Abstract
Context
The prevalence of severe obesity is increasing markedly, as is prevalence of comorbid conditions such as hypertension and type 2 diabetes mellitus; however, apart from bariatric surgery and pharmacotherapy, few clinical trials have evaluated the treatment of severe obesity.
Objective
To determine the efficacy of a weight loss and physical activity intervention on the adverse health risks of severe obesity.
Design, Setting, and Participants
Single-blind randomized trial conducted from February 2007 through April 2010 at the University of Pittsburgh. Participants were 130 (37% African American) severely obese (class II or III) adult participants without diabetes recruited from the community.
Interventions
One-year intensive lifestyle intervention consisting of diet and physical activity. One group (initial physical activity) was randomized to diet and physical activity for the entire 12 months; the other group (delayed physical activity) had the identical dietary intervention but with physical activity delayed for 6 months.
Main Outcome Measures
Changes in weight. Secondary outcomes were additional components comprising cardiometabolic risk, including waist circumference, abdominal adipose tissue, and hepatic fat content.
Results
Of 130 participants randomized, 101 (78%) completed the 12-month follow-up assessments. Although both intervention groups lost a significant amount of weight at 6 months, the initial-activity group lost significantly more weight in the first 6 months compared with the delayed-activity group (10.9 kg [95% confidence interval {CI}, 9.1–12.7] vs 8.2 kg [95% CI, 6.4–9.9], P=.02 for group×time interaction). Weight loss at 12 months, however, was similar in the 2 groups (12.1 kg [95% CI, 10.0–14.2] vs 9.9 kg [95% CI, 8.0–11.7], P=.25 for group×time interaction). Waist circumference, visceral abdominal fat, hepatic fat content, blood pressure, and insulin resistance were all reduced in both groups. The addition of physical activity promoted greater reductions in waist circumference and hepatic fat content.
Conclusion
Among patients with severe obesity, a lifestyle intervention involving diet combined with initial or delayed initiation of physical activity resulted in clinically significant weight loss and favorable changes in cardiometabolic risk factors.
The high and sustained prevalence of obesity is among the most significant public health problems for the 21st century.1 Rates of severe obesity have increased even faster during the past 3 decades,2,3 particularly among African American women.1 This has clinically important implications because of the increased prevalence of comorbid conditions, especially hypertension and type 2 diabetes mellitus,4,5 among persons with severe obesity.
In the face of the obesity epidemic, there is a dearth of evidence-based treatment guidelines for severe obesity. While bariatric surgery generally results in a large, sustained weight loss6,7 and can dramatically improve some comorbid conditions, notably diabetes,8 the long-term health effects are not fully understood. Moreover, bariatric surgery is performed in only 1% of severely obese adults annually, making it an unlikely public health solution for the rapidly increasing prevalence of severe obesity. With regard to nonsurgical treatment of persons with severe obesity, it is frequently stated, even in contemporary articles,9 that lifestyle intervention approaches are ineffective.
Axiomatic in the contemporary approaches to treatment of obesity is that clinically significant health benefits are obtained with modest weight loss ranging from as little as 5% to 10% of initial body weight.10 However, few published clinical trials have been specifically designed to determine the effectiveness of a lifestyle intervention in adults with severe obesity.11–13 As a result, practitioners lack evidence-based guidelines for treatment of the most severely obese persons, arguably the subgroup most seriously affected in the ongoing obesity epidemic.
We conducted a 1-year randomized trial to examine the effects of an intensive lifestyle intervention on weight loss in severely obese adults. We specifically examined whether adoption of a physical activity program in addition to a dietary intervention would promote additional weight loss compared with a dietary intervention alone and whether these changes would differ in African American and white individuals. We also examined changes in waist circumference, visceral abdominal fat, hepatic steatosis, and other cardiometabolic risk factors.
METHODS
Patient Recruitment
Participants were recruited via television and newspaper advertisements and mass mailings. The study was reviewed and approved by the human ethics committees of the University of Pittsburgh. Recruitment commenced in February 2007, the last participant was randomized in March 2009, and all data were available for analysis in April 2010. All participants provided written informed consent to participate in the study.
Inclusion/Exclusion Criteria
Participants were eligible if they were between the ages of 30 and 55 years and had severe obesity, defined as body mass index between 35 and 39.9 (calculated as weight in kilograms divided by height in meters squared) for class II obesity and 40 or greater for class III obesity. Race/ethnicity was self-reported. Participants had to be able to walk without assistance, commit to the schedule of intervention and assessment visits, and obtain medical clearance for intervention. Candidates were excluded if they had a history of cancer within the past 5 years, had a history of or were receiving current treatment for coronary artery disease, had enrolled within the past year in a formal weight reduction program, reported losing more than 5% of current body weight in the previous 6 months, had a history of bariatric surgery, or had uncontrolled hypertension, diabetes, or pregnancy during the previous 6 months.
