Abstract
Background
Sex steroid hormones have been proposed to play a role in the development of non-epithelial ovarian cancers (NEOC) but so far no direct epidemiological data are available.
Methods
A case-control study was nested within the Finnish Maternity Cohort, the world’s largest bio-repository of serum specimens from pregnant women. Study subjects were selected among women who donated a blood sample during a singleton pregnancy that led to the birth of their last child preceding diagnosis of NEOC. Case subjects were 41 women with sex-cord stromal tumors (SCST) and 21 with germ cell tumors (GCT). Three controls, matching the index case for age, parity at the index pregnancy, and date at blood donation were selected (n=171). Odds ratios (OR) and 95% confidence intervals (CI) associated with concentrations of testosterone, androstenedione, 17-OH-progesterone, progesterone, estradiol and sex hormone binding globulin (SHBG) were estimated through conditional logistic regression.
Results
For SCST, doubling of testosterone, androstenedione and 17-OH-progesterone concentrations were associated with about 2-fold higher risk of SCST [ORs and 95% CI of 2.16 (1.25–3.74), 2.16 (1.20–3.87), and 2.62 (1.27–5.38), respectively]. These associations remained largely unchanged after excluding women within 2, 4 or 6 years lag-time between blood donation and cancer diagnosis. Sex steroid hormones concentrations were not related to maternal risk of GCT.
Conclusions
This is the first prospective study providing initial evidence that elevated androgens play a role in the pathogenesis of SCST.
Impact
Our study may note a particular need for larger confirmatory investigations on sex steroids and NEOC.
Keywords: testosterone, androstenedione, 17-OH-progesterone, progesterone, estradiol, pregnancy, non-epithelial ovarian cancer, nested case-control study
Introduction
Non-epithelial ovarian cancers (NEOC) account for approximately 10% of all ovarian tumors and about 7% of the invasive ones (1). They are divided into two major distinct subtypes, sex cord-stromal tumors (SCST) and germ cell tumors (GCT) (1–3). SCST occur in women of all ages but increase in frequency during the 4th and 5th decades of age and have a median age at diagnosis of 52 years (4–6). In contrast, GCT occur predominantly in young women with the peak incidence around age 18 and are rarely observed after age 30 (7). The incidence rates of the two subtypes of NEOC also differ by race: SCST are twice as frequent in women of European and American background than in women from Asian descent (4), while GCT are more frequent in Asian women (8;9).
Because of the low incidence of NEOC, very few studies have investigated risk factors for their development. So far, only 6 studies, 5 of which included between 10 and 72 cases of SCST or GCT, have reported on the association of these tumors with traditional reproductive risk factors, such as parity, oral contraceptive use, ages at the first and last births, and time since last birth (1;10–14). Although the results from these studies are not entirely consistent, parous women appear to be at reduced risk of both SCST and GCT (1;10;12), but, there is some indication that the effect of other reproductive factors (1;11;12;15), particularly age at last birth (11;12) may differ in the two subtypes. It has also been suggested that exposure to high estrogens in utero may be associated with NEOC in the offspring (16;17) and, possibly, also in the mother (11).
The association of NEOC with concentrations of sex steroid hormones during the last pregnancy preceding the index diagnosis was explored in a case-control study, nested within the large, nation-wide Finnish Maternity Cohort (FMC). Early pregnancy (6–21 gestational weeks) concentrations of testosterone, androstenedione, 17-OH-progesterone (the precursor hormone for ovarian and adrenal synthesis of androgens (18;19)), progesterone, estradiol and sex hormone binding globulin (SHBG) were measured. To our knowledge, this is the first epidemiological investigation to directly assess the associations of endogenous sex steroid hormones with maternal risk of SCST and GCT.
Materials and Methods
Study population
The FMC is the world’s largest bio-repository of serum specimens from pregnant women. It was established in 1983 with the purpose of preserving for research serum samples drawn in the latter part of the first trimester, or the early weeks of the second trimester, from pregnant women during mandatory testing for systemic infections (20;21). After testing, leftover sera are put away for long-term storage at −25°C in a central repository. The repository contains more than 1.6 million samples donated by over 850,000 women, from more than 98% of all pregnancies in the country.
Selection of cases and controls
The design was a case-control study nested within the FMC. Eligible women were FMC members who donated a blood sample between gestational weeks 6 and 21 of a pregnancy which resulted in a singleton birth, and who were free of any invasive (except non-melanoma skin), or borderline ovarian cancer at the time of blood donation. Eligible cases were identified through a linkage with the Finnish Cancer Registry (FCR). The FCR started recording cancer cases in 1953. The registry covers the entire territory of Finland, which now comprises a population of about 5.4 million, almost entirely Caucasian. Reporting of new cancer cases is mandatory since 1961 and the coverage of the FCR is virtually complete with no losses to follow-up (22). Cases were women diagnosed with primary NEOC after recruitment into the cohort until February 2007 with information on gestational age at blood donation. If a case subject had more than one eligible sample preceding the index diagnosis, the one donated closest in time to the date of diagnosis was selected for the study. With one exception, the index sample was from the last pregnancy preceding the cancer diagnosis. A total of 85 potentially eligible cases were identified. Lists with up to 10 potentially eligible controls per each case, matched on age (± 6 months), date (± 3 months) and parity at index pregnancy were initially drawn.
A record linkage with the Finnish Population Registry led to the exclusion of 8 cases (5 whose pregnancy did not end with a child birth and 3 multiple births). A linkage of the working file with detailed FMC files was conducted to verify information on gestational age at blood donation and to check for sample availability. Twelve cases were further excluded: 2 who donated a blood sample outside gestational weeks 6 to 21 and 10 with no available sample. The same exclusion criteria were applied to the pool of controls. Three controls among those fully eligible per each case were selected at random. Three cases with no eligible controls were further excluded and for 12 cases, only two (n=10) or one (n=2) eligible control was available. For nine controls, a sample from the last but one pregnancy preceding index date was available. In total, 62 cases of NEOC and 171 controls (41 cases and 113 controls for SCST; 21 cases and 58 controls for GCT) were included in the study. Further data about maternal and child characteristics during index birth were obtained from a linkage with the Finnish Birth Registry (23). Information on invasive breast and ovarian cancers diagnosed among the first degree relatives of the study subjects was obtained through separate linkages with the Population and Cancer Registries.
