Abstract
Cebus apella is renowned for its dietary flexibility and capacity to exploit hard and tough objects. Cebus apella differs from other capuchins in displaying a suite of craniodental features that have been functionally and adaptively linked to their feeding behavior, particularly the generation and dissipation of relatively large jaw forces. We compared fiber architecture of the masseter and temporalis muscles between the tufted capuchin (C. apella; n = 12 ) and two “untufted” capuchins (C. capuchinus, n = 3; C. albifrons, n = 5). These three species share broadly similar diets, but tufted capuchins occasionally exploit mechanically challenging tissues. We tested the hypothesis that C. apella exhibits architectural properties of their jaw muscles that facilitate relatively large forces, including relatively greater physiologic cross-sectional areas (PCSA), more pinnate fibers, and lower ratios of mass to tetanic tension (Mass/P0). Results show some evidence supporting these predictions, as C. apella has relatively greater superficial masseter, whole masseter, and temporalis PCSAs, significantly so only for the temporalis following Bonferroni adjustment. Capuchins did not differ in pinnation angle or Mass/P0. As an architectural trade-off between maximizing muscle force and muscle excursion/contraction velocity, we also tested the hypothesis that C. apella exhibits relatively shorter muscle fibers. Contrary to our prediction, there are no significant differences in relative fiber lengths between tufted and untufted capuchins. Therefore, we attribute the relatively greater PCSAs in C. apella primarily to their larger muscle masses. These findings suggest that relatively large jaw-muscle PCSAs can be added to the suite of masticatory features that have been functionally linked to the exploitation of a more resistant diet by C. apella. By enlarging jaw-muscle mass to increase PCSA, rather than reducing fiber lengths and increasing pinnation, tufted capuchins appear to have increased jaw-muscle and bite forces without markedly compromising muscle excursion and contraction velocity. One performance advantage of this morphology is that it promotes relatively large bite forces at wide jaw gapes, which may be useful for processing large food items along the posterior dentition. We further hypothesize that this morphological pattern may have the ecological benefit of facilitating the dietary diversity seen in Cebus apella. Lastly, the observed feeding on large objects, coupled with a jaw-muscle architecture that facilitates this behavior, raises concerns about utilizing C. apella as an extant behavioral model for hominins that might have specialized on small objects in their diets.
Keywords: Tufted capuchins, Feeding behavior, Masseter, Temporalis, Fiber length, PCSA, Early hominin diet
Introduction
The tufted capuchin monkeys (Cebus apella) are renowned for their dietary flexibility and capacity to exploit mechanically challenging plant tissues, such as palm nuts and the bases of palm leaves (Izawa and Mizuno, 1977; Terborgh, 1983; Janson and Boinski, 1992; Lambert et al., 2004; Wright, 2005). Similar to other Cebus monkeys, C. apella selects foods of relatively low toughness when available (Wright, 2005). However, C. apella occasionally ingests tissues that are relatively tough (Wright, 2005), stiff, and hard (Wright, 2005; Chalk et al., 2008; Wright et al., 2008). Existing data indicate that the toughest tissues ingested and masticated by C. apella are between two and four times greater than the toughest tissues ingested by C. olivaceus (Wright, 2005). Indeed, the mechanical properties of the foods they ingest and masticate has led many anthropologists to use C. apella as a model for interpreting hominin diets (e.g., Kay, 1981; Teaford and Ungar, 2000; Ungar et al., 2008a).
Cebus apella displays a suite of craniodental features that has been functionally and adaptively linked to their feeding behavior. A comparative assessment of mechanical advantage has shown that C. apella has improved leverage of the masseter and temporalis muscles compared to other Cebus monkeys, resulting in the capacity for relatively increased force production at the anterior dentition (Wright, 2005). Other features include size- and shape-related aspects of the mandible that reflect the capacity to resist relatively large loads incurred during incision and chewing (e.g., Bouvier and Tsang, 1990; Cole, 1992; Daegling, 1992). Large, thick-enameled molars, relatively wide incisor tooth rows, and large canine and post-canine cross-sectional areas have also been associated with an increased reliance on both the anterior and postcanine dentition for processing mechanically challenging tissues (e.g., Kay, 1981; Anapol and Lee, 1994a; Wright, 2005). Likewise, C. apella displays dental microwear features that have been interpreted as consistent with a diet of seasonal hard objects (Teaford, 1985; Ungar et al., 2006).
Jaw-muscle morphology, in particular the fiber architecture of the muscles, also plays a crucial role in feeding mechanics. Fiber architecture is a critical determinant of a whole muscle’s contractile ability and has been theoretically (Gans and Bock, 1965; Gans, 1982) and empirically (Powell et al., 1984) related to the capacity of a muscle to generate force and excursion. Muscle fiber architecture describes the internal arrangement of muscle fibers relative to the force-generating axis of a muscle.1 Fibers may run parallel to, or be aligned at an angle, relative to the force-generating axis (i.e., parallel versus pinnate fibers). All else being equal, parallel-fibered muscles tend to be designed for producing large excursions, while pinnate-fibered muscles are best suited for producing large muscle forces (Gans, 1982). For a given muscle volume, this trade-off occurs because parallel fibers tend to be longer and comprise more sarcomeres in series. The more sarcomeres in series, the greater the distance through which a muscle can shorten or lengthen (Williams and Goldspink, 1978). Fiber length, therefore, is proportional to a muscle’s maximum excursion and, by extension, its contraction velocity.
By contrast, pinnate fibers tend to be shorter, but their geometry allows more fibers to be packed next to each other, although at an angle relative to the muscle’s force-generating axis. This angular deviation from the force-generating axis results in some loss of force attributable to pinnation, but even a highly pinnate muscle like the masseter (20°–30° of pinnation) is capable of transmitting nearly 90% of its contractile force along the force-generating axis of the muscle (Gans, 1982). Maximum isometric force is governed by the number and diameter of fibers aligned in parallel to each other. The greater number of fibers that can be packed adjacent to each other explains why pinnate fibered muscles tend to produce more force despite their angular deviation from the force-generating axis. The physiologic cross-sectional area (PCSA) of a muscle represents the cross-sectional areas of all fibers within a muscle and is therefore proportional to the maximum force a muscle can generate (Powell et al., 1984).
With only a few exceptions, previous investigators have relied either on muscle mass or bone proxies of muscle size to infer the maximum force production and excursion capabilities (i.e., contractile properties) of whole muscle in extant (e.g., Turnbull, 1970; Shea, 1983; Cachel, 1984) and fossil (e.g., Antón, 1996) primates. However, muscle mass and bone proxies generally provide poor estimates of muscle force for both masticatory (Bouvier and Tsang, 1990; Antón, 1999) and limb (Lieber, 2002) musculature. For masticatory muscles in particular, achieving accurate estimates of maximum muscle force and excursion capabilities is rendered difficult by the complex internal geometry of the muscle. Muscle fibers do not run the length of whole muscles (Ounjian et al., 1991), fibers of bi- and multi-pinnate muscles can vary widely in length throughout a muscle, and, assuming a constant muscle volume, fiber length is inversely related to muscle force (Gans and Bock, 1965). For pinnate-fibered muscles, mass or volume will tend to overestimate the maximum force-generating capacity of a muscle, while normalizing muscle mass by whole muscle length will underestimate its force-producing capacity. Therefore, the incorporation of such surrogate architectural data in biomechanical models could potentially limit or confound interpretations of species differences in masticatory function and performance (e.g., Bouvier and Tsang, 1990).
Here we conduct a comparative analysis of jaw-muscle fiber architecture to investigate the functional correlates of masticatory muscles in tufted capuchins. Cebus apella, C. capuchinus, and C. albifrons share broadly similar diets consisting of fruits, seeds, nectar, and invertebrates, with the key ecological distinction among these taxa being the ability of C. apella to occasionally exploit mechanically challenging tissues (Anapol and Lee, 1994; Wright, 2005). We compare fiber architecture of the masseter and temporalis muscles because as the two most powerful jaw-closing muscles, these muscles are important determinants of a monkey’s maximum bite force.
Hypotheses to be tested
We address two hypotheses relating the functional consequences of jaw-muscle fiber architecture to bite force production and jaw movements during feeding behaviors between C. apella on the one hand, and C. capuchinus and C. albifrons on the other.
Hypothesis 1
Muscles with larger cross-sectional areas, increased pinnation angles, and lower effective mass to tetanic tension ratios (M/P0) are functionally advantageous for generating greater muscle forces. For the jaw-closing muscles, this architectural configuration likely translates into larger maximum muscle and bite forces. Therefore, Hypothesis 1 predicts that C. apella will exhibit relatively greater PCSAs, increased pinnation angles, and small M/P0 ratios in the masseter and temporalis muscles compared to C. albifrons and C. capuchinus.
Hypothesis 2
Muscles with smaller PCSAs tend to be comprised of longer, less pinnate fibers given that fiber length is inversely proportional to PCSA. These longer-fibered muscles facilitate larger muscle excursions and increased contraction velocities. Therefore, Hypothesis 2 predicts that C. apella will exhibit relatively shorter fibers compared to C. albifrons and C. capuchinus. We have no ecological or behavioral basis for predicting that C. albifrons or C. capuchinus generate relatively greater excursions or contraction velocities compared to C. apella. Thus, we would initially interpret relatively longer fibers in untufted capuchins as an architectural tradeoff of their relatively smaller PCSAs compared to C. apella.
Materials and Methods
Samples
We evaluated the masseter and temporalis muscles of 12 Cebus apella, five C. albifrons, and three C. capuchinus.2 All but two specimens of C. apella were captive3, and all were dentally adult based on third molar eruption. Cebus material was provided courtesy of the Anthropological Institute and Museum (Zurich) and the National Museum of Natural History (Washington, DC). All tissue was previously fixed and stored either in formalin or ethanol. We subsequently stored the harvested muscles in 10% buffered formalin until use.
