Abstract
During male sexual differentiation, the transforming growth factor-β (TGF-β) signaling molecule anti-Müllerian hormone (AMH; also known as Müllerian inhibiting substance, MIS) is secreted by the fetal testes and induces regression of the Müllerian ducts, the primordia of the female reproductive tract organs. Currently, the molecular identity of downstream events regulated by the AMH signaling pathway remains unclear. We found that male-specific Wnt4 expression in mouse Müllerian duct mesenchyme depends upon AMH signaling, implicating the WNT pathway as a downstream mediator of Müllerian duct regression. Inactivation of β-catenin, a mediator of the canonical WNT pathway, did not affect AMH signaling activation in the Müllerian duct mesenchyme, but did block Müllerian duct regression. These data suggest that β-catenin mediates AMH signaling for Müllerian duct regression during male sexual differentiation.
Keywords: β-Catenin, AMH, MIS, Müllerian duct regression, Wnt4, Mouse
INTRODUCTION
Prior to sexual differentiation in amniotes, all embryos form the anlagen for both male and female reproductive tract organs, the Wolffian (mesonephric) and Müllerian (paramesonephric) ducts, respectively (Kobayashi and Behringer, 2003; Masse et al., 2009). At embryonic day (E) 11.5 in mice, the Müllerian duct forms as an invagination of the mesonephric (coelomic) epithelium of the anterior urogenital ridge, which subsequently elongates posteriorly lateral to the Wolffian duct and reaches the cloaca at E13.5 (Kobayashi and Behringer, 2003; Kobayashi et al., 2004; Guioli et al., 2007; Orvis and Behringer, 2007; Ma, 2009). Delaminating mesonephric (coelomic) epithelial cells adjacent to the extending Müllerian duct epithelium contribute to the surrounding Müllerian duct mesenchyme (Zhan et al., 2006; Guioli et al., 2007).
In mammals, sex is determined genetically (Waters et al., 2007; DeFalco and Capel, 2009; Sekido and Lovell-Badge, 2009): XY individuals become males, whereas XX individuals become females. The sex-determining gene on the Y chromosome Sry directs differentiation of the XY fetal gonad into a testis (Polanco and Koopman, 2007; DiNapoli and Capel, 2008; Sekido and Lovell-Badge, 2009). Subsequently, the testis secretes male-specific hormones that virilize the rest of the body (Nef and Parada, 2000). One of these male fetal hormones is anti-Müllerian hormone (AMH; also known as Müllerian inhibiting substance, MIS), a member of the transforming growth factor-β (TGF-β) family (Munsterberg and Lovell-Badge, 1991; Teixeira et al., 2001; Josso et al., 2006). AMH signaling is both essential and sufficient for Müllerian duct regression (Behringer et al., 1990; Tsuji et al., 1992; Behringer et al., 1994; Belville et al., 1999). Loss of Amh function in the mouse results in the differentiation of ectopic female reproductive tracts in males, including oviducts, uterus and vagina (Behringer et al., 1994; Belville et al., 1999). The presence of these female organs in an otherwise normal male causes infertility in Amh-null males because they disrupt the passage of spermatozoa out of the body. Ectopic expression of human AMH (hAMH) under the widely expressed mouse metallothionein1 promoter (Mt-hAMH) causes regression of the Müllerian ducts in female mice (Behringer et al., 1990). Thus, male-specific expression of AMH ensures elimination of the female reproductive tract organs in males for normal fertility.
Molecular components of the AMH signaling pathway have been identified (di Clemente et al., 2003). TGF-β family signaling is mediated by two receptors, type I and II, which phosphorylate receptor SMAD proteins (Massague et al., 2005; Kitisin et al., 2007; Moustakas and Heldin, 2009). The anti-Müllerian hormone type 2 receptor gene (Amhr2, also known as MISIIR) is expressed in the Müllerian duct mesenchyme and adjacent mesonephric (coelomic) epithelium (Baarends et al., 1994; di Clemente et al., 1994; Zhan et al., 2006; Arango et al., 2008). Inactivation of Amhr2 also causes complete retention of ectopic female reproductive tract organs in males, identical to Amh-null males (Mishina et al., 1996; Belville et al., 1999). Mt-hAMHtg/+; Amhr2−/− males are identical in phenotype to Amh- and Amhr2-null males, indicating that the AMH type II receptor is the only type II receptor that transduces the AMH signal (Mishina et al., 1999). Two type I receptors, ALK3 (BMPR1A – Mouse Genome Informatics) and ALK2 (ACVR1 – Mouse Genome Informatics), and three receptor SMADs, SMAD1, SMAD5 and SMAD8 (also known as SMAD9 – Mouse Genome Informatics), redundantly mediate AMH signaling (Jamin et al., 2002; Zhan et al., 2006; Orvis et al., 2008). Upon activation of AMH signaling, the receptor SMADs enter the nucleus and regulate transcription of downstream target genes. However, little is known about the downstream molecular regulation of AMH signaling. Although it was shown that matrix metalloproteinase 2 (Mmp2) mediates Müllerian duct regression ex vivo, the Müllerian duct regresses normally in male mice that lack Mmp2 activity in vivo (Roberts et al., 2002).