Laboratory analyses
Hormonal analyses were performed at the Clinical Chemistry Laboratory of Umeå University Hospital, Umeå, Sweden. The technicians performing the assays were unaware of the case, control, or quality control status of the specimens. Serum specimens of individually matched case and control subjects were always included in the same laboratory run. In addition to routine laboratory quality controls, a pool of serum from the cohort was created at the beginning of the study and 2 aliquots, undistinguishable from the test samples were inserted in each laboratory run. Sex steroids were quantified by the Liquid Chromatography Tandem Mass Spectrometry on an Applied Biosystems API4000 triple stage quadrupole mass spectrometer, which has demonstrated good laboratory performances as supported by other studies (24–27). Laboratory quality controls most closely corresponding to the levels observed in the population: 0.1 ng/mL for testosterone, 5.0 ng/mL for androstenedione, 5.0 ng/mL for 17-OH-progesterone, 75.0 ng/mL for progesterone, and 5.0 ng/mL for estradiol, showed inter-run coefficients of variation (CV) of 14.6%, 5.3%, 5.0%, 5.0% and 8.3%, respectively. Inter- and intra-run CV based on the blinded pool of quality controls were 3.6% and 7.8% for testosterone, 4.0% and 8.3% for androstenedione, 5.4% and 8.1% for 17-OH-progesterone, 3.5% and 7.0% for progesterone, and 5.5% and 6.3% for estradiol. SHBG was quantified with solid-phase competitive chemiluminescence assays on Immulite 2000 Siemens analyzer. The inter-run CV based on laboratory quality controls with concentrations of 41 nmol/L was 1.5%. Due to low sample volume, SHBG measurements were possible only 25 SCST cases and 43 matched controls, and 13 GCT cases and 27 matched controls. Bioavailable fractions of testosterone and estradiol concentrations were calculated by mass action models based on concentrations of total hormones in blood and their affinity constants for albumin and SHBG (28). No outliers, defined as concentrations exceeding 3 times the interquartile range, for any of the hormones were identified.
Statistical analysis
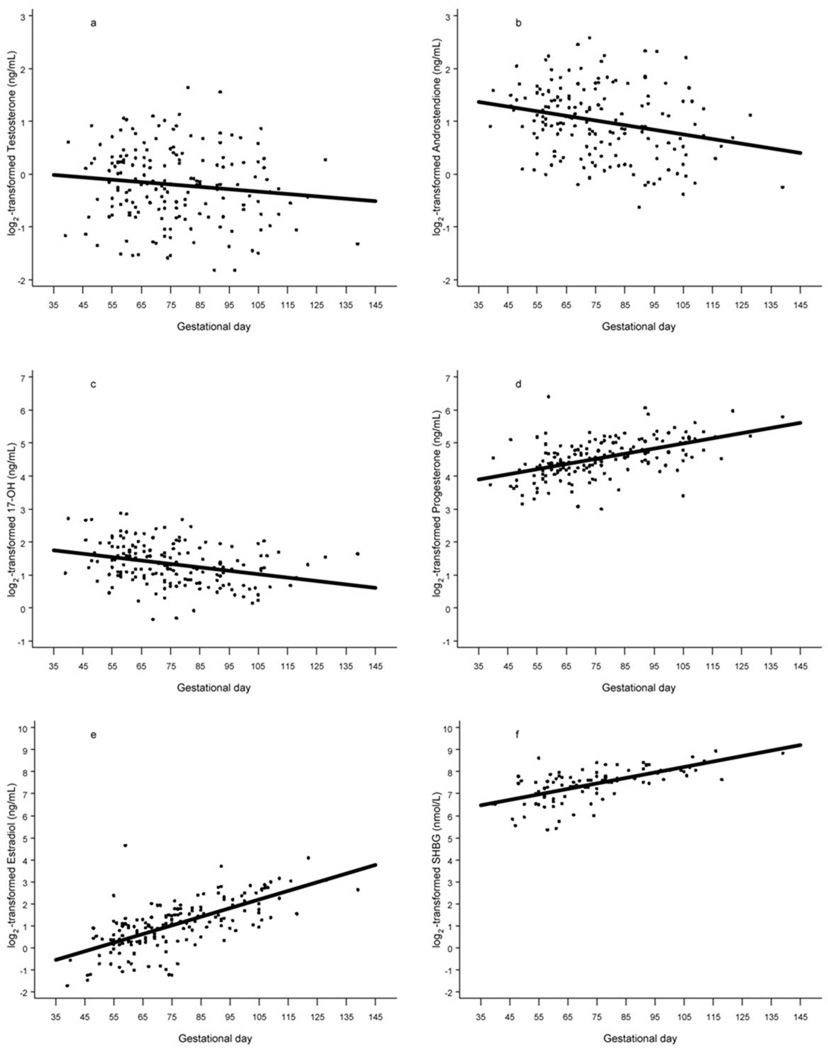
Prior to analysis, all original hormone values were log2-transformed to normalize their distributions. Hormone concentrations varied linearly with gestational age (Figure 1) and all statistical analyses were adjusted for gestational age.
Figure 1.
Scatterplot of log2-scale testosterone (a), androstenedione (b), 17-OH-progesterone(c), progesterone (d), estradiol (e), and sex hormone binding globulin (f) concentrations (controls combined) by gestational age. The solid line shows the progression of hormone concentrations during pregnancy, estimated by linear regression.
Pearson partial correlation coefficients were used to relate hormone concentrations to specific characteristics of interest [e.g., maternal age at sampling (last birth)]. Mixed effect models were used to compare mean hormone concentrations in cases and controls. The conditional logistic regression models (appropriate for the individually matched design) were used to compare differences between cases and controls and to calculate odds ratios (OR) and corresponding 95% confidence intervals (CI) for SCST and GCT. For the hormonal variables, ORs were calculated for a unit change of log2-transformed concentrations, which corresponds to a doubling of the concentrations. To ensure that study results were not influenced by the presence of yet undiagnosed, but hormonally active tumor, sensitivity analyses excluding women diagnosed within 2, 4 and 6 years after blood donation were conducted. For subjects with SHBG measurements, bioavailable fractions of testosterone and estradiol were also related to risk. Further subgroup analyses by ages at sampling (< 30 vs. ≥ 30 years) and cancer diagnosis (below and above 40 years), limited to multiparous women, to those who donated an eligible sample during the last pregnancy preceding index date, or with borderline tumors for SCST were also performed. Tests of homogeneity between the odds ratios in different subgroups were based on Chi-square statistics, calculated as the deviations of logistic regression coefficients observed in each of the subgroups, relative to the overall regression coefficient (29). The effect of potential confounders (e.g. maternal age at first birth, family history of breast/ovarian cancers, maternal smoking, the child’s sex, birth length and weight) was evaluated and variables that changed point estimates by more than 5% for two or more hormones were retained in the final models. The effect of adjustment for hormone concentrations included adjustment of testosterone models for androstenedione (and vice versa) and adjustment of androgen and progesterone models for 17-OH-progesterone (and vice versa) was also explored. All tests of statistical significance were two-sided and the ORs were considered significant if the p values were < 0.05.
The study was approved by the ethical committees of the National Institute for Health and Welfare, Finland, University of Umea, Sweden, and German Cancer Research Center, Germany.
Results
SCST and GCT cases and their respective control subjects were comparable in most pregnancy, maternal and child characteristics (Table 1). The median age at diagnosis was 39 years (range: 23.1–50.0) for SCST and 35 years (range: 21.1–51.4) for GCT. The majority of SCST were borderline tumors (33 cases, 80%), whereas most of GCT were invasive cancer (18 cases, 86%). The vast majority of SCST (85%, 35 cases) were granulosa cell tumors. The lag-time between blood donation and diagnosis was shorter for GCT than for SCST (4.1 vs. 6.8 years, respectively).
