This work shows that the Arabidopsis transcription factor BROTHER OF LUX ARRHYTHMO (BOA) is an activator in regulating the expression of CIRCADIAN CLOCK ASSOCIATED1 (CCA1). BOA forms a transcriptional feedback loop with CCA1 and regulates circadian rhythms in Arabidopsis.
Abstract
BROTHER OF LUX ARRHYTHMO (BOA) is a GARP family transcription factor in Arabidopsis thaliana and is regulated by circadian rhythms. Transgenic lines that constitutively overexpress BOA exhibit physiological and developmental changes, including delayed flowering time and increased vegetative growth under standard growing conditions. Arabidopsis circadian clock protein CIRCADIAN CLOCK ASSOCIATED1 (CCA1) binds to the evening element of the BOA promoter and negatively regulates its expression. Furthermore, the period of BOA rhythm was shortened in cca1-11, lhy-21 (for LATE ELONGATED HYPOCOTYL), and cca1-11 lhy-21 genetic backgrounds. BOA binds to the promoter of CCA1 through newly identified promoter binding sites and activates the transcription of CCA1 in vivo and in vitro. In transgenic Arabidopsis lines that overexpress BOA, the period length of CCA1 rhythm was increased and the amplitude was enhanced. Rhythmic expression of other clock genes, including LHY, GIGANTEA (GI), and TIMING OF CAB EXPRESSION1 (TOC1), was altered in transgenic lines that overexpress BOA. Rhythmic expression of BOA was also affected in mutant lines of toc1-1, gi-3, and gi-4. Results from these studies indicate that BOA is a critical component of the regulatory circuit of the circadian clock.
INTRODUCTION
The circadian system orchestrates diverse physiological and metabolic processes that confer a selective advantage in growth and development of many organisms, including plants (Green et al., 2002; Michael et al., 2003; Dodd et al., 2005; Locke et al., 2005; McClung, 2008; Salomé et al., 2008; Ni et al., 2009). Biological oscillations enable plants to anticipate daily changes of the environment and synchronize plant functions with environmental cues, such as day/night cycles and temperature changes (Green et al., 2002; Locke et al., 2005; Wijnen and Young, 2006; Gutiérrez et al., 2008; Pruneda-Paz et al., 2009).
In Arabidopsis thaliana, several components of the oscillator of the circadian clock have been identified, including the evening-phased pseudo-response regulator TIMING OF CAB EXPRESSION1 (TOC1) and two morning-expressed MYB transcription factors, CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) (Schaffer et al., 1998; Wang and Tobin, 1998; Strayer et al., 2000; Locke et al., 2006; Hamilton and Kay, 2008; McClung, 2008). A shared feature of circadian clocks in different organisms is that the rhythms are generated by the interactions of rhythmically expressed genes that form positive and negative feedback loops (Wijnen and Young, 2006; Hamilton and Kay, 2008). CCA1/LHY and TOC1 were identified as components of the feedback loop of the circadian clock of Arabidopsis (Alabadí et al., 2001). The transcription factors CCA1 and LHY function as negative regulators of TOC1 by directly binding to the evening element (EE) in the TOC1 promoter. Increased expression of TOC1 was predicted to close the feedback loop by activating the transcription of CCA1 and LHY (Green and Tobin, 1999; Alabadí et al., 2001, 2002; Mizoguchi et al., 2002).
In addition to CCA1, LHY, and TOC1, GIGANTEA (GI), LUX ARRHYTHMO/PHYTOCLOCK1 (LUX/PCL1), EARLY FLOWERING4 (ELF4), PSEUDO-RESPONSE REGULATOR7 (PRR7), PRR9, and CCA1 HIKING EXPEDITION (CHE) are Arabidopsis clock components that form interlocking loops with components of the TOC1 and CCA1/LHY loop (Locke et al., 2005, 2006; Wijnen and Young, 2006; Kolmos and Davis, 2007; Hamilton and Kay, 2008; McClung, 2008; Pruneda-Paz et al., 2009). GI was identified as a key component of the circadian clock in Arabidopsis and was modeled as a positive regulator of TOC1, the Y component in the three-loop Arabidopsis circadian network model proposed by others (Fowler et al., 1999; Park et al., 1999; Locke et al., 2005, 2006; Mizoguchi et al., 2005). LUX is a GARP protein (Hazen et al., 2005; Onai and Ishiura, 2005). CCA1 and LHY bind to the promoter of LUX and negatively regulate LUX expression; LUX was predicted to be a positive regulator of CCA1/LHY in a potential reciprocal regulatory loop (Hazen et al., 2005; Onai and Ishiura, 2005; Baudry and Kay, 2008). Similarly, PRR7, PRR9, and ELF4 also form interlocking regulatory loops with CCA1/LHY in the Arabidopsis circadian clock (Farré et al., 2005; Kikis et al., 2005; Locke et al., 2005, 2006; Wijnen and Young, 2006; Kolmos and Davis, 2007; McWatters et al., 2007; Hamilton and Kay, 2008; McClung, 2008). CHE represses CCA1 expression, while expression of CHE is negatively regulated by CCA1; CHE serves as a linker between TOC1 and CCA1 (Pruneda-Paz et al., 2009).
The functions of clock genes are also regulated by nontranscriptional processes (Schöning and Staiger, 2005). For example, the clock function of CCA1 is affected by proper phosphorylation (Daniel et al., 2004). GI functions through interaction with the F-box protein ZEITLUPE (ZTL) to maintain the high amplitude of TOC1 rhythms and thereby affects clock function (Somers et al., 2000; Más et al., 2003b; Locke et al., 2006; Kim et al., 2007). Furthermore, dynamic changes in chromatin structure are tightly connected to the Arabidopsis circadian clock, and transcription of clock genes is affected by chromatin remodeling (Perales and Más, 2007; Más, 2008). Epigenetic modifications of the circadian clock genes CCA1, LHY, TOC1, and GI induce expression changes in downstream genes and alter circadian-mediated physiological and metabolic pathways that greatly affect plant biomass (Ni et al., 2009).
However, clock control is pervasive and scientists are far from having a complete understanding of mechanisms that regulate rhythmicity (McClung, 2006; Wijnen and Young, 2006; Más, 2008). So far, only a few bona fide transcription factors have been identified as clock components, namely, CCA1/LHY, LUX, and CHE (Schaffer et al., 1998; Wang and Tobin, 1998; Hazen et al., 2005; Onai and Ishiura, 2005; Baudry and Kay, 2008; Pruneda-Paz et al., 2009), although a recent report indicates that TOC1 is capable of functioning as a transcription factor (Legnaioli et al., 2009). Whereas CCA1 and LHY were determined to be negative regulators of TOC1, GI, and LUX/PCL1, TOC1, GI, and potentially LUX were suggested to be positive regulators of CCA1 and LHY. However, neither TOC1 nor GI has been demonstrated to bind the promoter of CCA1 or LHY; similarly, there is no evidence for direct binding of LUX to the promoter of CCA1 and/or LHY (Strayer et al., 2000; Alabadí et al., 2001; Hwang et al., 2002; Hazen et al., 2005; Onai and Ishiura, 2005; McClung, 2006; Wijnen and Young, 2006; Perales and Más, 2007).
