Abstract
Thyroid hormones are essential hormones for regulating growth and development in humans and wildlife. Methods to monitor precise and low levels of these hormones in serum and tissues are needed to assess overall health, whether from disease considerations or possibly from environmental contaminant exposures. Common and routine methods typically rely upon radio-immunoassays, which can be expensive, and typically only measure T4 and T3, which can be a limitation in fully evaluating impacts on thyroid regulation. In this study we developed an liquid chromatography-tandem mass spectrometry method for the simultaneous analysis of five thyroid hormones including thyroxine (T4), 3,3′,5-triidothyronine (T3), 3,3′,5′-triiodothyronine (rT3; reverse T3), 3,3′-diiodothyronine (3,3′-T2), and 3,5-diiodothyronine (3,5-T2) in serum samples. The LC/MS-MS parameters were optimized and calibrated over a wide concentration range (1.0 to 500 ng/mL) with on-column detection limits of 1.5-7.0 pg. Using spiked bovine serum samples, mean method recoveries were calculated to be 81.3-111.9 % with RSDs of 1.2-9.6 % at spiking levels ranging from 10 to 100 ng/mL. This method was compared with measurements made by standard RIAs and to measurements made in a serum Standard Reference Material (SRM 1951b). Development of this method expands the capacity to measure thyroid hormones by including a larger suite of thyroid hormones, and has promising applications for measuring catabolism of thyroid hormones in vitro.
Keywords: Thyroid Hormones, Tandem mass spectrometry, Serum, Solid phase extraction, thyroxine
Introduction
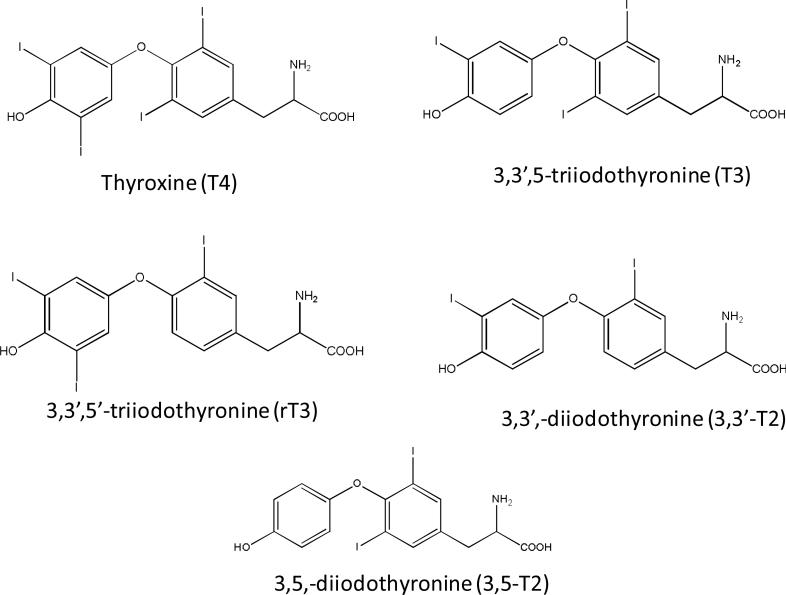
The thyroid hormones, thyroxine (T4) and 3,3′,5-triidothyronine (T3) (Chemical structure shown in Fig. 1), are tyrosine-based hormones produced by the thyroid gland. Synthesis of all circulating T4, and a small percentage of circulating T3, occurs on thyoglobulin molecules located within the thyroid in most mammals. In healthy human subjects, total serum T4 (including both protein bound and unbound) is present at about a 60-fold higher concentration than total T3. T3 is often referred to as the “active” hormone, and most T3 is produced by biotransformation (i.e. deiodination) of T4 (called the pro-hormone) by intracellular enzymes in peripheral tissues [1]. T4 and T3 are important in regulating a number of biological processes, including growth and development, carbohydrate metabolism, oxygen consumption, protein synthesis and fetal neurodevelopment [2]. Biotransformation of T4 also produces the inactive metabolite 3,5′,3′-triidothyronine (rT3) [2] (Fig.1), which may inhibit thyroid production through feedback mechanisms [3,4]. Thus, the molar ratio of T3 to rT3 is an important diagnostic marker for the metabolism and function of thyroid hormones in clinical chemistry. Further cleavage of iodine atoms from rT3 and T3 (regulated by cellular enzymes) results in the formation of several distinct diiodothyronines: 3,5-diiodothyronine (3,5-T2), 3,3′-diiodothyronine (3,3′-T2) and 3′,5′-diiodothyronine (3′,5′-T2) [4] (Chemical structures shown in Fig. 1). Although the absolute contribution of T2 isomers to the physiological function in humans is unclear, experimental data raise the possibility that T2 isomers may have many kinds of biological activities in different tissues [4, 5]. For example, the isomer 3,5-T2 has selective thyromimetic activity and can suppress thyroid stimulating hormone (TSH) levels [6]. In animals, the 3,3′-T2 and 3,5-T2 isomers induce a dose-dependent increase in resting metabolic rate, an increase accompanied by a parallel increase in the oxidative capacity of metabolically active tissues such as liver, skeletal muscle, brown adipose tissue, and heart [7, 8].
Fig. 1.
Chemical structure of thyroid hormones
Therefore, regulating the levels of T4, T3, rT3, and T2 isomers is an integral component of thyroid hormone (TH) homeostasis, which is important in understanding metabolism and function of thyroid hormones at the cellular level. Having the capacity to measure the different thyroid hormone analogues (from diodothyronine via triodothyronine via thyroxine) in serum and tissues can provide a better diagnostic tool and may explain other T4/T3-influenced metabolic abnormalities. In addition, methods for monitoring these hormones can be utilized to measure catalytic activity of enzymes (i.e. deiodinases) which facilitate thyroid hormone catalysis in tissues and provide more information on cellular metabolomicsA broad range of legacy and emerging environmental chemicals such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs), and brominated flame retardants (BFRs), have similar chemical structures to thyroid hormones and are known to impact thyroid regulation. Because they are similar in structure, these contaminants can competitively bind to serum thyroid hormone proteins, and may produce complex effects on thyroid hormone signaling [9-11]. Thus, the measurement of a full suite of thyroid hormone levels in human and animal tissues provides useful information to assess thyroid function, to monitor and diagnose hyper- or hypo-thyroidism, to adjust thyroid medications, and to understand the endocrine-disrupting effects of environmental chemicals.