Table 1.
Distribution of characteristics of non-epithelial ovarian cancer cases and their matched controls, median (min, max) or n (percentage) from the Finnish Maternity Cohort, 1983–2007*
| Characteristics | Sex cord-stromal tumors (SCST) | Germ cell tumors (GCT) | ||||
|---|---|---|---|---|---|---|
| Cases (41) | Controls (113) | p- value |
Cases (21) | Controls (59) | p- value |
|
| Age at index (last) birth (years)# |
30.8 (22.2–40.0) | 30.9 (21.7–40.9) | - | 29.7 (18.4–35.9) | 29.5 (18.0–36.1) | - |
| Parity at index pregnancy | ||||||
| 1 | 6 (15%) | 18 (16%) | - | 6 (29%) | 16 (27%) | - |
| 2 | 17 (41%) | 49 (43%) | 8 (38%) | 28 (47%) | ||
| 3+ | 18 (44%) | 46 (41%) | 7 (33%) | 15 (26%) | ||
| Age at first birth (years) | 25.8 (18.6–40.5) | 25.3 (16.4–41.4) | 0.21 | 25.8 (19.0–32.5) | 25.3 (17.9–33.3) | 0.58 |
| Gestational age (weeks) | 11 (7–16) | 11 (6–20) | 0.94 | 10 (7–15) | 10 (6–18) | 0.92 |
| Age at diagnosis (years) | 39.0 (23.1–50.0) | - | - | 34.8 (21.1–51.4) | - | - |
| Lag time (years) | 6.8 (0.1–19.4) | - | - | 4.1 (0.1–16.2) | - | - |
| Cancer type | ||||||
| Invasive | 8 (20%) | - | 18 (86%) | - | ||
| Borderline | 33 (80%) | - | 3 (14%) | - | ||
| Family history of ovary cancer | 0 (0%) | 1 (1%) | 0.99 | 0 (0%) | 1 (2%) | 0.99 |
| Family history of breast cancer | 1 (2%) | 1 (1%) | 0.71 | 1 (5%) | 4 (7%) | 0.59 |
| Smoking (yes) | 4 (10%) | 17 (16%) | 0.38 | 3 (15%) | 11 (20%) | 0.69 |
| Child sex | ||||||
| Male | 18 (45%) | 62 (56%) | 0.27 | 9 (45%) | 31 (55%) | 0.46 |
| Female | 22 (55%) | 48 (44%) | 11 (55%) | 25 (45%) | ||
| Child birth weight (g) | 3,535 (2,080–4,650) | 3,560 (1,900–4,670) | 0.29 | 3,775 (2,470–4,400) | 3,490 (2,025–5,000) | 0.21 |
| Child birth length (cm) | 50 (42–55) | 50 (43–57) | 0.39 | 51 (46–55) | 50 (44–55) | 0.26 |
| Hormones** | ||||||
| testosterone (ng/mL) | 1.09 (0.95–1.24) | 0.86 (0.79–0.93) | 0.01 | 0.91 (0.75–1.10) | 0.90 (0.80–1.01) | 0.92 |
| androstenedione (ng/mL) | 2.36 (2.08–2.67) | 1.96 (1.81–2.11) | 0.03 | 2.18 (1.86–2.55) | 2.09 (1.90–2.30) | 0.72 |
| 17-OH-progesterone (ng/mL) | 2.86 (2.55–3.21) | 2.42 (2.23–2.62) | 0.03 | 2.47 (2.13–2.87) | 2.67 (2.44–2.92) | 0.45 |
| progesterone (ng/mL) | 22.2 (20.4–24.2) | 23.6 (22.4–25.0) | 0.29 | 22.9 (20.3–25.9) | 22.6 (20.9–24.3) | 0.85 |
| estradiol (ng/mL) | 2.05 (1.79–2.34) | 2.00 (1.84–2.18) | 0.81 | 1.91(1.53–2.40) | 2.23 (1.95–2.56) | 0.33 |
Conditional logistic regression models were used to compare differences between cases and controls; mixed effect models were used to compare mean hormone concentrations in cases and controls;
Geometric means and (10th, 90th) percentile of hormone concentrations (adjustment for gestational age and maternal age);
Samples not from the last pregnancy preceding cancer diagnosis (3 SCST controls, 1 GCT case and 6 GCT controls).
Correlations of sex steroids hormones with each other and with gestational age and maternal age at sampling in the all control subjects (N=171) are presented in Table 2. The strongest correlations were observed between the two androgens (r=0.87) which were also directly correlated with 17-OH-progesterone (r=0.54 and 0.63 for testosterone and androstenedione, respectively). Progesterone was weakly positively correlated with androgens (r=0.21 and 0.26 for testosterone and androstenedione, respectively) and moderately with 17-OH-progesterone (r=0.50). Interestingly, in the case group of women with SCST weak inverse correlations of progesterone with androgens were observed (r=−0.18 and r=−0.10) and the correlation with 17-OH-progesterone was less pronounced (r=0.20). Estradiol was moderately positively correlated with other sex steroids in the controls (correlation coefficients ranging from 0.29 to 0.55), but in the SCST cases, only very weak correlations of estradiol with androgens and 17-OH-progesterone were observed (ranging from – 0.06 to 0.13). There was no correlation of maternal age with any of the studied hormones among controls.
Table 2.
Pearson partial correlations between hormones, maternal age at index pregnancy, and gestational day in all control subjects (n=171) from the Finnish Maternity Cohort, 1983–2007*
| Hormones | testosterone | androstenedione | 17-OH-progesterone | progesterone | estradiol | SHBG** |
|---|---|---|---|---|---|---|
| testosterone (ng/mL) | 0.38 (P=0.002) | |||||
| androstenedione (ng/mL) | 0.87 (p<0.0001) | 0.26 (P=0.01) | ||||
| 17-OH-progesterone (ng/mL) | 0.54 (p<0.0001) | 0.63 (p<0.0001) | 0.12 (P=0.26) | |||
| progesterone (ng/mL) | 0.21 (p=0.01) | 0.26 (p=0.001) | 0.50 (p<0.0001) | 0.37 (P=0.0002) | ||
| estradiol (ng/mL) | 0.55 (p<0.0001) | 0.49 (p<0.0001) | 0.29 (p=0.0001) | 0.50 (p<0.0001) | 0.67 (p<0.0001) | |
| maternal age at sampling (years) | 0.05 (p=0.56) | −0.02 (p=0.76) | 0.03 (p=0.66) | 0.06 (p=0.45) | −0.003 (p=0.97) | −0.002 (p=0.98) |
| gestational age (days) | −0.12 (p=0.12) | −0.25 (p=0.001) | −0.32 (p<0.0001) | 0.51 (p<0.0001) | 0.67 (p<0.0001) | 0.63 (p<0.0001) |
All correlations (except those with gestational day) were adjusted for gestational age;
data available for 95 subjects only.
