Abstract
Ethanol craving plays a major role in relapse drinking behavior. Relapse and ethanol craving are an important focus for the treatment of alcoholism. The ethanol deprivation effect (EDE) is a widely used animal model of alcohol craving. While the EDE is widely studied in rats, the molecular mechanisms underlying EDE are not clearly understood. The C57BL/6 inbred mouse strain is widely used for behavioral and molecular analyses of ethanol drinking but studies on the EDE have not been reported in this strain. In the present study, we characterized a simple behavioral protocol that rapidly and reliably induced EDE in C57BL/6 mice. Briefly, single-housed adult male C57BL/6NCrl and C57BL/6J mice were presented at the beginning of dark phase with two-bottle choice drinking containing either 10 % w/v ethanol or tap water for 18-hrs/day, as well as food ad libitum. Following ethanol drinking for 4 days or 14-days, mice were deprived of ethanol for a period of 4 days. To study EDE, mice were reinstated with two-bottles containing either ethanol (10 % w/v) or water. Mice were exposed to single or multiple ethanol deprivation cycles. Ethanol consumption (g/kg/18-hrs) and percent ethanol preference (% preference/18-hrs) was recorded for individual mice. C57BL/6NCrl mice consumed moderate amounts (4.78 ± 0.63 g/kg) of ethanol but showed robust EDE after ethanol drinking episodes (4 days or 14 days) as evidenced by increased ethanol consumption and ethanol preference following re-instatement of ethanol. While repeated ethanol deprivation in C57BL/6NCrl mice transiently increased ethanol consumption and ethanol preference, the magnitude of these behaviors was reduced as compared to the first deprivation cycle. In contrast, the C57BL/6J substrain consumed substantially higher levels (9.65 ± 0.90 g/kg) of ethanol but did not show a clear EDE after single or multiple ethanol deprivation cycles. In conclusion, we established a simple and reliable behavioral model to study EDE in C57BL/6NCrl mice. A reliable behavioral model to study EDE in inbred C57BL/6NCrl mice could greatly facilitate further studies on molecular mechanisms of ethanol craving behavior.
Keywords: ethanol, ethanol-deprivation effect, ethanol-preference, craving
1. Introduction
Craving, described as a “desire for previously experienced effects of psychoactive substances”, is thought to have a major role in relapse drinking behavior in previously abstinent alcoholics (Koob, 2000). Relapse drinking behavior is a ubiquitous problem for individuals recovering from alcoholism, since at least 60–80% of abstinent alcoholics will relapse during their lifetime (Barrick and Connors, 2002; Chiauzzi, 1991; Jaffe, 2002; Weiss et al., 2001). Relapse and ethanol craving are an important focus of therapeutic approaches towards treatment of alcoholism.
The ethanol deprivation effect (EDE) is a robust and widely used animal model of ethanol craving (Heyser et al., 1997; Sinclair and Li, 1989; Spanagel and Holter, 1999). In the EDE, animals previously self-administering ethanol will increase their ethanol intake following a period of deprivation. Renewed access to ethanol solutions after a period of deprivation for several days/weeks leads to a pronounced, although temporary, increase in voluntary ethanol intake. The EDE is observed in a variety of species including rats (McKinzie et al., 1998; Rodd-Henricks et al., 2000a; Sinclair and Senter, 1967), mice (Salimov et al., 1993), monkeys (Kornet et al., 1990; Sinclair, 1971), and humans (Burish et al., 1981; Mello and Mendelson, 1972). Short (12-h or less; Sinclair and Li, 1989) or long (up to 75 days; Sinclair et al, 1973) deprivation intervals both produce an EDE in rats. Further validation of the EDE as a model for relapse comes from studies showing that pharmacological agents known to be effective in treating alcoholism will also decrease the EDE in animal models (Heyser et al., 1998; Kornet et al., 1991; Spanagel and Zieglgansberger, 1997).
In spite of considerable data on the EDE in rat model systems, the molecular events underlying EDE are poorly understood. Behavioral studies using mouse models have produced valuable information regarding molecular aspects of ethanol consumption due to the availability of multiple inbred lines and transgenic over-expressing or null animals. While several studies have shown gene deletion effects on EDE in hybrid null mice (129/Sv x C57BL/6N) (Cowen et al., 2003; Sanchis-Segura et al., 2006), there are no studies characterizing EDE in the widely used C57BL/6 strain, which voluntarily consume large amounts of ethanol. Use of the C57BL/6 model for EDE would greatly aid mechanistic studies given the wide range of established pharmacological, behavioral, and genetic resources using this inbred mouse line.
