The recruitment of coat proteins for transport vesicles (COPI-, COPII-, and clathrin-coated) is mediated by the small GTPases of the ADP-ribosylation factor (ARF) family of which there are three SAR, 12 ARF, and six ARL genes in the Arabidopsis thaliana genome (Vernoud et al., 2003). These GTPases are themselves recruited by guanidine exchange factors located at the donor compartments, which convert them into active GTP-bound forms (Anders and Jürgens, 2008). The well-established inhibitor of ARF guanidine exchange factors is the fungal toxin brefeldin A (Robinson et al., 2008). Normally, the ARF-GTPases are located on membranes where transport vesicles are formed (Kawasaki et al., 2005; D'Souza-Schorey and Chavrier, 2006). According to the wealth of data from various eukaryotic organisms, these are the endoplasmic reticulum (ER) for COPII vesicles, Golgi apparatus for COPI vesicles, and the trans-Golgi network (TGN)/early endosome (EE) and the plasma membrane for clathrin-coated vesicles. However, a recent article in The Plant Cell (Böhlenius et al., 2010) reported that a barley (Hordeum vulgare) green fluorescent protein (GFP)-tagged ARF (Hv-ARFA1b/1c) localizes to a multivesicular body (MVB) when transiently expressed in onion epidermal cells. We take issue with this conclusion, in particular because no electron microscopy evidence was presented to confirm that the structure labeled was indeed multivesiculate. Here, we present evidence that Arabidopsis ARF1 localizes to the TGN and Golgi stack. We argue that multiple lines of evidence are required to make an accurate determination of protein localization, especially with respect to the endomembrane system, which has high potential for overexpression and tagging-induced artifacts (Millar et al., 2009; Moore and Murphy, 2009).
In all other higher plants so far investigated, principally Arabidopsis and tobacco (Nicotiana tabacum), the very similar At-ARFA1c (differing from the barley ARF by only four amino acids) locates to the Golgi apparatus/TGN. A Golgi localization was first demonstrated using an antibody generated against a full-length At-ARFA1c-glutathione S-transferase fusion protein (Pimpl et al., 2000) by both immunogold electron microscopy (Pimpl et al., 2000; Stierhof and El Kasmi, 2010) (Figures 1A and 1B) and immunofluorescence (Ritzenthaler et al., 2002) (Figures 1D, 1F, and 1G). The same result was obtained when ARF1-(X)FP constructs are expressed (Xu and Scheres, 2005; Stefano et al., 2006) (Figures 1D and 1E). Functional confirmation of an ARF1 localization at the Golgi apparatus comes additionally from several expression studies on tobacco cells with GDP-(T31N) or GTP-(Q71L) restricted ARF1 mutants, which lead to an abrogation of ER-to-Golgi transport and a redistribution of Golgi enzymes into the ER (Lee et al., 2002; Takeuchi et al., 2002; Stefano et al., 2006). The expression of these mutants (leading to an inhibition of COPI vesicle formation) is equivalent to treatment with brefeldin A, which in tobacco results in a fusion of Golgi membranes with the ER (Langhans et al., 2011). However, there is good evidence that ARF1 also locates to the TGN. This was originally suggested by Pimpl et al. (2003) who showed that a functional ARF1 was also required for post-Golgi sorting of soluble vacuolar proteins, implying (as in mammals) that ARF1 was required for clathrin-coated vesicle formation at the TGN. Visualization of a potential ARF1 localization at the TGN was provided later by immunofluorescence in Arabidopsis using lines expressing SYP61–cyan fluorescent protein and VHA-a1-GFP as TGN markers (Paciorek et al., 2005; Tanaka et al., 2009) and demonstration that a FM4-64 positive compartment, distinct from the Golgi apparatus, was labeled by ARF1-GFP (Xu and Scheres, 2005; Tanaka et al., 2009). A similar, non-Golgi ARF1-positive compartment was also identified by Stefano et al. (2006) and later confirmed as the TGN by immunogold electron microscopy by Stierhof and El-Kasmi (2010) (Figure 1A).
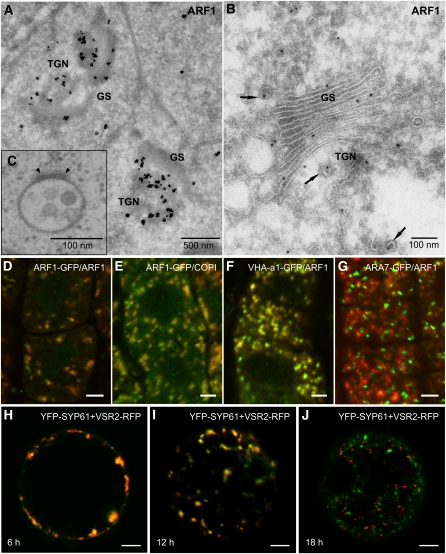
Figure 1.
