Abstract
Planar polarity describes the coordinated polarisation of cells or structures in the plane of a tissue. The patterning mechanisms that underlie planar polarity are well characterised in Drosophila, where many events are regulated by two pathways: the ‘core’ planar polarity complex and the Fat/Dachsous system. Components of both pathways also function in vertebrates and are implicated in diverse morphogenetic processes, some of which self-evidently involve planar polarisation and some of which do not. Here, we review the molecular mechanisms and cellular consequences of planar polarisation in diverse contexts, seeking to identify the common principles across the animal kingdom.
Keywords: Drosophila, Planar polarity, Vertebrate
Introduction
‘Planar polarity’ refers to any manifestation of polarity within a two-dimensional surface. Nübler-Jung (Nübler-Jung, 1987) introduced this term to describe the spatial organisation of polarised structures such as bristles on the insect cuticle. Planar polarity is a common property of animal tissues (Fig. 1) that is most obvious when cells are organised in epithelial sheets, where it is defined as polarity in a plane other than the apicobasal axis, but can also be seen in non-epithelial tissues. [For definitions of planar polarity, see Adler and others (Adler, 2002; Lewis and Davies, 2002; Lawrence et al., 2007; Wang and Nathans, 2007).]
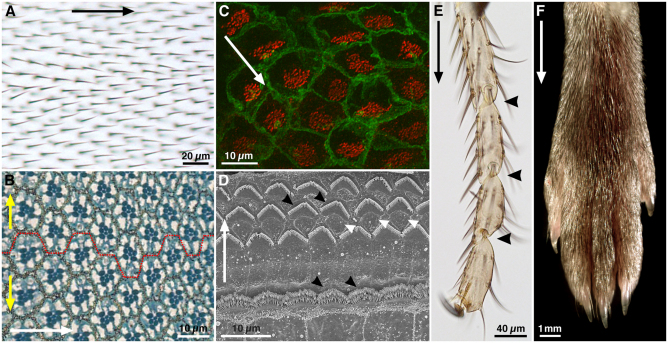
Fig. 1.
Examples of planar polarity in flies and mice. (A) Adult Drosophila wing surface. Planar polarity is evident in the organization of trichomes on the proximodistal (PD) axis (black arrow). (B) Sub-apical section through an adult Drosophila eye at the dorsoventral (DV) midline. Each eye facet comprises a group of ~20 cells (an ommatidium). In this section plane, the pigmented rhabdomeres (dark blue) of seven photoreceptors are in the centre of each ommatidium. Ommatidia are hexagonally tesselated and show mirror-image symmetry around the DV midline (broken red line), revealing axes of planar polarity on the anteroposterior (AP, white arrow) and DV (yellow arrows) axes. (C) Confocal image of mouse brain ependymal cells, with cell membranes stained for β-catenin (green) and cilia basal bodies for γ-tubulin (red). In each cell, basal bodies are displaced towards one side (white arrow), creating ‘translational’ polarity. (D) Scanning electron micrograph of an adult mouse organ of Corti. Planar polarity is seen on the mediolateral axis (white arrow), in the arrangement of the hair cell stereocilia (black arrowheads). Support cells between the hair cells (white arrowheads) show no overt morphological polarisation. (E) Tarsal joints of an adult Drosophila leg. Planar polarity is evident on the PD axis (black arrow) through the polarisation of bristles and the joints (black arrowheads). (F) Distal end of an adult mouse leg, showing the PD polarised arrangement of the fur. Images courtesy of Dr Henry Ho (C), Dr XuDong Wu (D) and Dr Cindy Lu (F), unpublished. (A,B,E) D.S., unpublished.
Planar polarity is most frequently studied at the level of individual cells, for example in the Drosophila wing, or in the organisation of multicellular structures, such as ommatidia in the fly eye or hair follicles in mammalian skin (Fig. 1A,B,F). This level of organisation is often referred to as ‘planar cell polarity’ (PCP). However, planar polarity also exists at the subcellular level, for example in the common orientation of cilia on a multiciliated cell (Fig. 1C), as well as in whole tissues, as in the common distal polarisation of fly wing hairs and mouse limb hairs (Fig. 1A,F). For these reasons, we prefer the more general term ‘planar polarity’.
This review aims to summarise our current knowledge of how planar polarity is established, emphasising the common mechanisms at work across the animal kingdom. We discuss how planar polarity arises in a range of contexts, in each case requiring polarised cell-cell interactions that align cells with their immediate neighbours and long-range patterning events that orient this polarisation with the axes of the tissue. For reasons of space, the only invertebrate considered is the well-studied dipteran Drosophila melanogaster. However, planar polarity has been studied in diverse insects (for a review, see Strutt, 2009), as well as in ascidians (e.g. Jiang et al., 2005), planarians (Almuedo-Castillo et al., 2011) and worms (for reviews, see Walston and Hardin, 2006; Segalen and Bellaïche, 2009). Using the example of the Drosophila wing, we define a framework for how planar polarity is established in epithelial tissues. To facilitate comparisons across species, we provide an operational definition for the term ‘planar polarity’, and in this light review a range of planar polarity processes identified in vertebrates. Finally, we consider the intriguing and recently discovered relationship between planar polarity and cilia function in vertebrates. As most planar polarised cells in Drosophila are non-ciliated, we discuss how these studies in vertebrates provide unique insights into planar polarity establishment.
The basics of planar polarity specification
Planar polarity studies began in the insect cuticle in the 1940s, and were followed by extensive genetic analysis in Drosophila (e.g. Gubb and García-Bellido), with the wing being particularly well characterised. A key advantage of the wing is its simplicity, with each cell in a monolayer epithelium adopting a polarity that is easily discerned by the presence of a single distally pointing trichome (a small hair, see Fig. 1A, Fig. 2B). To provide a framework for understanding planar polarity establishment, we first describe what has been learnt about this from the Drosophila wing, given the strong evidence that the principles seen in the wing are conserved across tissues and species.
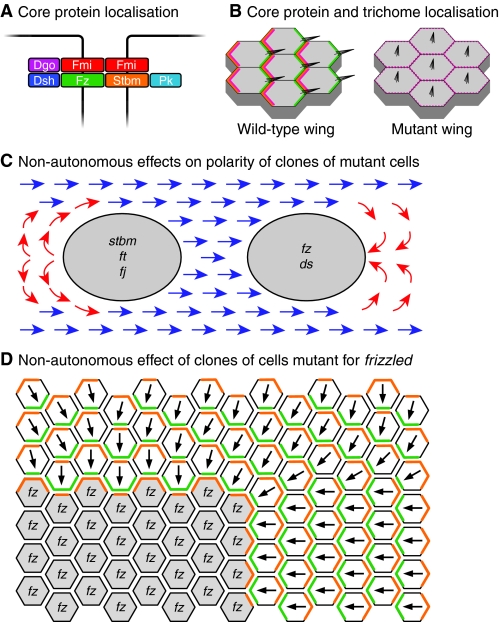
Fig. 2.
Properties of the core planar polarity proteins in Drosophila wing development. (A) Core protein arrangement at the adherens junction zone of epithelial cells in the Drosophila wing. An intercellular asymmetric junctional complex forms, with the transmembrane proteins Fz (green) and Fmi (red), and the cytosolic proteins Dsh (dark blue) and Dgo (purple) in one cell, associating with the transmembrane proteins Stbm (orange) and Fmi, and the cytosolic protein Pk (pale blue) in the adjacent cell. (B) Subcellular distribution of the core proteins and effectors in the pupal wing. From ~28 hours of pupal life, the core proteins show strong asymmetric subcellular distributions, with Fz, Dsh, Dgo and Fmi (green) being localised at distal cell edges (right), and Stbm, Pk and Fmi (orange) at proximal cell edges (left). The effectors Inturned, Fuzzy, Fritz and Multiple Wing Hairs are recruited proximally (pink), and locally inhibit trichome formation, such that trichomes (black) only emerge distally. Cells that lack or have uniform core protein activity show unpolarised effector protein localisation (pink) and trichome production in the cell centre. (C) Non-autonomous effects on trichome polarity (normal polarity shown in blue) in the wing caused by clones of cells lacking planar polarity gene function. Groups of cells lacking stbm, ft or fj activity (grey, left) cause trichomes proximal to the clone to invert their polarity (red arrows), whereas groups of cells lacking fz or ds function (grey, right) cause trichomes distal to the clone to invert their polarity. (D) Model of how a fz clone might alter trichome polarity in neighbouring wild-type cells. Cells lacking Fz (grey) can only form complexes containing Stbm (orange) inside the clone, interacting with Fz (green) in the neighbouring wild-type cells. The abnormal polarity of these junctional complexes around the edge of the clone then propagates to neighbouring cells. Dgo, Diego; ds, dachsous; Dsh, Dishevelled; fj, four-jointed; Fmi, Flamingo; ft, fat; Fz, Frizzled; Pk, Prickle; Stbm, Strabismus.
Two main cellular systems govern the cell-cell interactions that underlie the local alignment of cell polarity in the wing: the so-called ‘core’ planar polarity pathway (often just referred to as the ‘planar polarity pathway’ or ‘PCP pathway’) and the Fat/Dachsous (Ft/Ds) system. Both act to generate asymmetric cell-cell contacts through heterophilic interactions between cell-surface proteins, which exhibit asymmetric subcellular activities and/or distributions.
The core pathway
Six proteins have been placed in the core pathway in flies, owing to their similar activities and colocalisation to the adherens junction (AJ) region of cells, where they form a putative intercellular complex (Fig. 2A). From early in wing development, the core proteins exhibit asymmetric subcellular localisations that are particularly prominent when trichomes form. At this stage, the seven-pass transmembrane protein Frizzled (Fz) is confined to distal cell junctions along with the cytosolic proteins Dishevelled (Dsh) and Diego (Dgo), whereas the four-pass transmembrane protein Strabismus (Stbm, also known as Van Gogh; Vang – FlyBase) and the cytosolic protein Prickle (Pk) are localised proximally; the seven-pass transmembrane cadherin Flamingo (Fmi, also known as Starry Night; Stan – FlyBase) is present both distally and proximally (Fig. 2A,B) (for a review, see Strutt and Strutt, 2009). Complete loss of activity of any of the core proteins leads to a loss of planar polarity, with trichomes initiating in the cell centre (Fig. 2B) (Wong and Adler, 1993).
The core protein asymmetric localisations are thought to result from intracellular feedback interactions between proximally and distally localising components (Tree et al., 2002), whereas the cell-cell coordination of this asymmetry involves the formation of asymmetric intercellular contacts (Chen et al., 2008; Strutt and Strutt, 2008; Wu and Mlodzik, 2008). At the local level, the emergence of coordinated core protein asymmetry is likely to be self-organising, as the activation of core protein expression shortly before trichome formation (when morphogen-based cues are most probably absent) leads to the short-range coordination of polarity (Strutt and Strutt, 2002; Strutt and Strutt, 2007). Evidence that the core pathway plays an instructive role in polarity establishment comes from its directional non-autonomous effects on hair polarity (Gubb and García-Bellido, 1982; Vinson and Adler, 1987; Taylor et al., 1998) (Fig. 2C,D). Groups of cells that lack Fz induce neighbouring cells to point their hairs towards the mutant cells, whereas loss of Stbm causes neighbouring cells to point their hairs away. In both cases, the phenotype suggests that mutant cells and their normal neighbours can only assemble asymmetric junctional complexes of a particular polarity, and this polarity is then propagated to neighbouring cells, an interpretation supported by several theoretical models (e.g. Amonlirdviman et al., 2005; Klein and Mlodzik, 2005; Le Garrec et al., 2006).