Most clinical laboratories routinely measure free or total T4 and T3 by automated radioimmunoassay kits [12-14]. Diiodothyronines have also been measured by using immunoassays (IA) [8]. RIA or IA approaches have high sensitivity, but can sometimes lack specificity for thyroid hormones [15-20] due to either endogenous factors (e.g., abnormal binding proteins, dialyzable protein binding competitors, heterophile antibodies, autoantibodies) or in vitro factors (free fatty acids, assay antibodies, analogs, intrinsic dilution). Mass spectrometry (MS) could be a superb detection method for thyroid hormones with high specificity in comparison to RIA or IA. Methods based on gas chromatography/mass spectrometry (GC/MS) with selected ion monitoring (SIM) have been developed to measure total T4 and T3, but they require laborious sample cleanup and derivatization [21-23]. The iodine speciation methods by liquid chromatography-inductively coupled plasma-mass spectrometry (LC-ICP-MS) showed high sensitivity [24-26] for the measurement of thyroid hormones by monitoring the single isotope of iodine . However, this method monitors iodine alone and doesn't provide a molecular ion or fragment, allowing for the possibility of co-elution with other iodine containing molecules. Liquid chromatography (LC) coupled to MS in single ion monitoring mode, or tandem mass spectrometry (MS/MS) in multiple reaction monitoring (MRM) mode, have been used to detect total or free T4 or T3 [22, 27-35]. Thus these methods all have some limitations to direct measurements of a suite of thyroid hormones. In this study we investigated the mass spectrometric characteristics of a suite of five different thyroid hormones, including T4, T3, rT3, 3,5-T2 and 3,3′-T2, and report on a method for the simultaneous analysis of these thyroid hormones in serum samples using a solid phase extraction cleanup and a liquid chromatography-tandem mass spectrometry analysis. This method can be used to measure thyroid hormones in animal tissues and serum samples and provide more knowledge on active and inactive hormones present in biological samples.
Experimental
Chemicals and materials
3,3′,5,5′-Tetraiodo-L-thyronine (L-thyroxine, T4) with purity of ≥ 98% (HPLC), 3,3′,5-triiodothyroxine (T3) with purity of ≥ 97%, and 3,5-diiodo-L-thyronine (3,5-T2) with purity of 95% were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3,3′,5′-triiodothyronine (reverse T3, rT3) and 3,3′-diiodo-L-thyronine (3,3′-T2) with purity of 95% were purchased from USBiological (Swampscott, MA, USA). 13C6-labelled L-thyroxine (13C6-T4) with purity of 99% was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). SampliQ solid phase extraction (SPE) cartridges (3 mL, 60 mg of OPT polymer) were purchased from Agilent technologies (Santa Clara, CA, US). Bovine serum GIBCO™ was a product of New Zealand (Invitrogen Corporation, Grand Island, NY, USA). L-Ascorbic acid (Viltamicc, ACS Reagent) and DL-Dithiothreitol (for molecular biology, minimum 99% titration) were from Sigma-Aldrich Co. (St Louis, MO, USA), and anhydrous granular citric acid (GR, ACS) and acetic acid was from Em Science, A division of EM Industries, Inc. (Gibbstown, NJ, USA). Methanol (GR ACS) was from EMD Chemicals Inc. (Gibbstown, NJ, USA). Water was purified by a Milli-Q water purification system from Millipore Corporation (Billerica, MA, USA). Human serum Standard Reference Material® (SRM) 1951b (Lipids in Frozen Human Serum) was from the National Institute of Standard & Technology (Gaithersburg, MD, USA).
Standard stock solutions of all target analytes were prepared in methanol. Working solutions of all target analytes were prepared in a solvent mixture of methanol and water (v/v, 50/50) with various levels of 2, 10, 20, 100, 200, 500, and 1000 ng/mL. 13C-T4 was prepared in the mixture of methanol and water (v/v, 50/50) at a concentration of 100 ng/mL. Standard solutions were aliquoted into 0.5 mL volumes and were mixed with 0.5 mL of 100 ng/mL 13C-T4 solution, yielding a set of seven levels of calibration standards with the mass ratios of all unlabeled target analytes to 13C-T4 ranging from 0.01 to 10. A protection solution to prevent degradation of thyroid hormones during the sample process was prepared containing the antioxidants ascorbic acid, citric acid and dithiothreitol at concentrations of 25 g/L in water.
Sample Preparation
An aliquot of 0.5 mL of thawed serum was placed into a 10-mL glass centrifuge tube. 120 μL of protection solution containing ascorbic acid, citric acid and dithiothreitol was added to prevent the potential conversions of thyroid hormones [29]. One milliliter of acetone was added to the centrifuge tube and mixed thoroughly and then allowed to sit for at least 30 min to deproteinate the serum. 250 μL of 13C6-T4 (100 pg/μL) was spiked into each sample as an internal standard for quantification of all five thyroid hormones. After vortex mixing, all the samples were centrifuged at 3500 rpm for 5 min at 25°C. The supernatants were transferred to another 4-mL brown glass vial and the precipitates in the test tube were extracted twice more by using 1.0 mL of a mixture of acetone and water (v/v: 1:1). All the supernatants and the extracts were combined and the solution volume was reduced to 1.0 mL by evaporation under a N2 current. The concentrated extracts were loaded into the SampliQ solid phase extraction (SPE) cartridges, which were preconditioned sequentially with 3.0 mL of methanol and 5.0 mL of distilled water. The cartridges were first washed with 3.0 mL of 30% methanol in water, and then the target compounds and internal surrogate were eluted by 4.0 mL of 0.1% acetic acid in methanol. The eluent was collected in 4.0 mL amber glass vials and the volume was reduced to 200 μL under N2 for instrumental analysis.