Randomization
Participants were randomized into 2 study groups, with blocking according to sex, level of obesity (class II and III), and race/ethnicity (African American and white). Intervention assignment was blocked by race/ethnicity to ensure equal racial/ethnic representation across study groups. The study was single-blind; assessors for all outcomes were blinded to participant group assignment, and all outcomes data were kept blinded until final data entry for 12-month assessments was completed.
Treatment Groups
Participants were randomized to a1-year lifestyle intervention consisting of diet and physical activity. One group was randomized to diet and physical activity for the entire 12 months (initial physical activity), while the other group had the identical dietary intervention but with physical activity delayed for 6 months (delayed physical activity). The behavioral lifestyle intervention program was delivered with a combination of group, individual, and telephone contacts.14 During months 1 through 6, participants received 3 group meetings and 1 individual contact per month. During months 7 through 12, participants received 2 group sessions and 2 telephone contacts per month. All participants were prescribed a diet that we have shown to result in a sustained 8% to 10% weight loss in 12 months.15 Energy intake was reduced to 1200 to 2100 kcal/d based on initial body weight. Targeted macro-nutrient composition was 20% to 30% fat, 50% to 55% carbohydrate, and 20% to 25% protein. To facilitate dietary compliance and improve weight loss,14 liquid and prepackaged meal replacements were provided at no cost for all but 1 meal per day during months 1 through 3 and for only 1 meal replacement per day during months 4 through 6 of the intervention. Adherence to the dietary intervention was monitored by having participants record the time of meals as well as the type and caloric value of food consumed.
A progressive physical activity program was prescribed at the onset of treatment for participants in the initial-activity group and following the initial 6 months of the dietary intervention for those in the delayed-activity group. Moderate-intensity physical activity, similar in intensity to brisk walking, was prescribed and progressed to 60 minutes, 5 days perweek.16,17 To maximize adoption and maintenance of physical activity, participants were allowed to accumulate multiple 10-minute physical activity sessions per day, were provided with a pedometer and step goals of more than 10 000 steps per day, and were instructed to self-monitor their physical activity in a weekly diary. Participants received modest financial compensation for their participation to offset costs incurred for their participation in the study. To promote adherence to the behavior intervention, participants were provided with low-cost supplies related to the intervention (eg, pedometer, exercise videos) and were eligible to periodically receive small financial incentives for adherence to the behavioral goals of the intervention.
Primary and Secondary Outcomes
Body weight, height, and waist circumference were measured using standard protocols.18 Body fat and fat-free mass were determined either by dual-energy x-ray absorptiometry or by air-displacement plethysmography in 24 participants exceeding the weight capacity of the scanner (>136 kg). Computed tomography scans were performed at baseline and at 6 months to quantify abdominal adipose tissue and hepatic fat content as previously described.19 Other secondary outcomes included blood pressure and levels of fasting glucose, insulin, hepatic enzymes, and lipids including cholesterol and triglycerides. Insulin resistance was calculated using the homeostatic model assessment method.20 Multisensor physical activity monitors (Sensewear Pro3; BodyMedia, Pittsburgh, Pennsylvania) were worn between 7 and 11 consecutive days (mean, 7.2 [SD, 1.8] days; 23.1 [SD, 0.6] hours/d) at baseline, 6 months, and 12 months to provide objective measures of physical activity.
Statistical Analysis
Categorical variables were analyzed with χ2 tests. Intention-to-treat analysis of primary and secondary outcomes was performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina), with the type I error rate fixed at .05 (2-tailed). The Markov chain Monte Carlo method was used to impute missing data. A total of 10 imputations were generated. Separate mixed-effects models were fit for each of these outcomes, which were measured repeatedly at baseline, 6 months, and 12 months. Main effects of treatment group and time, as well as the treatment group × time interaction effect were examined in the mixed-effect models using the unstructured dependence structure. Least-square means were obtained from the mixed-effects models. Results from each imputation were then combined using the PROC MIANALYZE procedure in SAS.
Based on our results from a similar weight loss intervention study,15 an a priori power analysis was performed to determine the sample size necessary to detect significant weight loss in both groups combined at 12 months and difference at 6 months between the initial physical activity and delayed physical activity groups. Furthermore, a systematic review of the literature21 suggests that diet alone results in 2% to 3% less weight loss than what is achieved with the combination of diet plus physical activity. Therefore, assuming 6.5% weight loss in the initial-activity group and 9.0% in the delayed-activity group with an SD of 4.5% (effect size, 0.55) and with α fixed at .05 for a 2-tailed test and power set at 0.80, a sample size of 53 participants per experimental condition would be required to detect this difference in weight loss at 6 months. Assuming an attrition rate of 20%, this would require 64 participants per experimental condition (128 total participants). Thus, we randomized 130 participants in this study to provide a sufficient sample to detect these estimated differences in weight loss at 6 months.