How polarised core protein localisation becomes aligned with the axes of the wing is poorly understood. Positional fates on the dorsoventral (DV) and anteroposterior (AP) axes of the wing are largely specified by gradients of the morphogens Wingless (Wg, a member of the Wnt family) and Decapentaplegic (Dpp), respectively (for a review, see Strigini and Cohen, 1999). As Fz also acts as a Wnt receptor, an early suggestion was that a Wnt gradient might provide a polarising cue (e.g. Adler et al., 1997). However, the absence of planar polarity phenotypes upon loss of activity of multiple Wnts (e.g. Lawrence et al., 2002; Chen et al., 2008) caused this idea to fall out of favour. Nevertheless, it is intriguing to note that early in wing development, Fz distribution in cells is oriented towards the source of Wg (Aigouy et al., 2010). An alternative suggestion that core protein localisation is aligned with the axes of the wing by Ft/Ds activity (Ma et al., 2003) is not consistent with the findings that in some genetic conditions in which Ft activity is absent or unlikely to be polarised, core protein-dependent polarisation is largely normal (Matakatsu and Blair, 2006; Feng and Irvine, 2007). However, Ft/Ds can influence core protein polarisation in some contexts, for example by affecting the axis of cell division, cell dynamics and cell packing (Ma et al., 2008; Aigouy et al., 2010), and by influencing the polarisation of microtubules that are involved in vesicle-based transport of Fz distally (Shimada et al., 2006; Harumoto et al., 2010).
The Ft/Ds pathway
Although once considered an ‘upstream’ pathway that provides long-range patterning information to the core pathway (Yang et al., 2002; Ma et al., 2003), current data suggest that the Ft/Ds pathway is a parallel system for locally aligning cell polarity. Ft and Ds both encode cadherins that preferentially bind to each other at the cell surface (Strutt and Strutt, 2002; Ma et al., 2003; Matakatsu and Blair, 2004), and this interaction is modulated by phosphorylation of both extracellular domains by the Golgi protein Four-jointed (Fj) (Strutt et al., 2004; Brittle et al., 2010; Simon et al., 2010) (Fig. 3A,B). As with the core proteins Fz and Stbm, groups of cells that lack Ft, Ds or Fj activity show directional non-autonomous effects on the polarity of neighbouring cells (Adler et al., 1998; Zeidler et al., 2000; Strutt et al., 2002; Ma et al., 2003) (Fig. 2C). As Ft and Ds interact heterophilically, such effects are best explained by models in which Ft/Ds mediate polarised cell-cell interactions (Casal et al., 2006) (for reviews, see Lawrence et al., 2008; Strutt, 2009).
Fig. 3.
Fat and Dachsous interactions in the Drosophila wing. (A,B) Schematics of the interactions between Fat (Ft) and Dachsous (Ds) at adherens junctions of epithelial cells in the Drosophila imaginal discs. (A) Ft (cyan) and Ds (magenta) are large atypical cadherin molecules that interact heterophilically. (B) Ft/Ds heterophilic interactions are modulated by Four-jointed (Fj, yellow) activity in the Golgi, which phosphorylates the extracellular cadherin repeats in Ft and Ds as they traffic to the cell surface. Fj-mediated phosphorylation of Ft increases its binding affinity for Ds, whereas phosphorylation of Ds decreases its affinity for Ft.
Although the Ft/Ds system, like the core pathway, coordinates cell polarity through heterophilic interactions, neither Ft nor Ds exhibits strong asymmetric subcellular localisation (Strutt and Strutt, 2002; Ma et al., 2003). However, Ft and Ds activity does lead to the polarised subcellular distribution of Dachs (Fig. 4A), a downstream-acting atypical myosin (Mao et al., 2006; Rogulja et al., 2008). Hence, the uniform distribution of Ft and Ds may be accompanied by strongly polarised subcellular activity, perhaps mediated by the phosphorylation of the Ft intracellular domain induced by Ds binding (Feng and Irvine, 2009; Sopko et al., 2009). Alternatively, Ft/Ds asymmetries may be amplified by an unknown downstream mechanism.
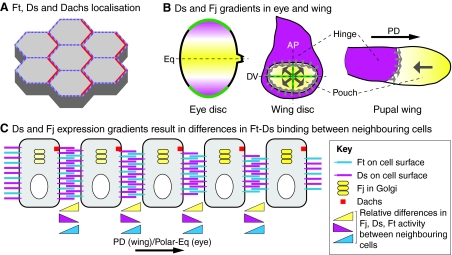
Fig. 4.
Properties of the Fat and Dachsous system in Drosophila. (A) A schematic of the apical surface of epithelial cells in the Drosophila wing. Fat (Ft, blue) and Dachsous (Ds, magenta) are uniformly distributed, but promote planar polarised asymmetry of Dachs (red). (B) Ds and Four-jointed (Fj) gradients. In the eye disc (left), the secreted morphogen Wg (green) is highly expressed at the dorsal and ventral poles, and activates Ds (magenta) expression while suppressing Fj (yellow) expression. The dotted line indicates dorsoventral (DV) midline, also known as the equator (Eq). In the third instar wing disc (middle) and early pupal wing (right), high levels of Ds are present outside the wing pouch, including the hinge. Fj shows graded expression (large grey arrows) within the wing pouch that is highest distally, probably owing to morphogen signalling from the DV and anteroposterior (AP) midlines (green). The sharp boundary between high Ds hinge expression and low Fj pouch expression might serve as a polarising cue (small grey arrows). (C) Model of how opposing gradients of Ds and Fj expression could lead to different levels of Ft/Ds binding activity across cells on the proximodistal (PD) axis of the wing disc or the polar-equatorial (Polar-Eq) axis of the eye disc. Ds levels decline left to right, Fj levels decline right to left and Ft levels are uniform. Triangles at the bottom indicate relative differences between Ds, Ft and Fj activity between pairs of cells. Fj cell-autonomously inhibits Ds activity and simultaneously enhances Ft activity. Thus, considering any cell, its left neighbour has higher Ds expression and activity and lower Ft activity than its right neighbour, promoting formation of more and stronger heterophilic Ft-Ds interactions on the left-right axis and fewer and weaker interactions on the right-left axis. The resulting difference in the strengths of Ft-Ds interactions on opposing cell edges leads to asymmetric distribution of Dachs (red).
The Ft/Ds pathway is aligned with the body axes via its coupling to the upstream morphogens that control the transcription of ds and fj. For example, in the Drosophila eye, a peripheral source of Wg activates ds and represses fj expression, creating opposing gradients of the two proteins (Zeidler et al., 1999; Yang et al., 2002; Simon, 2004) (Fig. 4B) and hence a gradient of Ft/Ds binding interactions across the tissue (Brittle et al., 2010; Simon et al., 2010) (Fig. 4C). In the wing, fj is expressed in a distal to proximal gradient (Zeidler et al., 2000; Strutt et al., 2004), most probably under the positive regulation of Wg and Dpp, whereas ds is largely confined to the wing hinge, with at best only locally graded expression (Strutt and Strutt, 2002; Ma et al., 2003; Matakatsu and Blair, 2004) (Fig. 4B). However, these gradients apparently play only a minor role in planar polarity patterning (Matakatsu and Blair, 2004; Simon, 2004), and a more potent cue is likely to be the boundaries between high and low ds and fj expression that occur between the wing pouch and the wing hinge, boundaries that also appear to be crucial for Ft/Ds-mediated control of growth (Zecca and Struhl, 2010).
Effectors and morphogenetic outputs of the core and Ft/Ds pathways
Factors that act downstream of the core and Ft/Ds pathways to mediate their effects on cell shape and behaviour are termed ‘effectors’. In general, effectors do not influence the asymmetric localisation or activity of the core proteins or of Ft and Ds, and they are often tissue- or cell-type specific, reflecting the diversity of potential readouts. The identification of effectors can be confounded if they have other cellular roles (such as being cytoskeletal modulators), and consequently their removal does not only affect planar polarity.
In the wing, the primary morphogenetic outcome of core pathway activity is the growth of the polarised trichome from the distal cell edge. This is mediated by a small group of proteins that apparently act exclusively as planar polarity effectors, influencing the polarity of both trichomes and bristles. These are Fuzzy (Fy), Inturned (In) and Fritz (Frtz) (Gubb and García-Bellido, 1982; Wong and Adler, 1993; Park et al., 1996; Collier and Gubb, 1997; Collier et al., 2005). In the pupal wing, they colocalise with Fmi, Stbm and Pk at the proximal cell edge (Fig. 2B) (Adler et al., 2004; Strutt and Warrington, 2008) and regulate the subcellular localisation of another effector, Multiple Wing Hairs (Mwh), that antagonises actin polymerisation and helps limit trichome formation to the distal cell edge (Strutt and Warrington, 2008; Yan et al., 2008).
Other proteins often regarded as core pathway effectors in Drosophila are the p21 GTPase RhoA (Rho1 – FlyBase) and its effector Drok (Rok – FlyBase) (Strutt et al., 1997; Winter et al., 2001), both of which regulate actin dynamics. In the wing, such a role has proved hard to substantiate, as loss of RhoA blocks cell division, which itself can lead to hair polarity defects (Adler et al., 2000), and loss of Drok causes twinned hairs with no polarity defect (Winter et al., 2001), and indeed loss of RhoA activity can also affect core protein localisation, suggesting a possible upstream function (Yan et al., 2009). In the eye, neither RhoA nor Drok affects ommatidial DV polarity, but both are required for regulation of ommatidial rotation (Winter et al., 2001; Strutt et al., 2002), which is an additional function of the core pathway in eye patterning.
Defining effectors of the Ft/Ds system is challenging, as this pathway mediates intertwined effects on both planar polarity and tissue growth (for reviews, see Lawrence et al., 2008; Reddy and Irvine, 2008). In the wing, the Ft/Ds pathway alters hair polarity by influencing core protein localisation (Strutt and Strutt, 2002; Ma et al., 2003), showing that the core pathway can serve as an effector of the Ft/Ds pathway. However, in the abdomen and larval cuticle, the Ft/Ds pathway may instead couple directly to effectors such as In, Fy and Frtz, as it can influence hair, bristle and denticle polarity independently of the core pathway (Casal et al., 2006; Repiso et al., 2010). Ft/Ds signalling also regulates the orientation of cell divisions along the proximal-distal axis of the wing (Baena-López et al., 2005), and in this case, seems to act via asymmetric localisation of a different effector, the atypical myosin Dachs (Mao et al., 2011b). Notably, loss of Dachs has negligible effects on hair polarity (Mao et al., 2006), and the core proteins do not affect the orientation of cell division (Baena-López et al., 2005), indicating that these act as parallel pathways below Ft/Ds. Furthermore, Ft/Ds regulation of planar polarity apparently depends on recruitment of the transcriptional repressor atrophin (Fanto et al., 2003), although its relationship to Dachs and/or to growth control remains unclear (for a review, see Sopko and McNeill, 2009).
The simplest way of viewing the relationship between the core pathway and the Ft/Ds pathway is to regard them as parallel but overlapping local alignment systems, both of which can independently respond to upstream patterning cues, but in particular contexts can also influence each other and share common downstream outputs (Fig. 5).
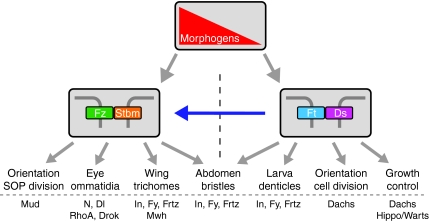
Fig. 5.
Relationships between the core and Ft/Ds pathways in Drosophila. Both the core and Ft/Ds pathways can respond independently to upstream morphogenetic patterning information (gradient in red). The core pathway (represented by heterophilic Fz-Stbm binding) interacts directly with effectors to control polarity of trichomes in the wing, the orientation of sensory organ precursor (SOP) divisions, and the DV polarity and rotation of ommatidia in the eye. In these contexts the Ft/Ds pathway can alter polarity via the core pathway (blue arrow) by an unknown mechanism. The Ft/Ds pathway can act independently of the core pathway to mediate effects on growth control, cell division orientation in the wing and eye discs, and the polarity of larval denticles. The Ft/Ds pathway acts in parallel with the core pathway to control trichome and bristle orientation in the abdomen. Example effectors mediating these effects are shown at the bottom. Dl, Delta; Drok, Rho kinase; Ds, Dachsous; Frtz, Fritz; Ft, Fat; Fy, Fuzzy; Fz, Frizzled; In, Inturned; Mud, Mushroom Body Defective; Mwh, Multiple Wings Hairs; N, Notch; Stbm, Strabismus.