Data collection
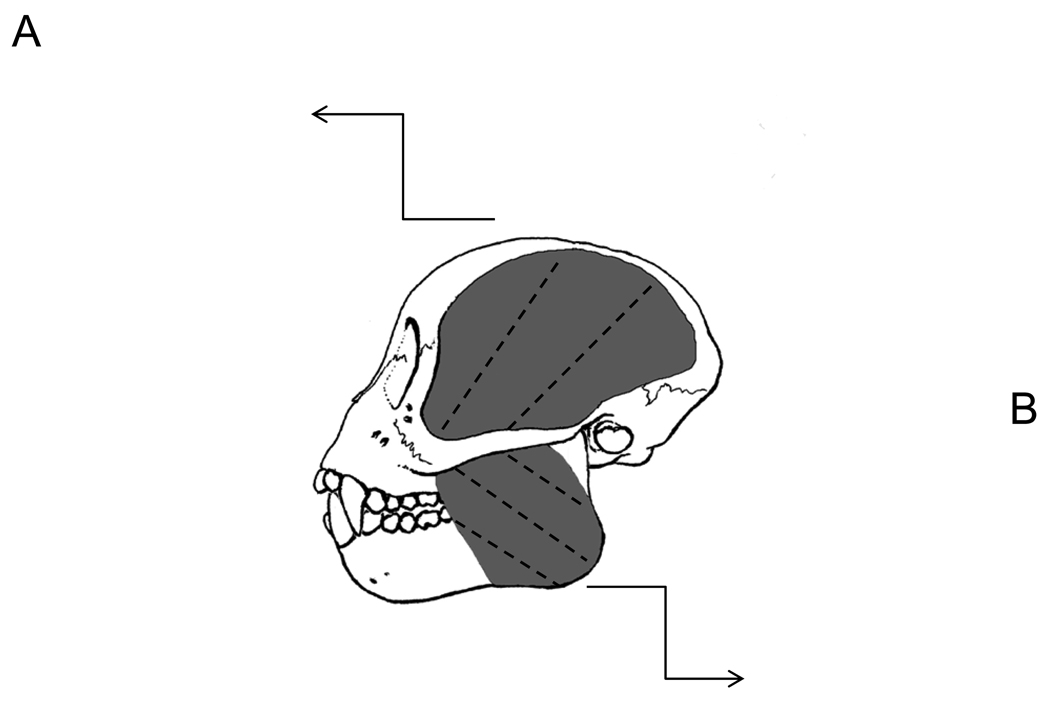
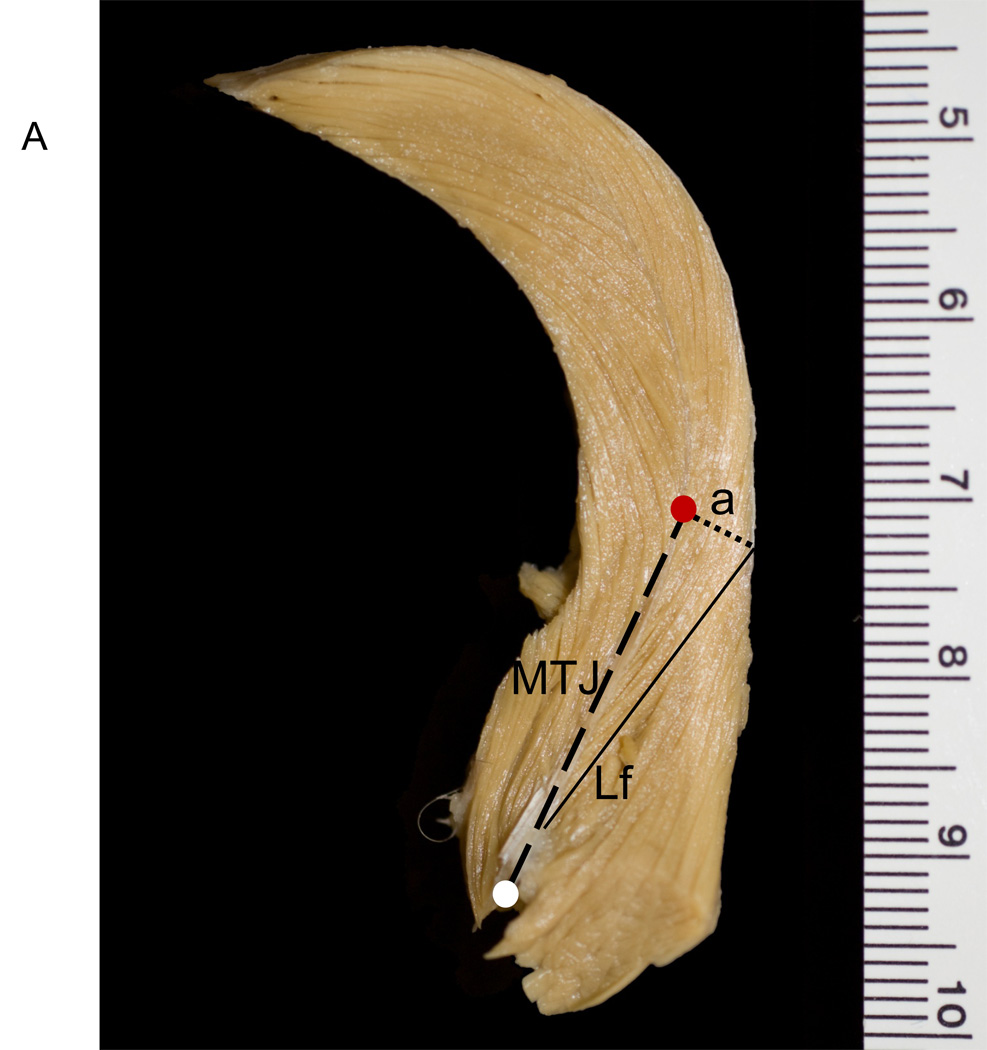
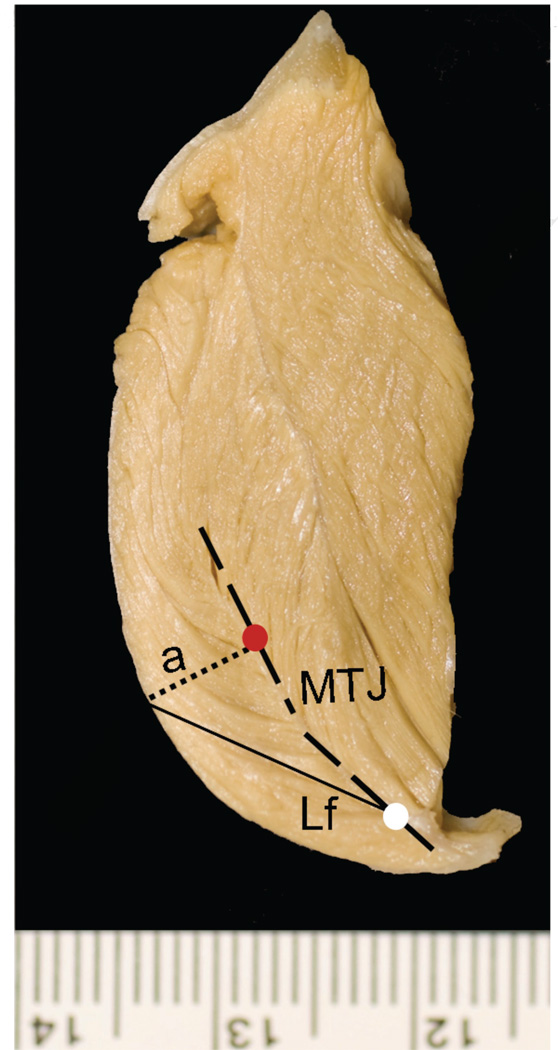
We removed the skin and superficial fascia overlying the jaw muscles. We measured whole masseter and temporalis muscle lengths from muscles in situ. Muscles then were dissected free from their bony attachments, trimmed of excess tendon and fascia, blotted dry, and weighed to the nearest 0.1 g. We also weighed the superficial and deep portions of the masseter. Following dissection of the muscles, we measured jaw length from the posterior edge of the condyle to infradentale. These and all other linear measurements were taken with digital calipers to the nearest 0.01 mm. Fiber length and pinnation angle were measured for both muscles following Taylor and Vinyard (2004) and Taylor et al. (2006). Depending on the size of the muscle, we sectioned the masseter and temporalis muscles along their lengths to produce a minimum of two and a maximum of five segments, each segment approximately 1.5 cm in thickness (Fig. 1). We oriented each segment to view fibers in cross section, pinned the segment to a styrofoam block, and then visualized the proximal and distal attachments of individual fibers to tendon (Fig. 1) with the use of a 5 diopter (2.25×) magnifier light. We selected anterior and posterior sampling sites for measurements along the length of the masseter (Fig. 1a), and proximal and distal sampling sites for the temporalis (Fig. 1b).
Figure 1.
Schematic of Cebus apella masseter and temporalis muscles. The temporalis and masseter were sectioned along their maximum lengths approximately every 1.5 cm, as depicted by the dashed lines in the central (lateral-view) schematic showing the muscles in situ. Cross-sections of a) left temporalis and b) left masseter. The thick black dashed line represents the central myotendinous junction in both muscles. The thin solid black line represents individual fiber lengths (NLf) running from the central tendon to the distal tendon. The black dotted line represents the perpendicular (a) to the central tendon. A minimum of six fibers was sampled from the anterior masseter and proximal temporalis (red circle), as well as the posterior masseter and distal temporalis (white circle). Pinnation angle was computed as the arcsine of a/NLf.
At each sampling site, we measured up to six adjacent fibers. For each fiber, we measured fiber length between the proximal and distal myotendinous junctions (Lf; Table 1 and Fig. 1). We calculated the angle of pinnation (θ) as the arcsine of a/Lf (Anapol and Barry, 1996; Table 1 and Fig. 1). Pinnation angle was computed for each fiber and then averaged across the total number of fibers. We calculated pinnation angles for the superficial masseter and temporalis muscles; deep masseter pinnation angles were not calculated.
Table 1.
Means (± standard errors) of jaw length and muscle architectural variables for Cebus apella, C. albifrons, and C. capuchinus and Mann-Whitney U-test results comparing C. apella to the two untufted capuchins.
| Cebus apella |
Cebus albifrons |
Cebus capuchinus |
C. apella vs. untufted capuchinsa,b |
|
|---|---|---|---|---|
| Masseter Ls (µ)c,d | 2.35 ±0.04 | 2.43 ±0.10 | 2.50 ±0.07 | NS |
| Temporalis Ls (µ)c,d | 2.46 ±0.07 | 2.40 ±0.06 | 2.44 ±0.05 | NS |
| Jaw length (mm) | 61.04 ±1.23 | 55.98 ±2.10 | 57.97 ±1.07 | C. apella > 0.0491* |
| Masseter length (mm) | 37.50 ±2.52 | 28.60 ±2.92 | 34.93 ±2.43 | C. apella > 0.0938 |
| Temporalis length (mm) | 64.13 ±2.61 | 49.50 ±2.16 | 57.57 ±1.91 | C. apella > 0.0128 |
| Superficial Masseter Muscle | ||||
| Mass (g) | 5.94 ±0.83 | 3.41 ±0.97 | 2.86 ±0.35 | C. apella > 0.0253* |
| NLf (mm)d | 11.12 ±1.07 | 8.58 ±0.97 | 10.18 ±2.73 | NS |
| PCSA (cm2)d | 4.87 ±0.60 | 3.31 ±0.86 | 2.84 ±0.57 | C. apella > 0.0510 |
| Deep Masseter Muscle | ||||
| Mass (g) | 1.04 ±0.18 | 0.53 ±0.32 | 0.59 ±0.10 | C. apella > 0.0345* |
| NLf (mm)d | 7.33 ±0.91 | 5.09 ±0.26 | 6.68 ±1.90 | NS |
| PCSA (cm2)d | 1.29 ±0.18 | 1.07 ±0.49 | 0.71 ±0.18 | NS |
| Whole Masseter Muscle | ||||
| Mass (g) | 6.97 ±0.95 | 3.83 ±1.09 | 3.44 ±0.44 | C. apella > 0.0109 |
| NLf (mm)d | 9.31 ±0.79 | 7.48 ±0.51 | 9.82 ±2.85 | NS |
| PCSA (cm2)d | 6.66 ±0.70 | 4.56 ±1.39 | 3.58 ±0.75 | C. apella > 0.0510 |
| Temporalis Muscle | ||||
| Mass (g) | 13.66 ±2.35 | 6.92 ±2.82 | 5.98 ±0.58 | C. apella > 0.0164 |
| NLf (mm)d | 14.02 ±1.34 | 12.48 ±1.39 | 14.37 ±3.19 | NS |
| PCSA (cm2)d | 8.84 ±1.06 | 4.88 ±1.57 | 4.20 ±0.72 | C. apella > 0.0075 |
Results based on two-tailed Mann-Whitney U-tests.