Testosterone, androstenedione and 17-OH-progesterone concentrations were significantly higher in SCST cases than in their controls, but no other significant differences in hormone levels between SCST or GCT cases and their controls were observed (Table 1). SHBG concentrations were marginally higher in SCST cases than in their controls (189 vs. 162 nmol/L, p = 0.08, with only 25 cases and 43 controls). Correspondingly, in conditional regression analyses (Table 3), doubling of androgen and 17-OH-progesterone concentrations were associated with about 2-fold increase in risk of SCST. Adjustment for maternal age at first birth, family history of breast/ovarian cancers, maternal smoking, and child sex increased risk estimates, with maternal age at first birth having the greatest impact (about 9% increase of androgen and 5% increase for 17-OH-progesterone estimates). The associations remained largely unchanged after excluding women with 2, 4 or 6 years lag-time between blood donation and cancer diagnosis (Table 3). Doubling of SHBG, bioavailable fractions of testosterone and estradiol were not statistically significantly associated with risk of SCST [OR and 95% CI of 1.88 (0.70–5.04), 1.18 (0.52–2.64), and 0.97 (0.31– 3.02), respectively].
Table 3.
ORs and 95% CIs for ovarian sex cord-stromal tumors (SCST) associated with doubling of hormone concentrations from the Finnish Maternity Cohort, 1983–2007*
| testosterone | androstenedione | 17-OH-progesterone | progesterone | estradiol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| SCST(41/113) | 1.90 (1.13–3.21) | 0.02 | 1.76 (1.03–3.03) | 0.04 | 2.07 (1.07–4.02) | 0.03 | 0.68 (0.31–1.48) | 0.33 | 1.07 (0.64–1.78 | 0.80 |
| Multivariate model* (41/113) | 2.16 (1.25–3.74) | 0.006 | 2.16 (1.20–3.87) | 0.01 | 2.62 (1.27–5.38) | 0.01 | 0.67 (0.29–1.53) | 0.34 | 1.04 (0.62–1.77) | 0.88 |
| Sensitivity analyses | ||||||||||
| Lag time ≥ 2 years (34/96) | 2.03 (1.13–3.64) | 0.02 | 1.85 (0.98–3.48) | 0.06 | 1.96 (0.90–4.28) | 0.09 | 0.58 (0.22–1.54) | 0.28 | 1.11 (0.62–1.99) | 0.72 |
| Lag time ≥ 4 years (31/88) | 2.30 (1.17–4.49) | 0.02 | 1.99 (0.98–4.08) | 0.06 | 2.53 (1.04–6.14) | 0.04 | 0.68 (0.25–1.86) | 0.45 | 1.01 (0.55–1.86) | 0.97 |
| Lag time ≥ 6 years (23/65) | 3.41 (1.43–8.16) | 0.01 | 2.54 (1.06–6.10) | 0.04 | 1.84 (0.67–5.06) | 0.24 | 0.47(0.14–1.63) | 0.23 | 0.95 (0.46–1.95) | 0.89 |
| Multiparous women (35/95) | 2.28(1.26–4.10) | 0.01 | 2.17 (1.18–4.00) | 0.01 | 2.49 (1.11–5.56) | 0.03 | 0.53 (0.22–1.29) | 0.16 | 0.96 (0.53–1.73) | 0.89 |
| Borderline tumors (33/96) | 2.01 (1.09–3.72) | 0.03 | 2.06 (1.09–3.88) | 0.03 | 2.58 (1.16–5.70) | 0.02 | 0.69 (0.27–1.77) | 0.44 | 1.09 (0.61–1.94) | 0.77 |
| Subgroup analyses | ||||||||||
| Age at sampling | ||||||||||
| < 30 years (15/40) | 3.21 (0.86–11.95) | 0.08 | 2.09 (0.54–8.07) | 0.29 | 4.77 (0.86–26.33) | 0.07 | 0.55 (0.15–1.99) | 0.36 | 0.77(0.31–1.90) | 0.57 |
| ≥30 years (26/73) | 1.80 (0.92–3.52) | 0.09 | 1.89 (0.95–3.76) | 0.07 | 2.01 (0.88–4.57) | 0.10 | 0.68 (0.21–2.28) | 0.54 | 0.98 (0.50–1.95) | 0.96 |
| Age at diagnosis ** | ||||||||||
| < 40 years (24/65) | 2.91 (1.30–6.50) | 0.01 | 3.14 (1.30–7.57) | 0.01 | 7.68 (1.68–35.13) | 0.01 | 0.66 (0.21–2.07) | 0.48 | 1.15 (0.58–2.29) | 0.69 |
| ≥40 years (17/48) | 1.45 (0.64–3.26) | 0.37 | 1.22 (0.51–2.93) | 0.66 | 1.00 (0.35–2.82) | 0.99 | 0.48 (0.11–2.07) | 0.33 | 0.88 (0.35–2.24) | 0.79 |
Adjustments for gestational age, maternal age at first birth, family history of breast/ovarian cancers, maternal smoking, and child sex;
The heterogeneity tests reached statistical significance only for 17-OH-progesterone (p=0.03) by age at diagnosis, <40 vs. ≥ 40 years.
Subgroup analyses by ages at sampling and diagnosis indicated somewhat stronger associations in women who were diagnosed before age 40 than after that age, but the heterogeneity tests reached statistical significance only for the effect of doubling of 17-OH-progesterone. In spite of statistical significance, however, these results should be interpreted with some caution as the heterogeneity tests were based on small numbers of subjects and were sensitive to selection of cut-off points. Analyses limited to multiparous women (35 cases) or borderline tumors (33 cases, 80%) yielded similar results to those reported overall. Adjustment of testosterone models for androstenedione did not alter the magnitude of the risk estimates, while adjustment of androstenedione models for testosterone completely abolished the association of androstenedione with risk (data not shown). Adjustment of androgen models for 17-OH-progesterone or of 17-OH-progesterone models for androgens resulted in substantial reduction of risk estimates (data not shown).
Sex steroids showed were not associated with maternal risk of GCT (Table 4). SHBG concentrations were associated with higher risk (not statistically significant), however only 13 case-control sets were included in this analysis, and the CI were large [OR and 95% CI of 2.01 (0.39–10.27)]. Analyses excluding case-control sets with lag-time to diagnosis of 2, 4, and 6 years and analyses limited to multiparous women (15 cases) or invasive tumors (18 cases, 86%) were similarly unremarkable.
Table 4.