RESULTS
Study Participants
The flow of study participants is shown in Figure 1. Retention rates for the initial physical activity group were 90% at 6 months and 73% at 12 months. Retention rates for the delayed physical activity group were 90% at 6 months and 83% at 12 months. These rates were not different between groups. The baseline characteristics of the study groups are shown in Table 1. There were no significant differences in demographics or characteristics contributing to outcomes between study groups among those enrolled in the study. Seventy-five percent of participants had class III obesity, and these proportions were similar in the study groups (Table 1). There were no racial/ethnic differences in body weight or body fat at baseline. Participants who dropped out were similar to those who completed intervention, except they were younger (44.1 years [SD, 6.9] vs 47.5 years [SD, 6.0], P=.02).
Figure 1.
Flow of Participant Recruitment, Screening, and Assessment
Table 1.
Baseline Characteristics of Initial Physical Activity and Delayed Physical Activity Groups
| Parameter | No. (%) | P Value | |
|---|---|---|---|
| Initial Activity (n = 67) | Delayed Activity (n = 63) | ||
| Age, mean (SD), y | 46.1 (6.5) | 47.5 (6.2) | .19 |
| Men | 10 (14.9) | 5 (7.9) | .21 |
| African American | 25 (37.3) | 23 (36.5) | .92 |
| BMI, mean (SD)a | 43.5 (4.8) | 43.7 (5.9) | .85 |
| Obesity severityb | |||
| Class II | 17 (25) | 15 (24) | .84 |
| Class III | 50 (75) | 48 (76) | .84 |
| Blood pressure medication | 22 (32.8) | 23 (36.5) | .99 |
| Lipid-lowering medication | 4 (6.0) | 6 (9.5) | .99 |
| Smoker | 8 (11.9) | 3 (4.8) | .99 |
Abbreviations: BMI, body mass index; CI, confidence interval.
Calculated as weight in kilograms divided by height in meters squared.
Class II defined as BMI between 35 and 39.9; class III defined as BMI of 40 or greater.
Weight Loss
The initial physical activity group received 75.1% (SD, 27.3%) of total intended intervention contact, which was not different compared with 71.9% (SD, 25.6%) for the delayed physical activity group (P=.49). Both groups lost a significant amount of weight at 6 months (10.9 kg [95% confidence interval {CI}, 9.1–12.7] vs 8.2 kg [95% CI, 6.4–9.9]) and 12 months (12.1 kg [95% CI, 10.0–14.2] vs 9.9 kg [95% CI, 8.0–11.7]) (Table 2, PFigure 2). The initial-activity group lost significantly more weight in the first 6 months compared with the delayed-activity group. While the magnitude of weight loss did not differ between groups at 12 months, the significant group×time interaction (=.02) reflects the greater weight loss at 6 months in the initial-activity compared with the delayed-activity group (Table 2).
Table 2.
Change in Body Weight, Body Composition, and Metabolic Parameters
| Outcome Variable | Assessment Period, Mean (95% CI)a |
P Value
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Time
|
Group
|
Group ×Time Interaction
|
|||||||
| Baseline | Month 6 | Month 12 | Month 6 vs Baseline | Month 12 vs Baseline | Baseline to Month 6 | Baseline to Month 12 | |||
| Weight, kg | |||||||||
| Initial activity (n = 67) | 120.58 (116.34–124.81) | 109.68 (105.66–113.69) | 108.46 (103.95–112.96) |
|
<.001 | <.001 | .30 | .02 | .25 |
|
| |||||||||
| Delayed activity (n = 63) | 117.37 (113.00–121.73) | 109.21 (105.11–113.31) | 107.50 (102.99–112.01) | ||||||
|
| |||||||||
| BMI | |||||||||
| Initial activity (n = 67) | 43.51 (42.21–44.81) | 39.62 (38.37–40.87) | 39.19 (37.74–40.64) |
|
<.001 | <.001 | .86 | .06 | .40 |
|
| |||||||||
| Delayed activity (n = 63) | 43.67 (42.33–45.02) | 40.59 (39.32–41.87) | 39.95 (38.50–41.40) | ||||||
|
| |||||||||
| Waist circumference, cm | |||||||||