In the present study, we evaluated the EDE in two closely related substrains of C57BL/6 mice, obtained from Charles River Laboratories (C57BL/6NCrl) and Jackson Laboratories (C57BL/6J). The inbred mice strain, C57BL/6, was identified many years ago as having a genetically influenced high preference for ethanol (McClearn and Rodgers, 1959). These findings were confirmed in several studies of ethanol preference drinking (Belknap et al., 1993; Fuller, 1964; Rodgers, 1972). The breeding stocks of C57BL/6 mice established at Jackson Laboratories and Charles River Laboratories have been separated for over 5 decades. Thus, genetic drift may have produced considerable behavioral differences between these two closely related inbred mouse populations. In line with this, an abstract by Mulligan et al. (2005) recently reported preliminary studies showing statistically significant differences in ethanol preference between C57BL/6NCrl and C57BL/6J mice. However, these mice did not differ in their response to ethanol-induced loss of righting reflex, sensitivity, acute functional tolerance to ethanol-induced hypnosis, saccharin and quinine preference, and ethanol metabolism (Mulligan et al., 2005).
In view of scarcity of behavioral data on EDE in mice, the present study evaluated ethanol preference and consumption after EDE in C57BL/6NCrl and C57BL/6J using the two-bottle (ethanol or water) choice paradigm. We show that a robust EDE can be observed in C57BL/6 mice but that results can be markedly affected by the source of the animals.
2. Materials and Methods
2.1. Animals
Male C57BL/6NCrl and C57BL/6J mice at 60–80 days of age were purchased from Charles River Laboratories (Wilmington, MA) and Jackson Laboratories (Bar Harbor, ME), respectively. All mice were habituated to the housing environment by initially group housing mice 4 per cage for 1 week followed by individually housing for 1 week. Cages and bedding (Harlan Sani-Chips ®, catalog #7090A, Harlan Teklad, Madison, WI) were replaced every week during the 6 hour window (see below) when ethanol solutions were not available to mice. Mice were housed in a temperature (75 ± 1°F) and light (12:12 h light–dark cycle; lights on at 06:00 h) controlled room having free access to rodent chow (catalog #7912; Harlan Teklad, Madison, WI) and water. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, 1996).
2.2. Two-bottle (ethanol or water) choice drinking
Two-bottle choice drinking in mice was initiated by positioning two bottles at the beginning of dark phase one containing 10 % w/v ethanol (Aaper Alcohol and Chemical Co., Kentucky, USA) and the other tap water. The drinking bottles were constructed from 10 ml plastic pipettes. The narrow end of the pipette was removed and replaced with a stainless steel ball valve sipper tube which allowed the mice to drink while minimizing fluid loss. The bottles were available for 18-hrs/day, as was ad libitum rodent chow. Mice were allowed free access to water for the remaining 6 hours/day from a standard water bottle. Bottle position was varied in double alternation fashion (L,L,R,R etc.) to control for arbitrary side preference. Mice were left undisturbed during drinking sessions, after which fluid consumption from the graduated drinking bottles was measured to nearest 0.1 ml. Following ethanol drinking, mice were deprived of ethanol for 4 days (unless stated otherwise), as described below. After this deprivation period, mice were again allowed access to the two-bottles containing either ethanol (10 % w/v) or water and fluid consumption was monitored every 18-hr as described above. Ethanol consumption was calculated as grams of ethanol per kilogram bodyweight per 18 hours. Percent ethanol preference for individual mice was obtained by dividing volume of ethanol consumption by total (ethanol plus water) fluid consumption for daily 18-hr drinking sessions. Separate bottles containing 10 % w/v ethanol or tap water were placed in an empty cage to account for loss due to evaporation. These values were subtracted from the amount of liquid consumed for each mouse to calculate corrected preference ratios and ethanol intake. Different experiments were designed to evaluate the effect of single or multiple ethanol deprivations on ethanol (4 days or 14 days-term) self-administration in inbred mice strains.
2.3. Experiment 1. Effect of single (4-days) or multiple (3 x 4 days) ethanol deprivations on ethanol self-administration in C57BL/6NCrl mice
Single housed C57BL/6NCrl mice (n = 26) were presented with two bottles containing either ethanol (10 % w/v) or tap water, 18-hrs/day for 4 consecutive days. On the fourth day graduated ethanol and tap water bottles were removed and mice had free access to standard water bottles for 4 days. After this period of ethanol deprivation, mice were again allowed access to the two graduated bottles containing either ethanol (10 % w/v) or water for 4 days (18-hrs/day).
In order to study the effect of multiple ethanol deprivations on ethanol consumption and ethanol preference, separate individually housed C57BL/6NCrl mice (n = 20) were presented with two-bottles containing either ethanol (10 % w/v) or water for 4-days (18-hrs/day). This was followed by ethanol-deprivation period of 4 days during which the animals only had access to water. This process of ethanol exposure and deprivation was repeated for 3 consecutive cycles, constituting multiple ethanol deprivations. Ethanol and water consumptions were recorded daily and volume corrected for loss due to evaporation using control bottles housed in similar position.
2.4. Experiment 2. Effect of multiple (3 x 4 days) ethanol deprivations on ethanol self-administration in C57BL/6J mice
Since C57BL/6J mice are a more commonly used inbred strain for studying behavioral effects of psychoactive drugs, we studied the effects of multiple ethanol self-administration and deprivation cycles in C57BL/6J mice from Jackson Laboratories. Individually housed C57BL/6J mice (n = 11) were exposed to 3 cycles of ethanol (10 % w/v, 4 days/18-hrs) exposure and 3 cycles of ethanol deprivations (4–7 days of ethanol deprivations) in a two-bottle choice paradigm as described in Experiment 1. Ethanol and water consumption were recorded daily after every 18-hrs to the nearest 0.1 ml accuracy.