ARF1 Localization, MVBs, and PVC Maturation.
(A) and (B) Immunogold electron microscopy with ARF1 antibodies. Bars = 500 nm in (A) and 100 nm in (B).
(A) Tokuyasu cryosection of high-pressure frozen/freeze-substituted Arabidopsis roots. Thawed sections were stained with 1-nm gold particles and silver enhanced Nanogold according to Stierhof and El-Kasmi (2010). Label is present over both the Golgi stack and TGN. (Micrograph courtesy of York-Dieter Stierhof, Tübingen.)
(B) Conventional cryosection of a maize (Zea mays) root cap cell (for details, see Pimpl et al., 2000). Gold particles (5 nm) are seen on vesicles (arrows) at both the cis- (c) and trans- (t) faces of the Golgi stack.
(C) Ultrastructure of an MVB. Section prepared from a high-pressure frozen/freeze-substituted/plastic-embedded sample of an Arabidopsis root. Note the almost perfect circular profile lacking vesicle budding profiles, as well as an electron-dense putative clathrin plaque (arrowheads) at the surface. Bar = 100 nm. (Micrograph courtesy of York-Dieter Stierhof, Tübingen.)
(D) to (G) Double immunofluorescence with antibodies directed against proteins indicated in each panel in 5-d-old root cells of the Arabidopsis seedlings. In all images, the GFP fusion proteins are shown in green and the endogenous ARF1 ([D], [F], and [G]) or COPI (E) proteins in red; merged signals are yellowish. The rabbit polyclonal anti-ARF1 antibody and anti-γ-COP antibody were diluted 1:800, and mouse monoclonal anti-GFP antibody was diluted 1:600. The secondary antibodies anti-rabbit CY3 (Sigma-Aldrich) and anti-mouse Alexa 488 (Invitrogen) were diluted 1:600. Bars = 4 μm.
(D) Endogenous ARF1 (detected by ARF1 antibody) colocalizes with signal from GFP antibody in a line expressing At-ARFA1c fused to enhanced GFP (ARF1-GFP).
(E) ARF1-GFP (detected by GFP antibody) colocalizes with COPI (detected by antibody against the Golgi marker γ-COP; Pimpl et al., 2000).
(F) ARF1 (detected by ARF1 antibody) also colocalizes with an anti-GFP signal in a line expressing the TGN marker VHA-a1-GFP.
(G) No significant colocalization was observed between the PVC/MVB marker GFP-ARA7 (detected by GFP antibody) and endogenous ARF1.
(H) to (J) Transient expression of fluorescently tagged TGN (YFP-AtSYP61) and PVC/MVB (AtVSR2-RFP) markers in tobacco mesophyll protoplasts. After short expression periods (6 h), both markers show complete colocalization. However, the two markers becoming increasingly separate with increasing length of expression. This can be interpreted as demonstrating the formation of the PVC/MVB at the TGN and its gradual maturation away from the TGN. Bars = 5 μm.
Böhlenius et al. (2010) based their conclusion that Hv-ARF1 localizes to MVBs on the observation of ARF1 colocalization with the Arabidopsis Rab GTPase ARA7/RabF2 in a transient expression assay in onion epidermal cells. We cannot confirm this observation with At-ARF1 in Arabidopsis roots. At-ARF1 antibodies that specifically colocalize with stably expressed ARF1-GFP (Figure 1 D) did not label structures which carry stably expressed ARA7-GFP (Figure 1G). On the other hand, Xu and Scheres (2005) localized a small proportion of the ARF1-GFP signal in Arabidopsis root cells to an ARA7-positive organelle. This may reflect localization of a portion of ARA7 to the TGN/EE since immunogold electron microscopy of plastic sections cut from high-pressure frozen Arabidopsis roots has confirmed the localization of ARA7 to the boundary membrane of MVBs (Haas et al., 2007). ARA7 also has been detected at the TGN in Arabidopsis roots (Stierhof and El Kasmi, 2010) and in a FM4-64-positive compartment, distinct from the Golgi apparatus (Dhonukshe et al., 2006). Taken together, all of the localization data so far available using different markers as well immunogold electron microscopy indicate that the reported colocalizations of ARF1 and ARA7 reflect the presence of both of these regulators at the TGN/EE rather than at the MVB.