Instrumental Analysis
Instrumental analysis were performed using an Agilent 6410 Triple Quad tandem mass spectrometer (LC-MS-MS) system equipped with Agilent 1200 Series Binary Pump SL and Agilent 1200 Autosampler. The injection volume for LC/MS/MS analysis was 5 μL. Data acquisition and analysis were performed using Agilent MassHunter Workstation software. For LC separation, 0.1% acetic acid and 10 mM ammonia acetate in deionized water was used as the aqueous mobile phase (A), and 0.1% acetic acid in methanol was used as the organic mobile phase (B). Three LC columns were examined, including Zorbax Eclipse XDB-C18 RRHT column (1.8 μm, 50 mm × 4.6 mm) (Agilent Technologies, Santa Clara, CA, US), Synergi 2.5 μ Polar-RP 100 A (2.5 μm, 50mm × 2.0 mm) (Phenomenex, Torrance, CA, US), and Restek Ultra II™ Biphenyl (5 μm, 50 × 2.1 mm) (Restek Cooperation, Bellefonte, PA, USA). The column temperature was set at 30°C and the flow rate was 0.25 mL/min. The LC gradient procedure and initial mobile phase compositions were varied to determine the optimal LC separation conditions. MS/MS responses of target analytes were evaluated by electrospray ionization (ESI) in both positive ion and negative ion modes, using the multiple reaction monitoring (MRM) mode. Nitrogen gas served as the nebulizer, dry and collision gas. The parameters settings included gas temperature at 350°C, gas flow of 11 L/min, nebulizer of 50 psi and capillary voltage of 4000 V by following instrumental parameters guidelines. Both fragmentor and collision energy were optimized for each compound by infusion of 1 ng/mL of the standard solutions in the mixture of methanol and water (1:1, v/v) at a flow rate of 0.25 mL/min. Two sets of tandem mass spectrometry parameters (Table 1) were established in positive ion mode and negative ion mode. The protonated molecule [M+H]+ from ESI in positive ion mode, or the deprotonated molecule [M-H]- from ESI in negative ion mode, was selected for collision induced dissociation. From the MS/MS spectra of each analyte, the two most intensive products ions were selected as MRM transitions for quantification and confirmation purposes. Since 13C-labelled T3, rT3 and T2 were not commercially available at the initiation of this study, and considering the similar chemical structures of all five thyroid hormones (Figure 1), we quantified the five target analytes by the internal standard 13C-T4.
Table 1.
MS/MS parameters and MRM transitions in both positive and negative modes
| Compounds detected (1.0-11 min) | MRM transitions | Fragmentor (V) | CE (V) |
|---|---|---|---|
| Positive ion mode | |||
| 3,3′-T2 or 3,5-T2 | 525.6-479.6 (Q)a 525.6-381.8 (C)b |
120 120 |
20 20 |
| T3 or rT3 | 651.8-605.9 (Q) 651.8-507.9 (C rT3) 651.8-478.7 (C T3) |
120 120 120 |
20 20 35 |
| 13C6-T4 T4 |
783.8-737.8 (Q) 777.7-731.5 (Q) 777.7-633.7 (C) |
160 160 160 |
25 25 35 |
| Negative ion mode | |||
| 3,3′-T2 or 3,5-T2 | 523.9-506.5 (Q) 523.9-126.8 (C) |
120 120 |
15 25 |
| T3 or rT3 rT3 |
649.9-632.9 (Q T3) 649.9-604.8 (Q rT3) 649.9-126.8 (C) |
120 160 160 |
15 35 35 |
|
13C6-T4 T4 |
782.0-611.0 (Q) 775.9-604.9 (Q) 775.9-574.8 (C) |
160 160 160 |
20 20 30 |
Q quantitation ions
C confirmation ions
Method calibration and application
The method precision and accuracy were evaluated by using bovine serum samples as a matrix. A 0.5 mL aliquot of bovine serum was spiked with five thyroid hormones at three levels: 10, 50 and 100 ng/mL. At each spiked level, triplicate experiments were performed by using the optimized sample preparation and instrumental analytical method. Thyroid hormones in bovine serum samples were also measured by radioimmunoassay (RIA) [36, 37] at the Duke University Hospital Clinical Laboratories and the RIA results were used to compare with the results from the optimized SPE procedure and LC-ESI-MS/MS method. The method was also used to measure thyroid hormones in a human serum Standard Reference Material (SRM 1951B), and the results were compared with published results in a previous study [29].