A missing component, X, was proposed as a positive regulator in a computer simulated three-loop model to connect the TOC1 and CCA1/LHY loop (Locke et al., 2006). Despite the recent identification of CHE as a direct negative regulator of CCA1 that connects TOC1 and CCA1, no transcription factor that can bind to the upstream regulatory sequences of CCA1 and/or LHY and function as a positive regulator of gene expression has been identified (Schöning and Staiger, 2005; McClung, 2006; Baudry and Kay, 2008; Pruneda-Paz et al., 2009).
Here, we report the identification of a clock gene, BROTHER OF LUX ARRHYTHMO (BOA). BOA binds to the CCA1 promoter, activates its expression, and forms a reciprocal transcriptional regulatory loop with CCA1. BOA also affects the expression of other clock genes, including LHY, TOC1, and GI. The expression of BOA is regulated by LHY, TOC1, and GI. We demonstrate that BOA is a component of the circadian clock in Arabidopsis.
RESULTS
BOA Is Rhythmically Expressed in Arabidopsis and Affects Flowering Time
In an effort to establish the impact of Arabidopsis transcription factors on plant development and stress responses, At5g59570 and other transcription factors were constitutively overexpressed in transgenic Arabidopsis. Plants that carried a transgene, in which the coding sequence of At5g59570 was under the control of cauliflower mosaic virus (CaMV) 35S promoter, exhibited a late flowering phenotype under long- and short-day conditions (Figures 1 and 2). At5g59570 encodes a protein of 298 amino acids with a conserved GARP DNA binding domain (DBD) located in the middle of the protein. The GARP DBD of At5g59570 protein is nearly identical to the DBD of circadian clock protein LUX/PCL1 (Hazen et al., 2005; Onai and Ishiura, 2005). As shown in Supplemental Figure 1B online and Figure 1B, expression of At5g59570 in nontransgenic plants showed characteristics of evening genes (i.e., accumulation of At5g59570 RNA was at its highest level at dusk and was at a very low level at dawn). The rhythmic expression pattern of At5g59570 is very similar to that of LUX/PCL1 (Hazen et al., 2005; Onai and Ishiura, 2005). In addition, At5g59570 is expressed in all tissue types examined, with strong expression in flowers (Figure 3A).
Figure 1.
Overexpression of BOA in Arabidopsis Caused Late Flowering.
(A) Rosette leaf numbers, surrogates for flowering time, of transgenic Arabidopsis plants overexpressing BOA (35S:BOA) or control plants (Col-0) maintained under either 16-h/8-h or12-h/12-h light/dark periods. Each bar represents the mean ± sd.
(B) qRT-PCR analysis of the accumulation of BOA transcripts in BOA overexpression transgenic plants (35S:BOA-8) that were entrained under a 12-h-light/12-h-dark period and released to continuous light (LL) at 21 d after sowing. Nontransgenic plants (Col-0) under the same conditions were included as controls. Plant samples were collected at 3-h intervals for RNA sample preparation. All the data was normalized against TUBULIN. Each time point represents the mean value of three repeats ± sd.
Figure 2.
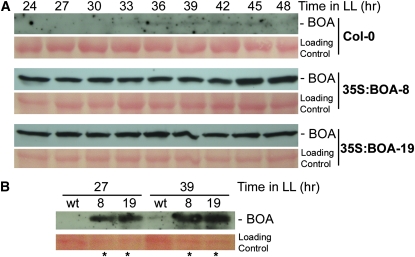
The Accumulation of BOA Protein in Transgenic Arabidopsis Lines Overexpressing BOA.
Tissues from transgenic plants carrying pC-35S:BOA were collected after being entrained under 12-light/12-dark conditions and subsequently released to continuous light (LL) conditions at 21 d after sowing. Plant samples were collected at time points as labeled and used for protein extraction. Thirty micrograms of each protein sample was resolved in 10% SDS-PAGE and blotted to nitrocellulose membrane. BOA protein was detected using anti-BOA antibody.
(A) Detection of BOA in two independent 35S:BOA lines (35S:BOA-8 and 35S:BOA-19). Col-0 is the wild-type control. The bottom image of each panel is the loading control visualized by Ponceau S staining prior to immunodetection.
(B) Comparison of BOA accumulation in transgenic lines with that in the Col-0 control. Bottom image is the loading control. 27, zeitgeiber time (ZT) 27; 39, ZT39; wt, Col-0; 8, 35S:BOA-8; and 19, 35S:BOA-19. Asterisks indicates that only 20 μg of protein rather than 30 μg of protein was loaded in each of those lanes. wt, wild type.
[See online article for color version of this figure.]
Figure 3.
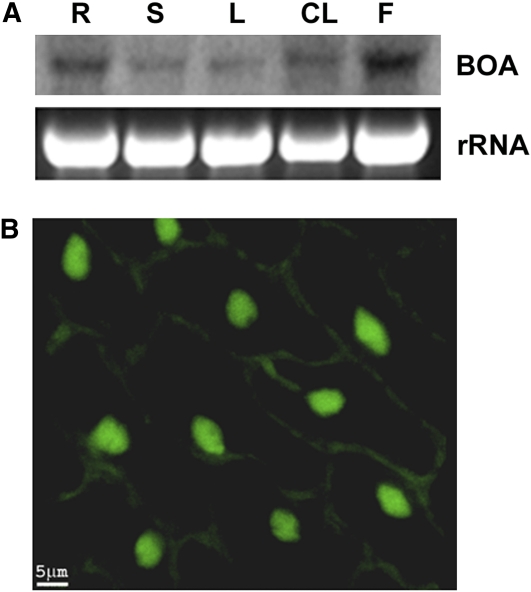
The Expression Profile of BOA.
(A) RNA gel blot analysis of BOA transcript levels in different tissue types. Top panel, phospho-image of the membrane probed with 32P labeled BOA coding sequence; bottom panel, image of rRNA in the denature gel stained with ethidium bromide (loading control). R, root; S, stem; L, leaf; CL, cauline leaf; F, flower. Samples were collected from plants maintained under 12-h-light/12-h-dark period starting from Zeitgeber time 8 (ZT8) in a time period of ~1.5 h.
(B) Subcellular localization of BOA flower petals of transgenic Arabidopsis plants carrying pC-35S:BOA:GFP. Bar = 5 μm.
[See online article for color version of this figure.]
To establish the subcellular location of At5g9570, transgenic Arabidopsis plants carrying a transgene encoding a fusion protein of At5g59570 and green fluorescence protein (GFP), under the control of the CaMV 35S promoter (pC-35S:BOA:GFP), were developed. As shown in Figure 3B, the BOA:GFP fusion protein was localized primarily to the nucleus in transgenic plants, with low GFP signal detected in the cytoplasm.
It was predicted that At5g59570 encodes a GARP family transcription factor that is closely related to LUX/PCL1 (see supplemental information in Hazen et al. [2005] for the phylogenic relationship between LUX [At3g46640] and At5g59570). At5g59570 is rhythmically expressed during the light/dark cycle and functions as a clock gene (as demonstrated below). Thus, At5g59570 was named the BROTHER OF LUX ARRHYTHMO. At5g59570 will be referred to as BOA hereafter.
CCA1 Interacts with the BOA Promoter and Regulates Its Expression
The expression of LUX is regulated by CCA1, which binds to the EE of the LUX promoter (Hazen et al., 2005). BOA and the gene immediately upstream of BOA, At5g59560 (SRR1) (Staiger et al., 2003), are in a head-to-head orientation and share an intergenic region of 2073 bp (between transcription start sites) that contains upstream regulatory sequences of both genes. A putative EE at the proximal position to BOA (located at −160 to −148 nucleotides relative to the transcription start site of BOA) was identified on the negative strand of the BOA promoter (see Supplemental Figure 2 online). BOA is rhythmically expressed with characteristics of evening genes (Figures 1B and 2; see Supplemental Figure 1B online), whereas the transcription of SRR1 (At5g59560) is not under clock control (Staiger et al., 2003). Thus, this EE is most likely responsible for regulating the expression of BOA.