| Initial activity (n = 67) | 124.35 (121.42–127.28) | 115.75 (112.76–118.75) | 114.15 (110.73–117.57) |
|
<.001 | <.001 | .22 | .01 | .27 |
|
| |||||||||
| Delayed activity (n = 63) | 121.70 (118.68–124.72) | 116.53 (113.52–119.54) | 113.41 (110.11–116.71) | ||||||
|
| |||||||||
| Abdominal subcutaneous fat, cm2 | |||||||||
| Initial activity (n = 67) | 718.00 (675.26–760.80) | 605.21 (566.69–643.74) | NAb |
|
<.001 | NA | .99 | .16 | NA |
|
| |||||||||
| Delayed activity (n = 63) | 717.56 (673.46–761.67) | 629.71 (589.93–669.49) | NAb | ||||||
|
| |||||||||
| Visceral fat, cm2 | |||||||||
| Initial activity (n = 67) | 199.39 (180.46–218.33) | 170.66 (155.77–185.55) | NAb |
|
.003 | NA | .34 | .58 | NA |
|
| |||||||||
| Delayed activity (n = 63) | 186.13 (166.61–205.66) | 163.23 (147.35–179.12) | NAb | ||||||
|
| |||||||||
| Liver fat, liver-spleen HU | |||||||||
| Initial activity (n = 66)c | 1.06 (1.00–1.12) | 1.18 (1.14–1.22) | NAb |
|
.004 | NA | .47 | .046 | NA |
|
| |||||||||
| Delayed activity (n = 63) | 1.09 (1.03–1.15) | 1.15 (1.11–1.20) | NAb | ||||||
|
| |||||||||
| Body fat, kg (n = 118) | |||||||||
| Initial activity (n = 67) | 60.40 (57.40–63.40) | 51.74 (48.89–54.59) | 51.23 (47.99–54.47) |
|
<.001 | <.001 | .60 | .008 | .27 |
|
| |||||||||
| Delayed activity (n = 63) | 59.24 (56.08–62.39) | 53.33 (50.34–56.33) | 51.90 (48.55–55.26) | ||||||
|
| |||||||||
| Fat-free mass, kg (n = 118) | |||||||||
| Initial activity (n = 62) | 58.73 (56.51–60.94) | 56.32 (54.08–58.55) | 56.00 (53.74–58.25) |
|
<.001 | <.001 | .18 | .52 | .49 |
|
| |||||||||
| Delayed activity (n = 56) | 56.55 (54.22–58.88) | 54.45 (52.14–56.77) | 54.18 (51.82–56.54) | ||||||
|
| |||||||||
| Systolic BP, mm Hg | |||||||||
| Initial activity (n = 67) | 135.43 (132.08–138.78) | 132.04 (128.14–135.94) | 120.60 (116.22–124.98) |
|
.37 | <.001 | .68 | .60 | >.99 |
|
| |||||||||
| Delayed activity (n = 63) | 134.43 (130.97–137.88) | 132.57 (128.59–136.54) | 119.60 (115.26–123.94) | ||||||
|
| |||||||||
| Diastolic BP, mm Hg | |||||||||
| Initial activity (n = 67) | 78.01 (75.97–80.05) | 75.68 (73.29–78.08) | 72.38 (70.31–74.46) |
|
.25 | <.001 | .50 | .67 | .82 |
|
| |||||||||
| Delayed activity (n = 63) | 77.00 (74.89–79.10) | 75.46 (73.03–77.89) | 71.74 (69.50–73.98) | ||||||
|
| |||||||||
| Mean arterial pressure, mm Hg | |||||||||
| Initial activity (n = 67) | 96.96 (94.61–99.31) | 94.12 (91.36–96.87) | 88.36 (85.68–91.04) |
|
.30 | <.001 | .55 | .51 | .98 |
|
| |||||||||
| Delayed activity (n = 63) | 95.95 (93.53–98.38) | 94.46 (91.71–97.21) | 87.30 (84.54–90.05) | ||||||
|
| |||||||||
| Alkaline phosphatase, U/L | |||||||||
| Initial activity (n = 67) | 86.46 (81.95–90.98) | 77.29 (72.71–81.88) | 70.20 (65.74–74.66) |
|
<.001 | <.001 | .96 | .56 | .23 |
|
| |||||||||
| Delayed activity (n = 63) | 86.30 (81.64–90.96) | 78.54 (73.63–83.45) | 72.98 (68.40–77.56) | ||||||
|
| |||||||||
| ALT, U/L | |||||||||
| Initial activity (n = 67) | 30.58 (28.42–32.74) | 28.79 (26.49–31.09) | 22.09 (19.95–24.23) |
|
.02 | <.001 | .86 | .63 | .31 |
|
| |||||||||
| Delayed activity (n = 63) | 30.30 (28.07–32.53) | 27.83 (25.49–30.17) | 23.33 (21.34–25.31) | ||||||
|
| |||||||||
| AST, U/L | |||||||||
| Initial activity (n = 67) | 25.81 (24.26–27.35) | 23.82 (22.09–25.55) | 23.71 (21.56–25.86) |
|
.049 | .32 | .41 | .72 | .55 |
|
| |||||||||
| Delayed activity (n = 63) | 24.87 (23.28–26.46) | 23.28 (21.53–25.03) | 23.77 (21.90–25.65) | ||||||
|
| |||||||||
| Total cholesterol, mg/dL | |||||||||
| Initial activity (n = 67) | 185.75 (178.05–193.44) | 181.64 (173.88–189.40) | 184.55 (175.88–193.22) |
|
.72 | .81 | .20 | .45 | .94 |
|
| |||||||||