ORs and 95% CIs for ovarian germ cell tumors (GCT) associated with doubling of hormone concentrations from the Finnish Maternity Cohort, 1983–2007*
| testosterone | androstenedione | 17-OH-progesterone | progesterone | estradiol | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| GCT (21/58) | 1.05 (0.54–2.08) | 0.88 | 1.19 (0.54–2.65) | 0.66 | 0.67 (0.27–1.67) | 0.39 | 1.13 (0.42–2.99) | 0.81 | 0.75 (0.42–1.36) | 0.35 |
| Multivariate model (21/58) | 1.04 (0.50–2.17) | 0.92 | 1.24 (0.53–2.91) | 0.62 | 0.66 (0.26–1.66) | 0.37 | 1.04 (0.38–2.84) | 0.94 | 0.68 (0.35–1.33) | 0.26 |
| Sensitivity analyses | ||||||||||
| Lag time ≥ 2 years (16/45) | 0.82 (0.34–1.96) | 0.66 | 1.05 (0.37–2.94) | 0.93 | 0.59 (0.21–1.67) | 0.32 | 1.08 (0.27–4.24) | 0.91 | 0.73 (0.31–1.74) | 0.48 |
| Lag time ≥ 4 years (11/31) | 0.60 (0.15–2.44) | 0.47 | 0.66 (0.13–3.29) | 0.62 | 0.45 (0.09–2.17) | 0.32 | 0.86 (0.10–7.32) | 0.89 | 0.59 (0.15–2.30) | 0.45 |
| Lag time ≥ 6 years (9/26) | 0.56 (0.13–2.36) | 0.43 | 0.59 (0.11–3.06) | 0.53 | 0.36 (0.06–2.09) | 0.25 | 0.89 (0.11–7.56) | 0.92 | 0.57 (0.13–2.52) | 0.42 |
| Multiparous women (15/42) | 0.94 (0.41–2.15) | 0.88 | 1.23 (0.47–3.22) | 0.67 | 0.69 (0.21–2.23) | 0.53 | 1.71 (0.36–8.20) | 0.50 | 0.61(0.25–1.51) | 0.29 |
| Invasive tumors (18/50) | 1.07 (0.49–2.35) | 0.87 | 1.21 (0.50–2.93) | 0.68 | 0.77 (0.30–2.01) | 0.59 | 1.00 (0.34–2.92) | 0.99 | 0.65 (0.32–1.32) | 0.23 |
None of the heterogeneity tests reached statistical significance and adjustment for gestational age, maternal age at first birth, family history of breast/ovarian cancers, maternal smoking, and child sex.
Discussion
This is the first prospective study to provide direct epidemiological evidence that elevated blood levels of sex steroid hormones during pregnancy, androgens in particular, may be related to an increased risk of developing SCST. Doubling of testosterone, androstenedione and 17-OH-progesterone (the precursor hormone for androgen synthesis) during the early part of the last pregnancy leading to child birth and preceding cancer diagnosis was associated with an approximately 2-fold greater risk of SCST. In contrast, none of the studied steroid hormones showed any association with risk of GCT.
Changes of sex steroids throughout pregnancy are summarized as below. Concentrations of testosterone are relatively stable throughout pregnancy, as demonstrated by longitudinal studies (30;31). One longitudinal study (31) shows that total testosterone levels remain relatively stable till the third trimester and increase 62% from 6th to 34th week of gestation, and the other longitudinal study (30) reports that total testosterone increases approximately 29% from 5th to 40th week of gestation. The free testosterone levels are within the range of non-pregnant women and gradually increase during the latter parts of pregnancy (31;32). Androstenedione levels only slightly increase throughout pregnancy in the longitudinal studies (30;31;33). 17-OH-progesterone levels reach their peak during the 5–6th week of pregnancy and then gradually decrease thereafter, but increase again during the latter parts of pregnancy (30;34). There are small variations in progesterone levels, similar to pre-pregnancy state (luteal phase of the menstrual cycle), from 3rd to 13th week of gestation and, progesterone levels increase significantly thereafter (30;34;35). Estradiol levels rise steadily from 3rd to 13th week of gestation (by 8th week of gestation its levels are above the range found during menstrual cycle) and then gradually increase thereafter (34–37). SHBG levels increase rapidly during the first half of pregnancy (till 25th week of gestation), then remained relatively constant until delivery (30;31). All studied hormones levels at 1 year postpartum drop down to below the first trimester values, similar to the pre-pregnancy state (31;33).
The 2-fold higher risk of SCST associated with elevated pre-diagnostic androgen concentrations is consistent with results from animal studies. Experiments in rodents have demonstrated that androgens may be involved in ovarian cancer induction, though a long induction period may be required (38–42). In particular, testosterone and dehydroepiandrosterone strongly promote carcinogenesis of spontaneous ovarian granulosa cell tumors (the predominant histological subtype in this study) in SWXJ-9 strain mice (38) and continuous administration with testosterone shortly after birth induced theca-cell ovarian tumors (also belonging to SCST) in the rat (39). Therefore, it is plausible that androgens are involved in the pathogenesis of SCST in humans.
The elevations in androgens and 17-OH-progesterone in the SCST case subjects are likely to be of maternal origin. Although the placenta produces androstenedione and testosterone from fetal precursors, these are rapidly converted to estrogens due to its potent aromatase capacity (43). 17-OH-progesterone is considered to reflect activities of corpus luteum, indicating ovarian steroidogenesis, particularly during the first trimester (36). Nevertheless, maternal 17-O-Hprogesterone during the latter parts of pregnancy could be derived from maternal and fetal adrenal glands as well as from the placenta sources (44), as the placenta does not secrete 17-OH-progesterone until 32 week of pregnancy (43;45) due to the lack of 17α-hydrolase activity. By contrast, the concentrations of progesterone and estradiol, which are predominantly of placental origin in the phase of pregnancy during which study samples were collected (45), were similar in case and control subjects and were not related to risk of SCST. The divergent correlations of androgens and 17-OH-progesterone with estradiol and progesterone concentrations in the SCST cases and in the control group further underscores the possibility of differences in sex steroid hormones metabolism between cases and controls.
Androgens measured during early pregnancy are likely to reflect pre-pregnancy exposure to the hormone because concentrations of androgens (testosterone and androstenedione) during early pregnancy and in the non-pregnant state are relatively similar (30;31). It is plausible to assume that women from general population, having normal pre-pregnancy androgens, continue to have normal androgens during early pregnancy. Similarly, this assumption is likely generalizable to women with polycystic ovary syndrome (PCOS) as evidence (37;43) has demonstrated that pregnant women with PCOS, having elevated pre-pregnancy androgens, continue to maintain higher androgens also during pregnancy, compared to normal pregnant women with normal androgen levels. Therefore, the observed increase in risk of SCST associated with doubling of testosterone in our study may be applicable also to the effect of elevated androgens / altered maternal androgen metabolism in the non-pregnant state.
The major strength of our study is its prospective design, which largely avoids selection and inverse causation biases which plague studies of a classical case-control design. Case and control subjects were tightly matched for age at sampling (last birth), parity at the index pregnancy, and date of sampling, allowing careful control for these important sources of potential confounding. SCST develop from the theca and granulosa cells, which are the major site of steroid hormones synthesis in the ovary (4;46), and these tumors are often hormonally active (5;47). To reduce the possibility that the observed associations with risk of SCST were influenced by hormonal secretion by a growing, but still undiagnosed tumour we limited the analyses to women whose samples were donated 2, 4 and 6 years prior to their cancer diagnosis. For testosterone, the more potent androgen, risk estimates remained strong and statistically significant, while those for androstenedione were slightly reduced and became of borderline significance. Thus, it is unlikely that the observed associations in our study were due to reverse causation bias.