The EE located in the promoter region of BOA shares an identical core sequence (in reverse orientation) with the EE located in the promoter region of LUX (Figure 4A). To determine if this element can bind CCA1, a glutathione S-transferase (GST) and CCA1 fusion protein (GST:CCA1) was produced in Escherichia coli (pGST:CCA1). Crude protein extracts prepared from bacterial strains carrying either pGST:CCA1 or the control vector pGEX-4T1 were used in electrophoretic mobility shift assays (EMSAs). As shown in Figure 4B, CCA1:GST binds to the EE located in the promoter of BOA. Furthermore, non-radio-labeled EE from either the promoter of BOA or the promoter of LUX competed for binding of CCA1 with similar efficacy. Probes in which nucleotide mutations were made in EEs of either BOA or LUX reduced their ability to compete with the EE of BOA (Figure 4B).
Figure 4.
CCA1 Binds to the Promoter of BOA.
(A) Nucleotide sequences of probes used in the EMSAs. Conserved EE sequences in BOA and LUX promoters are in bold. Mutations within the EEs of BOA and LUX promoters were made as described (Hazen et al., 2005). BOA-EE-, bottom strand EE of BOA promoter; LUX-EE+, upper strand EE of LUX promoter; BOA-EEm-, mutated EE of BOA promoter (bottom strand); LUX-EEm+, mutated EE of LUX promoter.
(B) CCA1 binds to EE of BOA. EMSAs were conducted using GST-CCA1 fusion protein. BOA-EE was radiolabeled and used as probe in the reactions. Unlabeled probes were included in reactions as competitors with molar ratios to radiolabeled probe as specified. The arrow indicates the protein and BOA-EE probe complex. Free, unbound radiolabeled probe.
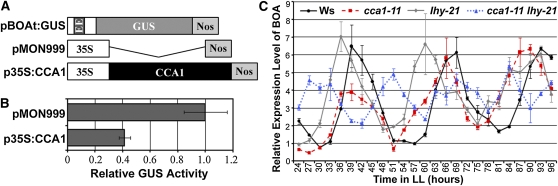
To establish the role of CCA1 as a regulator of BOA gene expression in vivo, an effector gene construct with CCA1 under the control of the CaMV 35S promoter (p35S:CCA1) was developed. A β-glucuronidase (GUS) reporter gene under the control of a truncated BOA promoter that includes the EE (pBOAt:GUS) was also constructed (sequence information of the truncated promoter is presented in Supplemental Figure 2 online). pBOAt:GUS was introduced into tobacco (Nicotiana tabacum) BY-2 protoplasts together with either p35S:CCA1 or the vector control (pMON999) (Figure 5A). The activity of the truncated BOA promoter in pBOAt:GUS was suppressed in samples cotransfected with p35S:CCA1 when compared with that of cotransfections with pMon999 (Figures 5A and 5B). Similarly, the expression of BOA was greatly suppressed in transgenic plants in which CCA1 was overexpressed (CCA1-OX), especially during the evening phase of the circadian cycle (see Supplemental Figure 3A online).
Figure 5.
CCA1 Regulates Expression of BOA.
(A) Diagram of gene constructs used in tobacco BY-2 protoplast transient assays. pBOAt:GUS, a truncated promoter of BOA (from nucleotides −207 to +43; see Supplemental Figure 2 online for sequence information) that includes EE was fused with the GUS gene. pMON999, a vector cassette control; p35S:CCA1, effector with coding sequence of CCA1 under the control of CaMV 35S promoter.
(B) The promoter activity of BOA was suppressed by CCA1 in transient assays. BY-2 protoplasts were cotransfected with either pMON999 + pBOAt:GUS + p35S::GFP or p35S:CCA1 + pBOAt:GUS + p35S::GFP. GUS activity data were normalized against the internal GFP control before comparison. Each bar represents the average value from three independent experiments with three repeats of each experiment ± sd.
(C) CCA1 and LHY affect the expression of BOA. The expression level of BOA in CCA1 and LHY T-DNA insertion lines cca1-11 and lhy-21 or cca1-11 lhy-21 and the control (Ws) was analyzed using qRT-PCR. Plants were entrained under 12-h-light/12-h-dark conditions and released to continuous light (LL), and samples were collected starting from 28 d after sowing. RNA samples were isolated from plant materials and were subjected to qRT-PCR analysis. All the data were normalized against TUBULIN. Each time point represents the mean value of three repeats ± sd.
[See online article for color version of this figure.]
The function of CCA1 and LHY are partially redundant (Mizoguchi et al., 2002). To further evaluate the role of CCA1 and LHY in regulating the expression of BOA, Arabidopsis T-DNA mutants with disrupted expression of either CCA1 (cca1-11) or LHY (lhy-21) or both CCA1 and LHY (cca1-11 lhy-21) (Hall et al., 2003) were obtained from the ABRC. The expression profile of BOA during the circadian cycles was determined by quantitative RT-PCR (qRT-PCR) in these lines. The period length was evaluated using the BRASS program to verify the significance of the data. As shown in Figure 5C, the period length of BOA expression in the mutant cca1-11 was shortened (23.25 ± 0.11 h versus 26.02 ± 0.26 h in wild-type plants). This result is similar to the effect of cca1-1 on expression of the CCR2 and TOC1: the promoters of both genes contain an EE (Green and Tobin, 1999; Alabadí et al., 2002; Mizoguchi et al., 2002; Lu et al., 2009). lhy-21 showed a similar effect on the rhythm of BOA (Figure 5C). The rhythm of BOA exhibits an even shorter period in the cca1-11 lhy-21 double mutant line (17.4 ± 0.05 h) and had a reduced amplitude (2.58 ± 0.45 versus 6.12 ± 1.04 in the wild-type plants) (Figure 5C). The effects on the rhythmic expression of TOC1, LUX, and CCR2 were similar to that on BOA in CCA1 and LHY double loss- of-function mutant lines (Alabadí et al., 2002; Mizoguchi et al., 2002; Hazen et al., 2005; Lu et al., 2009). These data strongly suggest that BOA is regulated by CCA1 and, most likely, LHY as well, especially considering that CCA1 and LHY share similar expression patterns, are able to bind to the same cis-element, and are capable of forming heterodimers in vivo (Lu et al., 2009).
BOA Binds to the Promoter of CCA1
It was predicted that LUX regulates the expression of CCA1 and LHY, although no direct evidence has been presented to support the prediction (Hazen et al., 2005). BOA shares an almost identical DBD with LUX (Hazen et al., 2005). To determine if BOA binds to the promoter of CCA1 and/or LHY in vivo, chromatin immunoprecipitation (ChIP) assays were conducted using leaf tissues of Arabidopsis plants (Columbia-0 [Col-0]) maintained in a growth chamber with a 12-light/12-dark cycle. As shown in Figure 6A, DNA sequences of the CCA1 promoter were enriched from the chromatin samples prepared from leaf samples collected during the night (ZT15) using anti-BOA antibodies. By contrast, the LHY promoter was not consistently enriched in similar assays, and no enrichment was observed for the promoter of the control gene IAA31. This strongly suggests that BOA directly binds to the promoter of CCA1 in vivo.