2.5. Experiment 3. Effect of single short term (4 days) ethanol deprivation after 14-days of ethanol self-administration in C57BL/6NCrl mice
Several previous studies have reported an EDE in rodents following long term ethanol self-administration (Holter et al., 1998; Spanagel and Holter, 1999). To study the effect of ethanol deprivation after a longer period of ethanol self-administration, C57BL/6NCrl mice (n= 9) were exposed to the two-bottle choice model containing either ethanol (10 % w/v) or water for 14 days (18-hrs/day). After stabilization of ethanol consumption from 14 days of self-administration, mice were deprived of ethanol for 4 days. This was by followed by reinstatement of drinking bottles containing ethanol (10 % w/v) or water for another 14 days.
3. Statistical analysis
Ethanol consumption was expressed as g/kg/18-hrs (mean ± SEM) and ethanol preference was expressed as % ethanol preference/18-hrs (mean ± SEM). Data was analyzed using repeated measures analysis of variance (ANOVA). Significant interactions were assessed by Bonferroni’s multiple comparison post-hoc test. A value of P < 0.05 was considered to be statistically significant in all cases.
4. Results
4.1. Short term (4 days) ethanol deprivation effect in C57BL/6NCrl mice
C57BL/6NCrl mice consumed a substantial amount of ethanol, 4.78 ± 0.63 g/kg/18-hrs, in the two-bottle (10% w/v ethanol or water) choice self-administration paradigm. Short-term ethanol deprivation produced a significant EDE. As depicted in Fig. 1a, four days of ethanol deprivation after 4 days of consecutive ethanol self-administration induced an increase (P < 0.05, days 1–4 vs. days 10 or 11; P < 0.01, days 2 or 3 vs. day 9 or 12, Bonferroni Multiple Comparison test) in ethanol consumption following reinstatement [F(7,207) = 11.16; P < 0.001]. Further, ethanol consumption (g/kg/18-hrs) significantly correlated with ethanol preference (r2 = 0.7126; P < 0.0001).
Fig. 1.
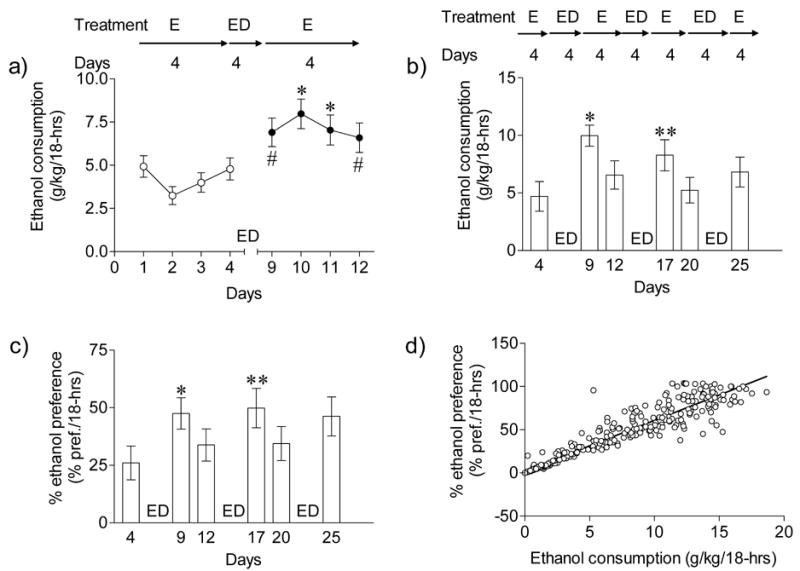
Effect of single or multiple repeated EDE after short term ethanol self-administration in C57BL/6NCr1 mice. a) Following 4 days of two-bottle choice drinking with 18-hrs ethanol access times, C57/BL/6NCr1 mice (n= 26) were deprived of ethanol for 4 days followed by re-exposure. Ethanol or total fluid intake per 18-hr sessions was recorded for 4-baseline days before and after ethanol deprivation. Ethanol consumption (g/kg/18-hrs) significantly increased in ethanol deprived animals compared to pre deprivation days (*P < 0.05, day’s 1–4 vs. day’s 10 or 11; #P < 0.01, day’s 2 or 3 vs. day 9 or 12, Bonferroni Multiple Comparison test). Separate single housed mice (n = 20) were exposed to repeated (3 cycles) two-bottles choice drinking periods lasting for 4 days followed by ethanol deprivations for 4 days. Ethanol consumption (g/kg/18-hrs) and ethanol preference (% pref./18-hrs) are shown for a day before and a day after ethanol deprivations. b) Ethanol consumption was significantly increased following repeated ethanol deprivations (*P < 0.001 day 4 vs. day 9; **P < 0.01 day 4 vs. day 17, Bonferroni Multiple Comparison test). Post deprivation ethanol consumption did not differ from baseline after the third deprivation cycle (P > 0.05, day 25 vs. day 4 or day 20, Bonferroni Multiple Comparison test), c) ethanol preference was elevated (*P < 0.05 day 4 vs. day 9; **P < 0.01 day 4 vs. day 17, Bonferroni Multiple Comparison test) following multiple ethanol deprivations, d) Mice display a significant correlation (r2 = 0.8988, P < 0.0001) between ethanol consumption (g/kg/18-hrs) and ethanol preference (% pref./18-hrs).