Other planar polarity systems in Drosophila
There are manifestations of planar polarity in Drosophila that are not mediated by either the core or Ft/Ds pathways, indicating that other planar polarity systems also exist. The best-studied is polarised cell rearrangement during the embryonic gastrulation process of germband extension, which is controlled by the pattern of transcription factor expression present in embryonic segments and also relies on local cell-cell interactions (for reviews, see Zallen, 2007; Bertet and Lecuit, 2009). Another example is the initial AP polarisation of ommatidial clusters in the eye disc as they are born from the morphogenetic furrow (Heberlein et al., 1993). An additional local cell alignment system dependent on the activities of the septate junction proteins Gliotactin and Coracle has also been proposed to act in parallel to the core and Ft/Ds systems in the wing (Venema et al., 2004).
Another intriguing alternative pathway for establishing planar polarity is seen in the egg chamber, where each follicle cell has basal actin filaments that are oriented on the DV axis (Gutzeit, 1990). This planar polarity depends on the activity of a ft homologue, fat2 (also known as fat-like and kugelei; kug – FlyBase) (Viktorinová et al., 2009), and of the receptor tyrosine phosphatase Dlar (Lar – FlyBase) (Bateman et al., 2001; Frydman and Spradling, 2001), and on components of the dystroglycan complex (Deng et al., 2003; Mirouse et al., 2009). These factors act non-autonomously, with clones that disrupt actin filament polarity also disrupting the polarity of neighbouring cells, suggesting roles in cell-cell communication. Furthermore, Dlar and Fat2 distributions are planar polarised, with Dlar found at both DV edges of each cell (Bateman et al., 2001) and Fat2 at only one end (Viktorinová et al., 2009). The involvement of novel factors, and the observation that this polarisation does not require the activity of the Ft/Ds or core pathways (Viktorinová et al., 2009), indicates that Fat homologues can coordinate planar polarity through novel mechanisms.
An operational definition for planar polarity
As we have seen, planar polarity is a well-defined phenomenon in Drosophila, which has permitted the characterisation of the underlying signalling events. However, as studies of these same pathways have expanded to tissues that do not show obvious planar organisation, ‘planar polarity’ events have become harder to recognise. Hence, processes are often labelled as involving planar polarity (or PCP) simply because one or more planar polarity proteins is reportedly involved. A particular source of confusion is that the core pathway was originally identified as a ‘non-canonical’ Wnt pathway, involving Frizzled family receptors and Dishevelled homologues in a β-catenin-independent signalling cascade (for reviews, see Strutt, 2003; Veeman et al., 2003a). Subsequently a plethora of non-canonical signalling processes have been designated as being ‘Wnt/PCP signalling’, even in the absence of evidence that they exhibit planar polarity at the cellular level. A related issue is that planar polarity may be indirectly affected by other defects in morphogenesis that are in fact due to signalling through the canonical Wnt pathway or some other uncharacterised pathway that involves known polarity proteins.
We suggest that when considering cellular organisation, a planar polarity process is defined as one in which cell-cell communication causes two or more cells to adopt coordinated polarity. Moreover, such a process should only be referred to as evidence of ‘PCP signalling’ or requiring the ‘planar polarity pathway’ when shown to be dependent upon planar polarity proteins that mediate these polarised cell-cell interactions. For some processes that involve the planar polarity proteins, further research will be required to establish whether they are true examples of planar polarity.
Planar polarity in diverse contexts
Although planar polarity is most obvious in two-dimensional epithelia such as the fly wing, it is prevalent throughout the animal kingdom, occurring in diverse contexts with many morphological outcomes. Multiple vertebrate orthologues of the major pathway components identified in flies have been found (see Table 1), and function via the same basic mechanisms, albeit with some modifications to accommodate the unique demands of each system. Here, we describe some of the best understood vertebrate planar polarised systems.
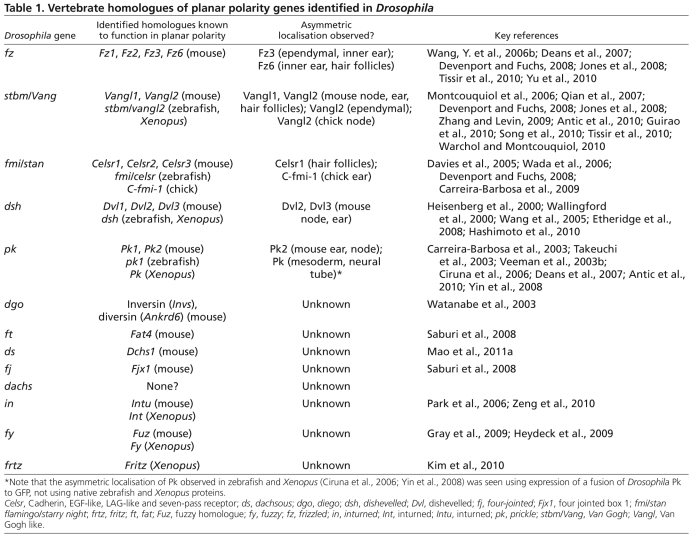
Table 1.
Vertebrate homologues of planar polarity genes identified in Drosophila
Arrays of polarised cells
The simplest manifestation of planar polarity is the alignment of a field of identical cells, as in the fly wing. A comparable vertebrate example is the node, where each epithelial cell extends a single primary cilium towards the posterior of the embryo (Fig. 6A). When all of the cells of the node are properly oriented, the cilia beat together and direct fluid flow to the left, thereby establishing the left-right (LR) axis of the embryo. Failure to uniformly align the cilia disrupts flow, and the embryos develop LR patterning defects (Antic et al., 2010; Borovina et al., 2010; Hashimoto et al., 2010; Song et al., 2010).
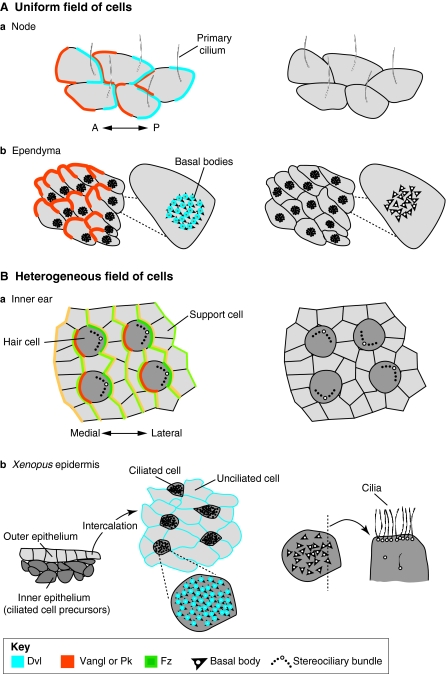
Fig. 6.
Organisation of vertebrate planar polarity in uniform and heterogeneous fields of cells. (A) Planar polarity in uniform fields of cells. In all panels, protein distributions are schematised on the left where known: Dvl (Dishevelled; blue), Vangl (Vang-like) or Pk (Prickle; orange), Fz (Frizzled; green). Surface views shown except where noted. The arrangements of polarised cells as seen in planar polarity mutants are illustrated on the right. In (a) the vertebrate node and (b) the ependymal lining of the brain, identically polarised cells point in one direction, as revealed by the asymmetric position of primary cilia in the node and patches of cilia in the ependyma (translational polarity). Planar polarity also exists at the subcellular level – i.e. rotational polarity – as revealed by the alignment of basal bodies (arrowheads) in individual ependymal cells (b). Cilia remain in the centre and/or fail to align with each other when planar polarity is not properly established. (B) In heterogeneous fields of cells, such as (a) the mouse inner ear and (b) Xenopus epidermis, polarised cells interdigitate with cells that show no outward polarisation. In the inner ear, core proteins are asymmetrically distributed in both hair cells (dark grey) and support cells (light grey); protein distributions are represented in lighter shades in support cells. In polarity mutants, hair cells are not uniformly oriented. In the epidermis, ciliated cells are born in the inner epithelium and then intercalate with unciliated cells in the outer epithelium (shown in cross-section), creating a mosaic of polarised and unpolarised cells in the mature epithelium (shown in surface view). Dvl and Vangl proteins may localise to basal bodies, which normally all point in the same direction. In planar polarity mutants, ciliary orientation is disrupted; defects in ciliogenesis and the apical localisation of the basal bodies have also been reported, as illustrated for one cell shown in cross-section on the right. A, anterior; P, posterior.
The emergence of planar polarity in the node involves both asymmetric positioning of the cilium within each cell and coordinated alignment of the polarised cells. Consistent with the parallels to the fly wing, this depends on expression of orthologues of the core pathway proteins Stbm, Pk and Dsh, which are asymmetrically distributed in the node prior to the posterior localisation of the basal body of the primary cilium (Fig. 6Aa). Moreover, as predicted from flies, Vang-like1 (Vangl1), Vang-like2 (Vangl2) and Prickle2 (Pk2) appear to be restricted to one side of the cell (Antic et al., 2010; Song et al., 2010), while Dishevelled2 (Dvl2) and Dishevelled3 (Dvl3) are on the other (Hashimoto et al., 2010). This localisation is probably instructive, as LR asymmetry defects occur when either Vangl or Dvl activity is severely reduced in multiple species, including mouse, frog and zebrafish (Zhang and Levin, 2009; Antic et al., 2010; Borovina et al., 2010; Hashimoto et al., 2010; May-Simera et al., 2010; Song et al., 2010). Moreover, in mouse, many cilia remain in the cell centre (Fig. 6Aa) (Antic et al., 2010; Hashimoto et al., 2010; Song et al., 2010), indicating that intrinsic planar polarity is lost, echoing the central position of hairs in mutant fly wing cells (Fig. 2B).
A related situation occurs in the ventricular lining of the brain, where multiciliated ependymal cells extend cilia that beat in a coordinated fashion to propel cerebrospinal fluid (Fig. 1C, Fig. 6Ab). These cells exhibit two forms of polarity (Mirzadeh et al., 2010). First, the basal bodies and hence cilia are aligned relative to each other within the cell (rotational polarity). Second, the entire patch of cilia is displaced to the anterior side of each cell (translational polarity, Fig. 6Ab). Both forms of polarity emerge together and correlate with the onset of coordinated beating in a uniform direction (Hirota et al., 2010).
During establishment of translational polarity, the patch of cilia migrates to a peripheral position and the cells align uniformly according to this asymmetry. The polarisation event actually depends on the prior asymmetric positioning of the primary cilium in the radial glia precursors of the ependymal cells. When the primary cilium is not present – as occurs in the absence of the ciliogenic protein Kinesin family member 3a (Kif3a) – the patches remain central (Fig. 6Ab) (Mirzadeh et al., 2010). The mechanism is poorly characterised, but Vangl2 and Fz3 are localised to ependymal cell boundaries under the influence of the Fmi orthologues Celsr2 (Cadherin, EGF-like, LAG-like and seven-pass receptor 2) and Celsr3, consistent with the intracellular signalling functions of the core pathway (Tissir et al., 2010). Furthermore, Vangl2 distribution is asymmetric (Fig. 6Ab), suggesting that polarised cell-cell interactions occur (Guirao et al., 2010). Surprisingly, inhibition of Dvl2 does not affect the positioning of this cilia patch (Hirota et al., 2010); however, this could be due to redundancy or to the insufficient reduction of activity in the radial glial precursors.
Rotational polarity involves alignment not of cells but of organelles (Fig. 6Ab), raising the issue of how the core proteins might function in this context. The correct intracellular alignment of the cilia requires multiple core pathway proteins (Guirao et al., 2010; Hirota et al., 2010; Tissir et al., 2010) and both Dvl2 and Vangl2 localise to basal bodies (Fig. 6Ab) (Hirota et al., 2010; Park et al., 2008; Ross et al., 2005). Strikingly, basal bodies also fail to reach the apical surface of a number of ciliated cell types upon reduction of Vangl, Dvl or Celsr activity (Park et al., 2008; Mitchell et al., 2009; May-Simera et al., 2010; Tissir et al., 2010) (Fig. 6Bb). This suggests a model in which core proteins might polarise intracellular trafficking events that move basal bodies within the cell, influencing both basal body delivery to the apical surface and their alignment on the planar axis.
Mixed arrays of polarised and unpolarised cells
Polarised cells often reside in epithelia that house a diverse array of cell types, such as in the vertebrate inner ear, where morphologically asymmetric hair cells interdigitate with support cells (Fig. 1D, Fig. 6Ba). Each hair cell is topped by a bundle of actin-rich stereocilia linked to one true cilium, the kinocilium. The entire bundle is positioned along one edge, placing the kinocilium on the perimeter of the cell, and fields of polarised hair cells are further organized according to the axes of each sensory epithelium. For example, in the cochlea, all hair cells point their bundles laterally.