Figure 6.
BOA Binds to the Promoter of CCA1 and Regulates Its Expression in Vivo and in Vitro.
(A) BOA binds to CCA1 promoter in vivo. Leaf samples from Col-0 plants maintained under 12-h-light/12-h-dark photoperiods were collected at Zeitgeber time 7 (ZT7) and 15 (ZT15) for chromatin preparation. Anti-BOA antibody was used to enrich BOA-bound chromatins (ChIP). Serum collected from rabbits prior to immunization (Prebleed) was used in control reactions. A mock ChIP without serum was included as negative control. DNA samples from ChIP assays and 5% of the amount of input chromatin were amplified using CCA1 promoter-specific primers. PCR products were resolved in 1.2% agarose gels.
(B) BOA activates transcription from the CCA1 promoter in vitro. In vitro transcription assays were conducted using rice whole-cell extract. Plasmid pCCA1t:GUS was used as reporter gene. RNA polymerase II inhibitor α-amanitin (5 μg/mL) was added to the control reaction. In vitro transcription assays with 500 ng (+) or 1 μg (++) of BOA protein or without BOA were included.
(C) Sequences of PBS4 probes used in EMSAs.
(D) BOA binds to PBS4 of CCA1. EMSAs were conducted using BOA purified from E. coli with or without nonlabeled competitor with molar ratios as labeled. Arrow indicates bands of the BOA-PBS4 complex.
(E) BOA activates CCA1 expression in vivo. Transgenic Arabidopsis lines 35S:BOA-8 and 35S:BOA-19 carrying pC-35S:BOA and wild-type plants (Col-0) were maintained under 12-h-light/12-h-dark photoperiods before they were released to continuous light (LL) for sample collection starting from 21 d after sowing. RNAs from those samples were subjected to qRT-PCR analysis to quantify CCA1 expression. Each data point reflects the mean of three repeats ± sd.
Like BOA, ARR10 is an Arabidopsis GARP family transcription factor. It was previously reported that the ARR10 protein binds to a conserved sequence of AGATHYK through key residues located in the three α-helices (Hosoda et al., 2002). The signature of key residues important for target DNA sequence recognition in the DBD of ARR10 is RxLWx37Ex2ASxLQKx5K (Hosoda et al., 2002). The DBD in BOA has a signature sequence of RxVWx37Ex2ASxLQKx5K, a single amino acid difference from that of ARR10, namely, a change of L to V at the second conserved residue (Hosoda et al., 2002). This indicates that BOA may bind to cis-elements with similar consensus sequences as that for ARR10. We scanned the promoter of CCA1 for the consensus sequences of ARR10 binding sites and identified four of such sequences: the sequences are designated as Putative BOA binding Site1 (PBS1), PBS2, PBS3, and PBS4 (see Supplemental Figure 4 online). Among these four putative sites, PBS3 includes two tandem repeats of the consensus sequences. To evaluate further whether BOA can bind to the CCA1 promoter or not through these putative binding sites, EMSAs were conducted using PBS4 as probe (Figure 6C). BOA specifically binds to PBS4 (Figure 6D). Similarly, BOA binds to PBS3 (see Supplemental Figure 5 online).
BOA Regulates Expression of CCA1
The expression of CCA1 can be positively or negatively regulated by other clock genes, including PRR7, PRR9, TOC1, and CHE (Alabadí et al., 2001; Farré et al., 2005; Pruneda-Paz et al., 2009). We conducted transient expression experiments in tobacco BY-2 protoplasts to determine if BOA regulates the activity of the CCA1 promoter; these experiments did not show that BOA has such an effect. We subsequently used a rice (Oryza sativa) whole-cell extract transcription assay system to evaluate the effect of BOA on regulating expression of the CCA1 promoter. A truncated version of CCA1 promoter, which includes PBS3 and PBS4, was fused with the GUS gene and used as a reporter (pCCA1t:GUS) in this experiment. As shown in Figure 6B, the primer extension results demonstrated that addition of purified BOA protein to the in vitro transcription reactions enhanced the accumulation of the reporter transcripts in a dose-dependent manner. The enhancement was suppressed by addition of RNA polymerase II inhibitor α-amanitin into the reaction mix (Figure 6B).
We also developed Arabidopsis transgenic lines in which BOA was overexpressed under the control of CaMV 35S promoter (pC-35S:BOA) (Figure 1B). In transgenic line 35S:BOA-8 (see Figure 2 for evidence of BOA protein overaccumulation), the amplitude of CCA1 rhythm was enhanced (23.14 ± 9.8 versus 11.96 ± 1.04 in wild-type plants), and the period length was increased (29.95 ± 0.08 h versus 25.15 ± 0.07 h in wild-type plants) when plants were released to continuous light conditions (Figure 6E). This result was confirmed in independent transgenic line 35S:BOA-19 (Figures 2 and 6E). Similar results were obtained when entrained 35S:BOA-8 plants were released to continuous dark conditions, after which amplitude of CCA1 rhythm was enhanced and period length was increased (26.85 ± 0.98 versus 24.95 ± 0.04 in wild-type plants) (see Supplemental Figure 6 online). These data suggest that the effect of BOA on regulating the expression of CCA1 is light independent. Furthermore, a weak allele of BOA (designated as boa-1) was isolated from ABRC line Cs819586 in which a T-DNA was inserted into the 3′ untranslated region of the BOA gene. The expression of BOA in boa-1 was rhythmic; however, the overall accumulation of BOA transcripts was reduced and the amplitude of BOA expression was dampened (see Supplemental Figure 1B online). In boa-1, the amplitude of CCA1 rhythm was modestly dampened with slightly shortened period (23.23 ± 0.1 versus 24.47 ± 0.08 in wild-type plants) (see Supplemental Figure 1A online). Taken together, these data indicate that BOA activates the expression of CCA1, presumably through direct binding of BOA to the CCA1 promoter.
Overexpression of BOA Alters Expression of Arabidopsis Clock Genes in the Core Loop of the Oscillator
It was previously proposed that CCA1/LHY and TOC1 form a feedback loop to serve as an oscillator of the circadian clock in Arabidopsis (Alabadí et al., 2001). Locke et al. (2006) demonstrated that the expression of GI is regulated by CCA1/LHY and plays a role in regulating the expression of TOC1. However, we could not consistently enrich the promoter of LHY in our ChIP assays using anti-BOA antibody; therefore, proof of regulation of LHY by BOA was incomplete. To evaluate the impact of BOA on the expression of these clock components, qRT-PCR analysis of these genes was conducted to compare their expression levels in transgenic lines overexpressing BOA and in wild-type plants. As was the case with CCA1, the period of LHY rhythm was longer in the 35S:BOA-8 line (29.94 ± 0.09 h) with enhanced amplitude (Figure 7A). The rhythmic expression of GI and TOC1 was likewise affected by overexpression of BOA; however, it is difficult to determine if those changes are caused by altering period length or phase (Figures 7B and 7C).
Figure 7.
BOA Affects Transcription of Key Arabidopsis Circadian Genes.
Transgenic Arabidopsis plants overexpressing BOA (35S:BOA-8) or wild type plants (Col-0) were entrained under 12-h-light/12-h-dark photoperiods prior to being released to continuous light and collected starting from 21 d after sowing. RNA samples were prepared from plant samples and subjected to qRT-PCR analysis to quantify the expression of LHY (A), GI (B), and TOC1 (C). Each data point reflects the mean of three repeats ± sd.
