic genes in the livers of mice fed the MCD diet.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days. Hepatic levels of (A) Col1a1, (B) CTGF, (C) TGFβ, and (D) TIMP-1 were quantified. Data are expressed as mean ± SEM. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet. #Significantly different from wild type mice fed the MCD diet.
Figure 6. Effect of MCP-1-deficiency on collagen and α-SMA protein levels in livers of mice fed the MCD diet.
MCP-1-deficient and wild type mice, both on the C57Bl/6J genetic background, were fed either control diet or MCD diet for 28 days. Representative photomicrographs showing liver sections stained for (A–D) Type 1 collagen (black) and (F–I) sirius red-stained liver sections from wild type mice fed control diet (A,F) or MCD diet (C,H) and from MCP-1-deficient mice fed control diet (B, G) or MCD diet (D, I). (E) Quantification of positive staining for Type 1 collagen was performed using morphometry as described in Methods. Data are expressed as mean ± SEM. (J) Western blot showing α-SMA protein levels in liver. N = 4 wild type mice fed control diet, 3 MCP-1−/− mice fed control diet, 8 wild type mice fed MCD diet, and 6 MCP-1−/− mice fed MCD diet. *Significantly different from mice of the same genotype fed control diet. #Significantly different from wild type mice fed the MCD diet.
Discussion
The MCD diet is widely utilized to evaluate mechanisms of NASH in rodents. However, key differences in the mechanism of hepatic steatosis among the multiple animal models of NASH make translation of the results to human disease challenging. We found that MCP-1 did not contribute to macrovesicular steatosis in mice fed the MCD diet, in striking contrast to models of NAFLD/NASH induced by feeding a high fat diet2,3,12. Indeed, triglyceride accumulation in livers of mice fed the MCD diet is mediated through multiple mechanisms, including an altered balance in fatty acid uptake and secretion of triglycerides, increased de novo lipogenesis, and increased triglyceride synthesis5,28. High levels of sucrose in the diet contribute to the increased lipogenesis and triglyceride synthesis, but not the abnormal fatty acid uptake and triglyceride secretion5. Indeed, simple replacement of sucrose with starch in the MCD diet formulation largely prevents hepatic steatosis in this model, with only minimal effect on loss of body weight5. The complex mechanisms of severe hepatic steatosis induced by the MCD diet may bypass the requirement of MCP-1 to induce steatosis. Interestingly, MCP-1-deficiency significantly reduced hepatic triglyceride levels and Oil Red O staining in livers of mice fed methionine/choline-sufficient control diet, which is high in sucrose like the MCD diet. This rather subtle change in baseline hepatic lipid levels in mice fed the control diet may reflect the defined role of MCP-1 in hepatic lipid accumulation, glucose regulation, and insulin resistance induced by diets high in sugar2,29.
In models of western diet-induced NAFLD, the role of MCP-1 in liver inflammation is potentially secondary to its role in promoting steatosis, although several studies suggest potential disconnects between lipid accumulation and liver inflammation in NAFLD. For example, the two diacylglycerol acyltransferases (DGATs) have different effects on the pathogenesis of NASH in mice fed the MCD diet. Inhibition of DGAT1 has no effect on triglycerides or inflammation but decreases fibrosis30, whereas inhibition of DGAT2 decreases triglyceride synthesis and steatosis but increases inflammation and fibrosis31. In mice fed a diet high in sucrose or fat, there was an increase in both steatosis and inflammation compared to control mice32. However, human consumption of fructose correlated with decreased steatosis, and increased inflammation and fibrosis33. The lack of effect of MCP-1-deficiency on the pathogenesis of steatosis and inflammation in mice fed the MCD diet is somewhat unexpected when compared to the effect of MCP-1-deficiency on liver pathology in mice fed a high fat diet. However, the mechanisms of liver inflammation in mice fed the MCD diet are complex and deletion of a single chemokine may not have profound effects.
Indeed, intracellular signaling pathways such as the JNK MAPK34, transcription factors such as CEBP/β35 and NF-κB24,36, and nuclear receptors such as FXR37 and PPARs38,39 contribute to hepatic expression of numerous proinflammatory mediators including ICAM-1, cytokines such as TNFα, IL-6 and MCP-1, as well as osteopontin and COX-224,25,35,40. As many of these mediators contribute to the pathogenesis of steatohepatitis in the MCD diet model, their expression was evaluated in the present study. Of importance, the exact role of some cytokines, such as TNFα and IL-6, remains unclear. More recently, we showed that coagulation protease signaling through protease activated receptor-1 (PAR-1) is a central amplifier of MCP-1 induction and the inflammatory response in livers of mice fed the MCD diet11.