Abundant evidence shows that planar polarity in the inner ear is achieved by a conserved core protein pathway. Mutations in orthologues of Fmi (Celsr1), Stbm (Vangl1, Vangl2), Fz (Fz2, Fz3, Fz6) and Dsh (Dvl1, Dvl2, Dvl3) (see Table 1) all cause hair cell misorientation (Fig. 6Ba) (Curtin et al., 2003; Montcouquiol et al., 2003; Wang et al., 2005; Wang, J. et al., 2006; Wang, Y. et al., 2006b; Deans et al., 2007; Etheridge et al., 2008; Torban et al., 2008; Song et al., 2010; Yu et al., 2010), and core proteins are asymmetrically localised (Fig. 6Ba) (Wang et al., 2005; Montcouquiol et al., 2006; Wang, J. et al., 2006; Wang, Y. et al., 2006b; Deans et al., 2007; Qian et al., 2007; Etheridge et al., 2008; Jones et al., 2008; Song et al., 2010). Most exciting is the observation that core proteins are also polarised in the support cells, which may be active participants in the planar polarisation process (Wang, Y. et al., 2006b; Deans et al., 2007; Song et al., 2010; Warchol and Montcouquiol, 2010), despite the fact they do not themselves have any asymmetrically positioned appendages.
Whether the predicted relationships are also conserved is harder to demonstrate as it is difficult to distinguish whether a protein is localised on the medial side of the hair cell or on the lateral side of the closely apposed support cell. A further complexity is that the cells of the organ of Corti are moving as the cochlea extends, thereby obscuring when and where the relevant cell-cell interactions occur (Chen et al., 2002; Yamamoto et al., 2009a). However, mosaic studies conclusively demonstrated that Fz6 and Pk2 do indeed mark opposite sides of cells (Wang, Y. et al., 2006b; Deans et al., 2007), just as Fz and Pk do in flies. Moreover, Vangl2 is required for proper localisation of other core components (Wang et al., 2005; Montcouquiol et al., 2006; Wang, J. et al., 2006; Wang, Y. et al., 2006b; Deans et al., 2007; Etheridge et al., 2008), indicating that the characteristic signalling events mediated by the core protein complex take place in this tissue. Indeed, ablation experiments in chick indicate that polarised Vangl2 in support cells may provide polarity information for newly regenerated hair cells, which consistently appear with the correct orientation (Warchol and Montcouquiol, 2010). The Ft/Ds pathway is also strongly implicated in inner ear patterning, as hair cell orientation is disrupted in mice with mutations in Fat4 and Dcsh1 mutations, which are the most conserved orthologues of Drosophila ft and ds, respectively (Saburi et al., 2008; Mao et al., 2011a). Although the effects on core protein distribution are unknown, Fat4 interacts genetically with both Vangl2 and Fjx1 (Saburi et al., 2008), the sole mammalian Fj orthologue, indicating that a conserved relationship exists between the core and a putative vertebrate Ft/Ds pathway.
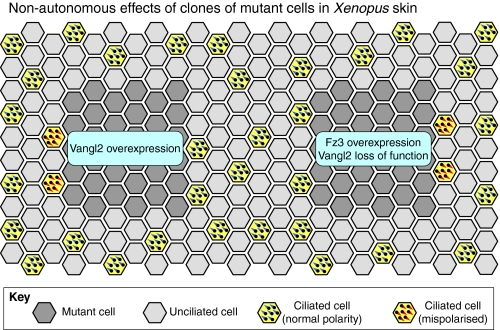
Additional evidence that polarity can be propagated by morphologically unpolarised cells comes from studies of the frog skin, where multiciliated epidermal cells are aligned along the AP axis of the animal (Fig. 6Bb). These ciliated cells are born in the inner epithelial layer and then migrate up to intercalate with unciliated cells in the outer layer (Drysdale and Elinson, 1992). Explant and transplant studies suggest that the outer epithelium is polarised first and passes this information to the ciliated cells as they arrive (Konig and Hausen, 1993; Mitchell et al., 2009). Planar polarity proteins seem to mediate this process: inhibition of a dsh orthologue or of Vangl2 prevents alignment of both the basal bodies within the cell and of the cell relative to its neighbours (Fig. 6Bb) (Park et al., 2008; Mitchell et al., 2009). Similar effects occur upon overexpression of Vangl2 or Fz3 (Mitchell et al., 2009). Of particular interest is that wild-type ciliated cells become misoriented when they are forced to intercalate into an outer layer where core pathway signalling has been disrupted. Furthermore, at the boundaries of transplants, cells orient their cilia towards low levels of Vangl2 and high levels of Fz3 (Fig. 7) (Mitchell et al., 2009), an effect also seen upon overexpression of Vangl2 in the chick inner ear (Sienknecht et al., 2011). Thus, as in flies, Vangl and Fz provide directional information that allows cells to achieve a uniform polarisation, providing some of the best evidence for non-autonomous signalling in vertebrates, and highlighting the ability of multiple cell types to participate in polarised cell-cell interactions.
Fig. 7.
Non-autonomous planar polarity phenotypes in the Xenopus epidermis. Grafting experiments in the Xenopus epidermis reveal that core proteins mediate non-autonomous cell-cell interactions that control the polarity of neighbouring tissue. Grafts of cells with altered core protein expression are indicated in dark grey. Surrounding cells are either multiciliated (yellow) or non-ciliated (light grey). Multiciliated cells are normally polarised to direct fluid flow posteriorly and ventrally (bottom left of the diagram, basal bodies shown in blue). However, in wild-type cells on the anterior edge of grafts that overexpress Vang-like2 (Vangl2), or on the posterior edge of grafts that overexpress Frizzled3 (Fz3) or that have reduced Vangl2 activity, cells produce cilia with inverted polarity (basal bodies shown in red). Anterior is towards the left.
Polarisation of multicellular structures
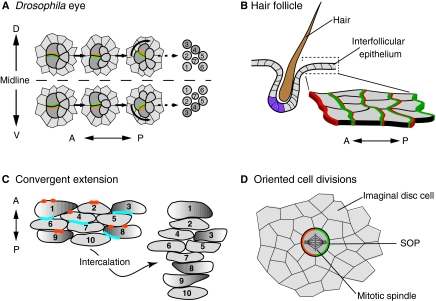
Planar polarity mechanisms also align asymmetrically organised groups of cells. For example, in the Drosophila eye, planar polarity is manifest through the orientation of the ommatidia – groups of about 20 cells that give rise to the facets of the adult eye (for a review, see Wolff and Ready, 1993) (Fig. 1B, Fig. 8A). Ommatidia initially have AP polarity that is independent of the core or Ft/Ds pathways, which subsequently specify mirror-image planar polarity on the DV axis. Notably, the DV polarity of each ommatidium is determined by core pathway activity in just two cells, the R3/R4 photoreceptor pair, and is read out through a Notch/Delta-dependent feedback loop that causes one cell to take on R4 fate and the other R3 fate (Zheng et al., 1995; Cooper and Bray, 1999; Fanto and Mlodzik, 1999; Tomlinson and Struhl, 1999). Mosaic analysis established that R3/R4 fate determination requires higher Fz activity in the presumptive R3 cell and higher Stbm activity in the presumptive R4 cell (Zheng et al., 1995; Wolff and Rubin, 1998; Tomlinson and Struhl, 1999). Consistent with this, Fz is localised to the R3 side of the R3/R4 cell boundary and Stbm to the R4 side (Strutt et al., 2002). As Fz and Stbm expression levels do not seem to vary along the DV axis (Zheng et al., 1995; Rawls and Wolff, 2003), a plausible (but unproven) model is that the subcellular localisation of the core proteins is the key factor in modulating Notch/Delta activity and hence in defining cell fates (Strutt et al., 2002).
Fig. 8.
Planar polarity organisation in multicellular structures and during morphogenesis. Planar polar organisation is also evident in multicellular structures, such as in (A) Drosophila ommatidia and (B) mammalian hair follicles. (A) In the developing fly eye, planar polarisation of core proteins (green and orange) determines which cells will be R3 and R4 photoreceptors (dark grey) and the direction of ommatidium rotation [which at this stage consists of the R3 and R4 photoreceptors, plus R7, R2 and R5 (light grey)]. (Right) The final adult orientation of R1-R7. (B) In mammalian skin, hair follicles comprise multiple cell types that differ in gene expression (purple) and morphology along the anteroposterior (AP) axis. Core proteins (green and orange) are asymmetrically localised across the epithelium, including in the interfollicular cells (as shown in the magnified view). Planar polarity also directs cell movements and oriented cell divisions that sculpt tissues during morphogenesis, as seen during (C) convergent extension in vertebrates and (D) sensory organ precursor (SOPs, dark grey) division in fly imaginal discs. (C) During convergent extension, cells extend along the mediolateral axis (illustrated by graded shading of a subset of cells) and intercalate with each other, causing elongation along the AP axis. Each cell is numbered before and after intercalation to highlight the changing cell-cell interactions. Dvl (Dishevelled) proteins (blue) are localised posteriorly, and Pk (Prickle; orange) anteriorly, although in some contexts, asymmetric localisation has been difficult to detect. (D) Asymmetric localisation of core proteins (green and orange) in SOPs determines the orientation of the mitotic spindle and hence cell division.
An absence of fz activity prior to photoreceptor differentiation leads to randomised DV polarity of ommatidia (Strutt and Strutt, 2002), indicating that long-range polarity coordination across the eye epithelium requires core protein activity in cells other than R3/R4. One hypothesis is that weak DV subcellular polarisation of the core proteins occurs throughout the epithelium, but only cells that take on the R3/R4 fate are primed to respond; the subcellular distribution of Fz/Stbm in cells other than the R3/R4 pair is unknown. Alternatively, asymmetry might only evolve in the R3/R4 pair. The Ft/Ds pathway also acts upstream of the core pathway in determining DV polarity of ommatidia (Zeidler et al., 1999; Rawls et al., 2002; Strutt and Strutt, 2002; Yang et al., 2002; Fanto et al., 2003), but how these pathways and long-range cues are integrated remains contentious (Fanto and McNeill, 2004).
Hair-follicle organisation in the mammalian skin is conceptually similar to that of ommatidia (Fig. 8B). Rodent skin is polarised, with body hairs directed towards the tail and limb hairs pointing distally (Fig. 1F). The polarised unit is the hair follicle, which consists of cells organised into an asymmetric structure, as revealed by differences in gene expression and morphology (Devenport and Fuchs, 2008). When the follicle is properly polarised, the hair emerges from the skin at an angle. Mutations in Celsr1, Vangl2 and Fz6 disrupt this orientation and create whorls and ridges in the fur (Guo et al., 2004; Devenport and Fuchs, 2008; Ravni et al., 2009). Moreover, these three proteins are asymmetrically distributed (Fig. 8B), and this distribution depends on cell-cell interactions mediated by them. Intercellular signalling events appear to propagate this polarity across the epithelium, as wild-type hair follicles can become misaligned when surrounded by mutant cells (Wang, Y. et al., 2006a; Devenport and Fuchs, 2008). How this uniform cell-to-cell planar polarity is translated into polarity of individual follicles is unknown.
An alternative process by which multicellular structures become polarised is seen in the notum and abdomen of Drosophila, where multicellular bristles become aligned on the same axis as surrounding trichome-bearing cells (Gubb and García-Bellido, 1982; Adler, 1992; Theisen et al., 1994). Trichome polarisation is probably analogous to wing planar polarisation, depending upon AP asymmetric localisation of core proteins. By contrast, bristle polarisation involves a different mechanism that acts through core pathway-dependent orientation of an initial asymmetric cell division (Gho and Schweisguth, 1998; Lu et al., 1999; Bellaïche et al., 2004). Consistent with the orientation of the trichomes in surrounding cells, the bristle cluster founder cell (the SOP) exhibits localisation of Fz posteriorly and Stbm anteriorly (Bellaïche et al., 2004), leading to the asymmetric distribution of factors that orient the spindle and specify different daughter cell fates (for a review, see Segalen and Bellaïche, 2009) (Fig. 8D).