Boldfaced p-values identify significant differences following the sequential Bonferroni adjustment.
Starred (*) p-values identify significant differences prior to the sequential Bonferroni adjustment. Unstarred p-values identify differences where 0.05 < p < 0.10. NS = non-significant.
Average sarcomere lengths exclude the C. apella specimen fixed at a wide jaw gape.
Ls, sarcomere length; NLf, normalized fiber length; PCSA (cm2), physiologic cross-sectional area.
To adjust for the variation in fiber length that occurs because muscles were fixed at varying degrees of jaw gape, we normalized measured fiber lengths to a standardized sarcomere length (Felder et al., 2005). Following fiber length measurements, muscle segments were chemically digested in 30% HNO3 and saline solution (Loeb and Gans, 1986) until the surrounding connective tissue was digested and fiber bundles were easily separable. Muscle segments were stored in 1 × PBS until manually dissected. Fiber bundles from each muscle or muscle segment were dissected in 1 × PBS under a dissection microscope (Nikon SMZ1500). Five to ten small fiber bundles were mounted on slides, cover-slipped with mounting medium (Cytoseal) and left to air dry. We measured sarcomere lengths (Ls; 0.01 µm) from these fiber bundles using laser diffraction, which relies on incident laser light diffracting through the I-band region of the sarcomere to estimate sarcomere length (Lieber et al., 1984). Raw fiber lengths were normalized to a resting fiber length (NLf) by dividing by a standard Ls of 2.41 µm, calculated as optimal Ls in macaque limb muscle (Walker and Schrodt, 1974).
Using the aforementioned measurements, we computed the following variables for C. apella, C. albifrons, and C. capuchinus:
Mean fiber lengths (NLf) for the superficial, deep, and whole masseter and temporalis muscles, estimated as the average of all fibers sampled from a muscle or muscle region. We used the average fiber length for each individual muscle in all subsequent calculations involving fiber length (see below).
- Physiological cross-sectional area (PCSA), computed as:
where 1.0564 gm/cm3 is the specific density of muscle (Mendez and Keys, 1960). - Muscle mass/predicted effective maximal tetanic tension (M/P0), computed following (Sacks and Roy, 1982):
This ratio can be used to estimate the extent to which a muscle’s mass is due to longer versus shorter, more pinnate fibers (Anapol and Barry, 1996). A higher ratio indicates longer fibers and a muscle dedicated to excursion/contraction velocity over force production.
Data analysis
Initially, we employed two-tailed Mann-Whitney U-tests to determine if C. albifrons and C. capuchinus exhibited significant differences for any of the above variables. Finding none, we combined these two taxa into a single group, referred to hereafter as “untufted capuchins.” We used two-tailed Mann-Whitney U-tests to determine if there were significant differences between tufted and untufted capuchins in jaw, sarcomere, and whole muscle lengths as well as absolute PCSAs and fiber lengths (NLf). To examine relative differences between groups in masseter and temporalis architectural variables, we created dimensionless shape ratios by dividing architectural variables by jaw length (Taylor and Vinyard, 2004). In addition, we examined ratios of fiber length (NLf/WT0.33) and PCSA (PCSA/WT) to muscle mass, which represent the tendency of a muscle toward excursion/contraction velocity or force, respectively (Lieber and Blevins, 1989). We used one-tailed Mann-Whitney U-tests to address Hypothesis 1 predicting that C. apella exhibits significantly greater relative PCSAs, greater pinnation angles, and lower M/P0 ratios compared to untufted capuchins. Similarly, we used one-tailed Mann-Whitney U-tests to test Hypothesis 2 predicting that C. apella has relatively shorter jaw-muscle fibers. We set an a priori α = 0.05 and minimized the potential for Type I error by employing the sequential Bonferroni adjustment (Rice, 1989). Because our small samples limit our statistical power to detect a significant difference, we highlight trends tending towards significance where 0.05 < p < 0.10.
Results
Gross morphology
The gross morphology of the masseter and temporalis muscles in Cebus is similar to that of other primates and non-primate mammals (Turnbull, 1970; Bouvier and Tsang, 1990; Antón, 1999; Taylor and Vinyard, 2004). The masseter is a multipinnate-fibered muscle with a large superficial region and a much smaller deep region. As in many anthropoid primates, the posterior deep masseter fibers run nearly orthogonal to the superficial masseter fibers and maintain a relatively transverse orientation on the jaw. The fan-shaped temporalis is a bipinnate muscle, with the superficial and deep portions separated by an intramuscular tendon.
Sarcomere-length normalization
Measured sarcomere lengths approximated the 2.41µm Ls used for normalization (Table 1). The maximum and minimum deviations from this standard across species were on the order of ±0.2 µm. The only exception was one C. apella, whose jaws were fixed in a wide gape, with measured sarcomere lengths of 3.76 µm (+1.35 µm) and 3.97 µm (+1.56 µm) for the masseter and temporalis, respectively. When we evaluated only those specimens whose jaws were fixed with the incisors in occlusion, average Ls for Cebus masseter and temporalis muscles was 2.41 µm (± 0.18) and 2.43 µm (± 0.20), respectively.
Regional variation
Fiber lengths (NLf) vary between the masseter and temporalis muscles and by masseter region ranging from 5.09 –7.33 mm for the deep masseter to 8.58–11.12 mm for the superficial masseter, and up to 12.48 –14.37 mm for the temporalis muscle (Table 1). As might be expected given the highly pinnate masseter muscle, masseter fibers are significantly (paired t-test, p < 0.05) shorter than temporalis fibers within species. Superficial masseter pinnation angles range between 14.3° in C. capuchinus to 19.8° in C. albifrons and are greater than pinnation angles observed for the temporalis muscle, which range between 7.6–12.4° across the three species (Table 2). Of the two jaw-closing muscles, the temporalis muscle contributes a greater percentage of mass and PCSA to their combined muscle volume and area. The deep masseter PCSA ranges between 21% and 27% of whole masseter PCSA (Table 1).
Table 2.
Means (± standard errors) for size-adjusted architectural variables for Cebus apella, C.albifrons, and C. capuchinus and Mann-Whitney U-test results of hypothesis tests comparing C.apella to the two untufted capuchins.
| Cebus apella |
Cebus albifrons |
Cebus capuchinus |
C. apellavs. untufted capuchinsa,b |
|
|---|---|---|---|---|
| Hypothesis 1: | ||||
| Superficial Masseter Muscle | ||||
| PCSA0.5/Jaw lengthc | 0.080 ±0.010 | 0.057 ±0.012 | 0.049 ±0.011 | C. apella > 0.0578 |
| PCSA/Muscle weight | 2.647 ±0.225 | 2.187 ±0.327 | 2.028 ±0.453 | C. apella > 0.0860 |
| Pinnation angle (°) | 18.07 ±2.54 | 19.87 ±0.58 | 14.33 ±3.77 | NS |
| Mass/P0 | 0.541 ±0.049 | 0.419 ±0.047 | 0.482 ±0.125 | NS |
| Deep Masseter Muscled | ||||
| PCSA0.5/Jaw length | 0.021 ±0.003 | 0.017 ±0.007 | 0.012 ±0.004 | C. apella > 0.0567 |
| PCSA/Muscle weight | 1.254 ±0.335 | 1.202 ±0.362 | 0.902 ±0.324 | NS |
| Mass/Po | 0.368 ±0.034 | 0.263 ±0.016 | 0.515 ±0.131 | NS |
| Whole Masseter Muscle | ||||
| PCSA0.5/Jaw length | 0.109 ±0.011 | 0.078 ±0.020 | 0.062 ± 0.014 | C. apella > 0.0199* |
| PCSA/Muscle weight | 3.446 ±0.230 | 2.815 ±0.561 | 2.415 ±0.565 | C. apella > 0.0592 |
| Mass/Po | 0.460 ±0.038 | 0.370 ±0.026 | 0.465 ±0.130 | NS |
| Temporalis Muscle | ||||
| PCSA0.5/Jaw length | 0.147 ±0.017 | 0.085 ±0.022 | 0.072 ±0.012 | C. apella > 0.0055 |
| PCSA/Muscle weight | 3.719 ±0.299 | 2.525 ±0.458 | 2.325 ±0.408 | C. apella > 0.0038 |
| Pinnation angle (°) | 12.42 ±1.46 | 10.18 ±0.99 | 7.60 ±2.30 | NS |
| Mass/Po | 0.660 ±0.061 | 0.582 ±0.065 | 0.666 ±0.145 | NS |
| Hypothesis 2: | ||||
| Superficial Masseter Muscle | ||||
| NLf/Jaw lengthc | 0.181 ±0.015 | 0.152 ±0.011 | 0.175 ±0.047 | NS |
| NLf/Muscle weight0.33 | 2.189 ±0.339 | 3.297 ±0.807 | 3.472 ±0.630 | NS |
| Deep Masseter Muscle | ||||
| NLf/Jaw length | 0.118 ±0.015 | 0.084 ±0.001 | 0.114 ± 0.029 | NS |
| NLf/Muscle weight0.33 | 7.850 ±1.323 | 10.026 ±4.778 | 13.667 ± 3.942 | NS |
| Whole Masseter Muscle | ||||
| NLf/Jaw length | 0.152 ±0.012 | 0.134 ±0.011 | 0.169 ±0.049 | NS |
| NLf/Muscle weight0.33 | 1.522 ±0.228 | 2.564 ±0.730 | 2.762 ±0.530 | NS |
| Temporalis Muscle | ||||
| NLf/Jaw length | 0.230 ±0.019 | 0.223 ±0.020 | 0.248 ±0.055 | NS |
| NLf/Muscle weight0.33 | 1.204 ±0.149 | 2.365 ±0.477 | 2.404 ±0.510 | C. apella < 0.0676 |
Results based on one-tailed Mann-Whitney U-tests. Boldfaced p-values identify significant differences following the sequential Bonferroni adjustment.
Starred (*) p-values identify significant differences prior to the sequential Bonferroni adjustment. Unstarred p-values identify differences where 0.05 < p < 0.10. NS = non-significant.