The very large overall size of Finnish Maternity Cohort (N ≈ 850,000) made it possible to study the hormonal determinants of non-epithelial ovarian tumors, which are rare forms of cancer, although the very small numbers of cases, particularly for GCT, was insufficient to detect moderate or weak hormone-risk associations. A limitation of our study is that no data on maternal pre-pregnancy hyperadrogenism / testosterone concentrations were available and the lack of sufficient sample for analyses of SHBG in all subjects. Study samples had been stored at relatively high temperature (−25°C), but we observed no correlations of hormone levels with time in storage and their concentrations varied with gestational age as expected, as also shown previously (48).
It is not feasible in the study design to select controls matched to cases on gestational age, even in much larger cohorts and will pose unreasonable, severe restrains on control selection. Therefore, the only alternative is to adjust for gestational age in the phase of data analysis to minimize the intra-person variability in sex steroids levels during pregnancy. One approach is adjusted for a linear term of gestational age, and the other is calculating residual values by the local linear regression (49). We adopt the former approach in the manuscript due to the following four reasons. Firstly, concentrations of all studied hormone varied linearly with gestational age (Figure 1). Secondly, the same approach has been extensively used in various epidemiological studies (50–53). Thirdly, in fact the former approach provides more straightforward interpretation in comparison with the presentation of residual values by the local linear regression, as the latter approach is required to calculate the difference (residual) between the assay value and the estimated mean hormone value determined for the day of gestation on which the blood sample was donated. Lastly, results obtained by both approaches give almost identical results. It is also possible that hormones levels adjusted for gestational age could be subject to measurement error. However the intra-person variability in biomarker levels from a single measurement is unlikely to be differential with respect to cases and controls and would most likely lead to an underestimation of the effect estimates, as supported by other studies (54–57).
Another relative limitation of the study might be that only a single blood sample was obtained for each pregnant woman. A single measurement for endogenous sex steroids in premenopausal women is suggested to reliably estimate average levels for serum androgen and SHBG over a 1–3 year period (58;59;55;60;61). To our knowledge, so far no study on the reproducibility of a single measurement for endogenous sex steroids in pregnant women has been reported and little is known about the intra-person variability in sex steroids during pregnancy. Although a lot of work has been done in relation to Down syndrome markers, so far only 3 studies (62–64), after exhausting literature searches, on the correlations between sex steroids levels during different period of the same pregnancy have been identified. Nevertheless, it is plausible to conclude that sex steroids hormones track during pregnancy as there are moderate-to-strong correlations (more than 0.40) between concentrations of sex steroids during the different period (62–64) of the same pregnancy. Firstly, correlations between the first and second trimester in both Caucasian women and Chinese women were strong for testosterone (0.83 for Boston and 0.62 for Shanghai), SHBG (0.85 for Boston and 0.67 for Shanghai), and estradiol (0.72 for Boston and 0.61 for Shanghai), and modest for progesterone (0.45 for Boston and 0.39 for Shanghai) (62). Secondly, correlations between the first and third trimester in white women were 0.53 for testosterone, 0.23 for SHBG, and 0.25 for estradiol; while in black women were more higher, 0.67 for testosterone, 0.43 for SHBG, and 0.35 for estradiol (63). Thirdly, correlations between the second and third trimester were 0.78 for estradiol and 0.60 for total estrogen (64). In addition, strong correlations during successive pregnancies of the same women for estrogen have been reported, e.g., pregnancy estradiol, measured between 8th and 17th of gestational weeks, were strongly correlated in successive pregnancies of the same women (0.78 for total estradiol and 0.73 for free estradiol) (65). It is plausible to assume that the correlations of hormone levels measured within the same pregnancy is most likely higher than those correlations of hormone levels measured in successive pregnancies.
Taken together, a single measurement for androgens (testosterone in particular) during early pregnancy can reliably represent cumulative exposure to the hormone as strong correlations during the different period of the same pregnancy for testosterone have been reported, e.g., correlations between the first and second trimester were 0.83 for Caucasian women in Boston and 0.62 for Chinese women in Shanghai (62) and, correlations between the first and third trimester were 0.67 in black women and 0.53 in white women (63). In addition, concentrations of androgens are relatively stable throughout pregnancy (30) (31) (33) and, androgens levels during early pregnancy and in the non-pregnant state are also relatively similar (30;31). The samples of the Finnish Maternity Cohort were donated around the first trimester—between the 6th and 21th weeks of gestation, which is likely to be the most relevant period for the exposure to early pregnancy androgens (testosterone in particular), also reflecting pre-pregnancy exposure to the hormone.
A completion of the first full-term pregnancy may induce substantial alternations in a women’s hormonal milieu as most studies have shown that in pregnant state, parous women have lower concentrations of a number of hormones, including estrogens and androgens (64;66–69), compared with nulliparous women. Effects of pregnancy on hormone levels may sustain in the non-pregnant state, e.g., before and after menopause. Although, some studies report that nulliparous women had similar (higher trend, but not statistically significant) androgens (testosterone and androstenedione) levels, in comparison to parous women, both in the pre-menopausal (70;71) and post-menopausal women (72–74). Most studies support of reductions in concentrations of sex steroids after the first full-term pregnancy both in the pre-menopausal women for androgens (dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS)) (75), progesterone (71), and estrogens (free estradiol) (76) and, also in the post-menopausal women for androgens (testosterone) (77), and estrogens (free estradiol (78) and estrone sulfate (79)).
Therefore, the effect of pregnancy on maternal risk of NEOC is most likely to be the result of the cumulative long-term exposure of androgens (testosterone in particular) throughout a woman’ life-cycle till cancer diagnosis, by promoting carcinogenesis of spontaneous ovarian granulosa cell tumors or inducing theca-cell ovarian tumors, as animal studies (38–42) has demonstrated that androgens may be involved in ovarian cancer induction after a long induction period.
In summary, this study is the first prospective epidemiological investigation providing initial direct evidence that elevated androgens, and testosterone in particular, play an important role in the pathogenesis of SCST long before the clinical onset of the disease. Our data suggests that SCST and GCT have distinct hormonal risk profiles, possibly reflecting differences in their pathogenesis. Our study may note a particular need for larger confirmatory investigations on sex steroids and NEOC.
Acknowledgements
The authors are indebted to Yelena Afanasyeva, Pirjo Kontiokari, Annika Uimonen, and Sara Kuusiniemi for their excellent technical assistance in the conduct of the study.
Funding
This research was supported by the National Cancer Institute at the National Institutes of Health (R01-CA120061).
Footnotes
Conflict of interest
None declared by any of the co-authors.