Some reports in rats have shown the EDE to increase with repeated bouts of deprivation (Rodd et al., 2003). To test this in a mouse model, we performed multiple short-term ethanol deprivations and re-exposures. This experiment produced a significant EDE in C57BL/6NCrl mice [F(5,119) = 13.88, P < 0.0001]. As shown in Fig. 1b, a short term (4 days) ethanol self administration followed by ethanol deprivation (4 days) produced a robust (2.1 fold) increase in ethanol consumption on day 9 vs. day 4 (P < 0.001, Bonferroni Multiple Comparison test). Multiple comparison testing showed that ethanol consumption was also increased significantly (1.9 fold) on day 17 compared with day 4. However, by the third cycle of deprivation there were no significant changes in ethanol consumption with drinking levels returned to near baseline (P > 0.05, day 25 vs. day 4 or day 20). Deprivation-induced increases in ethanol consumption were also associated with significant increases in ethanol preference [F(5,119) = 4.47, P = 0.0011] which mirrored the increases in ethanol consumption (P < 0.05, day 4 vs. day 9 or day 17, Bonferroni Multiple Comparison test). The first round of ethanol deprivation followed by ethanol reinstatement induced 1.8-fold increase in ethanol preference (P < 0.001, Bonferroni Multiple Comparison test). In subsequent rounds there were progressively smaller increases in ethanol preference following deprivation (Fig. 1c). Ethanol consumption (g/kg/18-hrs) was significantly correlated (r2 = 0.8998; P < 0.0001) with ethanol preference (Fig. 1d). As indicated by the correlation between consumption and preference, the increase in ethanol intake was not due to a general increase in total fluid consumption, which remained stable throughout the cycles of multiple ethanol deprivations [F(9,171) = 0.99, P = 0.45].
4.2. Short term (4 days) ethanol deprivation effect in C57BL/6J mice
C57BL/6J mice are a commonly used inbred mouse substrain for behavioral studies and are known to consume large amounts of ethanol (Metten et al., 1998; Phillips et al., 1994). In the present study, C57BL/6J mice consumed 9.65 ± 0.90 g/kg/18-hrs of ethanol in the two-bottle ethanol/water choice drinking paradigm. There was a significant difference in ethanol consumption between C57BL/6J mice and C57BL/6NCrl mice (unpaired t-test, P < 0.001). Surprisingly, short term ethanol deprivation in C57BL/6J mice did not result in significant increases in ethanol consumption or ethanol preference (Figures 2a and 2b). Repeated cycles of ethanol self-administration for 4 days followed by ethanol deprivation for 4 days produced ~1.4 fold increase (P < 0.05, day 4 vs. day 17, Bonferroni Multiple Comparison test) in ethanol consumption only after the second deprivation period [F(5, 65) = 4.591, P = 0.0038]. However, there was no significant increase in ethanol preference [F (5, 65) = 1.438, P = 0.22]. Ethanol consumption (g/kg/18-hrs) again correlated (r2 = 0.447; P < 0.0001) with ethanol preference since total fluid intake did not significantly change before or after ethanol deprivation periods.
Fig. 2.
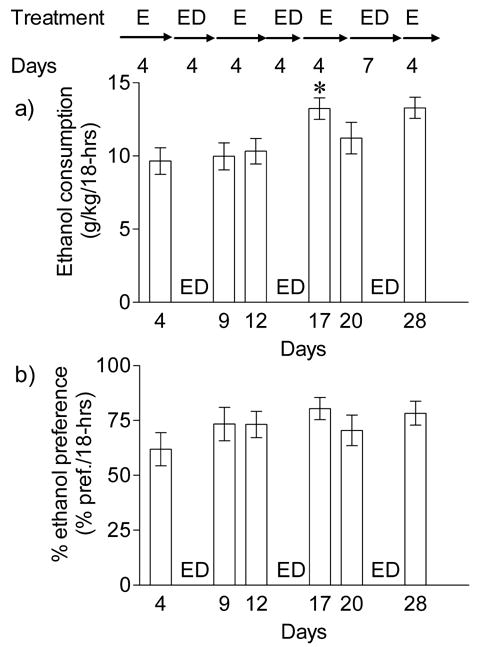
Effect of repeated short-term ethanol deprivation on ethanol self-administration in C57BL/6J mice. C57BL/6J mice (n = 11) were exposed to repeated (3 cycles) two-bottles choice drinking periods lasting for 4 days followed by ethanol deprivations for 4–7 days. Ethanol consumption (g/kg/18-hrs) and ethanol preference (% pref./18-hrs) are shown for a day before and a day after ethanol deprivations. a) Ethanol consumption (g/kg/18-hrs) was increased following second cycle of ethanol deprivation (*P < 0.05, day 4 vs. day 17, Bonferroni Multiple Comparison test), b) ethanol preference was not altered following repeated ethanol deprivations.