| Delayed activity (n = 63) | 192.94 (185.00–200.87) | 191.92 (183.90–199.95) | 192.09 (183.17–201.00) | ||||||
|
| |||||||||
| HDL-C, mg/dL | |||||||||
| Initial activity (n = 67) | 47.27 (44.63–49.90) | 44.89 (42.20–47.59) | 45.88 (42.86–48.90) |
|
<.001 | .12 | .45 | .35 | .87 |
|
| |||||||||
| Delayed activity (n = 63) | 48.71 (46.00–51.43) | 45.10 (42.24–47.96) | 47.07 (44.08–50.05) | ||||||
|
| |||||||||
| Triglycerides, mg/dL | |||||||||
| Initial activity (n = 67) | 127.51 (112.23–142.79) | 123.23 (109.35–137.11) | 115.53 (100.52–130.54) |
|
.27 | .20 | .77 | .20 | .83 |
|
| |||||||||
| Delayed activity (n = 63) | 124.29 (108.53–140.04) | 131.90 (116.71–147.08) | 114.54 (98.82–130.27) | ||||||
|
| |||||||||
| Glucose, mg/dL | |||||||||
| Initial activity (n = 67) | 93.69 (90.94–96.43) | 90.11 (87.49–92.73) | 92.51 (89.57–95.46) |
|
.07 | .78 | .80 | .57 | .50 |
|
| |||||||||
| Delayed activity (n = 63) | 93.17 (90.35–96.00) | 90.66 (87.85–93.47) | 93.65 (90.66–96.65) | ||||||
|
| |||||||||
| Insulin, uU/mL | |||||||||
| Initial activity (n = 67) | 17.07 (14.81–19.33) | 12.06 (10.36–13.75) | 12.33 (10.21–14.46) |
|
<.001 | .004 | .46 | .63 | .82 |
|
| |||||||||
| Delayed activity (n = 63) | 15.83 (13.37–18.30) | 11.61 (9.86–13.36) | 11.55 (9.22–13.88) | ||||||
|
| |||||||||
| HOMA-IR | |||||||||
| Initial activity (n = 67) | 4.03 (3.47–4.60) | 2.70 (2.30–3.11) | 2.87 (2.35–3.38) |
|
<.001 | .01 | .40 | .46 | .67 |
|
| |||||||||
| Delayed activity (n = 63) | 3.68 (3.07–4.28) | 2.65 (2.23–3.07) | 2.73 (2.16–3.29) | ||||||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HU, Hounsfield units; NA, not available.
SI conversion factors: To convert total cholesterol and HDL-C values from mg/dL to mmol/L, multiply by 0.0259; triglyceride values from mg/dL to mmol/L, by 0.0113; glucose values from mg/dL to mmol/L, by 0.0555; and insulin values from μIU/mL to pmol/L, by 6.945.
Means with multiple imputation used for missing data.
Measurement of parameters determined by computed tomography conducted only at baseline and 6 months.
Number of liver-fat measurements at baseline in the initial-activity group is 66, because 1 organ scan was of poor quality.
Figure 2. Absolute and Percentage Weight Loss in Initial Physical Activity and Delayed Physical Activity Groups.
Means estimated using an intention-to-treat analysis using multiple imputations. Error bars indicate 95% confidence intervals. Effects for absolute weight loss: time (0–6 and 0–12 months), P<.001; group, P=.99; group×time, P=.10 for 0 to 6 months and P=.14 for 0 to 12 months. Effects for percentage weight loss: time (0–6 and 0–12 months), P<.001; group, P=.99; group×time: P=.14 for 0 to 6 months and P=.25 for 0 to 12 months.
In a secondary analysis of study completers (Figure 3), 80% of initial-activity participants vs 60% of delayed-activity participants lost more than 5% of their baseline body weight at 6 months, whereas the percentage of participants achieving this magnitude of weight loss at 12 months was 78% in the initial-activity group vs 65% in the delayed-activity group. The heavier class III obese participants in our study lost significantly more weight than class II obese participants (10.9% [95% CI, 8.9%–13.0%] vs 7% [95% CI, 4.2%–9.9%] at 12 months; P=.047), although the intervention group did not confound these differences. The pattern of results comparing the intervention groups, however, was not affected when either obesity class or race/ethnicity was included in the analysis. Moreover, the intervention effects were consistent when outcomes were analyzed using baseline observations carried forward (eTable, available at http://www.jama.com) or with mixed-effects modeling using data missing at random.