Planar polarity-mediated changes in tissue shape
Planar polarity processes also shape three-dimensional tissues that do not exhibit obvious signs of planar polarity. In these cases, the orientation of cells reveals transient planar polarisation, as they move in a specific direction or divide with a specific orientation. When cell polarity is not coordinated along a specific axis, severe morphogenetic defects can arise, including failure of neural tube closure and polycystic kidney disease.
An important example of such a mechanism is convergent extension, during which cells within a tissue intercalate with each other to narrow and extend the tissue. For example, during neurulation of vertebrate embryos, cells in the neural plate migrate towards the midline and mediolaterally intercalate (Fig. 8C), permitting the neural plate to curl up and form the neural tube. When convergent extension fails, the neural plate remains short and broad, preventing the edges from meeting and closing. Convergent extension is also crucial for elongation of the body axis during gastrulation and of the cochlea during inner ear morphogenesis (for reviews, see Keller et al., 2000; Wallingford et al., 2002; Rida and Chen, 2009).
Convergent extension offers a prime example of a true planar polarity process that was revealed due to its dependence on planar polarity gene function. The manipulation of multiple core protein orthologues has resulted in the failure of body axis extension during gastrulation in both zebrafish and frogs (Heisenberg et al., 2000; Tada and Smith, 2000; Wallingford et al., 2000; Goto and Keller, 2002; Jessen et al., 2002; Park and Moon, 2002; Takeuchi et al., 2003; Veeman et al., 2003b). Mutation of these genes in mice results in craniorachischisis, where the neural tube remains open from hindbrain to tail (Kibar et al., 2001; Murdoch et al., 2001; Curtin et al., 2003; Wang, J. et al., 2006; Wang, Y. et al., 2006b).
Although there is ample evidence that planar polarity proteins are required for convergent extension, their effects at the cellular level remain unclear due to the wide variety of cell behaviours that contribute to this process. Imaging studies during zebrafish and frog gastrulation have revealed that core protein function is required for cells to elongate, align, directionally migrate and intercalate on the mediolateral and radial axes (Wallingford et al., 2000; Goto and Keller, 2002; Jessen et al., 2002; Veeman et al., 2003b; Goto et al., 2005; Ybot-Gonzalez et al., 2007; Yin et al., 2008). Importantly, these cell morphology changes probably involve direct cell-cell interactions, as transplantation experiments in zebrafish have shown that loss of core protein activity can non-autonomously affect neighbouring wild-type cells (Jessen et al., 2002; Veeman et al., 2003b).
Although intercalating cells behave as if they are planar polarised on the mediolateral axis, there are few reports that this polarity is accompanied by asymmetric distribution of planar polarity proteins. The use of fluorescently tagged proteins in some studies in zebrafish and frogs has failed to reveal such asymmetry (Wallingford et al., 2000; Carreira-Barbosa et al., 2003; Veeman et al., 2003b), possibly owing to non-physiological expression levels, whereas other studies have shown clear asymmetric localization of Pk and Dsh that is dependent on core pathway function (Ciruna et al., 2006; Yin et al., 2008) (Fig. 8C). However, during mouse neurulation, no asymmetry has been observed, even using a Dvl2-EGFP transgene that reveals Dvl2 asymmetry in the cochlea (Wang et al., 2005; Wang, J. et al., 2006). One explanation may be that convergent extension differs from other polarisation processes because the relevant cells are actively moving and shifting their cell-cell contacts. Thus, it may be difficult to capture transient asymmetric localisation of proteins. In support of this, Dsh protein localisation is variable in zebrafish mesodermal cells undergoing various cell shape changes (Yin et al., 2008).
Another important mechanism for altering tissue shape in a polarised manner is oriented cell division (for a review, see Lecuit and Le Goff, 2007). As already noted, in Drosophila the core proteins regulate bristle polarity by orienting an asymmetric division of the SOP cell (Fig. 8D). However, the oriented cell divisions that underlie changes in tissue shape are not mediated by core proteins in Drosophila (Baena-López et al., 2005). Instead, loss of either Ft or Ds results in truncated adult limbs, apparently owing to a failure to polarise Dachs distribution within the cell (Mao et al., 2011b), which causes a loss of oriented divisions in the growing tissue (Baena-López et al., 2005).
Vertebrate core proteins can also affect the orientation of cell divisions (Gong et al., 2004; Lake and Sokol, 2009; Segalen et al., 2010), and in contrast to Drosophila, this has been suggested to be a driving force in tissue elongation during gastrulation (Gong et al., 2004). However, recent results argue against this idea because blocking oriented cell division independently of core protein activity does not affect embryo length (Quesada-Hernández et al., 2010). In addition, a putative Ft/Ds pathway has been implicated in orienting cell divisions in the tubules of the mammalian kidney (Saburi et al., 2008), with mutations in Fat4 causing misoriented mitoses and dilated tubules. Interestingly, this is enhanced by reduction in Vangl2 dose, suggesting that in vertebrates the core and Ft/Ds systems might cooperate to orient cell divisions.
Other possible examples of planar polarity
The loss of core protein activity results in additional morphogenetic defects, possibly reflecting other requirements for planar polarity. However, it is often unclear which defects are primary and which are secondary to other planar polarity-dependent events. For example, abnormal heart looping is a read-out of earlier LR patterning abnormalities (e.g. Song et al., 2010), whereas outflow tract defects more likely reflect a direct failure in polarised cell migration (Phillips et al., 2005). In addition, a subset of core planar polarity proteins may have been co-opted to mediate polarised changes in cell morphology that do not depend on heterophilic cell-cell interactions. This might include Fz- and Celsr-dependent axon guidance, as well as neuronal migration (for a review, see Wada and Okamoto, 2009), although it is also possible that planar polarity proteins mediate axon-axon or neuron-neuron interactions that have not yet been described. These issues highlight the need for caution in labelling an event as ‘planar polarity’ simply because a known planar polarity gene is involved: many of these genes participate in multiple, independent pathways that are not all used for planar polarity per se.
Novel features of planar polarity in vertebrates
So far we have emphasised the evidence that strongly supports conservation of planar polarity processes. However, some notable differences between flies and vertebrates also exist, reflecting novel ways that vertebrates mobilise and modulate an underlying polarising mechanism.
Upstream signals
One of the least understood aspects of planar polarity is how cells align along a specific tissue axis. At the local level, direct cell-cell interactions are involved, as illustrated by the presence of non-autonomous effects in flies and vertebrates. Where flies and vertebrates may diverge is at the level of global regulation, i.e. in the long-distance cues that ensure that wing trichomes point distally or that ear hair cells point laterally. The Ft/Ds system partly serves such a function in flies, where gradients of morphogens align cells with the body axis by inducing gradients of ds and fj expression. To date, little is known about the roles of Ft and Ds vertebrate orthologues. However, the presence of planar polarity phenotypes in Fat4 and Dchs1 mutant mice suggests that at least some functions are conserved (Saburi et al., 2008; Mao et al., 2011a).
Aside from the Ft/Ds system, cell orientations may be determined by an as yet unidentified secreted factor (sometimes referred to as ‘Factor X’), which can bias the initial distributions or activity of core proteins. Given that Wnt family ligands bind to Fz family receptors, Wnts are obvious candidates for such factors. Although experimental data do not support such a role for Wnts in Drosophila (Lawrence et al., 2002; Chen et al., 2008), Wnts are strongly implicated in several planar polarity processes in vertebrates, including convergent extension and hair cell orientation (Heisenberg et al., 2000; Tada and Smith, 2000; Dabdoub et al., 2003; Qian et al., 2007).
Whether or not Wnts actually serve as orienting cues is unresolved. For example, the mild hair cell orientation defects in Wnt5a mutant mice (Qian et al., 2007) could instead reflect an effect on the expression of Fat4, Dchs1 or Fjx1, similar to the relationship between Wg and the Ft/Ds system in flies. Moreover, many studies that support a role for Wnts in planar polarity coordination rely on overexpression assays, in which a Wnt might inappropriately stimulate a Fz receptor. Nevertheless, it is widely thought that Wnts directly orient planar polarity in vertebrates, where the increased number of cells and greater distances may necessitate additional long-range cues.
Novel pathway components
Several molecules are required for planar polarity in vertebrates but not in flies, raising the issue of whether additional vertebrate-specific mechanisms exist. The best studied example is Protein Tyrosine Kinase 7 (Ptk7), the homologue of Off-track, which mediates axon guidance but not planar polarity in flies (Winberg et al., 2001). Ptk7 mutant mice suffer typical planar polarity defects such as craniorachischisis and misoriented hair cells (Lu et al., 2004; Yen et al., 2009; Paudyal et al., 2010). Whereas Ptk7 function remains unclear, two other proteins implicated in the activation of vertebrate planar polarity signalling – Collagen Triple Helix Repeat Containing 1 (Cthrc1) and Receptor Tyrosine Kinase-like Orphan Receptor 2 (Ror2) (Hikasa et al., 2002; Yamamoto et al., 2008) – are proposed to promote Wnt signalling through effects on Fz receptor activity (Shnitsar and Borchers, 2008; Yamamoto et al., 2008). Hence, the introduction of new planar polarity genes may reflect an expanded role for Wnt signalling in vertebrates.
Typical planar polarity phenotypes also arise in mice and zebrafish that lack Scribble (Montcouquiol et al., 2003; Murdoch et al., 2003; Wada et al., 2005). The Drosophila scribble gene is primarily required for apicobasal polarity (Bilder and Perrimon, 2000), possibly indicating that vertebrate cells rely more heavily on proper apicolateral localisation of the core proteins. However, in zebrafish Scribble is required for convergent extension of mesenchymal cells (Wada et al., 2005) that lack apicobasal polarity, suggesting additional functions for Scribble in planar polarity patterning.
Downstream effectors
Planar polarity signalling can lead to diverse cellular consequences, from directed migration of intracellular organelles to dynamic actin cytoskeleton changes. Hence, it is not surprising that downstream effectors vary between organisms and tissues. By analogy to the original observations in Drosophila (Strutt et al., 1997), the molecules most often assigned this role in vertebrates are small Rho family GTPases. Consistent with this, RhoA (Wünnenberg-Stapleton et al., 1999; Tahinci and Symes, 2003), Rac1 (Sugihara et al., 1998; Tahinci and Symes, 2003; Habas et al., 2003), Cdc42 (Djiane et al., 2000; Choi and Han, 2002), the RhoA effector Rok (Wei et al., 2001; Marlow et al., 2002; Ybot-Gonzalez et al., 2007) and the Rok target Myosin II (Skoglund et al., 2008) have all been described as being planar polarity effectors during gastrulation. Moreover, Rac1 contributes to the polarisation and morphogenesis of hair cells in the mouse inner ear (Grimsley-Myers et al., 2009), whereas Myosin II is implicated in convergent extension of the cochlea (Yamamoto et al., 2009b).
A direct connection between the core proteins and RhoA activation has been established in frogs through the identification of the formin Daam1, which binds to both Dsh and RhoA (Habas et al., 2001), leading to RhoA activation by a Daam1-interacting GEF (Tanegashima et al., 2008). However, as in Drosophila, it should be noted that Rho family proteins are central regulators of actin dynamics, and so the disruption of a particular planar polarity pathway-dependent process does not necessarily imply that Rho GTPases are acting directly as planar polarity effectors. Interestingly, although there is a Daam1 homologue in flies, it does not play a role in planar polarity (Matusek et al., 2006). These caveats should be considered before concluding that a morphogenetic process is controlled by ‘planar polarity’ or ‘Wnt/PCP’ signalling based on the involvement of Wnt/Frizzled, RhoA, Rac1 or Daam1 activity.
There is also no clear picture regarding the functions of the vertebrate homologues of the Drosophila effectors In, Fy and Frtz (see Table 1). Loss of their activity in frog and in mouse has only mild effects on gastrulation, but disrupts ciliogenesis, additionally causing defects in Hh signalling due to cilia loss (Park et al., 2006; Gray et al., 2009; Heydeck et al., 2009; Kim et al., 2010; Zeng et al., 2010). Furthermore, during frog gastrulation, Frtz loss affects the ability of cells to elongate but not their polarity (Kim et al., 2010). Interestingly, in some contexts proper ciliogenesis may be required for the morphogenetic response to the core proteins (see below). If so, the weak gastrulation defects seen upon loss of activity of In, Fy and Frtz homologues may be an indirect effect of their requirement for cilia formation. Thus, just as it has been difficult to dissociate the contributions of Wnt pathway components to polarity versus non-polarity events, defining the specific contributions even of conserved effectors is complicated by their activities in multiple pathways.