4.3. EDE in C57BL6.NCrl mice after 14 days of ethanol self-administration
To determine whether longer periods of ethanol pre-exposure might increase the magnitude of the EDE, we exposed C57BL/6NCrl mice to two-bottle choice drinking for 14 days prior to ethanol deprivation (Fig. 3). Ethanol consumption during the 14 days of pre-deprivation period remained stable [F(13, 125) = 0.74, P > 0.05, Fig. 3a], confirming that uncontrolled environmental parameters did not alter ethanol drinking appreciably. As shown in Fig. 3b, ethanol deprivation for 4 days following 14 days of ethanol self-administration caused a robust elevation in ethanol self-administration after ethanol reinstatement [F(3, 35) = 16.47, P < 0.001]. Ethanol consumption after deprivation (day 19) was ~1.8 fold greater than consumption prior to deprivation (P < 0.001, day 14 vs. day 19, Bonferroni Multiple Comparison test). Elevated ethanol consumption lasted for 4 days following ethanol deprivation but decreased to near pre-deprivation levels by day 32 (P > 0.05, day 32 vs. day 14; Fig. 3b). Ethanol deprivation also induced a significant (1.6 fold, P < 0.05, day 14 vs. day 19, Bonferroni Multiple Comparison test) increase in ethanol preference (Fig. 3c) after 14 days of ethanol self-administration [F(3,35) = 3.864, P = 0.022]. Total fluid consumption was not significantly different before and after the ethanol deprivation period (not shown).
Fig. 3.
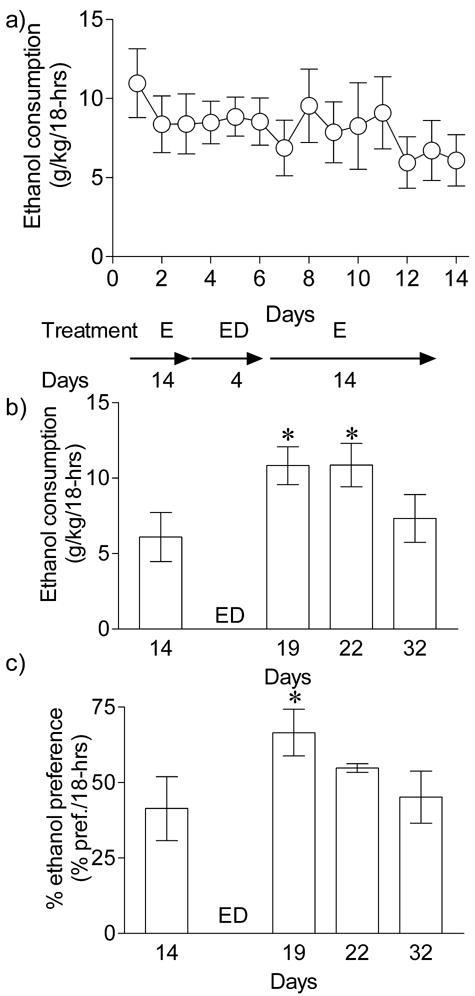
Ethanol deprivation effect after 14 day ethanol self-administration in C57BL/6NCr1 mice. a) Mice (n = 9) were allowed to self–administer ethanol (10 % w/v) for 14 days (18-hr sessions). The ethanol consumption remained stable during this period [F(13,125) = 0.74, P > 0.05, repeated measures ANOVA]. After 14 days of ethanol consumption mice were deprived of ethanol bottles for 4 days. After this short ethanol deprivation period, mice were reinstated with two-bottle (ethanol or water) choice drinking for 14 days. Ethanol consumption (g/kg/18-hrs) and ethanol preference (% pref./18-hrs) are shown for a day before ethanol deprivation and for days after ethanol reinstatements (day 19, day 22 and day 32). b) Ethanol consumption (g/kg/18-hrs) was markedly increased following ethanol deprivation for 4 days (*P < 0.001, day 14 vs. day 19 or day 22, Bonferroni Multiple Comparison test), c) ethanol preference was increased following repeated ethanol deprivations (*P < 0.05, day 14 vs. day 19, Bonferroni Multiple Comparison test). Ethanol consumption and ethanol preference were reduced to baseline ethanol self-administration levels by day 32 (P > 0.05, day 32 vs. day 14, Bonferroni Multiple Comparison test).
5. Discussion
The present study shows a significant ethanol deprivation effect in C57BL/6NCr1 mice, as evidenced by increased ethanol consumption and preference, following ethanol (10 % w/v) reinstatement using a two-bottle choice paradigm. This is the first report on the EDE in C57BL/6 mice, a widely used model for studies on ethanol drinking behavior. Our findings illustrate several differences compared to the EDE seen in rat models and also show that the EDE can vary across the same strain of mice derived from different suppliers.