Although several of these studies have clarified the mechanism whereby MCP-1 expression increases in mice fed the MCD diet, the results presented here indicate that MCP-1 is not required for the full manifestation of hepatic inflammation in mice fed the MCD diet. This is in contrast to other models of liver injury in which interventions targeting MCP-1 or its primary receptor CCR2 significantly reduce tissue macrophage accumulation12,15. The spectrum of inflammatory mediators induced in livers of mice fed the MCD diet is broad, and these other mediators may be sufficient to induce liver inflammation. Moreover, compensatory induction of other chemokines in MCP-1-deficient mice has been observed previously27, and could account for the lack of effect of MCP-1-deficiency on hepatic inflammation in mice fed the MCD diet. Of importance, MCP-1 clearly contributes to the pathogenesis of NAFLD in other models and mechanisms of increased MCP-1 expression in other models may be similar to the MCD diet.
The mechanism of liver fibrosis in the MCD diet model is largely considered to stem from the chronic inflammatory response elicited by steatosis and hepatocellular injury. Indeed, numerous studies indicate that interventions reducing steatosis also reduce inflammation and fibrosis41,42. It is initially counterintuitive that profibrogenic gene expression and collagen expression were reduced in MCP-1−/− mice fed the MCD diet with no effect on steatosis or inflammation. However, mediators produced secondary to steatosis could contribute to more distal processes in MCD diet-induced steatohepatitis without impacting steatosis. For example, we have shown that PAR-1-deficiency reduces MCP-1 expression, but not steatosis, in mice fed the MCD diet11. Moreover, other interventions have been shown to reduce fibrosis without impact on steatohepatitis in mice fed the MCD diet43. Our results indicate that the profibrogenic effects of MCP-1 in this model of steatohepatitis are independent of inflammation. However, the expression of TIMP-1, an inhibitor of metalloproteinases, was unaffected by MCP-1-deficiency. Of importance, the expression of TIMP-1 in this model is driven by TNF, which was also unaffected by MCP-1-deficiency44.
Multiple studies indicate that MCP-1 activates cultured hepatic stellate cells and portal fibroblasts15,45–47, and stellate cells are the principal cell type producing collagen in NASH15,48. Interestingly, we found that hepatic levels of α-SMA, an indicator of stellate cell activation, were reduced in livers of MCP-1−/− mice fed the MCD diet.
In summary, MCP-1-deficiency did not impact liver steatosis or inflammation in mice fed the MCD diet. However, MCP-1-deficiency reduced profibrogenic gene expression and liver fibrosis in this model. Of interest, MCP-1-deficiency selectively reduced the expression of several fibrogenic genes and collagen deposition in mice fed the MCD diet without impacting steatohepatitis. This is likely the result of direct effects of MCP-1 on stellate cell activation15. This result highlights an important distinction in the mechanism of steatosis in the MCD diet model of NASH compared to other models. The MCD diet model may represent a novel opportunity to investigate profibrogenic effects of MCP-1 outside the scope of its role in steatosis or inflammation.
Acknowledgments
Supported by American Heart Association Scientist Development Grant 0835121G (JPL) and by the National Institutes of Health P20 RR021940 (JPL) and R01DK081343 (GLG). The authors would like to thank Huina Cai, Ruipeng (Frank) Wang and Noriko Esterly for outstanding technical assistance.
Funding: Supported by American Heart Association Scientist Development Grant 0835121G (JPL) and by the National Institutes of Health P20 RR021940 (JPL) and R01DK081343 (GLG).
Abbreviations
- ALT
alanine aminotransferase
- CCR2
chemokine (C-C motif) receptor 2
- CTGF
connective tissue growth factor
- COX-2
cyclooxygenase-2
- DGAT
diacylglycerol acyltransferase
- GAPDH
glyceraldehyde 3 phosphate dehydrogenase
- H & E
hematoxylin and eosin
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- KC/GRO
keratinocyte chemoattractant/growth related gene
- MIP-2
macrophage inflammatory protein-2
- MCD
methionine-choline-deficient
- MCP-1
monocyte chemoattractant protein-1
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- α-SMA
α-smooth muscle actin
- Col1a1
pro-collagen 1a1
- PAR-1
protease-activated receptor-1
- TIMP-1
tissue inhibitor of metalloproteinase-1
- TGFβ
transforming growth factor-β
- TNFα
tumor necrosis factor α
Footnotes
Conflict of Interest: none declared.