Cilia: upstream or downstream?
One of the key differences in planar polarity signalling between flies and vertebrates is the unique involvement of cilia: in contrast to fly cells, primary cilia are present on most vertebrate cells, where they serve several functions (for reviews, see Gerdes et al., 2009; Goetz and Anderson, 2010). The realisation that cilia participate in planar polarity-dependent morphogenesis came from studies of the Bardet Biedl Syndrome (BBS) proteins, which are required for cilia formation due to their roles in intraflagellar transport (for a review, see Blacque and Leroux, 2006). Mice and zebrafish that lack BBS gene activity have both ciliogenesis defects and mild planar polarity defects, which are enhanced by loss of core protein activity (Ross et al., 2005; Gerdes et al., 2007; May-Simera et al., 2010).
Although the link between cilia and planar polarity is indisputable, the nature of this relationship is still unclear (for reviews, see Fischer and Pontoglio, 2009; Wallingford, 2010). In some contexts, cilia may serve as downstream effectors of planar polarity, as two mutations that cause cilia loss [intraflagellar transport protein 88 (Ift88) and Kif3a] cause polarity defects in the mouse ear and brain without affecting core protein distribution (Jones et al., 2008; Guirao et al., 2010). This mimics the loss of Drosophila effector proteins, and connects to the roles of vertebrate In, Fy and Frtz in ciliogenesis. However, such a role may be tissue or organism specific as Ift88 mutant zebrafish that lack cilia show normal convergent extension (Huang and Schier, 2009), as do mice that lack cilia (for a review, see Eggenschwiler and Anderson, 2007). One alternative interpretation is that cilia participate in an as yet unidentified parallel polarising pathway. Cilia have also been implicated in upstream signalling mechanisms, because their mechanical deflection in a specific direction can refine the alignment of cells in the frog skin and mouse ependyma (Mitchell et al., 2007; Guirao et al., 2010). Again, this is unlikely to be a universal role, given the normal polarisation of cells in the inner ear and node when cilia are absent or immotile (Nonaka et al., 2005; Jones et al., 2008). How planar polarity proteins function in the cilia is also a puzzle, with conflicting reports regarding which proteins are present in basal bodies and whether they are required for ciliogenesis (Ross et al., 2005; Park et al., 2008; Borovina et al., 2010; May-Simera et al., 2010; Song et al., 2010; Tissir et al., 2010), in addition to their well-documented functions in cilia orientation, asymmetric positioning of the cilium and the alignment of ciliated cells (Mitchell et al., 2009; Antic et al., 2010; Borovina et al., 2010; Hirota et al., 2010; Hashimoto et al., 2010; Song et al., 2010). More work is needed to determine whether a single conserved planar polarity pathway mediates all of these effects or whether a subset of polarity proteins promote cilia formation independent of their more traditional roles in planar polarity.
Conclusion
Planar polarity is a fundamental property of cells in many – if not most – tissues, and is absolutely required for proper morphogenesis of structures ranging in size from tiny hairs to whole organisms. Consequently, understanding the underlying mechanisms of planar polarity establishment is of intense interest. However, a lack of clarity in the field about what constitutes a planar polarity-dependent process presents a barrier to progress. With regard to this, we have put forward a practical definition of planar polarity as referring to processes in which heterophilic cell-cell interactions lead to mutually coordinated planar polarisation, which fits well with the known molecular mechanisms.
One of the best understood manifestations of planar polarity is when cells in a sheet adopt coordinated polarities, which provides the basis for almost all the morphogenetic processes reviewed here. A major challenge is to elucidate planar polarity processes that occur outside cell sheets, and which perhaps occur only transiently and only between a few cells – for example, during the exquisitely complex wiring of the mammalian nervous system. Other important issues include understanding the relationship between the core and Ft/Ds systems, and the roles of Ft/Ds-related proteins in vertebrates, a largely unexplored issue that is further complicated by the multiple functions of the Ft/Ds system in flies.
The remarkable conservation of the basic mechanisms that establish planar polarity tends to obscure the dearth of knowledge regarding how these pathways are deployed in a range of contexts. In both flies and vertebrates there is only a limited knowledge of the upstream patterning cues, which makes it difficult to identify conserved principles. Similarly, it will be important to identify bona fide downstream effectors and understand how they permit a vast array of morphological outcomes. As we have seen for cilia, which seem to act both up- and downstream of planar polarity proteins, many features of planar polarity specification are highly context specific, with upstream signals and downstream effectors varying from tissue to tissue. Although Drosophila is likely to continue to be a powerful organism for investigating the molecular activities of the core and Ft/Ds systems, most of the questions relating to upstream signals and effectors will be vertebrate specific. As such genes are likely to participate in many different developmental events, their discovery will require a major research endeavour. Based on our experience to date, such efforts will probably lead us to new and surprising examples of how planar polarisation influences animal development.
Acknowledgements
We thank Cindy Lu, XuDong Wu and Henry Ho for providing images shown in Fig. 1, and Amy Brittle, Helen Strutt, Sasha Krol and Henry Ho for helpful comments on the manuscript. D.S. is a Wellcome Trust Senior Research Fellow. Work on planar polarity in the laboratory of L.V.G. is supported by the NIH and the Lefler Center. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Adler P. N. (1992). The genetic control of tissue polarity in Drosophila. BioEssays 14, 735-741 [DOI] [PubMed] [Google Scholar]
- Adler P. N. (2002). Planar signalling and morphogenesis in Drosophila. Dev. Cell 2, 525-535 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Krasnow R. E., Liu J. (1997). Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol. 7, 940-949 [DOI] [PubMed] [Google Scholar]
- Adler P., Charlton J., Liu J. (1998). Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125, 959-968 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Liu J., Charlton J. (2000). Cell size and the morphogenesis of wing hairs in Drosophila. Genesis 28, 82-91 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Zhu C., Stone D. (2004). Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr. Biol. 14, 2046-2051 [DOI] [PubMed] [Google Scholar]
- Aigouy B., Farhadifar R., Staple D. B., Sagner A., Röper J.-C., Julicher F., Eaton S. (2010). Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773-786 [DOI] [PubMed] [Google Scholar]
- Almuedo-Castillo M., Salo E., Adell T. (2011). Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc. Natl. Acad. Sci. USA 108, 2813-2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amonlirdviman K., Khare N. A., Tree D. R. P., Chen W.-S., Axelrod J. D., Tomlin C. J. (2005). Mathematical modeling of planar cell polarity to understand domineering non-autonomy. Science 307, 423-426 [DOI] [PubMed] [Google Scholar]
- Antic D., Stubbs J. L., Suyama K., Kintner C., Scott M. P., Axelrod J. D. (2010). Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS ONE 5, e8999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-López L. A., Baonza A., García-Bellido A. (2005). The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 15, 1640-1644 [DOI] [PubMed] [Google Scholar]
- Bateman J., Reddy R. S., Saito H., Van Vactor D. (2001). The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 11, 1317-1327 [DOI] [PubMed] [Google Scholar]
- Bellaïche Y., Beaudoin-Massiani O., Stuttem I., Schweisguth F. (2004). The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development 131, 469-478 [DOI] [PubMed] [Google Scholar]
- Bertet C., Lecuit T. (2009). Planar polarity and short-range polarization in Drosophila embryos. Semin. Cell Dev. Biol. 20, 1006-1013 [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676-680 [DOI] [PubMed] [Google Scholar]
- Blacque O. E., Leroux M. R. (2006). Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell. Mol. Life Sci. 63, 2145-2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A., Superina S., Voskas D., Ciruna B. (2010). Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 12, 407-412 [DOI] [PubMed] [Google Scholar]
- Brittle A. L., Repiso A., Casal J., Lawrence P. A., Strutt D. (2010). Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr. Biol. 20, 803-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira-Barbosa F., Concha M. L., Takeuchi M., Ueno N., Wilson S. W., Tada M. (2003). Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development 130, 4037-4046 [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F., Kajita M., Morel V., Wada H., Okamoto H., Martinez Arias A., Fujita Y., Wilson S. W., Tada M. (2009). Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development 136, 383-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J., Lawrence P. A., Struhl G. (2006). Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133, 4561-4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Johnson J. E., Zoghbi H. Y., Segil N. (2002). The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495-2505 [DOI] [PubMed] [Google Scholar]
- Chen W.-S., Antic D., Matis M., Logan C. Y., Povelones M., Anderson G. A., Nusse R., Axelrod J. D. (2008). Asymmetric homotypic interactions of the atypical cadherin Flamingo mediate intercellular polarity signaling. Cell 133, 1093-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-C., Han J.-K. (2002). Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signalling pathway. Dev. Biol. 244, 342-357 [DOI] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M., Schier A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Gubb D. (1997). Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development 124, 4029-4037 [DOI] [PubMed] [Google Scholar]
- Collier S., Lee H., Burgess R., Adler P. (2005). The WD40 repeat protein Fritz links cytoskeletal planar polarity to Frizzled subcellular localization in the Drosophila epidermis. Genetics 169, 2035-2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. Y., Bray S. J. (1999). Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature 397, 526-530 [DOI] [PubMed] [Google Scholar]
- Curtin J. A., Quint E., Tsipouri V., Arkell R. M., Cattanach B., Copp A. J., Henderson D. J., Spurr N., Stanier P., Fisher E. M., et al. (2003). Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13, 1129-1133 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Donohue M. J., Brennan A., Wolf V., Montcouquiol M., Sassoon D. A., Hseih J. C., Rubin J. S., Salinas P. C., Kelley M. W. (2003). Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130, 2375-2384 [DOI] [PubMed] [Google Scholar]
- Davies A., Formstone C., Mason I., Lewis J. (2005). Planar polarity of hair cells in the chick inner ear is correlated with polarized distribution of c-flamingo-1 protein. Dev. Dyn. 233, 998-1005 [DOI] [PubMed] [Google Scholar]
- Deans M. R., Antic D., Suyama K., Scott M. P., Axelrod J. D., Goodrich L. V. (2007). Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J. Neurosci. 27, 3139-3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. M., Schneider M., Frock R., Castillejo-Lopez C., Gaman E. A., Baumgartner S., Ruohola-Baker H. (2003). Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130, 173-184 [DOI] [PubMed] [Google Scholar]
- Devenport D., Fuchs E. (2008). Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 10, 1257-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane A., Riou J.-F., Umbhauer M., Boucaut J.-C., Shi D.-L. (2000). Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127, 3091-3100 [DOI] [PubMed] [Google Scholar]
- Drysdale T., Elinson R. (1992). Cell migration and induction in the development of the surface ectodermal pattern of the Xenopus laevis tadpole. Dev. Growth Differ. 34, 51-59 [DOI] [PubMed] [Google Scholar]
- Eggenschwiler J. T., Anderson K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge S. L., Ray S., Li S., Hamblet N. S., Lijam N., Tsang M., Greer J., Kardos N., Wang J., Sussman D. J., et al. (2008). Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 4, e1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M., McNeill H. (2004). Planar polarity from flies to vertebrates. J. Cell Sci. 117, 527-533 [DOI] [PubMed] [Google Scholar]
- Fanto M., Mlodzik M. (1999). Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature 397, 523-526 [DOI] [PubMed] [Google Scholar]
- Fanto M., Clayton L., Meredith J., Hardiman K., Charroux B., Kerridge S., McNeill H. (2003). The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development 130, 763-774 [DOI] [PubMed] [Google Scholar]
- Feng Y., Irvine K. D. (2007). Fat and expanded act in parallel to regulate growth through warts. Proc. Natl. Acad. Sci. USA 104, 20362-20367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Irvine K. (2009). Processing and phosphorylation of the Fat receptor. Proc. Natl. Acad. Sci. USA 106, 11989-11994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Pontoglio M. (2009). Planar cell polarity and cilia. Semin. Cell Dev. Biol. 20, 998-1005 [DOI] [PubMed] [Google Scholar]
- Frydman H. M., Spradling A. C. (2001). The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128, 3209-3220 [DOI] [PubMed] [Google Scholar]