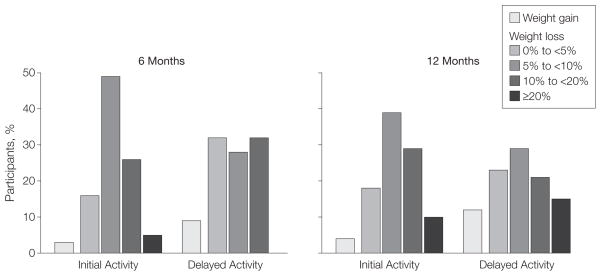
Figure 3. Percentage Weight Gain or Loss Among Participants Completing Intervention at 6 and 12 Months.
At 6 months, 61 of 67 participants in the initial physical activity group and 57 of 63 in the delayed physical activity group had completed the intervention; the corresponding numbers at 12 months were 49 of 67 in the initial-activity group and 52 of 63 in the delayed-activity group. The Cochran-Armitage trend test indicated a trend for higher weight loss (P=.04) in the initial-activity group compared with the delayed-activity group. No participants in the delayed-activity group lost more than 20% body weight at 6 months.
Body Composition, Waist Circumference, Visceral Adiposity, and Hepatic Fat
Both groups had a significant reduction in body fat and waist circumference at 6 and 12 months (Table 2). The initial physical activity group, however, had significantly greater reductions in body fat and waist circumference at 6 months compared with the delayed physical activity group. However, there were no differences in body fat or waist circumference at the completion of the subsequent 6 months, at which time both groups were engaged in physical activity. Both groups lost a significant but similar amount of visceral abdominal and subcutaneous abdominal adipose tissue in the first 6 months. Hepatic fat content was also decreased in both groups, although this decrease was significantly greater in the initial-activity group.
Physical Activity
The study groups engaged in similar amounts of physical activity at baseline as measured by the number of steps per day and the amount of time engaged in vigorous activity, the latter defined by minutes per week spent in activity with more than 6 metabolic equivalent tasks. At 6 months, the initial-activity group had significantly (P<.001) increased the number of steps per day from 7048 (SD, 2886) to 8475 (SD, 2927) and was engaged in approximately twice the amount of vigorous physical activity (71 [SD, 88 min/wk vs 34 [SD, 49] min/wk, respectively; P=.01). The delayed-activity group did not significantly increase their physical activity in the first 6 months. The initial-activity group maintained their amount of physical activity between 6 and 12 months, while the delayed-activity group significantly increased the number of daily steps (7047 [SD, 2597] steps to 7991 [SD, 2949] steps, P=.01) and minutes of vigorous activity (36 [SD, 45] to 53 [SD, 70], P=.03) completed per week from baseline to 12 months, at which time there was no difference in the amount of physical activity between the groups.
Other Health Outcomes
Table 2 shows changes in selected clinical measures of health, mostly related to cardiometabolic risk factors. Blood pressure was significantly and similarly reduced in both intervention groups. Levels of serum liver enzymes were reduced in both groups. Fasting insulin and insulin resistance improved significantly and similarly in both intervention groups. There was no difference in adverse events between groups. There was no significant measurable change in use of antihypertensive or lipid-lowering medications, nor was there a between-group difference in medication use.
COMMENT
This study is to our knowledge the first designed specifically to examine the effects of an intensive lifestyle intervention on weight loss, abdominal fat, hepatic steatosis, and other cardiometabolic risk factors in persons with severe obesity. Our results indicate that this non-surgical approach can be an effective treatment for severe obesity. The approximately 10% weight loss achieved is similar to that reported for overweight and class I obesity.22,23 Moreover, nearly 30% of participants achieved more than 10% weight loss, and 10% of participants achieved greater than 20% weight loss at 12 months. Adherence to the intervention by these severely obese participants was the same as that previously reported in overweight and obese participants.15,24 This is in accord with other studies that have reported significant diet-induced weight loss in severe obesity.11,25,26 Thus, our results directly counter the dogma that these severely obese individuals do not respond to life-style intervention. In addition, despite the slightly lower weight loss in African Americans, the interventions were effective in white as well as African American individuals, the latter of whom are at particular risk for type 2 diabetes and cardiovascular disease.27,28
Our intent is not to compare our results with those obtained with bariatric surgery, nor do we recommend that intensive lifestyle modification replace bariatric surgery. To the contrary, it is quite clear that bariatric surgery should continue to play an important role in the treatment of severe obesity. It should be pointed out, however, that many studies comparing surgery with conventional therapy for weight loss have implied that lifestyle intervention is synonymous with conventional therapy. We agree that conventional therapy is generally inadequate to treat severe obesity. In one of the few clinical trials focusing on the treatment of severe obesity, Ryan et al12 reported that severely obese adults randomized to an intensive medical weight loss program in a primary care setting lost a significant amount of weight compared with those receiving usual care; in that study, 21% of participants lost 10% or more of their weight. As is the case with many weight loss trials, however, the primary limitation of that study was that retention rates were relatively low. The more frequent and structured intervention contact in our study likely contributed to the relatively high adherence and retention and thus the degree of weight loss.