Is there experimental support for the speculation made by Böhlenius et al. (2010) that ARF1 at the MVB “mediates formation of vesicles with another destination, potentially in retrograde trafficking?” More precisely stated: Is there any evidence for (1) COPI- or clathrin-coated vesicle formation at the MVB and (2) recycling from MVB/late endosomes (LEs)? In the mammalian literature, there are numerous papers implicating the role of various coatomer subunits in endocytosis (Whitney et al., 1995; Razi et al., 2009). However, COPI seems mainly to be involved in early trafficking events (e.g., effecting transferrin internalization and recycling from EE; Daro et al., 1997) or in anterograde trafficking between EE and LE (Aniento et al., 1996). Although there is evidence for the participation of COPI in transport from the LE to vacuole in yeast (Gabriely et al., 2007), in no eukaryotic organism, including plants, have COPI proteins actually been demonstrated at the surface of MVB/LEs.
With respect to clathrin, to our knowledge, there are no reports for clathrin-coated vesicle formation on LEs in either mammalian or yeast cells. The same is true for plant MVBs, which in high-pressure frozen/plastic-embedded preparations have a circular profile normally without vesicle budding profiles (Figure 1C). However, plant MVBs have a densely labeled tuft at their surface, and this is probably the double-layered clathrin plaque related to the ESCRT-0 complex (Sachse et al., 2002; Wollert and Hurley, 2010). In addition, it should be noted that receptor recycling from endosomes is facilitated by the pentameric retromer complex (Bonifacino and Hurley, 2008). Retromer binds mainly to the tubular protrusions of the EE in mammalian cells (Mari et al., 2008), but in both yeast and plant cells, the situation is controversial, with evidence pointing to retromer at both the EE (Strochlic et al., 2007; Niemes et al., 2010) and MVB/LE (Jaillais et al., 2008; Balderhaar et al., 2010). However, it has recently been shown that retromer is recruited by the Rab GTPase Rab7 and not by an ARF-type GTPase (Seaman et al., 2009). Therefore, a role for ARF1 in retrograde trafficking from the MVB remains questionable.
The question now is, might such a colocalization reflect stable resident populations or a transitory, dynamic situation? Mammalian cell biologists usually regard multivesicular late endosomes to be the result of a gradual transformation (maturation) from pleiomorphic early endosomes (van Weering et al., 2010). Thus, when performing transient expression experiments as Böhlenius et al. (2010) have done, it cannot be ruled out that overlapping signals for ARF1 and ARA7 merely reflect an early stage in MVB formation from the TGN/EE. This can in fact be demonstrated in tobacco mesophyll protoplasts, which show a colocalization of TGN/EE and MVB/LE markers early in the expression period but a clear separation at a later time point (Figures 1H to 1J). The mechanism underlying this long delay in separation of markers is not clear. Although performed with plasmid concentrations that do not interfere with the appearance of Golgi stack markers, it cannot be ruled out that that the overexpression of the TGN and MVB markers temporarily perturbs their trafficking.
For reasons indicated above, attempting to localize a protein solely on the basis of transient expression of an (X)FP-tagged fusion construct can be misleading. Not only are such studies open to question because of their transient nature, but in such experiments, the proteins in question are sometimes highly overexpressed relative to their endogenous counterparts. This could be particularly troublesome when dealing with a regulatory protein like a GTPase whose overexpression could well perturb membrane flux leading to a temporary mislocalization of marker proteins. Ideally, the organelle/compartment to which a transiently expressed construct locates should be the same as that where a stably expressed construct resides and even better in a genetic background where the function of the tagged protein can be assessed. Therefore, to rule out problems of overexpression, the localization of the endogenous protein should also be determined by immunofluorescence and/or immunogold electron microscopy with a specific antibody.