- Gerdes J. M., Liu Y., Zaghloul N. A., Leitch C. C., Lawson S. S., Kato M., Beachy P. A., Beales P. L., DeMartino G. N., Fisher S., et al. (2007). Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 39, 1350-1360 [DOI] [PubMed] [Google Scholar]
- Gerdes J. M., Davis E. E., Katsanis N. (2009). The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M., Schweisguth F. (1998). Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature 393, 178-181 [DOI] [PubMed] [Google Scholar]
- Goetz S. C., Anderson K. V. (2010). The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Mo C., Fraser S. E. (2004). Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689-693 [DOI] [PubMed] [Google Scholar]
- Goto T., Keller R. (2002). The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev. Biol. 247, 165-181 [DOI] [PubMed] [Google Scholar]
- Goto T., Davidson L., Asashima M., Keller R. (2005). Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr. Biol. 15, 787-793 [DOI] [PubMed] [Google Scholar]
- Gray R. S., Abitua P. B., Wlodarczyk B. J., Szabo-Rogers H. L., Blanchard O., Lee I., Weiss G. S., Liu K. J., Marcotte E. M., Wallingford J. B., et al. (2009). The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat. Cell Biol. 11, 1225-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley-Myers C. M., Sipe C. W., Geleoc G. S., Lu X. (2009). The small GTPase Rac1 regulates auditory hair cell morphogenesis. J. Neurosci. 29, 15859-15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D., García-Bellido A. (1982). A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68, 37-57 [PubMed] [Google Scholar]
- Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J.-M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y.-G., et al. (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341-350 [DOI] [PubMed] [Google Scholar]
- Guo N., Hawkins C., Nathans J. (2004). Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. USA 101, 9277-9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit H. O. (1990). The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol. 53, 349-356 [PubMed] [Google Scholar]
- Habas R., Kato Y., He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell 107, 843-854 [DOI] [PubMed] [Google Scholar]
- Habas R., Dawid I. B., He X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17, 295-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T., Ito M., Shimada Y., Kobayashi T. J., Ueda H. R., Lu B., Uemura T. (2010). Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev. Cell 19, 389-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Shinohara K., Wang J., Ikeuchi S., Yoshiba S., Meno C., Nonaka S., Takada S., Hatta K., Wynshaw-Boris A., et al. (2010). Planar polarization of node cells determines the rotational axis of node cilia. Nat. Cell Biol. 12, 170-176 [DOI] [PubMed] [Google Scholar]
- Heberlein U., Wolff T., Rubin G. M. (1993). The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell 75, 913-926 [DOI] [PubMed] [Google Scholar]
- Heisenberg C. P., Tada M., Rauch G. J., Saude L., Concha M. L., Geisler R., Stemple D. L., Smith J. C., Wilson S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76-81 [DOI] [PubMed] [Google Scholar]
- Heydeck W., Zeng H., Liu A. (2009). Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev. Dyn. 238, 3035-3042 [DOI] [PubMed] [Google Scholar]
- Hikasa H., Shibata M., Hiratani I., Taira M. (2002). The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129, 5227-5239 [DOI] [PubMed] [Google Scholar]
- Hirota Y., Meunier A., Huang S., Shimozawa T., Yamada O., Kida Y. S., Inoue M., Ito T., Kato H., Sakaguchi M., et al. (2010). Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development 137, 3037-3046 [DOI] [PubMed] [Google Scholar]
- Huang P., Schier A. F. (2009). Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136, 3089-3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen J. R., Topczewski J., Bingham S., Sepich D. S., Marlow F., Chandrasekhar A., Solnica-Krezel L. (2002). Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 4, 610-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Munro E. M., Smith W. C. (2005). Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr. Biol. 15, 79-85 [DOI] [PubMed] [Google Scholar]
- Jones C., Roper V. C., Foucher I., Qian D., Banizs B., Petit C., Yoder B. K., Chen P. (2008). Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69-77 [DOI] [PubMed] [Google Scholar]
- Keller R., Davidson L., Edlund A., Elul T., Ezin M., Shook D., Skoglund P. (2000). Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 897-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z., Vogan K. J., Groulx N., Justice M. J., Underhill D. A., Gros P. (2001). Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251-255 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Shindo A., Park T. J., Oh E. C., Ghosh S., Gray R. S., Lewis R. A., Johnson C. A., Attie-Bittach T., Katsanis N., et al. (2010). Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329, 1337-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. J., Mlodzik M. (2005). Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 21, 155-176 [DOI] [PubMed] [Google Scholar]
- Konig G., Hausen P. (1993). Planar polarity in the ciliated epidermis of Xenopus embryos. Dev. Biol. 160, 355-368 [DOI] [PubMed] [Google Scholar]
- Lake B. B., Sokol S. Y. (2009). Strabismus regulates asymmetric cell divisions and cell fate determination in the mouse brain. J. Cell Biol. 185, 59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J., Struhl G. (2002). Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129, 2749-2760 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G., Casal J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G., Casal J. (2008). Do the protocadherins Fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat. Cell Biol. 10, 1379-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garrec J. F., Lopez P., Kerszberg M. (2006). Establishment and maintenance of planar epithelial cell polarity by asymmetric cadherin bridges: a computer model. Dev. Dyn. 235, 235-246 [DOI] [PubMed] [Google Scholar]
- Lecuit T., Le Goff L. (2007). Orchestrating size and shape during morphogenesis. Nature 450, 189-192 [DOI] [PubMed] [Google Scholar]
- Lewis J., Davies A. (2002). Planar cell polarity in the inner ear: how do hair cells acquire their oriented structure? J. Neurobiol. 53, 190-201 [DOI] [PubMed] [Google Scholar]
- Lu B., Usui T., Uemura T., Jan L., Jan Y.-N. (1999). Flamingo controls the planar polarity of sensory bristles and asymmetric division of sensory organ precursors in Drosophila. Curr. Biol. 9, 1247-1250 [DOI] [PubMed] [Google Scholar]
- Lu X., Borchers A. G. M., Jolicoeur C., Rayburn H., Baker J. C., Tessier-Lavigne M. (2004). PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430, 93-98 [DOI] [PubMed] [Google Scholar]
- Ma D., Yang C. H., McNeill H., Simon M. A., Axelrod J. D. (2003). Fidelity in planar cell polarity signalling. Nature 421, 543-547 [DOI] [PubMed] [Google Scholar]
- Ma D., Amonlirdviman K., Raffard R. L., Abate A., Tomlin C. J., Axelrod J. D. (2008). Cell packing influences planar cell polarity signaling. Proc. Natl. Acad. Sci. USA 105, 18800-18805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Rauskolb C., Cho E., Hu W. L., Hayter H., Minihan G., Katz F. N., Irvine K. D. (2006). Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 133, 2539-2551 [DOI] [PubMed] [Google Scholar]
- Mao Y., Mulvaney J., Zakaria S., Yu T., Morgan K. M., Allen S., Basson M. A., Francis-West P., Irvine K. D. (2011a). Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development 138, 947-957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Tournier A. L., Bates P. A., Gale J. E., Tapon N., Thompson B. J. (2011b). Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 25, 131-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow F., Topczewski J., Sepich D., Solnica-Krezel L. (2002). Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12, 876-884 [DOI] [PubMed] [Google Scholar]
- Matakatsu H., Blair S. S. (2004). Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development 131, 3785-3794 [DOI] [PubMed] [Google Scholar]
- Matakatsu H., Blair S. S. (2006). Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development 133, 2315-2324 [DOI] [PubMed] [Google Scholar]
- Matusek T., Djiane A., Jankovics F., Brunner D., Mlodzik M., Mihály J. (2006). The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 133, 957-966 [DOI] [PubMed] [Google Scholar]
- May-Simera H. L., Kai M., Hernandez V., Osborn D. P., Tada M., Beales P. L. (2010). Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish. Dev. Biol. 345, 215-225 [DOI] [PubMed] [Google Scholar]
- Mirouse V., Christoforou C. P., Fritsch C., St Johnston D., Ray R. P. (2009). Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev. Cell 16, 83-92 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mirzadeh Z., Han Y.-G., Soriano-Navarro M., García-Verdugo J. M., Alvarez-Buylla A. (2010). Cilia organize ependymal planar polarity. J. Neurosci. 30, 2600-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B., Jacobs R., Li J., Chien S., Kintner C. (2007). A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97-101 [DOI] [PubMed] [Google Scholar]
- Mitchell B., Stubbs J. L., Huisman F., Taborek P., Yu C., Kintner C. (2009). The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr. Biol. 19, 924-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Rachel R. A., Lanford P. J., Copeland N. G., Jenkins N. A., Kelley M. W. (2003). Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173-177 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Sans N., Huss D., Kach J., Dickman J. D., Forge A., Rachel R. A., Copeland N. G., Jenkins N. A., Bogani D., et al. (2006). Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265-5275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J. N., Doudney K., Paternotte C., Copp A. J., Stanier P. (2001). Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum. Mol. Genet. 10, 2593-2601 [DOI] [PubMed] [Google Scholar]
- Murdoch J. N., Henderson D. J., Doudney K., Gaston-Massuet C., Phillips H. M., Paternotte C., Arkell R., Stanier P., Copp A. J. (2003). Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Genet. 12, 87-98 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Yoshiba S., Watanabe D., Ikeuchi S., Goto T., Marshall W. F., Hamada H. (2005). De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 3, e268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nübler-Jung K. (1987). Insect Epidermis: disturbance of supracellular tissue polarity does not prevent the expression of cell polarity. Rouxs Arch. Dev. Biol. 196, 286-289 [DOI] [PubMed] [Google Scholar]
- Park M., Moon R. T. (2002). The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 4, 20-25 [DOI] [PubMed] [Google Scholar]
- Park T. J., Haigo S. L., Wallingford J. B. (2006). Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 38, 303-311 [DOI] [PubMed] [Google Scholar]
- Park T. J., Mitchell B. J., Abitua P. B., Kintner C., Wallingford J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. J., Liu J., Sharp E. J., Adler P. N. (1996). The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development 122, 961-969 [DOI] [PubMed] [Google Scholar]
- Paudyal A., Damrau C., Patterson V. L., Ermakov A., Formstone C., Lalanne Z., Wells S., Lu X., Norris D. P., Dean C. H., et al. (2010). The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev. Biol. 10, 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H. M., Murdoch J. N., Chaudhry B., Copp A. J., Henderson D. J. (2005). Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ. Res. 96, 292-299 [DOI] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X., Chen P. (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Hernández E., Caneparo L., Schneider S., Winkler S., Liebling M., Fraser S. E., Heisenberg C.-P. (2010). Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr. Biol. 20, 1966-1972 [DOI] [PubMed] [Google Scholar]
- Ravni A., Qu Y., Goffinet A. M., Tissir F. (2009). Planar cell polarity cadherin Celsr1 regulates skin hair patterning in the mouse. J. Invest. Dermatol. 129, 2507-2509 [DOI] [PubMed] [Google Scholar]
- Rawls A. S., Wolff T. (2003). Strabismus requires Flamingo and Prickle function to regulate tissue polarity in the Drosophila eye. Development 130, 1877-1887 [DOI] [PubMed] [Google Scholar]
- Rawls A. S., uinto J. B., Wolff T. (2002). The cadherins Fat and Dachsous regulate dorsal/ventral signaling in the Drosophila eye. Curr. Biol. 12, 1021-1026 [DOI] [PubMed] [Google Scholar]
- Reddy B. V., Irvine K. D. (2008). The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development 135, 2827-2838 [DOI] [PubMed] [Google Scholar]
- Repiso A., Saavedra P., Casal J., Lawrence P. A. (2010). Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat. Development 137, 3411-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rida P. C., Chen P. (2009). Line up and listen: planar cell polarity regulation in the mammalian inner ear. Semin. Cell Dev. Biol. 20, 978-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja D., Rauskolb C., Irvine K. D. (2008). Morphogen control of wing growth through the Fat signaling pathway. Dev. Cell 15, 309-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. J., May-Simera H., Eichers E. R., Kai M., Hill J., Jagger D. J., Leitch C. C., Chapple J. P., Munro P. M., Fisher S., et al. (2005). Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135-1140 [DOI] [PubMed] [Google Scholar]
- Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S. E., Harrison R., Mount R., Mcneill H. (2008). Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40, 1010-1015 [DOI] [PubMed] [Google Scholar]
- Segalen M., Bellaïche Y. (2009). Cell division orientation and planar cell polarity pathways. Semin. Cell Dev. Biol. 20, 972-977 [DOI] [PubMed] [Google Scholar]