TOC1 and GI Affect the Expression of BOA
Other authors (Alabadí et al., 2001) proposed that TOC1 and CCA1/LHY form a feedback regulatory loop. TOC1 regulates CCA1, at least in part, through its interaction with transcription factor CHE (Pruneda-Paz et al., 2009). As established above, CCA1 and BOA form a reciprocal regulatory loop. It is therefore important to determine if TOC1 affects the expression of BOA. toc1-1 is a missense mutation in the CCT motif of TOC1 that has a short period and an altered clock function (Millar et al., 1995; Schaffer et al., 1998; Dunlap, 1999; Strayer et al., 2000; Más et al., 2003a). As shown in Figure 8A, the period length of BOA rhythm was shortened in toc1-1 (22.46 ± 0.22 h in the mutant versus 24.82 ± 0.18 h in wild-type plants) with dampened amplitude (Figure 8A). Similar period changes of rhythm of CCA1 were observed in toc1-1 (Figure 8B), which is in agreement with a previous report (Alabadí et al., 2001). However, our data showed that the amplitude of CCA1 rhythm in toc1-1 was dampened (Figure 8B), whereas no effect on the amplitude of CCA1 rhythm was reported by Alabadí et al. (2001). The difference in the amplitude of CCA1 in toc1-1 observed by us and by Alabadí et al. (2001) may be related to differences in the ages of plant materials used in the assays. For instance, based on the publically available microarray data, the relative expression levels between TOC1 and CHE vary in plants and/or tissue types at different ages. The relative levels of these two proteins will affect their net impact on the expression of CCA1 since TOC1 and CHE interact with each other and their effects on regulating the expression of CCA1 are different (Alabadí et al., 2001; Pruneda-Paz et al., 2009). However, it is not known at this point if toc1-1 can serve as a cofactor and interact with CHE.
Figure 8.
Expression of BOA Affected by TOC1 and GI.
(A) Effect of TOC1 on the expression of BOA. TOC1-1 mutants (toc1-1) and control (C24) plants were maintained under 12-h-light/12-h-dark photoperiods and released to continuous light for sample collection starting from 20 d after sowing. qRT-PCR analyses were conducted to quantify the expression of BOA.
(B) Effect of TOC1 on the expression of CCA1. qRT-PCR analyses were conducted to quantify the expression of CCA1.
(C) Effect of GI on the expression of BOA. Plants of GI mutants (gi-3 and gi-4) and wild type control (Ler-0) were maintained as in (A). qRT-PCR analyses were conducted to quantify the expression of BOA. Each data point represents the mean of three repeats ± sd.
GI regulates the stability of TOC1 protein through an interaction with ZTL, the F-box protein that targets TOC1 for proteasomal degradation, thus maintaining the high amplitude of TOC1 rhythms (Somers et al., 2000; Más et al., 2003b; Kim et al., 2007; Somers and Fujiwara, 2009). Therefore, it is important to establish whether or not GI affects the expression of BOA. GI mutants gi-3 and gi-4 have point mutations at C3929T and G4750A, respectively, and cause early termination of translation, generating truncated proteins (Fowler et al., 1999). The expression of TOC1 is arrhythmic in these two mutant lines. Similarly, the expression of BOA was also arrhythmic in gi-4 and dramatically reduced in gi-3 (Figure 8C). All these data indicate that BOA is a clock gene that forms a reciprocal regulatory loop with CCA1 and is potentially part of a circadian oscillator that involves TOC1 and GI.
DISCUSSION
The regulation of circadian clocks in plants is far more complicated than simple feedback regulation of transcription, and even a full understanding of the feedback regulatory loops for generating rhythmicity remains to be established, especially considering that direct positive regulator(s) of CCA1 and/or LHY remain to be identified (Locke et al., 2006; Más, 2008; McClung, 2008).
In this report, we demonstrated that BOA forms a reciprocal regulatory loop with CCA1 and potentially with LHY. The expression of BOA is repressed by CCA1 through direct binding of CCA1 to the EE of the BOA promoter. Although we did not investigate the possibility that LHY is capable of binding to the promoter of BOA, a loss-of-function mutant of LHY (lhy-21) showed similar effects as that of CCA1 (cca-11) on the rhythm of BOA (Figure 5C). The amplitude of BOA expression is dramatically reduced, with a very short period in cca1-1 lhy-21 double mutants, similar to that of TOC1, LUX, and CCR2 in CCA1 and LHY double loss-of-function plants (Alabadí et al., 2002; Mizoguchi et al., 2002; Hazen et al., 2005; Lu et al., 2009). BOA is most likely regulated by LHY as well, especially considering that LHY is able to bind to the same cis-element as CCA1 does and interacts with CCA1 and is capable of forming a heterodimer with CCA1 in vivo (Lu et al., 2009). BOA itself binds to the PBS3 and PBS4 elements upstream of the CCA1 promoter and activates the transcription of CCA1 both in vitro and in vivo. However, the accumulation of CCA1 transcripts does not peak in the same phase as that of BOA protein under continuous light conditions. In addition to BOA, a set of positive and negative regulators, including LHY, CHE, TOC1, and LUX, and potentially other unidentified factors, are also involved in the regulation of CCA1 expression (Schaffer et al., 1998; Alabadí et al., 2001; Hazen et al., 2005; Pruneda-Paz et al., 2009), which may, in part, contribute to the phase difference between the peaking time of CCA1 transcripts and the peaking time of BOA protein. Unlike the CCA1 promoter, we were not able to consistently enrich the LHY promoter in ChIP experiments using anti-BOA antibody, although the amplitude and period of LHY rhythm were affected when BOA was overexpressed in transgenic plants (Figure 7). This observation suggests that BOA either directly or indirectly affects the expression of LHY. The possibility of direct binding of BOA to the promoter of LHY during certain stages of development and/or in specific tissue/organ types remains as our ChIP assays were conducted using chromatin samples prepared from a single developmental stage.
Although BOA was expressed in all tissue types tested, it showed strong expression in flowers (Figure 3). The expression profile of BOA and its preferred interaction with the promoter of CCA1 may contribute to the functional differences between CCA1 and LHY even though CCA1 and LHY play partially redundant roles in the circadian clock of Arabidopsis (Alabadí et al., 2002; Mizoguchi et al., 2002). CHE binds to the promoter of CCA1 and negatively regulates its promoter activity but not that of LHY (Pruneda-Paz et al., 2009). LHY negatively regulates the expression of CCA1 (Schaffer et al., 1998). In our ChIP experiments, we can consistently enrich only the promoter of CCA1 but not that of LHY in the same chromatin preparation; however, overexpression of BOA does affect the rhythm of both CCA1 and LHY. The configuration of circadian oscillator(s) in different developmental stages and/or tissue/organ or cell types may differ by relying more on a certain combination of clock genes to sustain the circadian rhythmicity. Under constant light, LUX is required for activation of CCA1 (Hazen et al., 2005). The expression of CCA1 and LHY is arrhythmic in mutant lines lux-1 and lux-2 after the plants are released to constant light (Hazen et al., 2005). The potential functional redundancy between BOA and LUX and their interrelationships with CCA1 and LHY need to be further explored.