Results indicate direction of difference between C. apella and the untufted capuchins.
To maintain dimensionless ratios, we used PCSA0.5/Jaw length and NLf/Muscle weight0.333
Pinnation angles for the deep masseter were small and difficult to measure. We therefore treated the deep masseter as a parallel-fibered muscle and did not incorporate pinnation angle in our estimate of PCSA.
Absolute comparisons
Linear measures and muscle masses are greatest in C. apella and smallest in C. albifrons (Table 1). C. albifrons and C. capuchinus are more similar in muscle mass than either is to C. apella. By contrast, C. albifrons is characterized by the smallest average body mass (2.74 kg), while C. apella (3.09 kg) and C. capuchinus (3.11 kg) are comparable in body size (Smith and Jungers, 1997). Thus, patterns of variation in jaw-muscle measures do not tightly correspond with differences in body size.
For the most part, muscle masses are significantly larger in C. apella compared to untufted capuchins (Table 1). Whole masseter muscle mass is 82–102% greater in C. apella compared to the other two Cebus species, while even greater percentage differences (97–128%) are apparent for temporalis muscle mass. Masseter and temporalis PCSA are 46% and 81% greater in C. apella compared to C. albifrons, and 86% and 110% greater in C. apella compared to C. capuchinus (Table 1). PCSA is always largest in C. apella and smallest in C. capuchinus (Table 1), but only temporalis PCSA is significantly larger in C. apella following Bonferroni correction (Table 1). There are no differences in absolute fiber length between tufted and untufted capuchins.
Hypothesis tests comparing relative differences between tufted and untufted capuchins
Hypothesis 1
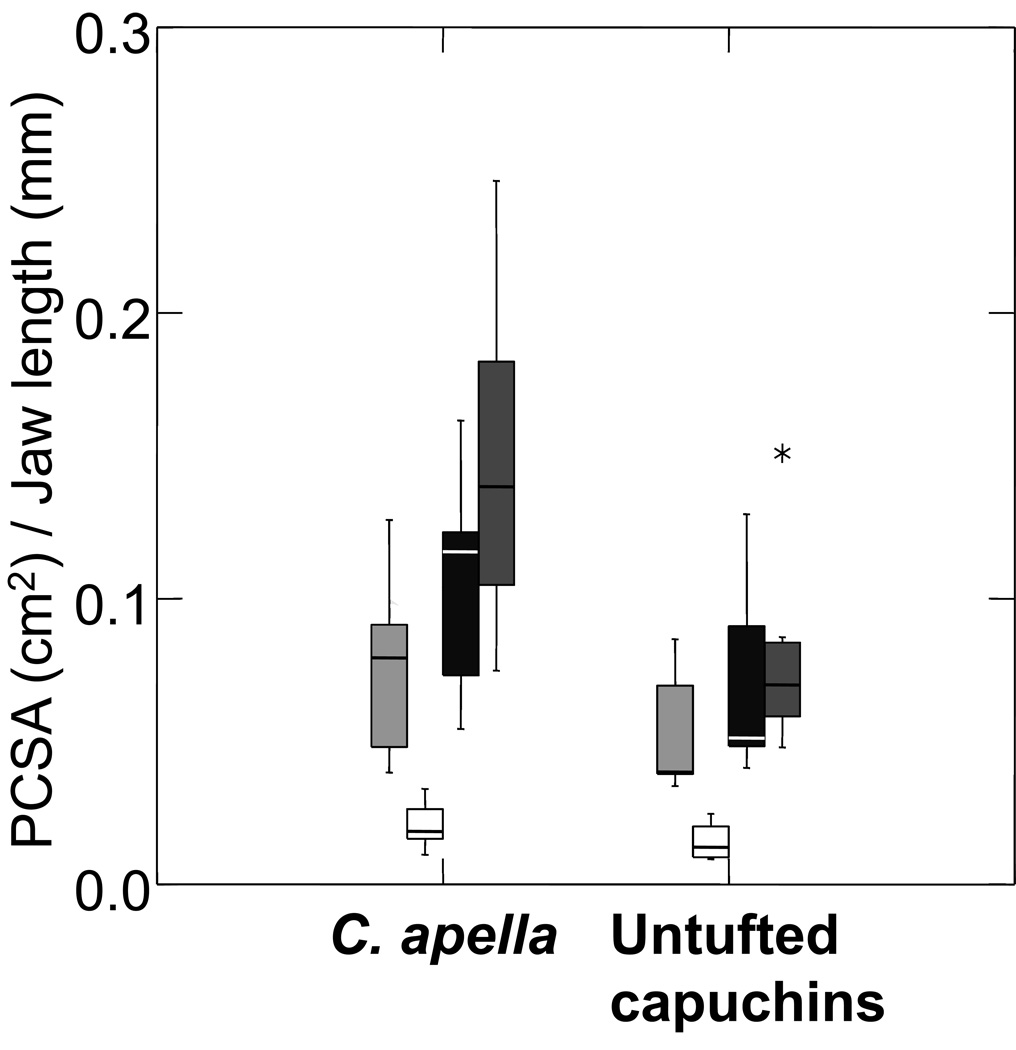
Cebus apella was predicted to exhibit architectural features of the jaw muscles that favor the production of relatively larger maximal muscle force. We observed a mosaic pattern of support for this prediction. Cebus apella has a significantly larger PCSA0.5/jaw length for the whole masseter and temporalis muscles, and displays similar trends for the superficial and deep masseter (Table 2a and Fig. 2a). However, only differences in the temporalis remain significant following Bonferroni adjustment. Differences in the ratio of PCSA/WT generally track ratios of PCSA0.5 /jaw length (Table 2). Pinnation angle did not differ significantly between tufted and untufted capuchins. Contrary to our predictions, C. apella trends towards higher mass to predicted effective maximal tetanic tension ratios (Mass/P0) for the superficial and deep masseter and temporalis muscles (Table 2).
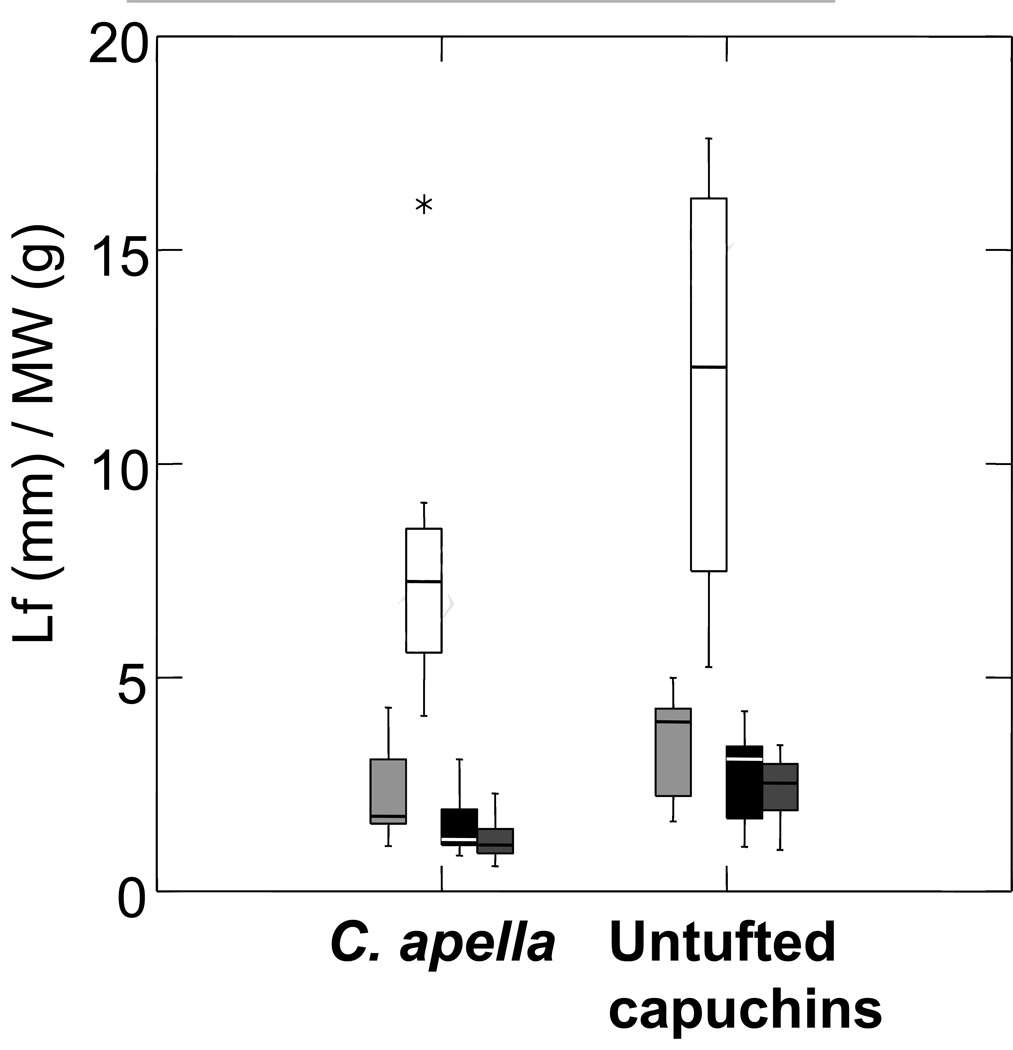
Figure 2.
Box plot comparing relative differences in a) PCSA and b) fiber length, between C. apella and untufted capuchins. From left to right, superficial masseter (light gray), deep masseter (white), whole masseter (black), and temporalis (dark gray). C. apella has relatively larger masseter and temporalis PCSAs compared to untufted capuchins, but only differences in relative temporalis PCSA are significant following Bonferroni adjustment. There are no differences in relative fiber length (or pinnation angle, see Table 2). These findings indicate that there is no architectural tradeoff between jaw-muscle PCSA and fiber length.
Hypothesis 2
We predicted that C. apella would have relatively shorter muscle fibers compared to untufted capuchins as an architectural tradeoff between PCSA and fiber length. However, we found no significant differences in NLf/Jaw length between capuchins (Table 2). The ratios of NLf/WT0.33 for all muscles and muscle regions are lower in C. apella (Table 2 and Fig. 2b) but none of these differences is significant. Thus, our prediction of an architectural trade-off between relative PCSA and NLf is not supported.
Discussion
Comparative assessment of capuchin jaw-muscle architecture
The results from Hypothesis 1 demonstrate that C. apella has relatively greater temporalis and likely masseter physiological cross-sectional areas (PCSA) compared to the untufted capuchins (Table 2). Contrary to our prediction for Hypothesis 2, C. apella does not display an architectural tradeoff between relative PCSA and fiber length (NLf; Table 2). By increasing relative PCSA without significantly decreasing relative NLf or pinnation angle, C. apella is effectively capable of increasing relative maximum force production without compromising maximum excursion or contraction velocity. In the absence of marked differences in relative fiber lengths or pinnation angles, C. apella differs from untufted capuchins primarily in jaw-muscle masses (Table 1). Therefore, increased PSCAs via enlarged jaw-muscle masses can be added to the complex of masticatory apparatus features in C. apella that differentiate this species from its more gracile congeners.