Results and discussion
LC separation
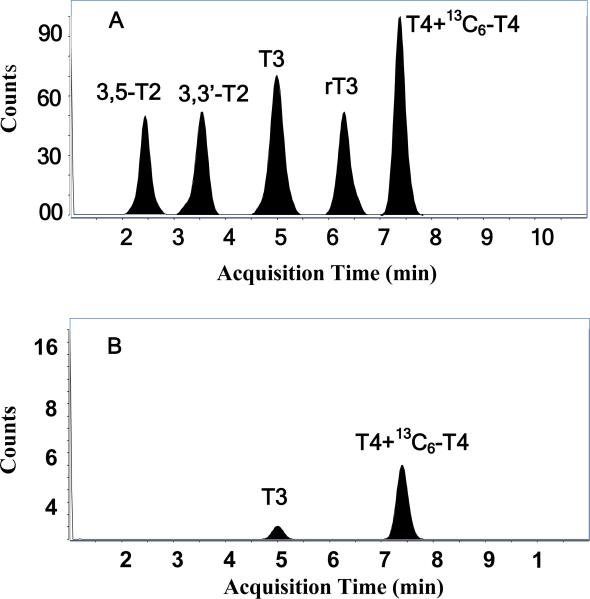
Three LC columns were tested for the separation of the five target hormones. At the start of the experiments, a long LC gradient procedure was applied in order to test chromatographic resolution of the five thyroid hormones in each of the three LC columns. The LC pump was ramped from 40% B to 80% B in 30 min, and then to 100% B in 5 min, and back to 40 % B in 5 min, and then maintained for 10 min. The total LC run time for one sample analysis was 50 min. Total ion current chromatographs from the three LC columns showed that three LC columns could all supply good baseline resolution of all five thyroid hormones. Compared with that from Synergi 2.5 μ Polar-RP 100 A column, both the Allure® Biphenyl column and Zorbax Eclipse XDB-C18 RRHT column could resolve all five thyroid hormones, but both columns were found to retain the hormones for longer times resulting in longer elution times (28 and 24 minutes, respectively) with wider peaks. The Allure® Biphenyl phase in Restek Ultra II™ Biphenyl column provided highly effective separation of all five thyroid hormones. However, π-π interactions resulted in the widening peaks, which may decrease the instrumental sensitivities for quantitative analysis. The Synergi 2.5 μ Polar-RP 100 A column separated all target analytes in 12 minutes, but resulted in a tailing peak for the first eluting hormone, 3,5-T2, using this gradient mobile phase. Due to the shorter elution times on the Synergi 2.5 μ Polar-RP 100 A column, we further optimized the mobile phase to improve the peak shape and resolution of 3,5-T2. The final modified LC gradient program developed was as follows: The LC pump was ramped from 50% B to 80% B in 10 min, and to 100 % B in 5 min, and then back to 40 % B in 1 min, and maintained for 9 min. The total run time is then only 25 min to complete a sample analysis. By using this modified LC program, sharper peaks for 3,5-T2 and all other four hormones were obtained, and the baseline separation of all five thyroid hormones were completed within a fairly short time (10 min) (Fig. 2A). A Synergy polar RP LC column was previously reported to separate rT3, T3 and T4 within three minutes [35] and the measurement of T3 or T4 was finished over ten minutes by using Zorbax Eclipse XDB-C18 RRHT column [28, 29]. Since the study by Yue et al. [35] focused on the measurement only two thyroid hormones (T3 and/or T4 only), they were able to detect and resolve these two hormones in a short run time; however, inclusion of the full suite of 5 thyroid hormones requires a longer LC run. Thus here, we selected the Synergy polar RP column and used a 25 min LC gradient program to simultaneously monitor 3,5-T2, 3,3′-T2, T3, rT3 and T4 based on the faster analysis time and good peak resolution.
Fig. 2.
Total ion current chromatograph by using the LC-ESI-/MS/MS method in positive ion mode. A: 50 ng/mL standard; B: human serum sample
MS/MS spectra characteristics and parameters optimization
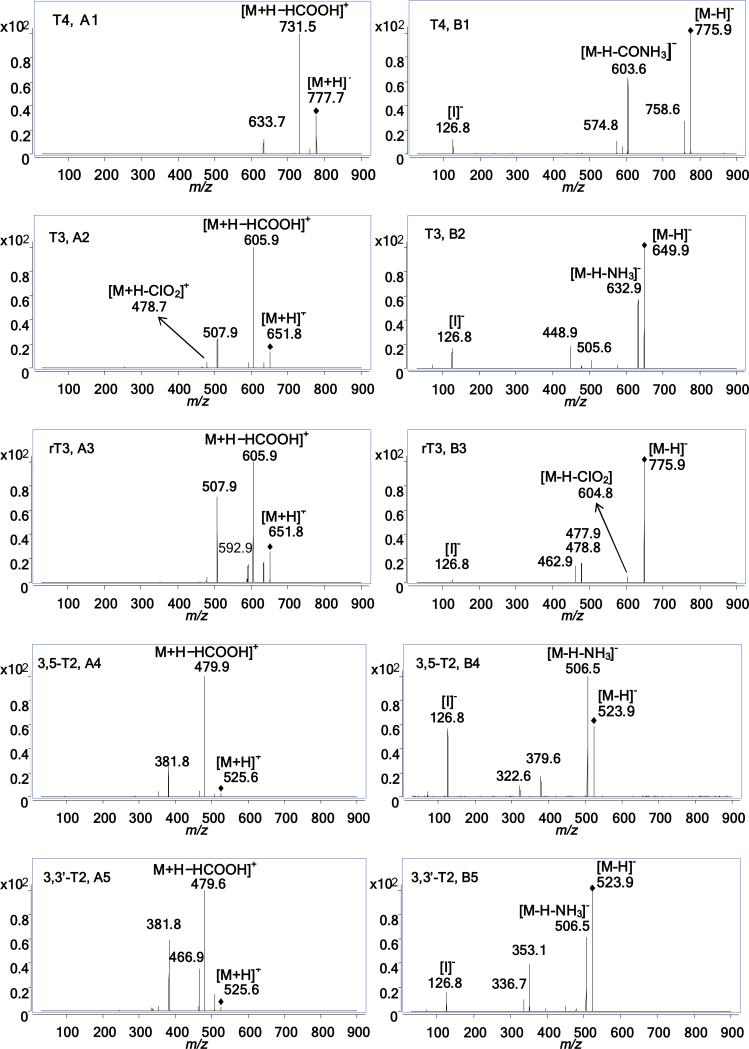
Under positive ion mode, protonated molecules [M+H]+ at m/z 777.7 for T4, m/z 651.8 for T3 and rT3, and m/z 525.6 for 3,5-T2 and 3,3′-T2, respectively, were selected as the precursor ions for further fragmentation. ESI-MS/MS spectra in positive ion mode demonstrated that the dominant fragment ion of T4 was m/z 731.5 (Fig. 3A1), for T3 and rT3 the dominant fragment ion was m/z 605.9 (Fig. 3A2 and A3), and for 3,5-T2 and 3,3′-T2 the dominant fragment ion was m/z 479.6 (Fig.3A4 and A5). These most abundant ions, [M+H−HCOOH]+, represent the loss of HCOOH in all thyroid hormone isomers. The second dominant ion was m/z 633.7 for T4, corresponding to [M+H-HIO]+ or [M+H-H3IN]+ (Fig. 3A1). Similarly, the second most abundant fragment ions for both T3 and rT3, (m/z 507.9), and for 3,5-T2 and 3,3′-T2, (m/z 381.8) also represented two possible compositional losses of either HIO or H3IN for these isomers (Fig. 3A2- A5). However, the relative abundance of m/z 507.9 from T3 was lower than that from rT3, and the relative abundance of m/z 381.8 in 3,5-T2 was lower than that from 3,3’-T2. The mass spectrometric characteristics of the two T3 isomers and the two T2 isomers in this study are similar to previous observations [38, 39]. Also interesting to note, when CE values increased from 20 v to 35 v, m/z 478.7, [M+H-CIO2]+, became the second most abundant fragment ion for T3. Therefore, in this study we selected a different secondary transition for T3 (Table 1).