Using a 4 day (Fig. 1) or 14 day (Fig. 3) ethanol two-bottle choice baseline, we found that C57BL/6NCrl mice showed very consistent 50–100% increases in ethanol consumption or preference following a 4 day deprivation period. The magnitude of these responses is very similar to findings in rat models. Similar to studies in rats, we observed that the deprivation effect was transient, tending to return to basal drinking levels within 3 days of reinstituting two-bottle choice drinking (Figs. 1–3). However, most studies on the EDE in rats have utilized a much longer ethanol self-administration baseline or longer deprivation periods than those used in our studies (Heyser et al., 1997; Rodd et al., 2003; Spanagel and Holter, 1999). We found that an EDE can be established in mice rapidly with 4 days of ethanol self-administration or deprivation without the need for long-term ethanol self-administration. However, a 14 day period of ethanol consumption prior to the EDE did show a trend toward a more prolonged period of elevated ethanol drinking following deprivation (Fig. 3 vs. Fig. 1). Our protocol thus provides a facile method for rapid studies on the EDE in mouse models.
Repeated EDE cycles closely resemble human alcohol drinking patterns (Finney and Moos, 1991; McMillen, 1997; Nezlek et al., 1994). The drinking pattern of human alcoholics is correspondingly segmented by multiple periods of abstinence and intake (Burish et al., 1981; Hilbrom, 1990; McMillen, 1997). This cyclic pattern of consumption and deprivation may have severe consequences in humans since multiple previous detoxifications are associated with a reduction in the response to treatment of withdrawal symptoms and heavier drinking during outpatient detoxification (Malcolm et al., 2000). Work on the EDE in rat models has generally shown that repeated cycles of deprivation cause an increase in the magnitude of the deprivation effect (see below). A repeated deprivation-access cycle of oral ethanol self-administration in P rats increased the magnitude and prolonged expression of EDE (Rodd et al., 2003). Repeated cycles of ethanol access and forced abstinence to a single concentration (10 % v/v) of ethanol resulted in ethanol intakes of greater than 10 g/kg/day and more prolonged expression (4 consecutive days of increase intake) of an EDE in P rats (Rodd-Henricks et al., 2000b). The expression of an EDE in HAD rats given a single concentration of ethanol (10 % v/v) was dependent upon exposure to repeated cycles of deprivation and ethanol access (Rodd-Henricks et al., 2000b). Surprisingly, our data suggested that repeated deprivation cycles in C57BL/6 mice did not increase the deprivation effect, and actually tended to show decrements in post-deprivation ethanol consumption over time (Fig. 1b). This might be due to a number of procedural differences in our method for inducing EDE in mice as compared to the methods used in rats. For example, mice in our studies had a daily 6-hour abstinence from ethanol during the light phase to allow basic animal housekeeping without disturbing the two-bottle choice drinking bottles, unlike the commonly used method in rats with 24-hour access to ethanol bottles. Although such procedural variance might explain differences between our studies and those in rats, there remains the possibility that rats, in general, exhibit a more robust EDE despite usually having lower tendencies to self-administer ethanol than the C57BL/6 mice used in this work.
One additional surprising finding of the studies reported here was that ethanol deprivation in C57BL/6J mice did not produce significant increases in ethanol consumption and ethanol preference. The C57BL/6NCrl mice consistently showed an EDE despite having basal ethanol consumption rates considerably lower than the C57BL/6J mice. One explanation for the differences in EDE between these two substrains is a possible “ceiling effect” in the Jackson Lab mice, such that deprivation can no longer produce increases in ethanol intake. This is reflected in the very high percentage of daily drinking observations having ethanol preference ratios > 0.75 in the C57BL/6J mice (60.8 %, Fig. 2) while the C57BL/6NCrl line has only 36.22 % (Fig. 3) with drinking preferences exceeding 0.75 [Figs. 2 and 3; chi-square test (χ2 = 50.26, P < 0.0001)]. This difference in drinking patterns between the Jackson Lab and Charles Rivers C57BL/6 substrains has been observed by others (R. Spanagel, personal communication). Regardless of the mechanism, our findings may explain why investigators have found it difficult to demonstrate the EDE in C57BL/6, since C57BL/6J mice are most often used for ethanol drinking studies due to their high basal intake (H. Becker, personal communication).
One alternative interpretation of the EDE seen in C57BL/6NCrl mice, or any other EDE two-bottle choice model, could be that the deprivation period decreases “aversion” for ethanol rather than increasing “craving”. This is of concern since the C57BL/6NCrl show preference ratios for ethanol slightly below or mildly above fifty-percent, thus representing little “preference” for ethanol (see Figs. 1 and 3). Operant models would be needed to fully characterize the contribution of increased incentive for ethanol caused by the deprivation period. Even in two-bottle models where preference exceeds fifty percent, an increase in preference caused by a deprivation period could always have a contribution by decreased aversion to ethanol. Conversely, assuming that ethanol preference in a two-bottle method likely represents a balance between incentive reward and aversion, any relative increase in preference could represent an increase in incentive for ethanol’s pharmacological effect. There are numerous reports showing the EDE in rat models with operant procedures, thus validating the basic premise of the EDE. Future studies will be required to determine to what extent the EDE in C57BL/6NCrl mice indeed represents increased incentive for ethanol.