Unfortunately, there has been relatively little previous work on capuchin jaw muscles, thereby limiting our ability to evaluate these results in terms of both absolute dimensions and relative differences among Cebus species. Much of the earlier research is descriptive (e.g., Starck, 1933; Ross, 1995), with some of these contributions including jaw-muscle weights for capuchins (e.g., Schumacher, 1961; Turnbull, 1970). Two studies consider jaw-muscle fiber architecture, but only in Cebus apella. Bouvier and Tsang (1990) estimated dry weights, fiber lengths, and PCSA for the masseter and temporalis (n = 3), while Anapol et al. (2008) recently published PCSAs for the three jaw adductors (n = 4) as part of a scaling analysis of primate jaw-muscle architecture.
Our fiber length estimates for C. apella overlap with estimates in Bouvier and Tsang (1990), although our average values were 29% to 40% percent shorter for the temporalis and superficial masseter, respectively.5 Bouvier and Tsang (1990) published dry weights for the jaw muscles making direct comparisons difficult. If we apply a correction factor of 4.71 for wet:dry weight based on rat masseter (Norton et al., 2001; see also Scanlon (1982) or Cartee et al. (1996) for similar correction factor values in other vertebrate skeletal muscle), Bouvier and Tsang (1990) present similar muscle weights for the temporalis (adjusted mass = 17.0 g) and masseter (adjusted mass = 6.8 g). If we similarly adjust their PCSA estimates to account for dry muscle weight, pinnation angle (based on our pinnation angle estimates), and the specific density of muscle, their adjusted PCSA estimates for the temporalis (8.9 cm2) and masseter (3.9 cm2) are comparable to those presented in Table 2.5 The differences between studies in both the methodology for estimating PCSA and sample composition caution against placing too much emphasis on specific similarities or differences beyond the broadly comparable results.
Anapol et al. (2008) conducted an interspecific allometric analysis of PCSA across primates testing several hypotheses predicting how primate jaw muscles scale with size. Their PCSA estimates for C. apella are lower for both the temporalis (2.8 cm2) and masseter (1.7 cm2) when compared to both our estimates (Table 1) and the adjusted values for Bouvier and Tsang (1990). Given the relatively small sample sizes in these studies, the differences in architectural estimates emphasize the need for continued evaluation of primate jaw-muscle architecture. When comparing PCSA estimates across the platyrrhines in their analysis, Anapol et al. (2008) found that C. apella has a relatively large jaw-muscle PCSA. Only Chiropotes, another hard-object feeding primate (Kinzey and Norconk, 1990), has a markedly larger relative PCSA estimate for their jaw muscles (Anapol et al., 2008). This interspecific comparison supports previous interpretations that C. apella is capable of producing relatively large masticatory forces among platyrrhines.
Sarcomere length adjustments and estimating optimal muscle forces in Cebus
We normalized measured fiber lengths to a standard sarcomere length of 2.41 µm in order to compare specimens that were fixed in varying jaw postures. Prior to normalization, we measured average sarcomere lengths (Ls) of 2.41 µm for the masseter and 2.43 µm for the temporalis in specimens whose jaws were fixed in occlusion. Earlier studies demonstrate that maximum forces are generated when the jaw is opened beyond incisal occlusion (Nordstrom and Yemm, 1974; Thexton and Hiiemae, 1975; Mackenna and Türker, 1978). Based on this prior work, it seems unlikely that our estimated optimal sarcomere length of 2.41µm, taken from Rhesus macaque limb muscle (Walker and Schrodt, 1974), is the sarcomere length at which capuchins generate maximum jaw-muscle forces. Determining this optimal fiber length for Cebus jaw muscles requires establishing muscle length-tension relationships in vivo and is beyond the scope of this work. Our probable underestimation of optimal sarcomere length is unlikely to affect the interspecific results reported here. On the other hand, efforts requiring accurate estimates of jaw-muscle forces, such as might be used in finite element models, will be impacted by this error.
Potential pathways for jaw-muscle enlargement in Cebus paella
Jaw muscle enlargement in C. apella could have occurred through multiple, non-mutually exclusive, pathways. First, the enlarged muscle mass of C. apella compared to its congeners may have resulted from an increase in the number of muscle fibers. Increasing fiber number, or muscle hyperplasia, is considered a relatively uncommon physiologic mechanism for enlarging muscle mass during an individual’s lifetime (McDonagh and Davies, 1984; Eriksson et al., 2006; Folland and Williams, 2007; but see Antonio and Gonyea, 1993). In fact, some researchers consider hyperplasia as evidence of muscle pathology during regeneration (Eriksson et al., 2006). Given these results, we speculate that hyperplasia in the jaw muscles of tufted capuchins would have evolved via natural selection related to generating larger bite forces. This evolutionary hypothesis is bolstered by both the marked variation in absolute and relative jaw-muscle masses and PCSAs across primates (Schumacher, 1961; Turnbull, 1970; Cachel, 1979; Ross, 1995; Anapol et al., 2008; Perry and Wall, 2008); Taylor et al., n.d.), as well as the observation of heritable variation in masseter weight and PCSA among inbred mouse strains (Taylor et al., 2008).
Alternatively, increasing muscle mass as a response to forceful recruitment throughout an individual’s lifetime typically involves enlarging the diameter of existing fibers or muscle hypertrophy (Folland and Williams, 2007). While we lack the necessary data to discern the relative roles of hypertrophy versus hyperplasia, two anecdotal pieces of evidence suggest that hypertrophy is not fully responsible for the observed differences in jaw-muscle mass between tufted and untufted capuchins. First, muscle hypertrophy typically involves increases in pinnation angle given that the larger fibers must be spatially accommodated within an existing myotendinous aponeurosis (Narici and Maganaris, 2006). The jaw muscles of tufted capuchins, however, did not exhibit significantly greater pinnation angles (Table 2).
Second, our comparisons involved almost exclusively captive specimens. If we assume the captive capuchins were maintained on roughly similar diets, then we can speculate that the observed differences in jaw-muscle mass and PCSAs partly reflect heritable variation. It does not necessarily follow that heritable variation only reflects differences in fiber number between species, but we speculate that fiber number would be among the traits that express significant heritable variation among primate species. We can note that inclusion of two wild-caught C. apella specimens in our sample did not change our ultimate findings as we observed similar, significant outcomes when comparing only captive tufted and untufted capuchins. Elsewhere, we have demonstrated significant architectural differences in the jaw muscles of captive tree-gouging and nongouging callitrichids (Taylor and Vinyard 2004, 2008; Taylor et al., in press), providing a cautious optimism for observing significant functional and potentially adaptive variation in muscle architecture from captive specimens.
Finally, it is possible that the jaw muscles of C. apella are enlarged compared to untufted capuchins because they include a greater proportion of larger-diameter, fast-contracting Type II fibers. The morphological, contractile, and metabolic properties of Type II fibers make them better suited for generating force compared to Type I fibers (Close, 1972; Bodine et al., 1987). These physiological differences have led previous investigators to link the predominance of Type II fibers in the jaw muscles of primates to feeding behaviors that require the generation of rapid, forceful bites (Rowlerson et al., 1983; Andreo et al., 2002; Hoh, 2002; Wall et al., 2008). This third pathway could involve environmentally-induced (i.e., plastic) changes in fiber type and size related to increased mechanical loading and enhanced neuromuscular activity (Pette and Staron, 2000) and/or evolutionary (i.e., heritable) changes in fiber type (Komi et al. 1977; Staron 1997; Rivero and Barrey 2001).
In a study of C. apella, Andreo et al. (2002) found that the masseter and temporalis muscles of tufted capuchins include a predominance of Type II fibers. While this finding might initially point to a physiological explanation for the larger jaw-muscle mass in C. apella, most primate jaw muscles studied to date comprise a significant percentage of Type II fibers (Rowlerson et al., 1983; Andreo et al., 2002). Furthermore, Andreo et al. (2002) did not clearly define from where they sampled within the muscles. For example, they reported that temporalis samples were taken from the anterior and posterior regions of the muscle. However, Wall et al. (2008) noted substantial differences in fiber type composition between the superficial and deep portions of the temporalis in papionins, with the superficial portion being composed almost entirely of Type II fibers and exhibiting higher levels of EMG recruitment during forceful chewing. Thus, the results from Andreo et al. (2002) may partly reflect sampling of areas within the jaw muscles that predominately consist of Type II fibers.
At present, we cannot identify specific mechanisms for increasing jaw-muscle mass in Cebus apella. To do so, we first need to conduct a comparative analysis to determine if tufted and untufted capuchins differ in the presence and percentages of different fiber types in their jaw muscles. Subsequently, comparisons of fiber size, per fiber type, will help determine whether the larger jaw muscles of tufted capuchins are the result of fiber hypertrophy and/or fiber hyperplasia. These data would significantly improve our understanding of the physiological processes that could account for the enlarged jaw muscles of tufted capuchins and the evolutionary mechanisms involved in this transition. Furthermore, these data would improve our ability to use C. apella as a model species for studying how primate jaw muscles evolve in response to shifts in dietary demands.
The functional and ecological implications of enlarged jaw-muscle mass in Cebus apella
We speculate that the increased jaw-muscle PCSAs in C. apella are functionally related to generating relatively large bite forces as a key element of a feeding biological role (Bock and von Wahlert, 1965) that includes breaching exceptionally stiff and tough foods. Previous research has shown that C. apella has a relatively robust mandible (Bouvier, 1986; Cole, 1992; Daegling, 1992), improved leverage for biting (Anapol and Lee, 1994b; Wright, 2005; Norconk et al., in press), relatively large incisors (Eaglen, 1984; Rosenberger, 1992), and relatively thick dental enamel (Kay, 1981; Dumont, 1995; Shellis et al., 1998; Martin et al., 2003). These craniodental specializations have been interpreted to support the hypothesis that tufted capuchins have a masticatory apparatus adapted for hard-object feeding (Kinzey, 1974). We argue that relative increase in PSCAs, via enlarged jaw-muscle masses, can be added to the complex of masticatory apparatus features in C. apella that may be functionally linked to exploiting these resistant foods.
Tufted capuchins do not exhibit the predicted architectural trade-off between PCSA and fiber length (NLf) because they have increased PCSA primarily by enlarging their masticatory muscles. By increasing relative PCSA without markedly decreasing NLf or increasing pinnation angle, C. apella is effectively capable of increasing relative force production without necessarily compromising muscle excursion and hence jaw gapes (Herring and Herring, 1974). Additionally, this architectural configuration may allow C. apella to maintain bite forces at large gapes as well as the jaw gape where maximal bite force is achieved. These last two functional outcomes follow from observations that most jaw-muscle sarcomeres stretch with increasing gape, and this stretching eventually decreases their contractile force as sarcomeres are lengthened past the plateau of the length-tension curve (Nordstrom et al., 1974; Nordstrom and Yemm, 1974; Hertzberg et al., 1980; Eng et al., 2007).