Fig. 3.
MS/MS spectra of five thyroid hormone isomers with fragmentor set at 120 v for 3,5-T2, 3,3’-T2, T3 and rT3 and 160 v for T4, and CE values set at 25 v for all compounds. A1-A5 from positive ion mode, B1-B5 from negative ion mode
Under negative ion mode, deprotonated molecules [M-H]- at m/z 775.9 for T4, m/z 649.9 for T3 and rT3, and m/z 523.9 for 3,5-T2 and 3,3′-T2, respectively, were selected as the precursor ions for further fragmentation. ESI-MS/MS spectra in negative ion mode demonstrated that the dominant fragment ion for T4 was m/z 604.9, [M-H-CIO2]- , (Fig. 3 B1), for T3 it was m/z 632.9, [M-H-NH3]- , (Fig. 3 B2), and for 3,5-T2 and 3,3′-T2 it was m/z 506.5 [M-H-NH3]- (Fig. 3 B4 and B5). In the case of rT3, when CE values increased to 35 v, it was observed that m/z 604.8 become a dominant fragment ion, and this was selected as the primary transition for rT3 (Table 1) to differentiate the two isomers. The second dominant ion was m/z 574.8 for T4 (Fig. 3B1), and m/z 126.8 [I]-, for T3, rT3, 3,5-T2 and 3,3′-T2, respectively, (Fig. 3B2 to B5). These fragments were then chosen as the secondary transitions (Table 1). However, the relative abundance of m/z 126.8 from T3 and 3,5-T2 was higher than those from rT3 and 3,3′-T2, respectively.
Iodination substitution patterns on the aromatic rings of thyroid hormone isomers resulted in the varied mass spectrometric patterns of the two T3 isomers and the two T2 isomers. This phenomenon was also observed in previous studies [38, 39]. From chemical structures of these isomers (Fig. 1) and varied fragmentation patterns (Fig. 3 A2 to A5, B2 to B5), iodine atoms in outer aromatic rings of rT3 and 3,3’-T2 isomers may favor the formation of [M+H-HIO]+ or [M+H-H3IN]+ ions in positive ion mode, contrasted with T3 and 3,5-T2, which may inhibit the loss of iodine in negative ion mode. The varied patterns of MS/MS fragment ions of both isomer pairs of 3,5-T2 and 3,3´-T2 and rT3 and T3 were used to differentiate T3 from rT3, 3,5-T2 from 3,3′-T2 by direct ESI-/MS/MS without LC separation [38, 39]. However, co-existing fragment ions in MS/MS spectra of T2 and T3 isomers could interfere with the accurate quantification for these isomers. The interference between two T2 isomers and between two T3 isomers was eliminated by the LC separation in this study, however, the characteristic patterns of MS/MS spectra of T3 and rT3, were used to set different MRM transitions for T3 and rT3 (Table 1). .
The optimum fragmentor and CE voltages generally increased with increasing number of iodine atoms attached to the thyroid hormones (Table 1 and Fig. 1). This suggests that the greater the number of iodine atoms that are attached, the higher the energy needed to fragment the protonated molecules [M+H]+, or deprotonated molecules [M-H]- , to obtain valuable MS/MS for identifying and quantifying these hormones. The trend was similar to that of tandem mass spectra of PBDEs [40], which have similar chemical structures to thyroid hormones. The reason is that enthalpy of formation of these chemicals, like PBDE, increases with an increasing number of halogens on the phenyl ring [41].
Instrumental Calibration
After LC-MS/MS conditions were established, the instrument was calibrated by a set of calibration standard solutions. The results showed good linearity (R2: 0.997 to 0.9995) for LC-ESI-MS/MS method within the range of 1.0 to 500 ng/mL in positive ion mode and 50-500 ng/mL in negative ion mode. The instrumental detection limits were calculated, using three time the signal-to-noise ratio, as 0.34-1.4 ng/mL (on column 1.5-7.0 pg) in positive ion mode and 4.9-14.6 ng/mL (24.5-73.0 pg) in negative ion mode (Table 2). The LC-ESI-/MS/MS method in negative ion mode had 7 to 43 times higher detection limits than in positive ion mode. For positive ion mode, the T3 detection limit was 1.5 pg, which is similar to the value of 1.0 pg reported for total T3 in serum samples [29]. The detection limit of T4 was 2.5 pg using this method, which is slightly lower than the 6 pg value reported for total T4 measurements in serum samples from a previous study [22]. Negative ion mode was used to measure total T4 and T3 in serum samples with detection limits of 15 pg in one study [30], which is much lower than our data (Table 2), but they also indicated that negative ion mode had lower sensitivity than positive ion mode (Table 2). Tai et al. [28] applied LC-MS in both positive and negative ion modes to measure T4 in human serum without specifying which ionization mode had higher sensitivity. In this study, it was found that positive ion mode has much higher sensitivity than negative ion mode and the positive ion mode was then selected for application in all subsequent analyses.
Table 2.