McClung (2006) placed TOC1 and LUX at similar positions in the model of the Arabidopsis circadian clock. Computational models indicate there may be an additional clock component that links TOC1 and CCA1/LHY and serves as a positive regulator to CCA1 and/or LHY (Locke et al., 2006; McClung, 2008; Pruneda-Paz et al., 2009). CHE is a negative regulator of CCA1 that is partially redundant with LHY in repression of CCA1 gene expression (Pruneda-Paz et al., 2009). CHE physically interacts with TOC1 and has been proposed to serve as a link between TOC1 and CCA1 (Pruneda-Paz et al., 2009). TOC1 might also regulate CCA1 by other mechanisms in addition to those that involve CHE as suggested by others, and a positive regulatory connection between TOC1 and CCA1 remains to be identified (Locke et al., 2006; Robertson and Webb, 2009). Overexpression of BOA affects the rhythms of TOC1 and GI. Likewise, gene expression of BOA was reduced in toc1-1 (Figure 8A). Data shown in Figure 8C indicate that the level of expression of BOA is reduced and/or arrhythmic in GI mutant lines. These data strongly indicate that clock function of BOA involves TOC1 and GI. Thus, we propose that BOA is a good candidate to serve as a positive regulatory link between TOC1 and CCA1.
Pruneda Paz et al. (2009) reported that TOC1 directly interacts with CHE and negatively regulates the expression of CCA1 and suggested that the relative amount of these two gene products may play a role in regulating clock function. TOC1 is capable of functioning as a transcription factor (Legnaioli et al., 2009). Thus, the interlocking regulatory relationships among different clock genes are likely to be more complicated than what was previously modeled. With the addition of BOA and CHE, the existing computational model of Arabidopsis circadian clock will continue to be updated.
METHODS
Plasmids
BOA coding sequence was amplified from a cDNA library prepared from Arabidopsis thaliana Col-0 seedlings using RT-PCR methods with primer set BOA-cD-5′-BglII/BOA-cD-3′-BglII (see Supplemental Table 1 online for primer sequences). The PCR product was digested with BglII, cloned into pMon999 (a plasmid from Monsanto Company containing the enhanced CaMV 35S promoter and Nos terminator) through BamHI and BglII sites, resulting in p35S:BOA. Similarly, BOA coding sequence lacking a stop codon was generated through PCR using primers BOA-cD-5′-BglII/BOA-cD-3′w/o*BglII and cloned into a pMon999-based plasmid p35S:GFP (Dai et al., 2006) through the BglII site, generating plasmid p35S:BOA:GFP in which the in-frame fusion protein BOA:GFP is under the control of CaMV 35S promoter. The 35S:BOA and 35S:BOA:GFP fusion genes were released from p35S:BOA and p35S:BOA:GFP using PstI/SmaI restriction sites, cloned into pCambia 1300 (http://www.cambia.org) using the same set of restriction sites, resulting in pC-35S:BOA and pC-35S:BOA:GFP, respectively. CCA1 coding sequence was amplified from an Arabidopsis cDNA library using RT-PCR with primers CCA1-cD-5′ Bm/CCA1-cD-3′ Nt. The PCR product was digested with BamHI and NotI restriction enzymes, cloned into pGEX4T-1 (Amersham Biosciences) through the same set of restriction sites, resulting in pGST:CCA1. CCA1 coding sequence was amplified from pGST:CCA1 using PCR with primers CCA1-5′ATG Bm/CCA1-cD-3′ Bm, digested with BamHI, and cloned into pMon999 through restriction sites BglII/BamHI, resulting in p35S:CCA1. The truncated CCA1 promoter with PBS3 and PBS4 binding sites plus 48-bp upstream sequences was amplified from Arabidopsis genomic DNA using PCR with primers CCA1-PBS3+48H3/CCA1-P3′-NC. The PCR product was digested with HindIII and NcoI and cloned into pE::GUS (Yin et al., 1997) to replace the E fragment of RTBV promoter using the same set restriction enzymes, resulting in pCCA1t:GUS. Similarly, truncated BOA promoter that contains the putative EE plus 49 bp of upstream sequences of EE was amplified from Arabidopsis genomic DNA using primers BOA-EE+49H3/BOA-p3′-NC and cloned into pE::GUS through HindIII/NcoI restriction sites to generate the pBOAt:GUS reporter gene.
Arabidopsis Plant Materials, Growth Conditions, and Transformation
Experiments were performed on Arabidopsis. Arabidopsis Col-0 ecotype was used to develop transgenic lines carrying pC-35S:BOA and pC-35S:BOA:GFP. A floral dip method was employed to generate transgenic lines (Bent, 2006). Seeds of Arabidopsis mutant lines cca1-11 (Cs9378) (Hall et al., 2003), lhy-21 (Cs9379) (Hall et al., 2003), cca1-11 lhy-21 (Cs9380) (Hall et al., 2003), toc1-1 (Cs3756) (Somers et al., 1998), gi-3 (cs51) (Fowler et al., 1999), and gi-4 (Cs181) (Fowler et al., 1999) were obtained from the ABRC. CCA1 overexpression line was provided by E.M. Tobin (Wang and Tobin, 1998). Plants of various mutant lines and controls were maintained in Conviron growth chambers (MPTS 120) with 70% humidity and ~200 μmol fluorescence light with a 12-light/12-dark period at 22°C unless the photoperiod is specified. Super Fine Geminating Mix (Farfard) was used as growth medium. Plants were maintained in Sanyo Chambers at 22°C and low-density constant light when they were released to constant light. For all time course studies, 30 to 40 seedlings were pooled as one sample for RNA isolation.
RNA Gel Blot Analysis
RNA samples isolated from different tissue types were resolved in 1.2% agarose denaturing gels, stained with ethidium bromide, imaged using an AlphaImager system (Alpha Innotech), and blotted to Hybond N+ membranes. Probe with sequences of the coding region of BOA was amplified from p35S:BOA using primers BOA-cD-5′-BglII and BOA-cD-3′-BglII in a PCR with deoxynucleotide triphosphate containing 32P-labeled dCTP. The membranes were hybridized with 32P labeled BOA probe at 68°C in 0.5 M NaHPO4, pH 7.2, and 7% SDS for 16 h, followed by three 30-min washes at 68°C in buffer containing 0.1% SDS plus 1× SSC, 0.5× SSC, or 0.1× SSC, respectively. Washed membrane was exposed to a phosphor screen (Molecular Dynamics) and signal detected on a Typhoon 9410 phosphor imager (Molecular Dynamics).
RT-PCR Analysis
RNA samples prepared using a Qiagen RNA isolation kit and treated with DNase I were subjected to RT-PCR analysis. RT-PCRs were conducted using the One-Step RT-PCR kit (Invitrogen) with specific primer sets as listed (see Supplemental Table 1 online). For each sample, 0.5 μg of DNase I–treated total RNA was used in the reaction. RT-PCRs were performed using a C-1000 thermocycler (Bio-Rad). RT-PCR products were resolved in 1.2% agarose gels and documented using a gel imaging system (Alpha Innotech). The RT-PCR program was as follows: 30 min at 50οC; 2 min at 95οC; 22 to 23 cycles of 30 s at 94οC, 30 s at 55οC and 1 min at 68οC, followed by one step at 68οC for 5 min.