We do not know whether other primates that consume hard and/or tough diets have modified their jaw-muscle architecture in a similar manner. Most of the previous work comparing primate jaw-muscle mass and architectural properties has focused on describing broader interspecific patterns (Schumacher, 1961; Turnbull, 1970; Cachel, 1979; Ross, 1995; Anapol et al., 2008; Perry and Wall, 2008), rather than phylogenetically-restricted comparisons that provide insights into the microevolutionary shifts in muscle architecture considered here. Thus, future work on jaw-muscle architecture is required to clarify whether primates routinely or only rarely enlarge their muscle masses in response to novel feeding challenges.
Stepping outside of mammals, comparative studies in other vertebrates have documented the evolution of increased bite force accomplished primarily by enlarging jaw-muscle masses rather than altering other aspects of muscle architecture. In a comparative study of finches, van der Meij and Bout (2004, 2006) demonstrated that fringillid finches have achieved relatively large bite forces compared to estrildid finches primarily by increasing the mass of their jaw-closing muscles. Increased relative bite force likely affords fringillids greater dietary diversity through improved husking efficiency and access to hard, close-shelled seeds (van der Meij and Bout, 2004, 2006, 2008). In a second comparative study, clariid catfish with enlarged jaw-closing muscles, but only minor differences in fiber length and pinnation angle, were modeled to have greater bite forces relative to a related species maintaining a primitive, smaller adductor mandibulae (Herrell et al., 2002; van Wassenbergh et al., 2005). Contrary to the finches, the relative increase in bite force in these catfish does not appear to impact their feeding ecology (Huysentruyt et al., 2004). Alternatively, the enlarged jaw-muscle masses are interpreted to increase acceleration of the jaw during closing and facilitate prey capture at large gapes (van Wassenbergh et al., 2005). While these broader comparative data support enlarging jaw-muscle mass as an evolutionary mechanism, the multiple performance outcomes challenge a single, definitive functional interpretation for the similar morphological changes seen in C. apella.
Physiological responses to altered activity levels may also contribute to the observed differences in muscle architecture among capuchins. Even though we speculated above that these zoological animals shared broadly similar diets in captivity, this remains unproven. Studies comparing rabbits fed different diets show that the group masticating the more obdurate diet increased their masseter mass without changes in fiber length (Taylor et al., 2006) as well as had larger muscle fibers (Kiliaridis et al., 1988). Our results do not point to either an evolutionary mechanism or a physiological response as the predominant contributing factor in the larger PCSA and enlarged jaw muscles of C. apella. Furthermore, both factors could have played a role as they are not mutually exclusive for the individuals in this study. The analyses of fiber type and fiber size outlined above would help to define the relative influences of evolutionary and physiological factors in establishing the differences in jaw-muscle architecture between tufted and untufted capuchins.
The jaw-muscle architecture of tufted capuchins suggests it is possible to increase jaw-muscle force without compromising masticatory gapes. Given this possibility, it is reasonable to ask, what are the advantages of altering fiber length and pinnation angle to increase muscle and bite forces? Prior to speculating, we should note the possibility that some species that have increased maximum muscle PCSA by shortening fibers and increasing pinnation may suffer no ecological consequences during feeding from decreased jaw gapes or reduced bite forces at large gapes. That said, one of the basic tenets of muscle architecture is that for a given volume, long-fibered, parallel muscles facilitate excursion and contraction velocity while short-fibered, pinnate muscles facilitate force production (Gans and Bock, 1965; Gans, 1982). An implication of this architectural contrast is that there are performance tradeoffs and hence different costs for maintaining either architectural configuration. For excursion and contraction velocity, the cost of short fibers relates to reduced maximum contractile velocities and excursions (Lieber, 2002). Force production, however, can be improved (at least theoretically) by simply adding muscle fibers without decreasing fiber length or increasing pinnation as suggested for C. apella. One potential cost to this additive strategy is an increase in metabolic expenditure as more fibers would need to contract to produce a given force compared to an optimal architectural configuration. While we know the metabolic cost to contract a muscle fiber varies with the type of contraction (Potma et al., 1994; Sih and Stuhmiller, 2003), muscle temperature (Steinen et al., 1996; He et al., 2000), and particularly the fiber type (Kushmerick et al., 1992; Bottinelli et al., 1994), we know little about the metabolic costs of mastication relative to extractable energy intake. Thus, it is possible that contracting more muscle fibers to achieve a given muscle force will have a significant metabolic cost to an individual. This question deserves further attention.
If the metabolic costs of feeding are sufficiently high, then we would predict that tufted capuchins routinely bite large, resistant food items and/or require this functional capacity at some critical point during their annual feeding cycle (e.g., to consume fallback foods). These feeding behaviors would suggest a functional benefit to maintaining a less economical architectural configuration. The metabolic costs might be further exacerbated if the jaw muscles of C. apella are comprised predominately of metabolically-expensive, Type II fibers (Andreo et al., 2002).
Field data suggest that tufted capuchins incorporate both large gapes and forceful biting, often along the posterior dentition, in their “destructive foraging” behaviors (Terborgh, 1983: 99; see also Izawa, 1979; Wright, 2004). Tufted capuchins consume larger fruits and seeds than sympatric C. olivaceous in the Iwokrama reserve in central Guyana (Wright, 2004; Norconk et al., in press). Similarly, C. apella consumes a slightly higher percentage of larger food items than C. albifrons at Cocha Cashu in Peru (Terborgh, 1983). Terborgh also reports that C. apella spends more than 44% of its foraging time breaking open thick and hard to moderately hard substrates as compared to 32% in C. albifrons. Within tufted capuchins, the importance of strength during foraging is emphasized by a size-related increase in substrates breached with adult males breaking open larger branches more frequently during foraging than adult females who break open larger branches more often than subadults and juveniles (Terborgh, 1983). Finally, during the dry season, C. apella falls back to consuming a large percentage of moderately-sized (~4 cm) but extremely hard palm nuts, which they frequently crack on their posterior dentition. By comparison, C. albifrons is incapable of breaking open these nuts unassisted and must adopt an alternative foraging strategy relying on insect infestation to weaken the palm nuts prior to accessing them (Terborgh, 1983). Collectively, the field data support the hypothesis that tufted capuchins rely on forceful bites often at wide jaw gapes during foraging.
The architectural configuration of tufted capuchin jaw muscles may have the ecological consequence of facilitating dietary diversity by providing C. apella the capacity to access relatively large, resistant foods. Capuchins are recognized for their dietary diversity (Freese and Oppenheimer, 1981; Terborgh, 1983; Robinson, 1986; Brown and Zunino, 1990; Fragaszy et al., 1990, 2004; Janson and Boinski, 1992), but the extent of dietary variation among capuchins remains uncertain. Simplistically comparing the number of plant foods eaten (data from Appendix I in Fragaszy et al., 2004) indicates that C. apella consumes the greatest number of plant foods (580), with the closest untufted capuchin, C. capucinus, consuming only 87% (507) of the number of plant foods. Multiple factors likely contribute to this dietary diversity, several of which involve masticatory morphology. Cebus apella ingests a diet comprising a wider range of mechanical properties, as they have been observed to occasionally process extremely tough (Wright, 2005), stiff, and hard foods (Wright, 2005; Chalk et al., 2008; Wright et al., 2008). The increased range of mechanical properties that C. apella can consume has been described as a niche broadening mechanism (Wright, 2005). Similarly, tufted capuchins process a larger range of food sizes (Terborgh, 1983; Wright, 2004; Norconk et al., in press) that may contribute to dietary diversity. Finally, reduced processing time through larger bite forces may boost dietary diversity through improving foraging efficiency and making some challenging items economical for routine processing (see Benkman and Pulliam, 1988; van der Meij and Bout, 2006). For example C. apella processes palm nuts significantly faster than C. albifrons at Cocha Cashu through their ability to generate increased bite forces (Terborgh, 1983). Collectively, the jaw muscle architecture of C. apella, as part of their overall masticatory apparatus configuration, may enhance the range of dietary items they can routinely consume in their environment.
The craniodental specializations of tufted capuchins support the hypothesis that this species has undergone selection to improve their ability to exploit stiff and tough tissues. We have no direct evidence, however, to point to a single selective scenario that improves feeding performance by increasing jaw-muscle PCSA and maintaining excursion abilities. We can reasonably rule out that larger jaw-muscle mass is simply an allometric correlate of increased body size (e.g., Hurov et al., 1988; van der Meij and Bout, 2004) given that C. apella exhibits larger jaw-muscles yet is similar in size to C. capuchinus (Smith and Jungers, 1997). We suggest, as many others have previously, that the total morphological pattern (e.g., Le Gros Clark, 1955) of the tufted capuchin masticatory apparatus likely relates to feeding on hard/tough objects. We challenge the more specific conclusion that Cebus apella is “morphologically adapted for small, tough object feeding” (Kinzey, 1974: 200), as the jaw-muscle architecture appears to facilitate ingesting large objects at wide gapes. This conclusion is consistent with observed feeding behaviors of C. apella in the wild.
Lastly, this particular aspect of feeding on large hard/tough objects may differ from behaviors predicted for early hominins. Although still much a matter of debate (Scott et al., 2005; Ungar et al., 2008b), a number of investigators have hypothesized that the masticatory apparatus of early hominins were designed for consuming a diet of hard foods, either routinely or when preferred foods were scarce (Kay, 1985; Grine and Kay, 1988; Laden and Wrangham, 2005; Lucas et al., 2008). An alternative, though not necessarily contradictory hypothesis, is that the postcanine megadontia characteristic of early hominins is an adaptation to repetitive loading of tough, but small and/or thin food items (Jolly, 1970; Lucas et al., 1986). Ingesting small food items is consistent with their markedly high mandibular rami, which are not favorable for generating relatively wide jaw gapes (Herring and Herring, 1974). The behavioral data demonstrating that C. apella feeds on large hard/tough objects, coupled with a jaw-muscle architecture that facilitates this behavior, suggest some level of caution is warranted when relying on C. apella as an extant behavioral model for hominins that might have specialized on small objects in their diets.
Acknowledgements
We are grateful to Carel van Schaik of the Anthropological Institute and Museum (Zürich) and Richard W. Thorington and Linda Gordon of the Smithsonian Institution for providing the specimens for dissection. We thank Barth Wright for sharing his insights on capuchin feeding behaviors. Grant sponsorship was provided by the US National Institutes of Health (R24 HD050837-01) and the National Science Foundation (BCS 0452160 and BCS 0552285). We also thank several anonymous reviewers for providing thoughtful comments on previous versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The masseter and temporalis muscles have multiple lines of action. For ease of consistency and interpretation, we restricted our analysis to the long axis of the muscle in situ (e.g. Taylor and Vinyard, 2004).