On-column method detection limits (pg) for total T4 and T3 in this study and reported by other researchers
| Positive ion mode (pg) | Negative ion mode (pg) | References | |
|---|---|---|---|
| 3,5-T2 | 3.5 | 24.5 | This study |
| 3,3'-T2 | 4.5 | 71.5 | This study |
| T3 | 1.5 | 73.0 | This study |
| rT3 | 7.0 | 68.5 | This study |
| T4 | 2.5 | 68.5 | This study |
| T4 | DLa: 6 QLb: 0.5 ng/g (30 μL LC injection) |
15 | de Brabandere et al.[22] Hopley et al. [27] Soukhova et al.[30] |
| T3 | 1 | 15 | Soukhova et al.[30] Tai et al.[29] |
DL detection limits
QL quantification limits
Method recoveries in spiked serum matrix
In spiked bovine serum samples, the recoveries of all five thyroid hormones ranged from 81.3 to 111.9 % with RSDs of 1.2-9.6%, which were calculated from concentration data shown in Table 3, indicating that the sample preparation method and LC-ESI-MS/MS conditions employed in this study are reliable to measure thyroid hormones. For the sample preparation method, deproteinated serum samples were cleaned-up by SampliQ SPE cartridge, which is simple and effective method for the purification and enrichment of thyroid hormones in serum samples, and also allowed reducing the final sample to 200 μL for LC-ESI-MS/MS analysis to enhance detection abilities for lower levels of detection for thyroid hormones. The data in Table 3 shows that no interferences were observed in the measurement of all five thyroid hormones in bovine serum samples by LC-ESI-/MS/MS in positive ion mode in one LC retention time channel (from 1 to 11 min). Also, no matrix enhanced and/or inhibitory effects were found based on the analysis of post treated bovine serum samples spiked with the five hormones at levels of 50 ng/mL each.
Table 3.
Mean measured values (ng/mL) and relative standard deviations (RSD) by triplicate analysis of spiked bovine serum samples
| 3,5-T2 | 3,3′-T2 | T3a | rT3 | T4a | |
|---|---|---|---|---|---|
| 100 ng/mL | |||||
| Intraday Mean | 106.3 | 105.5 | 115.8 | 106.9 | 167.4 |
| RSD | 6.0 | 2.1 | 4.1 | 4.3 | 1.2 |
| 50 ng/mL | |||||
| Intraday Mean | 49.1 | 49.0 | 50.1 | 51.3 | 113.0 |
| RSD | 5.3 | 9.6 | 4.2 | 2.6 | 2.6 |
| Interday Mean | 50.4 | 49.0 | 51.1 | 52.2 | 118.6 |
| RSD | 4.6 | 2.0 | 3.3 | 8.4 | 1.3 |
| 10 ng/mL | |||||
| Intraday Mean | 9.6 | 9.01 | 10.0 | 8.1 | 77.1 |
| RSD | 5.4 | 8.0 | 5.4 | 10.1 | 3.0 |
T4 and T3 values are the sum of spiked levels and endogenous levels.
72.4±7.0 ng/mL of T4 and 3.68 ±0.25 ng/g T3 were detected in bovine serum samples, and the values were different from those in Table 4 due to serum samples were from different batches.
Comparison with RIA
Levels of thyroid hormones in bovine serum samples were analyzed using both the developed LC-ESI-MS/MS method and a standard RIA method used in clinical laboratories. Both results are reported in Table 4. From the LC-ESI-MS/MS analysis, T3 levels were 3.74 ± 0.34 ng/mL, T4 levels were 83.6 ±5.41 ng/mL, and the levels of 3,5-T2, 3,3’-T2 and rT3 were lower than the method detection limits (Table 4). RIA methods reported a value of 3.24 ±0.21 ng/mL of T3 and 104.0 ± 4.58 ng/mL of T4 in the same sample of bovine serum (Table 4). T4 levels measured by RIA are higher than those measured using our LC-ESI-MS/MS method in this study. Although RIA could supply fast measurement of T4 and T3 with good sensitivity and excellent precision there could have been a few inaccuracies caused by interferences from endogenous immunoglobulins/antibodies in the RIA [42], which could lead to a falsely high or low result. A recent study showed free thyroid hormone concentrations measured by LC-ESI-MS/MS correlate to a greater degree with thyroid-stimulating hormone values compared to concentrations measured by immunoassay across the patient populations [43]. This study went on to suggest that LC-ESI-MS/MS is a more reliable, specific and attractive technique compared to immunoassays for the measurement of thyroid hormones not only for clinical disease diagnosis, but also for the study on subtle effects of environmental chemicals on thyroid function.
Table 4.
Comparison of results (ng/mL) from LC-ESI-/MS/MS and RIA methods on bovine serum
| Rep | 3,5-T2 | 3,3'-T2 | T3 | rT3 | T4 | |
|---|---|---|---|---|---|---|
| Bovine serum | ||||||
| LC/MS/MS | 1 | <0.74 | <0.92 | 3.86 | <1.4 | 77.4 |
| 2 | <0.74 | <0.92 | 4.01 | <1.4 | 86.4 | |
| 3 | <0.74 | <0.92 | 3.36 | <1.4 | 87.1 | |
| mean ±sd | <0.74 | <0.92 | 3.74±0.34 | <1.4 | 83.6±5.41 | |
| RIA | 1 | naa | na | 3.40 | na | 100 |
| 2 | na | na | 3.37 | na | 103 | |
| 3 | na | na | 3.03 | na | 109 | |
| mean ±sd | na | na | 3.24±0.21 | na | 104±4.58 | |
| SRM 1951b | ||||||
| This study | 1 | <0.74 | <0.92 | 1.17 | <1.4 | 78.3 |
| 2 | <0.74 | <0.92 | 1.33 | <1.4 | 76.6 | |
| 3 | <0.74 | <0.92 | 1.14 | <1.4 | 78.6 | |
| mean ±sd | <0.74 | <0.92 | 1.21±0.102 | <1.4 | 77.5±1.08 | |
| The study by Tai et al. [29] | 1 | na | na | 0.940 | na | na |
| 2 | na | na | 0.900 | na | na | |
| 3 | na | na | 0.897 | na | na | |
| mean ±sd | na | na | 0.912±0.0024 | na | na | |
na not available
Analysis of human serum SRM
A human serum Standard Reference Material (NIST SRM 1951b, Lipids in Frozen Human Serum) was also analyzed for the suite of thyroid hormones. Tai et al [29] reported on values for T3 in this material. As shown in Fig 2B, both T3 and T4 were detected, while 3,5-T2, 3,3′-T2 and rT3 were lower than our method detection limits. The mean concentration of T3 was 1.21 ± 0.1 ng/mL (Table 4). Although NIST has no reported levels for thyroid hormones in this human serum Standard Reference Material, Tai et al. [29] reported mean T3 levels of 0.912 ng/mL with RSDs of 2.63 %. The slight differences in measurements between these two LC-ESI-MS-MS studies could be due to differences in the sources of the T3 standards used. A value of 77.5±1.1 (n=3) ng/mL of T4 and an RSD of 1.4 % was measured in SRM 1951b. T4 levels have not been previously reported for this SRM.