- Segalen M., Johnston C. A., Martin C. A., Dumortier J. G., Prehoda K. E., David N. B., Doe C. Q., Bellaiche Y. (2010). The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and Zebrafish. Dev. Cell 19, 740-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Yonemura S., Ohkura H., Strutt D., Uemura T. (2006). Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10, 209-222 [DOI] [PubMed] [Google Scholar]
- Shnitsar I., Borchers A. (2008). PTK7 recruits dsh to regulate neural crest migration. Development 135, 4015-4024 [DOI] [PubMed] [Google Scholar]
- Sienknecht U. J., Anderson B. K., Parodi R. M., Fantetti K. N., Fekete D. M. (2011). Non-cell-autonomous planar cell polarity propagation in the auditory sensory epithelium of vertebrates. Dev. Biol. 352, 27-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A. (2004). Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development 131, 6175-6184 [DOI] [PubMed] [Google Scholar]
- Simon M. A., Xu A., Ishikawa H. O., Irvine K. D. (2010). Modulation of Fat:Dachsous binding by the cadherin domain kinase Four-Jointed. Curr. Biol. 20, 811-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P., Rolo A., Chen X., Gumbiner B. M., Keller R. (2008). Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135, 2435-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Hu J., Chen W., Elliott G., Andre P., Gao B., Yang Y. (2010). Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466, 378-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopko R., McNeill H. (2009). The skinny on Fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr. Opin. Cell Biol. 21, 717-723 [DOI] [PubMed] [Google Scholar]
- Sopko R., Silva E., Clayton L., Gardano L., Barrios-Rodiles M., Wrana J., Varelas X., Arbouzova N., Shaw S., Saburi S., et al. (2009). Phosphorylation of the tumor suppressor Fat is regulated by its ligand Dachsous and the kinase Discs Overgrown. Curr. Biol. 19, 1112-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M., Cohen S. M. (1999). Formation of morphogen gradients in the Drosophila wing. Semin. Cell Dev. Biol. 10, 335-344 [DOI] [PubMed] [Google Scholar]
- Strutt D. (2003). Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development 130, 4501-4513 [DOI] [PubMed] [Google Scholar]
- Strutt D. (2009). Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb. Perspect. Biol. 1, a000489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D., Strutt H. (2007). Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302, 181-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D., Warrington S. J. (2008). Planar polarity genes in the Drosophila wing regulate the localisation of the FH3 domain protein Multiple Wing Hairs to control the site of hair production. Development 135, 3103-3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D., Johnson R., Cooper K., Bray S. (2002). Asymmetric localisation of Frizzled and the determination of Notch-dependent cell fate in the Drosophila eye. Curr. Biol. 12, 813-824 [DOI] [PubMed] [Google Scholar]
- Strutt D. I., Weber U., Mlodzik M. (1997). The rôle of RhoA in tissue polarity and Frizzled signalling. Nature 387, 292-295 [DOI] [PubMed] [Google Scholar]
- Strutt H., Strutt D. (2002). Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev. Cell 3, 851-863 [DOI] [PubMed] [Google Scholar]
- Strutt H., Strutt D. (2008). Differential stability of Flamingo protein complexes underlies the establishment of planar polarity. Curr. Biol. 18, 1555-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Strutt D. (2009). Asymmetric localisation of planar polarity proteins: mechanisms and consequences. Semin. Cell Dev. Biol. 20, 957-963 [DOI] [PubMed] [Google Scholar]
- Strutt H., Mundy J., Hofstra K., Strutt D. (2004). Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development 131, 881-890 [DOI] [PubMed] [Google Scholar]
- Sugihara K., Nakatsuji N., Nakamura K., Nakao K., Hashimoto R., Otani H., Sakagami H., Kondo H., Nozawa S., Aiba A., et al. (1998). Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 17, 3427-3433 [DOI] [PubMed] [Google Scholar]
- Tada M., Smith J. C. (2000). Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127, 2227-2238 [DOI] [PubMed] [Google Scholar]
- Tahinci E., Symes K. (2003). Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev. Biol. 259, 318-335 [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Nakabayashi J., Sakaguchi T., Yamamoto T. S., Takahashi H., Takeda H., Ueno N. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674-679 [DOI] [PubMed] [Google Scholar]
- Tanegashima K., Zhao H., Dawid I. B. (2008). WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 27, 606-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Abramova N., Charlton J., Adler P. N. (1998). Van Gogh: a new Drosophila tissue polarity gene. Genetics 150, 199-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen H., Purcell J., Bennett M., Kansagara D., Syed A., Marsh J. L. (1994). dishevelled is required during wingless signalling to establish both cell polarity and cell identity. Development 120, 347-360 [DOI] [PubMed] [Google Scholar]
- Tissir F., Qu Y., Montcouquiol M., Zhou L., Komatsu K., Shi D., Fujimori T., Labeau J., Tyteca D., Courtoy P., et al. (2010). Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat. Neurosci. 13, 700-707 [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Struhl G. (1999). Decoding vectorial information from a gradient: sequential rôles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development 126, 5725-5738 [DOI] [PubMed] [Google Scholar]
- Torban E., Patenaude A. M., Leclerc S., Rakowiecki S., Gauthier S., Andelfinger G., Epstein D. J., Gros P. (2008). Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc. Natl. Acad. Sci. USA 105, 3449-3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree D. R. P., Shulman J. M., Rousset R., Scott M. P., Gubb D., Axelrod J. D. (2002). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signalling. Cell 109, 371-381 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Axelrod J. D., Moon R. T. (2003a). A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 5, 367-377 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Hallagan Louie S., Moon R. T. (2003b). Zebrafish Prickle, a modulator of noncanonical Wnt/Fz signalling, regulates gastrulation movements. Curr. Biol. 13, 680-685 [DOI] [PubMed] [Google Scholar]
- Venema D. R., Zeev-Ben-Mordehai T., Auld V. J. (2004). Transient apical polarization of Gliotactin and Coracle is required for parallel alignment of wing hairs in Drosophila. Dev. Biol. 275, 301-314 [DOI] [PubMed] [Google Scholar]
- Viktorinová I., König T., Schlichting K., Dahmann C. (2009). The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136, 4123-4132 [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Adler P. N. (1987). Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329, 549-551 [DOI] [PubMed] [Google Scholar]
- Wada H., Okamoto H. (2009). Roles of planar cell polarity pathway genes for neural migration and differentiation. Dev. Growth Differ. 51, 233-240 [DOI] [PubMed] [Google Scholar]
- Wada H., Iwasaki M., Sato T., Masai I., Nishiwaki Y., Tanaka H., Sato A., Nojima Y., Okamoto H. (2005). Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development 132, 2273-2285 [DOI] [PubMed] [Google Scholar]
- Wada H., Tanaka H., Nakayama S., Iwasaki M., Okamoto H. (2006). Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development 133, 4749-4759 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B. (2010). Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol. 22, 597-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B., Rowning B. A., Vogeli K. M., Rothbächer U., Fraser S. E., Harland R. M. (2000). Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81-85 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Fraser S. E., Harland R. M. (2002). Convergent extension: the molecular control of polarised cell movement during embryonic development. Dev. Cell 2, 695-706 [DOI] [PubMed] [Google Scholar]
- Walston T. D., Hardin J. (2006). Wnt-dependent spindle polarization in the early C. elegans embryo. Semin. Cell Dev. Biol. 17, 204-213 [DOI] [PubMed] [Google Scholar]
- Wang J., Mark S., Zhang X., Qian D., Yoo S.-J., Radde-Gallwitz K., Zhang Y., Lin X., Collazo A., Wynshaw-Boris A., et al. (2005). Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37, 980-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hamblet N. S., Mark S., Dickinson M. E., Brinkman B. C., Segil N., Fraser S. E., Chen P., Wallingford J. B., Wynshaw-Boris A. (2006). Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133, 1767-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Nathans J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647-658 [DOI] [PubMed] [Google Scholar]
- Wang Y., Badea T., Nathans J. (2006a). Order from disorder: self-organization in mammalian hair patterning. Proc. Natl. Acad. Sci. USA 103, 19800-19805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Guo N., Nathans J. (2006b). The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147-2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M. E., Montcouquiol M. (2010). Maintained expression of the planar cell polarity molecule Vangl2 and reformation of hair cell orientation in the regenerating inner ear. J. Assoc. Res. Otolaryngol. 11, 395-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D., Saijoh Y., Nonaka S., Sasaki G., Ikawa Y., Yokoyama T., Hamada H. (2003). The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development 130, 1725-1734 [DOI] [PubMed] [Google Scholar]
- Wei L., Roberts W., Wang L., Yamada M., Zhang S., Zhao Z., Rivkees S. A., Schwartz R. J., Imanaka-Yoshida K. (2001). Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 128, 2953-2962 [DOI] [PubMed] [Google Scholar]
- Winberg M. L., Tamagnone L., Bai J., Comoglio P. M., Montell D., Goodman C. S. (2001). The transmembrane protein Off-track associates with Plexins and functions downstream of Semaphorin signaling during axon guidance. Neuron 32, 53-62 [DOI] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R., Axelrod J. D., Luo L. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar polarity signalling to the actin cytoskeleton. Cell 105, 81-91 [DOI] [PubMed] [Google Scholar]
- Wolff T., Ready D. F. (1993). Pattern formation in the Drosophila retina. In The Development of Drosophila melanogaster (ed. Bate M., Martinez-Arias A.), pp. 1277-1326 Cold Spring Harbor: Cold Spring Harbor Press; [Google Scholar]
- Wolff T., Rubin G. (1998). strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development 125, 1149-1159 [DOI] [PubMed] [Google Scholar]
- Wong L. L., Adler P. N. (1993). Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123, 209-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Mlodzik M. (2008). The Frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev. Cell 15, 462-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wünnenberg-Stapleton K., Blitz I. L., Hashimoto C., Cho K. W. Y. (1999). Involvement of the small GTPases XRhoA and Xrnd1 in cell adhesion and head formation in early Xenopus development. Development 126, 5339-5351 [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okano T., Ma X., Adelstein R. S., Kelley M. W. (2009a). Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development 136, 1977-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Okano T., Ma X., Adelstein R. S., Kelley M. W. (2009b). Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development 136, 1977-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Nishimura O., Misaki K., Nishita M., Minami Y., Yonemura S., Tarui H., Sasaki H. (2008). Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 15, 23-36 [DOI] [PubMed] [Google Scholar]
- Yan J., Huen D., Morely T., Johnson G., Gubb D., Roote J., Adler P. N. (2008). The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 180, 219-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Lu Q., Fang X., Adler P. N. (2009). Rho1 has multiple functions in Drosophila wing planar polarity. Dev. Biol. 333, 186-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-h., Axelrod J. D., Simon M. A. (2002). Regulation of Frizzled by Fat-like cadherins during planar polarity signalling in the Drosophila compound eye. Cell 108, 675-688 [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P., Savery D., Gerrelli D., Signore M., Mitchell C. E., Faux C. H., Greene N. D. E., Copp A. J. (2007). Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen W. W., Williams M., Periasamy A., Conaway M., Burdsal C., Keller R., Lu X., Sutherland A. (2009). PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 136, 2039-2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Kiskowski M., Pouille P.-A., Farge E., Solnica-Krezel L. (2008). Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol. 180, 221-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Smallwood P. M., Wang Y., Vidaltamayo R., Reed R., Nathans J. (2010). Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 137, 3707-3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A. (2007). Planar polarity and tissue morphogenesis. Cell 129, 1051-1063 [DOI] [PubMed] [Google Scholar]
- Zecca M., Struhl G. (2010). A feed-forward circuit linking Wingless, Fat-Dachsous signaling, and the Warts-Hippo pathway to Drosophila wing growth. PLoS. Biol. 8, e1000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler M. P., Perrimon N., Strutt D. I. (1999). The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr. Biol. 9, 1363-1372 [DOI] [PubMed] [Google Scholar]
- Zeidler M. P., Perrimon N., Strutt D. I. (2000). Multiple roles for four-jointed in planar polarity and limb patterning. Dev. Biol. 228, 181-196 [DOI] [PubMed] [Google Scholar]
- Zeng H., Hoover A. N., Liu A. (2010). PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev. Biol. 339, 418-428 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Levin M. (2009). Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis 47, 719-728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Zhang J., Carthew R. W. (1995). frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development 121, 3045-3055 [DOI] [PubMed] [Google Scholar]