Two-Step qRT-PCR Analysis
For qRT-PCR, 1.0 μg of total RNA was treated with DNase I (Ambion) and used for first-strand cDNA synthesis using A-MLV reverse transcriptase system (NEB). qPCR was conducted using SYBR advantage qPCR premix (Clontech) with specific primer sets listed in Supplemental Table 1 online. Amplification was performed in the StepOnePlus Real-Time PCR system (Applied Biosystems) with a reaction program as follows: 2 min at 95οC; 40 cycles of 30 s at 95οC, 30 s at 55οC, 30 s at 72οC, and 5 min at 72οC. Melt curve was determination as follows: 180 cycles, each cycle persisting for 10 s and the first cycle at 60οC, with an increase of 0.2οC each cycle after cycle 2. TUBULIN control reactions of each sample were included in same plate for data normalization. Three replicates were included in each time point/treatment. At least two overlapping samples were included in each plate to normalize the data among different plates. Data analysis was performed as described (Dai et al., 2008).
Immunoblot Analysis
Accumulation of BOA protein in wild-type or transgenic plants was analyzed using immunoblots. Protein samples were prepared from pooled plant tissues collected at each time point and quantified using the Dc protein assay kit (Bio-Rad). Thirty micrograms of each protein sample was resolved via 10% SDS-PAGE and blotted onto nitrocellulose (NC) membrane (Protran BA83; Whatman). Before immunoanalysis, NC membranes were stained with Ponceau S (Sigma-Adlrich) and imaged using a digital camera. Rabbit polyclonal antibodies against the peptide representing amino acids 204-298 of BOA was obtained from Monsanto Company. Membranes were incubated with rabbit anti-BOA antibody with a 1:4000 dilution in 1× PBS plus 5% skim milk overnight followed by 4 × 10 min of washing steps using 1± PBS plus 0.1% Triton X-100. After washing, the membranes were incubated with horseradish peroxidase–conjugated goat anti-rabbit second antibody (SouthernBiotech) for 2 h followed by 4 × 10 min washing steps. After washing, the horseradish peroxidase substrates (SuperSignal; Thermo Scientific) were applied to the membranes. Signals on the membrane were detected by exposing to x-ray film.
ChIP
The ChIP assays were performed by following a published protocol (Nagaki et al., 2003) with rabbit polyclonal antibodies raised against the C-terminal region of BOA (from amino acids 204 to 298) and prebleed (gift from the Monsanto Company). Chromatin was prepared from Col-0 plants maintained under 12-light/12-dark conditions and harvested at 20 d after sowing. The DNA samples from ChIP assays were subjected to PCR analysis using primers CCA1-P5′H3/CCA1-P3′ (see Supplemental Table 1 online). The PCR products were resolved in 1% agarose gels and imaged using a gel document system.
EMSAs
EMSAs were performed as previous described (Dai et al., 2004). For experiments of binding of CCA1 to the EE of BOA, CCA1 protein crude extracts were prepared from BL21(DE3) strains that either carry pGEX4T-1 control or pGST:CCA1 after isopropyl-β-d-thiogalactopyranoside induction and used in the EMSA. Nonspecific competitors poly(dI-dC) and poly(dA-dT) were included in each reaction. Specific competitors and mutants were included as labeled. For experiments to determine if BOA binds to PBS4 of the CCA1 promoter, purified BOA protein (a gift from the Monsanto Company) was used in the assays. Dried gels were exposed to a phosphor screen. Signal on the phosphor screen was detected using a phosphor imager.
BY-2 Protoplast Transient Assays
Tobacco (Nicotiana tabacum) BY-2 protoplast transient assays were performed as described (Dai et al., 2004). Protoplasts isolated from BY-2 suspension cells were cotransfected with either pBOAt:GUS/p35S:CCA1 or pBOAt:GUS/pMON999 control using a Gene Pulser II device with RF Module (Bio-Rad). The experiments were repeated three times with three replicates in each experiment. p35S::GFP was included in each transfection and served as an internal control. GUS activity of each sample was normalized against the GFP control prior to data comparison. GFP and GUS activity of each sample was quantified using a SpectraMAX Gemini florescence spectrophotometer (Molecular Devices).
Rice Whole-Cell Extract in Vitro Transcription Assay
In vitro transcription assays using rice whole-cell extract were conducted as described (Liu et al., 2007). RNA samples from in vitro transcription reactions with or without addition of purified BOA protein were subjected to primer extension analysis using reverse transcriptase from NEB. Samples from in vitro transcription reactions containing RNA polymerase II inhibitor α-aminitin were also included. 32P-labeled primer complementary to GUS coding sequence was used in the primer extension experiments. Samples of primer extension reactions were resolved in 5% denaturing PAGE gels. Dried gels were exposed to a phosphor screen for signal detection.
Period Length and Amplitude Calculation
Period length and amplitude was calculated using BRASS software downloaded from http://millar.bio.ed.ac.uk/PEBrown/BRASS/BrassPage.htm.
Microscopy
The subcellular localization of BOA:GFP fusion protein was visualized using a Zeiss LSM 510 multiphoton microscope at the Danforth Center Integrated Microscopy Core Facility.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: AT5G59570 (BOA), AT5G59560 (SRR1), AT2G46830 (CCA1), AT1G01060 (LHY), AT5G61380 (TOC1), AT1G22770 (GI), AT3G46640 (LUX).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Expression of CCA Is Suppressed in boa-1.
Supplemental Figure 2. The Nucleotide Sequences of the Proximal Region of the BOA Promoter.
Supplemental Figure 3. Transcription of BOA Is Suppressed in Transgenic Lines Overexpressing CCA1.
Supplemental Figure 4. The Nucleotide Sequence of the Proximal Region of the CCA1 Promoter with Predicted Putative BOA Binding Sites Highlighted.
Supplemental Figure 5. BOA Binds to PBS3 of the CCA1 Promoter.
Supplemental Figure 6. BOA Activates CCA1 Expression in Vivo after Plants Are Released to Constant Dark.
Supplemental Table 1. List of Primers Used.
Supplementary Material
Acknowledgments
We thank Monsanto Company for financial support of this work and for providing purified BOA protein and polyclonal antibodies against BOA. We thank Marie Petracek for critical discussion and Sarah Yongstrom, Yunjing Wang, and Jeremy Tate for technical assistance. We thank ABRC for the T-DNA insertion lines of CCA1, LHY, GI, and TOC1 and those labs who generously donated these lines to ABRC. We thank E.M. Tobin for the CCA1-OX line.