References
- 1.Horn-Ross PL, Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 U.S. case-control studies. VI. Nonepithelial cancers among adults. Collaborative Ovarian Cancer Group. Epidemiology. 1992;3:490–495. doi: 10.1097/00001648-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev. 2008;34:427–441. doi: 10.1016/j.ctrv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Guillem V, Poveda A. Germ cell tumours of the ovary. Clin Transl Oncol. 2007;9:237–243. doi: 10.1007/s12094-007-0045-0. [DOI] [PubMed] [Google Scholar]
- 4.Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34:1–12. doi: 10.1016/j.ctrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee-Jones L. Ovary: Sex cord-stromal tumors. Atlas Genet Cytogenet Oncol Haematol. 2003 November [Google Scholar]
- 7.Lee-Jones L. Ovary: Germ cell tumors. Atlas Genet Cytogenet Oncol Haematol. 2003 August [Google Scholar]
- 8.Koulouris CR, Penson RT. Ovarian stromal and germ cell tumors. Semin Oncol. 2009;36:126–136. doi: 10.1053/j.seminoncol.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Dallenbach P, Bonnefoi H, Pelte MF, Vlastos G. Yolk sac tumours of the ovary: an update. Eur J Surg Oncol. 2006;32:1063–1075. doi: 10.1016/j.ejso.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Adami HO, Hsieh CC, Lambe M, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344:1250–1254. doi: 10.1016/s0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 11.Albrektsen G, Heuch I, Kvale G. Full-term pregnancies and incidence of ovarian cancer of stromal and germ cell origin: a Norwegian prospective study. Br J Cancer. 1997;75:767–770. doi: 10.1038/bjc.1997.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Zamorano LM, Salazar-Martinez E, De Los Rios PE, Gonzalez-Lira G, Flores-Luna L, Lazcano-Ponce EC. Factors associated with non-epithelial ovarian cancer among Mexican women: a matched case-control study. Int J Gynecol Cancer. 2003;13:756–763. doi: 10.1111/j.1525-1438.2003.13604.x. [DOI] [PubMed] [Google Scholar]
- 13.dos SS, I, Swerdlow AJ. Ovarian germ cell malignancies in England: epidemiological parallels with testicular cancer. Br J Cancer. 1991;63:814–818. doi: 10.1038/bjc.1991.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce EA, Costaggini I, Vitonis A, et al. The epidemiology of ovarian granulosa cell tumors: a case-control study. Gynecol Oncol. 2009;115:221–225. doi: 10.1016/j.ygyno.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 15.The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med. 1987;316:650–655. doi: 10.1056/NEJM198703123161102. [DOI] [PubMed] [Google Scholar]
- 16.Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 17.Walker AH, Ross RK, Haile RW, Henderson BE. Hormonal factors and risk of ovarian germ cell cancer in young women. Br J Cancer. 1988;57:418–422. doi: 10.1038/bjc.1988.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 19.Hirsutism . In: Clinical Gynecologic Endocrinology and Infertility. 5th ed. Speroff L, Glass RH, Kase NG, editors. Baltimore, Maryland: Williams. & Wilkins; 1994. pp. 483–513. [Google Scholar]
- 20.Koskela P, Anttila T, Bjorge T, Brunsvig A, Dillner J, Hakama M, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer. 2000;85:35–39. doi: 10.1002/(sici)1097-0215(20000101)85:1<35::aid-ijc6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Lehtinen M, Koskela P, Ogmundsdottir HM, et al. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. Am J Epidemiol. 2003;158:207–213. doi: 10.1093/aje/kwg137. [DOI] [PubMed] [Google Scholar]
- 22.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33:365–369. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 23.Gissler M, Teperi J, Hemminki E, Merilainen J. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75–80. doi: 10.1177/140349489502300113. [DOI] [PubMed] [Google Scholar]
- 24.Schlusener MP, Bester K. Determination of steroid hormones, hormone conjugates and macrolide antibiotics in influents and effluents of sewage treatment plants utilising high-performance liquid chromatography/tandem mass spectrometry with electrospray and atmospheric pressure chemical ionisation. Rapid Commun Mass Spectrom. 2005;19:3269–3278. doi: 10.1002/rcm.2189. [DOI] [PubMed] [Google Scholar]
- 25.Lopez de Alda MJ, az-Cruz S, Petrovic M, Barcelo D. Liquid chromatography-(tandem) mass spectrometry of selected emerging pollutants (steroid sex hormones, drugs and alkylphenolic surfactants) in the aquatic environment. J Chromatogr A. 2003;1000:503–526. doi: 10.1016/s0021-9673(03)00509-0. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann M, Harwood T, Gaston-Parry O. A new quantitative LC tandem mass spectrometry assay for serum 25-hydroxy vitamin D. Steroids. 2010;75:1106–1112. doi: 10.1016/j.steroids.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Kunisue T, Fisher JW, Fatuyi B, Kannan K. A method for the analysis of six thyroid hormones in thyroid gland by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1725–1730. doi: 10.1016/j.jchromb.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem. 1991;37:667–672. [PubMed] [Google Scholar]
- 31.Kerlan V, Nahoul K, Le Martelot MT, Bercovici JP. Longitudinal study of maternal plasma bioavailable testosterone and androstanediol glucuronide levels during pregnancy. Clin Endocrinol (Oxf) 1994;40:263–267. doi: 10.1111/j.1365-2265.1994.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 32.Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293–298. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- 33.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 35.Tulchinsky D, Hobel CJ. Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17 alpha-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. Am J Obstet Gynecol. 1973;117:884–893. doi: 10.1016/0002-9378(73)90057-4. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimi T, Strott CA, Marshall JR, Lipsett MB. Corpus luteum function in early pregnancy. J Clin Endocrinol Metab. 1969;29:225–230. doi: 10.1210/jcem-29-2-225. [DOI] [PubMed] [Google Scholar]
- 37.Lobo RA. Endocrinology of pregnancy. In: Lobo RA, Mishell DR Jr, Paulson RJ, Shoupe D, editors. Infertility, contraception, and reproductive endocrinology. 4th ed. New York: Blackwell Science, Inc.; 1997. pp. 183–207. [Google Scholar]
- 38.Beamer WG, Shultz KL, Tennent BJ. Induction of ovarian granulosa cell tumors in SWXJ-9 mice with dehydroepiandrosterone. Cancer Res. 1998;48:2788–2792. [PubMed] [Google Scholar]
- 39.Horning ES. Carcinogenic action of androgens. Br J Cancer. 1958;12:414–418. doi: 10.1038/bjc.1958.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capen CC. Mechanisms of hormone-mediated carcinogenesis of the ovary. Toxicol Pathol. 2004;32 Suppl 2:1–5. doi: 10.1080/01926230490462075. [DOI] [PubMed] [Google Scholar]
- 41.Capen CC, Beamer WG, Tennent BJ, Stitzel KA. Mechanisms of hormone-mediated carcinogenesis of the ovary in mice. Mutat Res. 1995;333:143–151. doi: 10.1016/0027-5107(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 42.Rainey WE, Sawetawan C, McCarthy JL, et al. Human ovarian tumor cells: a potential model for thecal cell steroidogenesis. J Clin Endocrinol Metab. 1996;81:257–263. doi: 10.1210/jcem.81.1.8550761. [DOI] [PubMed] [Google Scholar]