Not all specimens preserved both the masseter and temporalis muscles.
The two wild specimens were from Paraguay.
2.3 kg/cm2 (converted from 22.5 N cm−2), is an estimate of the specific tension of muscle (Powell et al., 1984).
Based on our reading of Bouvier and Tsang (1990), whole masseter muscles (i.e., deep and superficial portions) were removed and weighed, but only superficial masseter fiber lengths were measured. If we apply a similar protocol to our data for estimating PCSA, then our “masseter” PCSA estimate is broadly similar at 5.6 cm2.
References
- Anapol F, Barry K. Fiber architecture of the extensors of the hindlimb in semiterrestrial and arboreal guenons. Am. J. Phys. Anthropol. 1996;99(3):429–447. doi: 10.1002/(SICI)1096-8644(199603)99:3<429::AID-AJPA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Anapol F, Lee S. Morphological adaptation to diet in platyrrhine primates. Am. J. Phys. Anthropol. 1994a;94:239–261. doi: 10.1002/ajpa.1330940208. [DOI] [PubMed] [Google Scholar]
- Anapol F, Lee S. Morphological adaptations to diet in platyrrhine primates. Am. J. Phys. Anthropol. 1994b;94:239–261. doi: 10.1002/ajpa.1330940208. [DOI] [PubMed] [Google Scholar]
- Anapol F, Shahnoor N, Ross CF. Scaling of reduced physiologic cross-sectional area in primate muscles of mastication. In: Vinyard CJ, Ravosa MJ, Wall CE, editors. Primate Craniofacial Function and Biology. New York: Springer; 2008. pp. 201–216. [Google Scholar]
- Andreo JC, Oliveira JA, Navarro JA, Roque DD, Roque JS, Buchain RL. Histoenzymology and morphometry of the masticatory muscles of tufted capuchin monkey (Cebus apella Linnaeus, 1758) Okajimas Folia Anat. Jpn. 2002;79(1):33–41. doi: 10.2535/ofaj.79.33. [DOI] [PubMed] [Google Scholar]
- Antón SC. Tendon associated bone features of the masticatory system in Neandertals. J. Hum. Evol. 1996;31:391–408. [Google Scholar]
- Antón SC. Macaque masseter muscle: internal architecture, fiber length and cross-sectional area. Int. J. Primatol. 1999;20:441–462. [Google Scholar]
- Antonio J, Gonyea WJ. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exer. 1993;25:1333–1345. [PubMed] [Google Scholar]
- Benkman CW, Pulliam HR. The comparative feeding rates of North American sparrows and finches. Ecology. 1988;69:1195–1199. [Google Scholar]
- Bock WJ, von Wahlert G. Adaptation and the form-function complex. Evolution. 1965;19:269–299. [Google Scholar]
- Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J. Neurophysiol. 1987;57(6):1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Reggiani C, Stienen GJM. Myofibrillar APTase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. J. Physiol. 1994;481:663–675. doi: 10.1113/jphysiol.1994.sp020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M. Biomechanical scaling of mandibular dimensions in New World monkeys. Int. J. Primatol. 1986;7:551–567. [Google Scholar]
- Bouvier M, Tsang SM. Comparison of muscle weight and force ratios in New and Old World monkeys. Am. J. Phys. Anthropol. 1990;82(4):509–515. doi: 10.1002/ajpa.1330820410. [DOI] [PubMed] [Google Scholar]
- Brown AD, Zunino GE. Dietary variability in Cebus apella in extreme habitats: evidence for adaptability. Folia Primatol. (Basel) 1990;54:187–195. doi: 10.1159/000156443. [DOI] [PubMed] [Google Scholar]
- Cachel S. Growth and allometry in primate masticatory muscles. Arch. Oral Biol. 1984;29(4):287–293. doi: 10.1016/0003-9969(84)90102-x. [DOI] [PubMed] [Google Scholar]
- Cachel SM. A functional analysis of the primate masticatory system and the origin of the anthropoid post-orbital septum. Am. J. Phys. Anthropol. 1979;50:1–18. doi: 10.1002/ajpa.1330500102. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Bohn EE, Gibson BT, Farrar RP. Growth hormone supplementation increases skeletal muscle mass of old male Fischer 344/brown Norway rats. J. Gerontol. 1996;51A:B214–B219. doi: 10.1093/gerona/51a.3.b214. [DOI] [PubMed] [Google Scholar]
- Chalk J, Wright BW, Lucas PW, Verderane MP, Fragaszy D, Visalberghi E. The mechanical properties of foods processed by Cebus libidinosus at Boa Vista, Brazil. Am. J. Phys. Anthropol. Suppl. 2008;46:77. [Google Scholar]
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 1972;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cole TM. Postnatal heterochrony of the masticatory apparatus in Cebus apella and Cebus albifrons. J. Hum. Evol. 1992;23:253–282. [Google Scholar]
- Daegling D. Mandibular morphology and diet in the genus Cebus. Int. J. Primatol. 1992;13:545–570. [Google Scholar]
- Dumont ER. Enamel thickness and dietary adaptation among extant primates and chiropterans. J. Mammal. 1995;76:1127–1136. [Google Scholar]
- Eaglen RH. Incisor size and diet revisited: the view from a platyrrhine perspective. Am. J. Phys. Anthropol. 1984;64:263–275. doi: 10.1002/ajpa.1330640308. [DOI] [PubMed] [Google Scholar]
- Eng CM, Ward SR, Winters TM, Kingsbury TD, Vinyard CJ, Taylor AB. Mechanics of the masticatory apparatus favor muscle force production at wide jaw gapes in tree-gouging marmosets. Am. J. Phys. Anthropol. Suppl. 2007;44:107. [Google Scholar]
- Eriksson A, Lindstrom M, Carlsson L, Thornell LE. Hypertrophic muscle fibers with fissures in power-lifters: fiber splitting or defect regeneration? Histochem. Cell Biol. 2006;126:409–417. doi: 10.1007/s00418-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J. Exp. Biol. 2005;208(Pt 17):3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- Folland JP, Williams AG. The adaptations to strength training. Morphological and neurological contributions to increased strength. Sports Med. 2007;37:145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Visalberghi E, Fedigan LM. Variability and adaptability in the genus Cebus. Cambridge: Cambridge University Press; 2004. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Visalberghi E, Robinson JG. Variability and adaptability in the genus Cebus. Folia Primatol. (Basel) 1990;54:114–118. doi: 10.1159/000156434. [DOI] [PubMed] [Google Scholar]
- Freese CH, Oppenheimer JR. The capuchin monkeys, genus Cebus. In: Coimbra-Filho A, Mittermeier RA, editors. Ecology and behavior of neotropical primates. vol. 1. Rio de Janeiro: Academia Brasileira de Ciências; 1981. pp. 331–390. [Google Scholar]
- Gans C. Fiber architecture and muscle function. Exerc. Sport Sci. Rev. 1982;10:160–207. [PubMed] [Google Scholar]
- Gans C, Bock W. The functional significance of muscle architecture -- a theoretical analysis. Ergebn. Anat. EntwGesch. 1965;38:115–142. [PubMed] [Google Scholar]
- Grine FE, Kay RF. Early hominid diets from quantitative image analysis of dental microwear. Nature. 1988;333(6175):765–768. doi: 10.1038/333765a0. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophysics Journal. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrell A, Adriaens D, Verraes W, Aerts P. Bite performance in clariid fishes with hypertrophied jaw adductors as deduced by bite modeling. J. Morphol. 2002;253:196–205. doi: 10.1002/jmor.1121. [DOI] [PubMed] [Google Scholar]
- Herring SW, Herring SE. The superficial masseter and gape in mammals. Am. Naturalist. 1974;108:561–576. [Google Scholar]
- Hertzberg SR, Muhl ZF, Begole EA. Muscle sarcomere length following passive jaw opening in the rabbit. Anat. Rec. 1980;197:435–440. doi: 10.1002/ar.1091970407. [DOI] [PubMed] [Google Scholar]
- Hoh JF. 'Superfast' or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J. Exp. Biol. 2002;205(Pt 15):2203–2210. doi: 10.1242/jeb.205.15.2203. [DOI] [PubMed] [Google Scholar]
- Hurov J, Henry-Ward W, Phillips L, German R. Growth allometry of craniomandibular muscles, tendons, and bones in the laboratory rat (Rattus norvegicus): relationships to oromotor maturation and biomechanics of feeding. Am. J. Anat. 1988;182:381–394. doi: 10.1002/aja.1001820409. [DOI] [PubMed] [Google Scholar]
- Huysentruyt F, Adriaens D, Teugels GG, Devaere S, Herrell A, Verraes W, Aerts P. Diet composition in relation to morphology in some African anguilliform clariid catfishes. Belg. J. Zool. 2004;134:41–46. [Google Scholar]
- Izawa K. Foods and feeding behavior of wild black-capped capuchins (Cebus apella) Primates. 1979;20:57–76. [Google Scholar]
- Izawa K, Mizuno A. Palm-fruit cracking behavior of wild black-capped capuchin (Cebus apella) Primates. 1977;18:773–792. [Google Scholar]
- Janson CH, Boinski S. Morphological and behavioral adaptations for foraging in generalist primates: the case of the cebines. Am. J. Phys. Anthropol. 1992;88(4):483–498. doi: 10.1002/ajpa.1330880405. [DOI] [PubMed] [Google Scholar]
- Jolly C. The seed-eaters: a new model of hominid differentiation based on a baboon analogy. Man. 1970;5:5–26. [Google Scholar]
- Kay RF. The nut-crackers -- a new theory of the adaptations of the ramapithecines. Am. J. Phys. Anthropol. 1981;55:141–151. [Google Scholar]
- Kay RF. The dental evidence for the diet of Australopithecus. A. Rev. Anthropol. 1985;14:315–341. [Google Scholar]
- Kiliaridis S, Engstrom C, Thilander B. Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Arch. Oral Biol. 1988;33:187–193. doi: 10.1016/0003-9969(88)90044-1. [DOI] [PubMed] [Google Scholar]
- Kinzey WG. Ceboid models for the evolution of hominoid dentition. J. Hum. Evol. 1974;3:187–193. [Google Scholar]
- Kinzey WG, Norconk MA. Hardness as a basis of fruit choice in two sympatric primates. Am. J. Phys. Anthropol. 1990;81:5–15. doi: 10.1002/ajpa.1330810103. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am. J. Physiol. 1992;263:C598–C606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Laden G, Wrangham R. The rise of the hominids as an adaptive shift in fallback foods: plant underground storage organs (USOs) and australopith origins. J. Hum. Evol. 2005;49(4):482–498. doi: 10.1016/j.jhevol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lambert JE, Chapman CA, Wrangham RW, Conklin-Brittain NL. Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. Am. J. Phys. Anthropol. 2004;125(4):363–368. doi: 10.1002/ajpa.10403. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The Fossil Evidence for Human Evolution: An Introduction to the Study of Palaeoanthropology. Chicago: University of Chicago Press; 1955. [Google Scholar]
- Lieber RL. Skeletal Muscle, Structure, Function, and Plasticity. Baltimore: Lippincott Williams and Wilkins; 2002. [Google Scholar]