Our suggestion that C57BL/6J mice might not exhibit an EDE due to a ceiling effect raises interesting points regarding the mechanisms and interpretation of the EDE. Data demonstrating that both HAD and LAD rats or P and NP rats exhibit an EDE would seem to negate a direct casual relationship between basal ethanol consumption and the EDE. These strains were selectively bred for extreme differences in ethanol consumption (cf. Murphy et al., 2002). Voluntary ethanol consumption in P rats is ~ 5 g/kg/day and NP rats is ~ 0.5 g/kg/day. HAD rats consume ~ 6.5 g/kg/day and LAD rats consume ~ 0.5 g/kg/day (cf. Murphy et al., 2002). Both P and HAD rats display an EDE (Rodd-Henricks et al., 2000b). In the present study basal consumption in C57BL/6J mice was ~ 10 g/kg/18-hrs and C57BL/6NCrl mice consumed ~ 4.5 g/kg/18-hrs. Although mice have been reported in some studies to metabolize ethanol approximately twice as fast as rats (Able, 1982), it remains possible that C57BL/6J mice achieve higher brain ethanol concentrations than do any of the rat strains mentioned above. Thus, a ceiling effect may contribute to the lack of an EDE for C57BL/6J mice in our studies.
Other factors, such as genetic differences in signaling events related to the genesis of the EDE or environmental factors from differing suppliers, could obviously also explain our observed differences in EDE between C57BL/6J and C57BL/6NCrl mice. There are more than 50 years of potential genetic drift between these two substrains. Mulligan et al. (2005) recently reported significant differences in ethanol preference and brain gene expression between C57BL/6NCrl and C57BL/6J mice. Our own microarray studies confirm substantial differences in gene expression in nucleus accumbens of these two highly related substrains (Wolstenholme, Khisti and Miles, unpublished results). Although molecular studies on such genetically similar substrains might contribute to our understanding of mechanisms underlying genetic variance in drinking behavior, these studies will be complicated by the existence of substantial “random” genetic variance between the two subtypes, unrelated to the difference in drinking behavior.
Much effort has been expended developing animal models that mimic the magnitude of ethanol intake achieved in human alcoholism. However, our findings suggest that “craving” for ethanol can be robustly observed in the absence of a long history of excessive ethanol intake. This hypothesis assumes that the EDE does indeed faithfully model some aspects of craving relevant to ethanol relapse behavior. Relevant to this, we did not measure blood ethanol levels in our animals so we do not know whether “pharmacologically relevant” ethanol concentrations were obtained. This was purposely done to avoid any distress or pain to mice that might alter two-bottle choice ethanol consumption. In the two-bottle choice method, mice tend to consume most ethanol during the first few hours of the dark phase (Rhodes et al., 2005). We have verified this observation with the C57BL/6NCrl mice, finding that the average consumption was 2.7 g/kg within the first 4 hours after placement of ethanol solutions. Thus, despite the C57BL/6NCrl mice consuming only 5–10 g/kg of ethanol per 18 hours, it is possible that significant blood ethanol levels were developed during the early portion of the drinking period. It has been assumed in most studies on the EDE, that ethanol consumption and subsequent withdrawal generate brain plasticity that accounts for the EDE. Supporting this is the fact that the EDE requires several days of withdrawal to be expressed. In our studies, we found that deprivation periods of less than 4 days did not produce a significant EDE (data not shown). Thus, despite the relatively low blood ethanol concentrations achieved in most rodent models, it seems highly likely that some pharmacological action of ethanol combined with withdrawal, is responsible for increasing ethanol consumption post-withdrawal. Regardless, it remains a possibility that some other pre-ingestive factors, unrelated to ethanol pharmacological actions, influence the EDE in rodent models.
As mentioned previously, the EDE has been found in a number of outbred strains of rats (Heyser et al., 1997; Holter and Spanagel, 1999; Rodd-Henricks et al., 2000a; Sinclair and Li, 1989), monkeys (Kornet et al., 1991; Sinclair, 1971), and human social drinkers (Burish et al., 1981). It has been suggested that the EDE can serve as a model of relapse to alcohol (Li, 2000; Spanagel and Holter, 2000), primarily because the increase in ethanol-drinking behavior after periods of abstinence parallels the clinical literature on the priming effect of alcohol in humans (Ludwig and Wikler, 1974; Ludwig et al., 1974). Pharmacological studies using EDE in rats have further validated its use as an animal model of craving relevant to alcoholism (Spanagel and Holter, 2000). New and existing anti-craving medications test successfully in the EDE model. For example, two medications currently on the market for the prevention of relapse in alcoholism, naltrexone and acamprosate, both block the EDE in rat models (Heyser et al., 1997). Our findings suggest that mouse EDE models such as that with C57BL/6NCrl mice used in these studies, might indeed be a valuable tool for future studies on the molecular mechanisms of craving and drinking behavior.