The addition of physical activity, regardless of whether initiated early in the program or delayed, promoted greater weight loss. This effect was statistically significant for the group×time interaction for body weight from baseline to 6 months, although the group×time interaction did not reach statistical significance when the data were analyzed as calculated weight change. Although weight loss was not statistically different between groups after physical activity had begun in the delayed-activity group, physical activity may have contributed to the ability to sustain weight loss from 6 to 12 months.
Our results are consistent with studies in overweight and class I obese participants reporting that the addition of physical activity modestly but significantly induces greater weight loss and is important to maintain weight loss.29 Moreover, these severely obese adults did not present with any particular physical limitations that precluded them from initiating a physical activity program at the onset of the weight loss intervention. This suggests that physical activity could also play an important role in the long-term maintenance of weight loss following bariatric surgery, which is in accord with previous associations between physical activity and degree of weight loss following bariatric surgery.30 Additional studies are clearly needed, however, to examine the long-term effects of physical activity on weight loss in severe obesity.
Another clinically relevant finding was the significant reduction in abdominal fat and hepatic steatosis. Abdominal fat assessed by imaging methods or by surrogate waist circumference is regarded as more strongly associated with type 2 diabetes and cardiovascular disease risk than is generalized obesity.31–34 Moreover, a large prospective cohort analysis revealed that higher waist circumference strongly predicts mortality.35 Hepatic steatosis is strongly associated with insulin resistance36,37 and a higher risk of cardiovascular disease.38 Although this lifestyle intervention did not achieve the degree of weight loss typically observed following bariatric surgery, this magnitude of weight loss was associated with significant improvements in insulin resistance, blood pressure, and levels of plasma triglycerides. Moreover, the greater reductions in waist circumference and degree of hepatic steatosis with the addition of physical activity indicate that the benefits of physical activity extend beyond effects on generalized obesity.
Our study also has several limitations. Participants were mostly women, and although groups were randomized according to sex, it is difficult to determine sex-specific responses. Additional studies should examine the effects of sustained intensive lifestyle intervention on long-term weight loss among severely obese persons and on the use of antihypertensive and lipid-lowering medications.
In conclusion, intensive lifestyle interventions using a behavior-based approach can result in clinically significant and meaningful weight loss and improvements in cardiometabolic risk factors in severely obese persons. It is also clear that physical activity should be incorporated early in any dietary restriction approach to induce weight loss and to reduce hepatic steatosis and abdominal fat. Our data make a strong case that serious consideration should be given by health care systems to incorporating more intensive lifestyle interventions similar to those used in our study. Additional studies are clearly needed to determine long-term efficacy and cost-effectiveness of such approaches.
Acknowledgments
Funding/Support: This study was funded by the Commonwealth of Pennsylvania Department of Health. Dr Brown was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant T32 DK07052.
Role of the Sponsors: Neither the Commonwealth of Pennsylvania Department of Health nor the National Institutes of Health had any role in design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00712127
Author Contributions: Dr Goodpaster had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Goodpaster, DeLany, Kuller, Vockley, McTigue, Jakicic.
Acquisition of data: Goodpaster, DeLany, Otto, South-Paul, Thomas, Brown, Hames, Jakicic.
Analysis and interpretation of data: Goodpaster, DeLany, Kuller, McTigue, Hames, Lang, Jakicic.
Drafting of the manuscript: Goodpaster, DeLany, Thomas, Jakicic.
Critical revision of the manuscript for important intellectual content: Goodpaster, DeLany, Otto, Kuller, Vockley, South-Paul, Brown, McTigue, Hames, Lang, Jakicic.
Statistical analysis: DeLany, Lang, Jakicic.
Obtained funding: Goodpaster, Jakicic.
Administrative, technical, or material support: Otto, Vockley, South-Paul, Brown, Hames.
Study supervision: Goodpaster, Otto, Jakicic.
Additional Contributions: We thank David E. Kelley, MD (Merck), for his valuable input on the study. Dr Kelley received no compensation for his contributions.
Financial Disclosures: Dr Jakicic reported serving on the scientific advisory board for Free and Clear, serving as a consultant to Proctor & Gamble Inc, BodyMedia Inc, and the University of Pittsburgh Medical Center Health Plan; and receiving research funding from Bodymedia Inc and the Beverage Institute for Health & Wellness. No other authors reported disclosures.