Reference List
- 1.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rull A, Rodriguez F, Aragones G, et al. Hepatic monocyte chemoattractant protein-1 is upregulated by dietary cholesterol and contributes to liver steatosis. Cytokine. 2009;48:273–279. doi: 10.1016/j.cyto.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Tous M, Ferre N, Rull A, et al. Dietary cholesterol and differential monocyte chemoattractant protein-1 gene expression in aorta and liver of apo E-deficient mice. Biochem Biophys Res Commun. 2006;340:1078–1084. doi: 10.1016/j.bbrc.2005.12.109. [DOI] [PubMed] [Google Scholar]
- 5.Pickens MK, Yan JS, Ng RK, et al. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009;50:2072–2082. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Haukeland JW, Damas JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Westerbacka J, Kolak M, Kiviluoto T, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 9.Marsillach J, Oliveras-Ferraros C, Beltran R, et al. Serum concentrations of extracellular fatty acid synthase in patients with steatohepatitis. Clin Chem Lab Med. 2009;47:1097–1099. doi: 10.1515/CCLM.2009.259. [DOI] [PubMed] [Google Scholar]
- 10.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 11.Luyendyk JP, Sullivan BP, Guo GL, et al. Tissue factor deficiency and protease activated receptor-1 deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol. 2010;176:177–186. doi: 10.2353/ajpath.2010.090672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195–2201. doi: 10.1161/ATVBAHA.108.168633. [DOI] [PubMed] [Google Scholar]
- 13.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dambach DM, Watson LM, Gray KR, et al. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 15.Seki E, De MS, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuziel WA, Morgan SJ, Dawson TC, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2 (gene targeting/monocyte chemoattractant protein 1/trafficking/peritonitis/granuloma) Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamara E, Galastri S, Aleffi S, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230–238. doi: 10.1016/j.jhep.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Ramm GA, Shepherd RW, Hoskins AC, et al. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology. 2009;49:533–544. doi: 10.1002/hep.22637. [DOI] [PubMed] [Google Scholar]
- 19.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergheim I, Guo L, Davis MA, et al. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Aleksunes LM, Yeager RL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 23.Kim ND, Moon JO, Slitt AL, et al. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Ip E, Dela PA, et al. COX-2 induction in mice with experimental nutritional steatohepatitis: Role as pro-inflammatory mediator. Hepatology. 2006;43:826–836. doi: 10.1002/hep.21108. [DOI] [PubMed] [Google Scholar]
- 25.Mas E, Danjoux M, Garcia V, et al. IL-6 deficiency attenuates murine diet-induced non-alcoholic steatohepatitis. PLoS One. 2009;4:e7929. doi: 10.1371/journal.pone.0007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppe SW, Sahai A, Malladi P, et al. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira AM, Rollins BJ, Faunce DE, et al. The effect of MCP-1 depletion on chemokine and chemokine-related gene expression: evidence for a complex network in acute inflammation. Cytokine. 2005;30:64–71. doi: 10.1016/j.cyto.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Rinella ME, Elias MS, Smolak RR, et al. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–1076. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura Y, Sugimoto M, Murayama T, et al. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Yang L, McCall S, et al. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schattenberg JM, Singh R, Wang Y, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 35.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, et al. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–1117. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 36.Dela PA, Leclercq I, Field J, et al. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–1674. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Wang J, Liu Q, et al. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Ip E, Farrell GC, Robertson G, et al. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 39.Leclercq IA, Lebrun VA, Starkel P, et al. Intrahepatic insulin resistance in a murine model of steatohepatitis: effect of PPARgamma agonist pioglitazone. Lab Invest. 2007;87:56–65. doi: 10.1038/labinvest.3700489. [DOI] [PubMed] [Google Scholar]
- 40.Sahai A, Malladi P, Melin-Aldana H, et al. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol. 2004;287:G264–G273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- 41.Titos E, Ferre N, Lozano JJ, et al. Protection from hepatic lipid accumulation and inflammation by genetic ablation of 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2010;92:54–61. doi: 10.1016/j.prostaglandins.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 Promotes Steatohepatitis via Induction of Interleukin-1beta in Mice. Gastroenterology. doi: 10.1053/j.gastro.2010.03.052. print copy in press 2010 Mar. Available from: URL: http://www.ncbi.nlm.nih.gov/pubmed/20347818?dopt=Citation. [DOI] [PMC free article] [PubMed]
- 43.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2008;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marra F, Romanelli RG, Giannini C, et al. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 46.Kruglov EA, Nathanson RA, Nguyen T, et al. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–G771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sprenger H, Kaufmann A, Garn H, et al. Differential expression of monocyte chemotactic protein-1 (MCP-1) in transforming rat hepatic stellate cells. J Hepatol. 1999;30:88–94. doi: 10.1016/s0168-8278(99)80011-7. [DOI] [PubMed] [Google Scholar]
- 48.Washington K, Wright K, Shyr Y, et al. Hepatic stellate cell activation in nonalcoholic steatohepatitis and fatty liver. Hum Pathol. 2000;31:822–828. doi: 10.1053/hupa.2000.8440. [DOI] [PubMed] [Google Scholar]