- 43.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 44.Tulchinsky D, Simmer HH. Sources of plasma 17alpha-hydroxyprogesterone in human pregnancy. J Clin Endocrinol Metab. 1972;35:799–808. doi: 10.1210/jcem-35-6-799. [DOI] [PubMed] [Google Scholar]
- 45.Speroff L, Fritz M, editors. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams. & Wilkins; 2005. The endocrinology of pregnancy; pp. 259–318. [Google Scholar]
- 46.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 47.Young RH, Scully RE. Endocrine tumors of the ovary. Curr Top Pathol. 1992;85:113–164. doi: 10.1007/978-3-642-75941-3_5. [DOI] [PubMed] [Google Scholar]
- 48.Holl K, Lundin E, Kaasila M, et al. Effect of long-term storage on hormone measurements in samples from pregnant women: The experience of the Finnish Maternity Cohort. Acta Oncol. 2008;47:406–412. doi: 10.1080/02841860701592400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleveland WS, Loader C. Smoothing by local regression: Principles and Methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. 1st ed. New York: Springer; 1996. pp. 113–120. [Google Scholar]
- 50.Agborsangaya CB, Surcel HM, Toriola AT, et al. Serum 25-hydroxyvitamin D at pregnancy and risk of breast cancer in a prospective study. Eur J Cancer. 2010;46:467–470. doi: 10.1016/j.ejca.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Toriola AT, Surcel HM, Calypse A, et al. Independent and joint effects of serum 25-hydroxyvitamin D and calcium on ovarian cancer risk: a prospective nested case-control study. Eur J Cancer. 2010;46:2799–2805. doi: 10.1016/j.ejca.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Holl K, Lundin E, Surcel HM, et al. Endogenous steroid hormone levels in early pregnancy and risk of testicular cancer in the offspring: a nested case-referent study. Int J Cancer. 2009;124:2923–2928. doi: 10.1002/ijc.24312. [DOI] [PubMed] [Google Scholar]
- 53.Chen T, Lundin E, Grankvist K, et al. Maternal hormones during early pregnancy: a cross-sectional study. Cancer Causes Control. 2010;21:719–727. doi: 10.1007/s10552-009-9500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136:1400–1413. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 55.Kotsopoulos J, Tworoger SS, Campos H, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–946. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tworoger SS, Hankinson SE. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control. 2006;17:889–899. doi: 10.1007/s10552-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 57.Hankinson SE, Manson JE, London SJ, Willett WC, Speizer FE. Laboratory reproducibility of endogenous hormone levels in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1994;3:51–56. [PubMed] [Google Scholar]
- 58.Muti P, Trevisan M, Micheli A, et al. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1996;5:917–922. [PubMed] [Google Scholar]
- 59.Micheli A, Muti P, Pisani P, et al. Repeated serum and urinary androgen measurements in premenopausal and postmenopausal women. J Clin Epidemiol. 1991;44:1055–1061. doi: 10.1016/0895-4356(91)90007-v. [DOI] [PubMed] [Google Scholar]
- 60.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 61.Lukanova A, Lundin E, Akhmedkhanov A, et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. Int J Cancer. 2003;104:636–642. doi: 10.1002/ijc.10990. [DOI] [PubMed] [Google Scholar]
- 62.Lagiou P, Samoli E, Okulicz W, et al. Maternal and cord blood hormone levels in the United States and China and the intrauterine origin of breast cancer. Ann Oncol. 2010 doi: 10.1093/annonc/mdq565. in press. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Graubard BI, Klebanoff MA, et al. Maternal hormone levels among populations at high and low risk of testicular germ cell cancer. Br J Cancer. 2005;92:1787–1793. doi: 10.1038/sj.bjc.6602545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panagiotopoulou K, Katsouyanni K, Petridou E, Garas Y, Tzonou A, Trichopoulos D. Maternal age, parity, and pregnancy estrogens. Cancer Causes Control. 1990;1:119–124. doi: 10.1007/BF00053162. [DOI] [PubMed] [Google Scholar]
- 65.Bernstein L, Lipworth L, Ross RK. Trichopoulos D. Correlation of estrogen levels between successive pregnancies. Am J Epidemiol. 1995;142:625–628. doi: 10.1093/oxfordjournals.aje.a117685. [DOI] [PubMed] [Google Scholar]
- 66.Allen NE, Roddam AW, Allen DS, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92:1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wuu J, Hellerstein S, Lipworth L, et al. Correlates of pregnancy oestrogen, progesterone and sex hormone-binding globulin in the USA and China. Eur J Cancer Prev. 2002;11:283–293. doi: 10.1097/00008469-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Lof M, Hilakivi-Clarke L, Sandin SS, de AS, Yu W, Weiderpass E. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health. 2009;9:10. doi: 10.1186/1472-6874-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Troisi R, Hoover RN, Thadhani R, et al. Maternal, prenatal and perinatal characteristics and first trimester maternal serum hormone concentrations. Br J Cancer. 2008;99:1161–1164. doi: 10.1038/sj.bjc.6604639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill P, Garbaczewski L, Kasumi K, Wynder EL. Plasma hormones in parous, nulliparous and postmenopausal Japanese women. Cancer Lett. 1986;33:131–136. doi: 10.1016/0304-3835(86)90017-0. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Closas M, Herbstman J, Schiffman M, Glass A, Dorgan JF. Relationship between serum hormone concentrations, reproductive history, alcohol consumption and genetic polymorphisms in pre-menopausal women. Int J Cancer. 2002;102:172–178. doi: 10.1002/ijc.10651. [DOI] [PubMed] [Google Scholar]
- 72.Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal androgen concentrations. J Womens Health (Larchmt) 2005;14:704–712. doi: 10.1089/jwh.2005.14.704. [DOI] [PubMed] [Google Scholar]
- 73.McTiernan A, Wu L, Barnabei VM, et al. Relation of demographic factors, menstrual history, reproduction and medication use to sex hormone levels in postmenopausal women. Breast Cancer Res Treat. 2008;108:217–231. doi: 10.1007/s10549-007-9588-6. [DOI] [PubMed] [Google Scholar]
- 74.Chavez-MacGregor M, Van Gils CH, van der Schouw YT, Monninkhof E, van Noord PA, Peeters PH. Lifetime cumulative number of menstrual cycles and serum sex hormone levels in postmenopausal women. Breast Cancer Res Treat. 2008;108:101–112. doi: 10.1007/s10549-007-9574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musey VC, Collins DC, Brogan DR, et al. Long term effects of a first pregnancy on the hormonal environment: estrogens and androgens. J Clin Endocrinol Metab. 1987;64:111–118. doi: 10.1210/jcem-64-1-111. [DOI] [PubMed] [Google Scholar]
- 76.Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74:741–745. [PubMed] [Google Scholar]
- 77.Lamar CA, Dorgan JF, Longcope C, Stanczyk FZ, Falk RT, Stephenson HE., Jr Serum sex hormones and breast cancer risk factors in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2003;12:380–383. [PubMed] [Google Scholar]
- 78.Chubak J, Tworoger SS, Yasui Y, Ulrich CM CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev. 2004;13:1296–1301. [PubMed] [Google Scholar]
- 79.Hankinson SE, Colditz GA, Hunter DJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses' Health Study (United States) Cancer Causes Control. 1995;6:217–224. doi: 10.1007/BF00051793. [DOI] [PubMed] [Google Scholar]