Acknowledgments
This work was supported by a grant (AA014717) from the National Institute on Alcohol Abuse and Alcoholism to MFM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Able EL. Behavioral teratology of alcohol (animal model studies of the fetal alcohol syndrome) In: Able EL, editor. Fetal Alcohol Syndrome Animal Studies. III. Boca Raton, FL: CRC Press; 1982. pp. 59–81. [Google Scholar]
- Barrick C, Connors GJ. Relapse prevention and maintaining abstinence in older adults with alcohol-use disorders. Drugs Aging. 2002;9(8):583–594. doi: 10.2165/00002512-200219080-00004. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112(4):503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42(11):1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Chiauzzi EJ. Preventing relapse in addictions: a biopsychological approach. New York: Pergamon Press; 1991. [Google Scholar]
- Cowen MS, Schroff KC, Gass P, Sprengel R, Spanagel R. Neurobehavioral effects of alcohol in AMPA receptor subunit (GluR1) deficient mice. Neuropharmacology. 2003;45(3):325–333. doi: 10.1016/s0028-3908(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Finney JW, Moos RH. The long-term course of treated alcoholism: I. Mortality, relapse and remission rates and comparisons with community controls. J Stud Alcohol. 1991;52(1):44–54. doi: 10.15288/jsa.1991.52.44. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Measurement of Alcohol Preference in Genetic Experiments. J Comp Physiol Psychol. 1964;57:85–88. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18(2):125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21(5):784–791. [PubMed] [Google Scholar]
- Hilbrom ME. Alcohol withdrawal seizures and binges versus chronic drinking. In: Port RJ, Mattson RH, Cramer JA, Diamond I, editors. Alcohol and Seizures: Basic Mechanisms and Clinical Concepts. Philadelphia: FA Davis; 1990. pp. 206–215. [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9(1):41–48. [PubMed] [Google Scholar]
- Holter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology (Berl) 1999;145(4):360–369. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- Jaffe SL. Treatment and relapse prevention for adolescent substance abuse. Pediatr Clin North Am. 2002;49(2):345–352. doi: 10.1016/s0031-3955(01)00008-6. vi. [DOI] [PubMed] [Google Scholar]
- Koob GF. Animal models of craving for ethanol. Addiction. 2000;95(Suppl 2):S73–S81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Van Ree JM. The effect of interrupted alcohol supply on spontaneous alcohol consumption by rhesus monkeys. Alcohol Alcohol. 1990;25(4):407–412. [PubMed] [Google Scholar]
- Kornet M, Goosen C, Van Ree JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology (Berl) 1991;104(3):367–376. doi: 10.1007/BF02246038. [DOI] [PubMed] [Google Scholar]
- Li TK. Clinical perspectives for the study of craving and relapse in animal models. Addiction. 2000;95(Suppl 2):S55–S60. doi: 10.1080/09652140050111645. [DOI] [PubMed] [Google Scholar]
- Ludwig AM, Wikler A. “Craving” and relapse to drink. Q J Stud Alcohol. 1974;35(1):108–130. [PubMed] [Google Scholar]
- Ludwig AM, Wikler A, Stark LH. The first drink: psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30(4):539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22(3):159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Mcclearn GE, Rodgers D. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Mckinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, Mcbride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22(7):1584–1590. [PubMed] [Google Scholar]
- Mcmillen BA. Toward a definition of a valid model of alcoholism: multiple animal models for multiple diseases. Alcohol. 1997;14(4):409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med. 1972;34(2):139–164. doi: 10.1097/00006842-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, Mcclearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9(12):983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Boehm SL, Ponomatarev I, Levin PS, Bergeson SE. Genetic and behavioral analysis of disparate alcohol preferences in two closely related inbred strains of C57BL/6 mice. Alcohol Clin Exp Res. 2005;29(Suppl 5):91A–91A. [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, Mcbride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32(5):363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Pilkington CJ, Bilbro KG. Moderation in excess: binge drinking and social interaction among college students. J Stud Alcohol. 1994;55(3):342–351. doi: 10.15288/jsa.1994.55.342. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18(4):931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, Mcbride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28(9):1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Mckinzie DL, Murphy JM, Mcbride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000a;24(6):747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Mckinzie DL, Shaikh SR, Murphy JM, Mcbride WJ, Lumeng L, Li TK. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000b;24(1):8–16. [PubMed] [Google Scholar]
- Rodgers DA. A psychological interpretation of alcoholism. Ann N Y Acad Sci. 1972;197:222–225. doi: 10.1111/j.1749-6632.1972.tb28155.x. [DOI] [PubMed] [Google Scholar]
- Salimov R, Salimova N, Klodt P, Maisky A. Interaction between alcohol deprivation and morphine withdrawal in mice. Drug Alcohol Depend. 1993;34(1):59–66. doi: 10.1016/0376-8716(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26(4):1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect in monkeys. Psychon Sci. 1971;25:1–22. [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6(6):505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increases preference for ethanol in rats following deprivation. Psychon Sci. 1967;8:11–12. [Google Scholar]
- Sinclair JD, Walker S, Jordan W. Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. Q J Stud Alcohol. 1973;34(3):744–757. [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34(2):231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Pharmacological validation of a new animal model of alcoholism. J Neural Transm. 2000;107(6):669–680. doi: 10.1007/s007020070068. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18(2):54–59. [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]