References
- Anders N., Jürgens G. (2008). Large ARF guanine nucleotide exchange factors in membrane trafficking. Cell. Mol. Life Sci. 65: 3433–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F., Gu F., Parton R.G., Gruenberg J. (1996). An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 133: 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderhaar H.J., Arlt H., Ostrowicz C., Bröcker C., Sündermann F., Brandt R., Babst M., Ungermann C. (2010). The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J. Cell Sci. 123: 4085–4094 [DOI] [PubMed] [Google Scholar]
- Böhlenius H., Mørch S.M., Godfrey D., Nielsen M.E., Thordal-Christensen H. (2010). The multivesicular body-localized GTPase ARFA1b/1c is important for callose deposition and ROR2 syntaxin-dependent preinvasive basal defense in barley. Plant Cell 22: 3831–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Hurley J.H. (2008). Retromer. Curr. Opin. Cell Biol. 20: 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro E., Sheff D., Gomez M., Kreis T., Mellman I. (1997). Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J. Cell Biol. 139: 1747–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., Baluska F., Schlicht M., Hlavacka A., Samaj J., Friml J., Gadella T.W., Jr. (2006). Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10: 137–150 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. (2006). ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7: 347–358 [DOI] [PubMed] [Google Scholar]
- Gabriely G., Kama R., Gerst J.E. (2007). Involvement of specific COPI subunits in protein sorting from the late endosome to the vacuole in yeast. Mol. Cell. Biol. 27: 526–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas T.J., Sliwinski M.K., Martínez D.E., Preuss M., Ebine K., Ueda T., Nielsen E., Odorizzi G., Otegui M.S. (2007). The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 19: 1295–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Gaude T. (2008). Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Nakayama K., Wakatsuki S. (2005). Membrane recruitment of effector proteins by Arf and Rab GTPases. Curr. Opin. Struct. Biol. 15: 681–689 [DOI] [PubMed] [Google Scholar]
- Langhans M., Förster S., Helmchen G., Robinson D.G. (February 28, 2011). Differential effects of the brefeldin A analogue (6R)-hydroxy-BFA in tobacco and Arabidopsis. J. Exp. Bot. http://dx.doi.org/10.1093/jxb/err007 [DOI] [PubMed]
- Lee M.H., Min M.K., Lee Y.J., Jin J.B., Shin D.H., Kim D.H., Lee K.H., Hwang I. (2002). ADP-ribosylation factor 1 of Arabidopsis plays a critical role in intracellular trafficking and maintenance of endoplasmic reticulum morphology in Arabidopsis. Plant Physiol. 129: 1507–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M., Bujny M.V., Zeuschner D., Geerts W.J., Griffith J., Petersen C.M., Cullen P.J., Klumperman J., Geuze H.J. (2008). SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9: 380–393 [DOI] [PubMed] [Google Scholar]
- Millar A.H., Carrie C., Pogson B., Whelan J. (2009). Exploring the function-location nexus: using multiple lines of evidence in defining the subcellular location of plant proteins. Plant Cell 21: 1625–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore I., Murphy A. (2009). Validating the location of fluorescent protein fusions in the endomembrane system. Plant Cell 21: 1632–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S., Langhans M., Viotti C., Scheuring D., San Wan Yan M., Jiang L., Hillmer S., Robinson D.G., Pimpl P. (2010). Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y.D., Kleine-Vehn J., Morris D.A., Emans N., Jürgens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Pimpl P., Hanton S.L., Taylor J.P., Pinto-daSilva L.L., Denecke J. (2003). The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15: 1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl P., Movafeghi A., Coughlan S., Denecke J., Hillmer S., Robinson D.G. (2000). In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12: 2219–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M., Chan E.Y., Tooze S.A. (2009). Early endosomes and endosomal coatomer are required for autophagy. J. Cell Biol. 185: 305–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C., Nebenführ A., Movafeghi A., Stussi-Garaud C., Behnia L., Pimpl P., Staehelin L.A., Robinson D.G. (2002). Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Langhans M., Saint-Jore-Dupas C., Hawes C. (2008). BFA effects are tissue and not just plant specific. Trends Plant Sci. 13: 405–408 [DOI] [PubMed] [Google Scholar]
- Sachse M., Urbé S., Oorschot V., Strous G.J., Klumperman J. (2002). Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13: 1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N., Harbour M.E., Tattersall D., Read E., Bright N. (2009). Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 122: 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G., Renna L., Chatre L., Hanton S.L., Moreau P., Hawes C., Brandizzi F. (2006). In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery. Plant J. 46: 95–110 [DOI] [PubMed] [Google Scholar]
- Stierhof Y.D., El Kasmi F. (2010). Strategies to improve the antigenicity, ultrastructure preservation and visibility of trafficking compartments in Arabidopsis tissue. Eur. J. Cell Biol. 89: 285–297 [DOI] [PubMed] [Google Scholar]
- Strochlic T.I., Setty T.G., Sitaram A., Burd C.G. (2007). Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J. Cell Biol. 177: 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Ueda T., Yahara N., Nakano A. (2002). Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 31: 499–515 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Kitakura S., De Rycke R., De Groodt R., Friml J. (2009). Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr. Biol. 19: 391–397 [DOI] [PubMed] [Google Scholar]
- van Weering J.R., Verkade P., Cullen P.J. (2010). SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 21: 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V., Horton A.C., Yang Z., Nielsen E. (2003). Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 131: 1191–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J.A., Gomez M., Sheff D., Kreis T.E., Mellman I. (1995). Cytoplasmic coat proteins involved in endosome function. Cell 83: 703–713 [DOI] [PubMed] [Google Scholar]
- Wollert T., Hurley J.H. (2010). Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464: 864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Scheres B. (2005). Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epiderwmal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]