Conclusions
The method developed in this study used solid phase extraction for sample preparation and LC-ESI-MS/MS to measure five different thyroid hormones in serum samples. SPE and tandem mass spectrometry eliminated complex sample matrix interferences and demonstrated high method sensitivity and specificity. The method showed good accuracy and precision based on the analysis of five thyroid hormones, at three different concentrations, spiked into bovine serum samples. The method also showed comparable results with a routine RIA methodFuture research will incorporate 13C-labelled T3, rT3 and T2 internal standards as they become commercially available. . This method holds great promise for evaluating catalytic activity of endogenous deiodinases in tissues which metabolize thyroid hormones via a deiodination route. Furthermore, this method could be used to examine effects of environmental contaminants on thyroid hormone regulation along multiple pathways.
Acknowledgements
This study was funded by a Grant from the National Institute of Health (NIH): 1R01-ES016099. We thank Restek Corporation for supplying the Restek Ultra II™ Biphenyl LC column.
References
- 1.Lum SM, Nicoloff JT, Spencer CA, Kaptein EM. Peripheral tissue mechanism for maintenance of serum triiodothyronine values in a thyroxine-deficient state in man. J Clin Invest. 1984;73:570–575. doi: 10.1172/JCI111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 3.Kohrle J, Spanka M, Irmscher K, Hesch RD. Flavonoid effects on transport, metabolism, and action of thyroid hormones. Prog Clin Biol Res. 1988;280:323–340. [PubMed] [Google Scholar]
- 4.Kelly GS. Peripheralmetabolism of thyroid hormones: a review. Altern Med Rev. 2000;5:306–333. [PubMed] [Google Scholar]
- 5.Goglia F. Biological effects of 3,5-diiodothyronine (T2). Biochem (Mosc) 2005;70:203–213. doi: 10.1007/s10541-005-0097-0. [DOI] [PubMed] [Google Scholar]
- 6.Ball SG, Sokolov J, Chin WW. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J Mol Endocrinol. 1997;19:137–147. doi: 10.1677/jme.0.0190137. [DOI] [PubMed] [Google Scholar]
- 7.Lanni A, Moreno M, Lombardi A, Goglia F. Calorigenic effect of diiodothyronines in the rat. J Physiol (London) 1996;494:831–837. doi: 10.1113/jphysiol.1996.sp021536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horst C, Rokos H, Seitz HJ. Rapid stimulation of hepatic oxygen consumption by 3,5-diiodo-L-thyronine. Biochem J. 1989;261:945–950. doi: 10.1042/bj2610945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoeller RT. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol. 2005;242:10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Legler J. New insights into the endocrine disrupting effects of brominated flame retardants. Chemosphere. 2008;73:216–222. doi: 10.1016/j.chemosphere.2008.04.081. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwagi K, Furuno N, Kitamura S, Ohta S, Sugihara K, Utsumi K, Hanada H, Taniguchi K, Suzuki K, Kashiwagi A. Disruption of thyroid hormone function by environmental chemicals. J Health Sci. 2009;55:147–160. [Google Scholar]
- 12.Ekins R. Measurement of free hormones in blood. Endocr Rev. 1990;11:5–46. doi: 10.1210/edrv-11-1-5. [DOI] [PubMed] [Google Scholar]
- 13.Midgley JEM. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem. 2001;47:1353–1363. [PubMed] [Google Scholar]
- 14.Stockigt JR. Free thyroid hormone measurement: a critical appraisal. Endocrinol Metab Clin North Am. 2001;30:265–289. doi: 10.1016/s0889-8529(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 15.Murthy JN, Yatscoff RW, Soldin SJ. Cyclosporine metabolite cross-reactivity in different cyclosporine assays. Clin Biochem. 1998;31:159–163. doi: 10.1016/s0009-9120(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 16.Soldin SJ, Steele BW, Witte DL, Wang E, Elin RJ. Lack of specificity of cyclosporine immunoassays. Arch Pathol Lab Med. 2003;127:19–22. doi: 10.5858/2003-127-19-LOSOC. [DOI] [PubMed] [Google Scholar]
- 17.Ekins R, Midgley JEM, Moon CR, Wilkins TA. Validity of analog free thyroxin immunoassays. Clin Chem. 1987;33:2137–2152. [PubMed] [Google Scholar]
- 18.Sapin R, d'Herbomez M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531–1535. doi: 10.1373/49.9.1531. [DOI] [PubMed] [Google Scholar]
- 19.Steele BW, Wang EW, Klee GG, Thienpont LM, Soldin SJ, Sokoll LJ. Analytic bias of thyroid function tests: analysis of a college of American pathologists fresh frozen serum pool by 3900 clinical laboratories. Arch Pathol Lab Med. 2005;129:310–317. doi: 10.5858/2005-129-310-ABOTFT. [DOI] [PubMed] [Google Scholar]
- 20.Fritz KS, Wilcox RB, Nelson JC. Quantifying spurious free T4 results attributable to thyroxine-binding proteins in serum dialysates and ultrafiltrates. Clin Chem. 2007;53:985–988. doi: 10.1373/clinchem.2007.085316. [DOI] [PubMed] [Google Scholar]