References
- Alabadí D., Oyama T., Yanovsky M.J., Harmon F.G., Más P., Kay S.A. (2001). Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883 [DOI] [PubMed] [Google Scholar]
- Alabadí D., Yanovsky M.J., Más P., Harmer S.L., Kay S.A. (2002). Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Curr. Biol. 12: 757–761 [DOI] [PubMed] [Google Scholar]
- Baudry A., Kay S. (2008). Clock control over plant gene expression. Advances in Botanical Research, Kader J.-C., Michel D., (San Diego: Academic Press; ), pp. 69–105 [Google Scholar]
- Bent A. (2006). Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 343: 87–103 [DOI] [PubMed] [Google Scholar]
- Dai S., Wei X., Alfonso A.A., Pei L., Duque U.G., Zhang Z., Babb G.M., Beachy R.N. (2008). Transgenic rice plants that overexpress transcription factors RF2a and RF2b are tolerant to rice tungro virus replication and disease. Proc. Natl. Acad. Sci. USA 105: 21012–21016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Zhang Z., Bick J., Beachy R.N. (2006). Essential role of the Box II cis element and cognate host factors in regulating the promoter of Rice tungro bacilliform virus. J. Gen. Virol. 87: 715–722 [DOI] [PubMed] [Google Scholar]
- Dai S., Zhang Z., Chen S., Beachy R.N. (2004). RF2b, a rice bZIP transcription activator, interacts with RF2a and is involved in symptom development of rice tungro disease. Proc. Natl. Acad. Sci. USA 101: 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel X., Sugano S., Tobin E.M. (2004). CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 3292–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A.N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J.M., Millar A.J., Webb A.A. (2005). Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633 [DOI] [PubMed] [Google Scholar]
- Dunlap J.C. (1999). Molecular bases for circadian clocks. Cell 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Farré E.M., Harmer S.L., Harmon F.G., Yanovsky M.J., Kay S.A. (2005). Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 15: 47–54 [DOI] [PubMed] [Google Scholar]
- Fowler S., Lee K., Onouchi H., Samach A., Richardson K., Morris B., Coupland G., Putterill J. (1999). GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 18: 4679–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.M., Tingay S., Wang Z.Y., Tobin E.M. (2002). Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 129: 576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.M., Tobin E.M. (1999). Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc. Natl. Acad. Sci. USA 96: 4176–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R.A., Stokes T.L., Thum K., Xu X., Obertello M., Katari M.S., Tanurdzic M., Dean A., Nero D.C., McClung C.R., Coruzzi G.M. (2008). Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc. Natl. Acad. Sci. USA 105: 4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Bastow R.M., Davis S.J., Hanano S., McWatters H.G., Hibberd V., Doyle M.R., Sung S., Halliday K.J., Amasino R.M., Millar A.J. (2003). The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell 15: 2719–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton E.E., Kay S.A. (2008). SnapShot: Circadian clock proteins. Cell 135: 368–368. e1 [DOI] [PubMed] [Google Scholar]
- Hazen S.P., Schultz T.F., Pruneda-Paz J.L., Borevitz J.O., Ecker J.R., Kay S.A. (2005). LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc. Natl. Acad. Sci. USA 102: 10387–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K., Imamura A., Katoh E., Hatta T., Tachiki M., Yamada H., Mizuno T., Yamazaki T. (2002). Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14: 2015–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Chen H.C., Sheen J. (2002). Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 129: 500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikis E.A., Khanna R., Quail P.H. (2005). ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. 44: 300–313 [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Fujiwara S., Suh S.S., Kim J., Kim Y., Han L., David K., Putterill J., Nam H.G., Somers D.E. (2007). ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449: 356–360 [DOI] [PubMed] [Google Scholar]
- Kolmos E., Davis S.J. (2007). ELF4 as a central gene in the circadian clock. Plant Signal. Behav. 2: 370–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnaioli T., Cuevas J., Mas P. (2009). TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 28: 3745–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dai S., Beachy R.N. (2007). Role of the C-terminal domains of rice (Oryza sativa L.) bZIP proteins RF2a and RF2b in regulating transcription. Biochem. J. 405: 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J.C., Kozma-Bognár L., Gould P.D., Fehér B., Kevei E., Nagy F., Turner M.S., Hall A., Millar A.J. (2006). Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol. Syst. Biol. 2: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J.C., Southern M.M., Kozma-Bognar L., Hibberd V., Brown P.E., Turner M.S., Millar A.J. (2005). Extension of a genetic network model by iterative experimentation and mathematical analysis. Mol. Syst. Biol. 1: 2005.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.X., Knowles S.M., Andronis C., Ong M.S., Tobin E.M. (2009). CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 150: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más P. (2008). Circadian clock function in Arabidopsis thaliana: time beyond transcription. Trends Cell Biol. 18: 273–281 [DOI] [PubMed] [Google Scholar]
- Más P., Alabadí D., Yanovsky M.J., Oyama T., Kay S.A. (2003a). Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 15: 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más P., Kim W.Y., Somers D.E., Kay S.A. (2003b). Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426: 567–570 [DOI] [PubMed] [Google Scholar]
- McClung C.R. (2006). Plant circadian rhythms. Plant Cell 18: 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.R. (2008). Comes a time. Curr. Opin. Plant Biol. 11: 514–520 [DOI] [PubMed] [Google Scholar]
- McWatters H.G., Kolmos E., Hall A., Doyle M.R., Amasino R.M., Gyula P., Nagy F., Millar A.J., Davis S.J. (2007). ELF4 is required for oscillatory properties of the circadian clock. Plant Physiol. 144: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael T.P., Salomé P.A., Yu H.J., Spencer T.R., Sharp E.L., McPeek M.A., Alonso J.M., Ecker J.R., McClung C.R. (2003). Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302: 1049–1053 [DOI] [PubMed] [Google Scholar]
- Millar A.J., Carré I.A., Strayer C.A., Chua N.H., Kay S.A. (1995). Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267: 1161–1163 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Wheatley K., Hanzawa Y., Wright L., Mizoguchi M., Song H.R., Carré I.A., Coupland G. (2002). LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell 2: 629–641 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Wright L., Fujiwara S., Cremer F., Lee K., Onouchi H., Mouradov A., Fowler S., Kamada H., Putterill J., Coupland G. (2005). Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17: 2255–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Talbert P.B., Zhong C.X., Dawe R.K., Henikoff S., Jiang J. (2003). Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163: 1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z., Kim E.D., Ha M., Lackey E., Liu J., Zhang Y., Sun Q., Chen Z.J. (2009). Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 457: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai K., Ishiura M. (2005). PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells 10: 963–972 [DOI] [PubMed] [Google Scholar]
- Park D.H., Somers D.E., Kim Y.S., Choy Y.H., Lim H.K., Soh M.S., Kim H.J., Kay S.A., Nam H.G. (1999). Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285: 1579–1582 [DOI] [PubMed] [Google Scholar]
- Perales M., Más P. (2007). A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell 19: 2111–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda-Paz J.L., Breton G., Para A., Kay S.A. (2009). A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science 323: 1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F.C., Webb A.A. (2009). Revolutionary functional genomics liberates CHE. Nat. Chem. Biol. 5: 276–277 [DOI] [PubMed] [Google Scholar]
- Salomé P.A., Xie Q., McClung C.R. (2008). Circadian timekeeping during early Arabidopsis development. Plant Physiol. 147: 1110–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R., Ramsay N., Samach A., Corden S., Putterill J., Carré I.A., Coupland G. (1998). The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93: 1219–1229 [DOI] [PubMed] [Google Scholar]
- Schöning J.C., Staiger D. (2005). At the pulse of time: Protein interactions determine the pace of circadian clocks. FEBS Lett. 579: 3246–3252 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Fujiwara S. (2009). Thinking outside the F-box: Novel ligands for novel receptors. Trends Plant Sci. 14: 206–213 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Schultz T.F., Milnamow M., Kay S.A. (2000). ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101: 319–329 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Webb A.A., Pearson M., Kay S.A. (1998). The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125: 485–494 [DOI] [PubMed] [Google Scholar]
- Staiger D., Allenbach L., Salathia N., Fiechter V., Davis S.J., Millar A.J., Chory J., Fankhauser C. (2003). The Arabidopsis SRR1 gene mediates phyB signaling and is required for normal circadian clock function. Genes Dev. 17: 256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer C., Oyama T., Schultz T.F., Raman R., Somers D.E., Más P., Panda S., Kreps J.A., Kay S.A. (2000). Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289: 768–771 [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Tobin E.M. (1998). Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Wijnen H., Young M.W. (2006). Interplay of circadian clocks and metabolic rhythms. Annu. Rev. Genet. 40: 409–448 [DOI] [PubMed] [Google Scholar]
- Yin Y., Chen L., Beachy R. (1997). Promoter elements required for phloem-specific gene expression from the RTBV promoter in rice. Plant J. 12: 1179–1188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.