Trial Personnel: Research coordinators: Jackie Welsch-Thobaben, RN, Department of Health and Physical Activity, University of Pittsburgh; Nicole Helbling, MS, RN, Jennifer Gabany, CRNP, Division of Endocrinology, Department of Medicine, University of Pittsburgh. Study facilitators: Tracey Murray, BS, Barbara Elnyczky, BS, Rebecca Danchenko, MS, Susan Harrier, BS, Diane Casile, MA, Department of Health and Physical Activity; Steve Anthony, MS, Angela Laslavic, MS, Kristin Valchar, Division of Endocrinology, Department of Medicine. Computed tomography scan analysis: Peter J Chomentowski, PhD, Division of Endocrinology, Department of Medicine. Research lab processing: Kelly McCoy, BS, Division of Endocrinology, Department of Medicine. Research nutritionist: Linda Semler, MS, RD, Department of Health and Physical Activity. Interventionist: Christine Pellegrini, PhD, Jessica Unick, PhD, Meghan McGuire, BS, L. Denise Edmonds, PhD, David O. Garcia, MS, Physical Activity and Weight Management Research Center, Department of Health and Physical Activity. Data management: Debra Martin, MS, Courteney Sashin, MS, Epidemiology Data Center, University of Pittsburgh School of Public Health; Michael McDermott, Physical Activity and Weight Management Research Center. Clinical support: Clinical Translational Research Center, University of Pittsburgh. Participant recruitment: Janet Bonk and Jennifer Rush, University of Pittsburgh School of Public Health; Susan Harrier, Diane Casile, Christine Pellegrini, Jessica Unick, David O. Garcia, Kelliann Davis, Physical Activity and Weight Management Research Center.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163(18):2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 3.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown CD, Higgins M, Donato KA, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8(9):605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 5.Park YW, Zhu S, Palaniappan L, Heshka S, Camethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flickinger EG, Pories WJ, Meelheim HD, Sinar DR, Blose IL, Thomas FT. The Greenville gastric bypass: progress report at 3 years. Ann Surg. 1984;199 (5):555–562. doi: 10.1097/00000658-198405000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–485. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monkhouse SJ, Morgan JD, Bates SE, Norton SA. An overview of the management of morbid obesity. Postgrad Med J. 2009;85(1010):678–681. doi: 10.1136/pgmj.2009.082271. [DOI] [PubMed] [Google Scholar]
- 10.Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346(8):591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JW, Brinkman VL, Hamilton CC. Weight loss and 2-y follow-up for 80 morbidly obese patients treated with intensive very-low-calorie diet and an education program. Am J Clin Nutr. 1992;56(1 suppl):244S–246S. doi: 10.1093/ajcn/56.1.244S. [DOI] [PubMed] [Google Scholar]
- 12.Ryan DH, Johnson WD, Myers VH, et al. Non-surgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170(2):146–154. doi: 10.1001/archinternmed.2009.508. [DOI] [PubMed] [Google Scholar]
- 13.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 14.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168 (14):1550–1560. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes Relat Metab Disord. 1995;19(12):893–901. [PubMed] [Google Scholar]
- 17.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA. 1999;282(16):1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 18.Wing RR, Jakicic J, Neiberg R, et al. Look AHEAD Research Group. Fitness, fatness, and cardiovascular risk factors in type 2 diabetes: Look AHEAD study. Med Sci Sports Exerc. 2007;39(12):2107–2116. doi: 10.1249/mss.0b013e31815614cb. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly J, Blair S, Jakicic J, Manore MM, Rankin JW, Smith BK American College of Sports Medicine. American College of Sports Medicine position stand: appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 22.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 23.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13(suppl 2):39–46. [PubMed] [Google Scholar]
- 24.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JW, Conley SB, Nicholas AS. One hundred pound weight losses with an intensive behavioral program: changes in risk factors in 118 patients with long-term follow-up. Am J Clin Nutr. 2007;86(2):301–307. doi: 10.1093/ajcn/86.2.301. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144(9):625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 27.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89(6):2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 28.Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord. 2002;26 (9):1205–1210. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- 29.Jakicic JM. The effect of physical activity on body weight. Obesity (Silver Spring) 2009;17(3s suppl 3):S34–S38. doi: 10.1038/oby.2009.386. [DOI] [PubMed] [Google Scholar]
- 30.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86(11):5412–5419. doi: 10.1210/jcem.86.11.8027. [DOI] [PubMed] [Google Scholar]
- 32.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 33.Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26(8):2341–2344. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283(6):E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170(15):1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 36.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang JH, Stein DT, Barzilai N, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293(6):E1663–E1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 38.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103(12):3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]