- Lieber RL, Blevins FT. Skeletal muscle architecture of the rabbit hindlimb: functional implications of muscle design. J. Morphol. 1989;199:93–101. doi: 10.1002/jmor.1051990108. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys. J. 1984;45(5):1007–1016. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: The University of Chicago Press; 1986. [Google Scholar]
- Lucas PW, Constantino PJ, Wood BA. Inferences regarding the diet of extinct hominins: structural and functional trends in dental and mandibular morphology within the hominin clade. J. Anat. 2008;212(4):486–500. doi: 10.1111/j.1469-7580.2008.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PW, Corlett RT, Luke DA. Postcanine tooth size and diet in anthropoid primates. Z. Morphol. Anthropol. 1986;76(3):253–276. [PubMed] [Google Scholar]
- Mackenna BR, Türker KS. Twitch tension in the jaw muscles of the cat at various degrees of mouth opening. Arch. Oral Biol. 1978;23:917–920. doi: 10.1016/0003-9969(78)90297-2. [DOI] [PubMed] [Google Scholar]
- Martin LB, Olejniczak AJ, Maas MC. Enamel thickness and microstructure in pitheciin primates, with comments on dietary adaptations of the middle Miocene hominoid Kenyapithecus. J. Hum. Evol. 2003;45:351–367. doi: 10.1016/j.jhevol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- McDonagh MJN, Davies CTM. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur. J. Appl. Physiol. 1984;52:139–155. doi: 10.1007/BF00433384. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Narici M, Maganaris C. Muscle architecture and adaptations to functional requirements. In: Bottinelli R, Reggiani C, editors. Skeletal Muscle Plasticity in Health and Disease: From Genes to Whole Muscle. Amsterdam, The Netherlands: Springer; 2006. pp. 265–288. [Google Scholar]
- Norconk MA, Wright BW, Conklin-Brittain NL, Vinyard CJ. Mechanical and nutritional properties of food as factors in platyrrhine dietary adaptations. In: Garber PA, Estrada A, Bicca-Marques JC, Heymann E, Strier K, editors. South American Primates: Testing New Theories in the Study of Primate Behavior, Ecology, and Conservation. New York: Springer; In press. [Google Scholar]
- Nordstrom SH, Bishop M, Yemm R. The effect of jaw opening on the sarcomere length of the masseter and temporal muscles of the rat. Arch. Oral Biol. 1974;46:139–146. doi: 10.1016/0003-9969(74)90209-x. [DOI] [PubMed] [Google Scholar]
- Nordstrom SH, Yemm R. The relationship between jaw position and isometric active tension produced by direct stimulation of the rat masseter muscle. Arch. Oral. Biol. 1974;19:353–359. doi: 10.1016/0003-9969(74)90176-9. [DOI] [PubMed] [Google Scholar]
- Norton M, Verstegeden A, Maxwell LC, McCarter RM. Constancy of masseter muscle structure and function with age in F344 rats. Arch. Oral Biol. 2001;46:139–146. doi: 10.1016/s0003-9969(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Ounjian M, Roy RR, Eldred E, Garfinkel A, Payne JR, Armstrong A, Toga AW, Edgerton VR. Physiological and developmental implications of motor unit anatomy. J. Neurobiol. 1991;22:547–559. doi: 10.1002/neu.480220510. [DOI] [PubMed] [Google Scholar]
- Perry JMG, Wall CE. Scaling of the chewing muscles in Prosimians. In: Vinyard CJ, Ravosa MJ, Wall CE, editors. Primate Craniofacial Function and Biology. New York: Springer; 2008. pp. 217–240. [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000;50(6):500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Potma EJ, Stienen GJM, Barends JPF, Elzinga G. Myofibrillar ATPase activity and mechanical performance of skinned fibres from rabbit psoas muscle. J. Physiol. 1994;474:303–317. doi: 10.1113/jphysiol.1994.sp020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 1984;57(6):1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Robinson JG. Seasonal variation in use of time and space by the wedge-capped capuchin monkey, Cebus olivaceus: implications for foraging theory. Smithson. Contr. Zool. 1986;431:1–56. [Google Scholar]
- Rosenberger AL. Evolution of feeding niches in New World monkeys. Am. J. Phys. Anthropol. 1992;98:275–306. doi: 10.1002/ajpa.1330880408. [DOI] [PubMed] [Google Scholar]
- Ross CF. Muscular and osseous anatomy of the primate anterior temporal fossa and the functions of the postorbital septum. Am. J. Phys. Anthropol. 1995;98:275–306. doi: 10.1002/ajpa.1330980304. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Mascarello F, Veggetti A, Carpene E. The fibre-type composition of the first branchial arch muscles in Carnivora and Primates. J. Muscle Res. Cell Motil. 1983;4(4):443–472. doi: 10.1007/BF00711949. [DOI] [PubMed] [Google Scholar]
- Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J. Morphol. 1982;173(2):185–195. doi: 10.1002/jmor.1051730206. [DOI] [PubMed] [Google Scholar]
- Scanlon PF. Wet and dry weight relationships of mallard (Anas platyrhynchos) tissues. Bull. Environ. Cont. Toxicol. 1982;29:615–617. doi: 10.1007/BF01669630. [DOI] [PubMed] [Google Scholar]
- Schumacher GH. Funktionelle Morphologie der Kaumuskulatur. Jena: Gustav Fischer; 1961. [Google Scholar]
- Scott RS, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, Walker A. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436(7051):693–695. doi: 10.1038/nature03822. [DOI] [PubMed] [Google Scholar]
- Shea BT. Size and diet in the evolution of African ape craniodental form. Folia Primatol. (Basel) 1983;40(1–2):32–68. doi: 10.1159/000156090. [DOI] [PubMed] [Google Scholar]
- Shellis RP, Beynon AD, Reid DJ, Hiiemae KM. Variations in molar enamel thickness among primates. J. Hum. Evol. 1998;35:507–522. doi: 10.1006/jhev.1998.0238. [DOI] [PubMed] [Google Scholar]
- Sih BL, Stuhmiller JH. The metabolic cost of force generation. Med. Sci. Sports Exer. 2003;35:623–629. doi: 10.1249/01.MSS.0000058435.67376.49. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J. Hum. Evol. 1997;32(6):523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Starck D. Die Kaumuskulatur der Platyrrhinen. Gegenbaurs Morphol. Jahrb. 1933;72:212–285. [Google Scholar]
- Steinen GJM, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J. Physiol. 1996;493:299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AB, Jones KE, Kunwar R, Ravosa MJ. Dietary consistency and plasticity of masseter fiber architecture in postweaning rabbits. Anat. Rec. A. Discov. Mol. Cell Evol. Biol. 2006;288(10):1105–1111. doi: 10.1002/ar.a.20382. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Vinyard CJ. Comparative analysis of masseter fiber architecture in tree-gouging (Callithrix jacchus) and nongouging (Saguinus oedipus) callitrichids. J. Morphol. 2004;261(3):276–285. doi: 10.1002/jmor.10249. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Vinyard CJ, Payseur BA. Variation in masseter muscle fiber architecture in five strains of inbred mice: implications for heritability of fiber architecture. Am. J. Phys. Anthropol. Suppl. 2008;46:204–205. [Google Scholar]
- Teaford MF. Molar microwear and diet in the genus Cebus. Am. J. Phys. Anthropol. 1985;66:363–370. doi: 10.1002/ajpa.1330660403. [DOI] [PubMed] [Google Scholar]
- Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc. Natl. Acad. Sci. 2000;97:13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborgh J. Five New World Primates. Princeton: Princeton University Press; 1983. [Google Scholar]
- Thexton AJ, Hiiemae KM. The twitch-contraction characteristics of opossum jaw musculature. Arch. Oral Biol. 1975;20:743–748. doi: 10.1016/0003-9969(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Turnbull WD. Mammalian masticatory apparatus. Fieldiana Geol. 1970;18:1–356. [Google Scholar]
- Ungar PS, Grine FE, Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS One. 2008a;3:e2044. doi: 10.1371/journal.pone.0002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar PS, Grine FE, Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS One. 2008b;3(4):e2044. doi: 10.1371/journal.pone.0002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar PS, Grine FE, Teaford MF, El Zaatari S. Dental microwear and diets of African early Homo. J. Hum. Evol. 2006;50(1):78–95. doi: 10.1016/j.jhevol.2005.08.007. [DOI] [PubMed] [Google Scholar]
- van der Meij MAA, Bout RG. Scaling of jaw muscle size and maximal bite force in finches. J. Exp. Biol. 2004;207:2745–2743. doi: 10.1242/jeb.01091. [DOI] [PubMed] [Google Scholar]
- van der Meij MAA, Bout RG. Seed husking time and maximal bite force in finches. J. Exp. Biol. 2006;209:3329–3335. doi: 10.1242/jeb.02379. [DOI] [PubMed] [Google Scholar]
- van der Meij MAA, Bout RG. The relationship between shape of the skull and bite force in finches. J. Exp. Biol. 2008;211:1668–1680. doi: 10.1242/jeb.015289. [DOI] [PubMed] [Google Scholar]
- van Wassenbergh S, Aerts P, Adriaens D, Herrell A. A dynamic model of mouth closing movements in clariid catfishes: the role of enlarged jaw adductors. J. Theor Biol. 2005;234:49–65. doi: 10.1016/j.jtbi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat. Rec. 1974;178:63–81. doi: 10.1002/ar.1091780107. [DOI] [PubMed] [Google Scholar]
- Wall CE, vinyard CJ, Williams SH, Johnson KR, Hylander WL. Specializations of the superficial anterior temporalis in baboons for mastication of hard foods. In: Vinyard CJ, Ravosa MJ, Wall CE, editors. Primate Craniofacial Function and Biology. New York: Springer; 2008. pp. 113–124. [Google Scholar]
- Williams PE, Goldspink G. Changes in sarcomere length and physiolgical properties in immobilized muscle. J. Anat. 1978;127:459–468. [PMC free article] [PubMed] [Google Scholar]
- Wright BW. Ph.D. Dissertation. University of Illinois: Urbana-Champaign; 2004. Ecological Distinctions in Diet, Food Toughness, and Masticatory Anatomy in a Community of Six Neotropical Primates in Guyana, South America. [Google Scholar]
- Wright BW. Craniodental biomechanics and dietary toughness in the genus Cebus. J. Hum. Evol. 2005;48(5):473–492. doi: 10.1016/j.jhevol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Wright BW, Wright KA, Chalk J, Verderane MP, Fragaszy D, Visalberghi E, Izar P, Ottoni EB. Fallback foraging as a way of life: dietary variability of and skeletal morphology in tufted capuchins. Am. J. Phys. Anthropol Suppl. 2008;46:225. doi: 10.1002/ajpa.21116. [DOI] [PubMed] [Google Scholar]