- 21.Hantson A-L, De Meyer M, Guérit N. Simultaneous determination of endogenous and 13C-labelled thyroid hormones in plasma by stable isotope dilution mass spectrometry. J Chromatogr B. 2004;807:185–192. doi: 10.1016/j.jchromb.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 22.De Brabandere VI, Hou P, Stöckl D, Thienpont LM, De Leenheer AP. Isotope dilution-liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of serum thyroxine a potential reference method. Rapid Commun Mass Spectrom. 1998;12:1099–1103. doi: 10.1002/(SICI)1097-0231(19980831)12:16<1099::AID-RCM290>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Thienpont LM, Fierens C, De Leeheer AP, Przywara L. Isotope dilution-gas chromatography/mass spectrometry and liquid chromatography/electrospray ionization-tandem mass spectrometry for the determination of triiodo-L-thyronine in serum. Rapid Commun Mass Spectrom. 1999;13:1924–1931. doi: 10.1002/(SICI)1097-0231(19991015)13:19<1924::AID-RCM734>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Michalke B, Schramel P, Witte H. Iodine speciation in human serum by reversed-phase liquid chromatography–ICP–mass spectrometry. Biol Trace Elem Res. 2000;78:81–91. doi: 10.1385/BTER:78:1-3:81. [DOI] [PubMed] [Google Scholar]
- 25.Simon S, Tietge JE, Michalke B, Degitz S, Schramm K-W. Iodine species and the endocrine system: thyroid hormone levels in adult Danio rerio and developing Xenopus laevis. Anal Bioanal Chem. 2002;372:481–485. doi: 10.1007/s00216-001-1211-9. [DOI] [PubMed] [Google Scholar]
- 26.Takatera K, Watanabe T. Speciation of iodo amino acids by high-performance liquid chromatography with inductively coupled plasma mass spectrometric detection. Anal Chem. 1993;65:759–762. doi: 10.1021/ac00072a018. [DOI] [PubMed] [Google Scholar]
- 27.Hopley CJ, Stokes P, Webb KS, Baynhm M. The analysis of thyroxine in human serum by an ‘exact matching’ isotope dilution method with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1033–1038. doi: 10.1002/rcm.1441. [DOI] [PubMed] [Google Scholar]
- 28.Tai S, Sniegoski LT, Welch MJ. Candidate references methods for total thyroxine in human serum: use of isotope-dilution liquid chromatography-mass spectrometry with electrospray ionization. Clin Chem. 2002;48:637–642. [PubMed] [Google Scholar]
- 29.Tai S, Bunk DM, White ET, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of total 3,3,’5-triiodothyronine in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2004;76:5092–5096. doi: 10.1021/ac049516h. [DOI] [PubMed] [Google Scholar]
- 30.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometry for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358:113–118. doi: 10.1016/j.cccn.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Uytfanghe K, Stöckl D, Thienpont LM. Development of a simplified sample pretreatment procedure as part of an isotope dilution-liquid chromatography/tandem mass spectrometry candidate reference measurement procedure for serum total thyroxine. Rapid Commun Mass Spectrom. 2004;18:1539–1540. doi: 10.1002/rcm.1510. [DOI] [PubMed] [Google Scholar]
- 33.van Uytfanghe K, Stöckl D, Ross HA, Thienpont LM. Use of frozen sera for FT4 standardization: investigation by equilibrium dialysis combined with isotope dilution mass spectrometry and immunoassay. Clin Chem. 2006;52:1817–1820. doi: 10.1373/clinchem.2006.070425. [DOI] [PubMed] [Google Scholar]
- 34.Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Yue B, Rockwood AL, Sandrock T, Laulu SL, Kushnir MM, Meikle AW. Free thyroid hormones in serum by direct equilibrium dialysis and online solid-phase extraction–liquid chromatography/tandem mass spectrometry. Clin Chem. 2008;54:642–651. doi: 10.1373/clinchem.2007.098293. [DOI] [PubMed] [Google Scholar]
- 36.Klee GG. Clinical usage recommendations and analytic performance goals for total and free triiodothyronine measurements. Clin Chem. 1996;42:155–159. [PubMed] [Google Scholar]
- 37.Nelson JC, Wilcox RB. Analytic performance of free and total thyroxine assays. Clin Chem. 1996;42:146–154. [PubMed] [Google Scholar]
- 38.Zhang Y, Conrad AH, Conrad GW. Detection and quantification of 3,5,3′-triiodothyronine and 3,3′,5′-triiodothyronine by electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 2005;16:1781–1786. doi: 10.1016/j.jasms.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Conrad AH, Thoma R, Conrad GW. Differentiation of diiodothyronines using electrospray ionization tandem mass spectrometry. J Mass spectrum. 2006;41:162–168. doi: 10.1002/jms.971. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Li QX. Application of mass spectrometry in the analysis of polybrominated diphenyl ethers. Mass Spectrom Rev. 2009 doi: 10.1002/mas.20263. DOI: 10.1002/mas.20263. [DOI] [PubMed] [Google Scholar]
- 41.Zeng X, Freeman PK, Vasilev YV, Voinov VG, Simonich SL, Barofsky DF. Theoretical calculation of thermodynamic properties of polybrominated diphenyl ethers. J Chem Eng Data. 2005;50:1548–1556. [Google Scholar]
- 42.Ismail AAA. Interference from endogenous antibodies in automated immunoassays: what laboratorians need to know. J Clin Pathol. 2009;62:673–678. doi: 10.1136/jcp.2008.055848. [DOI] [PubMed] [Google Scholar]
- 43.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009;55:1380–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]