Previous studies indicate that the AMH and WNT signaling pathways might interact mutually during Müllerian duct regression. In both sexes, Wnt7a expression in the Müllerian duct epithelium is required for Amhr2 expression in the adjacent Müllerian duct mesenchyme (Parr and McMahon, 1998). Wnt7a-null males do not regress the Müllerian duct system, leading to the development of female reproductive tract organs. These findings indicate that Wnt7a induces the Müllerian duct mesenchyme to become competent to respond to AMH. AMH signaling also regulates the expression of WNT pathway components. Sexually dimorphic expression patterns of two secreted Frizzled-related genes, Sfrp2 and Sfrp5, are regulated by AMH signaling (Cox et al., 2006). Activation of the canonical WNT pathway induces accumulation of β-catenin (CTNNB1 – Mouse Genome Informatics) in the nucleus, where β-catenin regulates transcription of downstream target genes with other cofactors, including LEF1 (van Amerongen and Nusse, 2009; Verheyen and Gottardi, 2010). The canonical WNT pathway can directly regulate Lef1 transcription, which is independent of LEF1 function (Filali et al., 2002). AMH signaling induces the accumulation of β-catenin and LEF1 in the nucleus of Müllerian duct mesenchyme cells (Allard et al., 2000). However, Müllerian duct regression occurs normally in compound Sfrp2- and Sfrp5-null mutant males (Cox et al., 2006), and the role of β-catenin and LEF1 during Müllerian duct regression has not been studied. Therefore, the functions of the WNT/β-catenin pathway for Müllerian duct regression remain unclear.
We have examined the relationship between AMH signaling and the WNT/β-catenin pathway during Müllerian duct regression. Male-specific expression of Wnt4 was regulated by AMH signaling in the Müllerian duct mesenchyme. Inactivation of β-catenin in the Müllerian duct mesenchyme did not alter the sexually dimorphic expression pattern of Wnt4, suggesting that β-catenin is dispensable for activation of the AMH signaling pathway in the Müllerian duct mesenchyme. However, loss of β-catenin in the Müllerian duct mesenchyme caused complete retention of ectopic female reproductive tract organs in males, indicating a requirement of β-catenin for Müllerian duct regression during male sexual differentiation. Thus, the WNT/β-catenin pathway mediates Müllerian duct regression downstream of the AMH signaling pathway.
MATERIALS AND METHODS
Mice
Amhr2+/− (Mishina et al., 1996), Mt-hAMHtg/+ (Behringer et al., 1990), R26RlacZ/+ (Soriano, 1999), Mox2Cre/+ (More-Cre) (Tallquist and Soriano, 2000), Wnt4+/− (Stark et al., 1994) and Wnt7a+/− (Parr and McMahon, 1995) mice were maintained on a C57BL/6J congenic background. Amhr2Cre/+ mice were maintained on a C57BL/6J × 129/SvEv mixed background (Jamin et al., 2002). Ctnnb1flox/flox mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained on a C57BL/6J × 129X1/SvJ × 129S1/Sv mixed background (Brault et al., 2001). Using Amhr2-Cre expression in the male germ-line, Amhr2Cre/+; Ctnnb1del/+ mice were generated by mating Amhr2Cre/+; Ctnnb1flox/+ males with C57BL/6J females. Amhr2Cre/+; Ctnnb1flox/del mice were generated by mating Ctnnb1flox/flox females with Amhr2Cre/+; Ctnnb1del/+ males. Littermate males were used for controls.
Wnt4 conditional null mice were generated as follows. Genomic fragments at the Wnt4 locus were PCR subcloned into pBlueScript KS(−) (Stratagene) using 129/SvEv genomic DNA as a template. The primers used for the 5′ arm were Sal-5arm-Fw1, ATCGGTCGACATCTCCCTGTTACATTCAGAGCCTGAGGGA and Cla-loxP-BglII-5arm-Rv2, ATCGATCGATATAACTTCGTATAGCATACATTATACGAAGTTATAGATCTGGGAACTGGGTTCACTCCAACAAAGCCTGC; for the floxed exon2 were Cla-exon2-Fw1, ATCGATCGATAGCTGCTTTGCCCAAACTGTCAATCTCTAA and RI-loxP-exon2-Rv2, ATCGGAATTCATAACTTCGTATAGCATACATTATACGAAGTTATGCCGCATACAGACCCTAGATGCCCAGAGTT; and for the 3′ arm were RI-3arm-Fw1, ATCGGAATTCCCTGGGTGTCTGGGAAAATGATATTTCTAG and Not-3arm-Rv2, ATCGGCGGCCGCTGGCTGCACAGGTCTGAGTCCTATGTGTCCT. The primers used for the 5′ Southern blot probe were RI-5probe-Fw1, ATCGGAATTCTTGGTTTGCTTTGTTCATCTGTGAAATGAG and Xba-5probe-Rv2, ATCGTCTAGACGGCCGGAACGACGGTGGCAGAGACACTGC; and for the 3′ Southern probe were Xba-3probe-Fw1, ATCGTCTAGAAGCCTTGGGGTACTCTGGGAAGAAAACCAG and 3probe-Rv2-RI, ATCGGAATTCGAACTCAGTCTTTTCTCACTGACT. After combining sequence-confirmed PCR products of the 5′ arm, floxed exon 2 and 3′ arm, FRT-PGK-neo-bpA-FRT and MC1-TK cassettes were inserted at an EcoRI site and between XhoI and SalI sites, respectively. Gene targeting was performed using AB1 ES cells as previously described (Behringer et al., 1994; Shawlot and Behringer, 1995). After screening 142 ES cell clones, five correctly targeted clones were obtained. Three clones were injected into C57BL/6J blastocysts and germ line transmission was obtained from one clone. The resulting Wnt4flox-neo/+ mice were crossed with Rosa26-Flpe mice (Farley et al., 2000) to remove the FRT-PGK-neo-bpA-FRT selection cassette and subsequently intercrossed to maintain homozygous Wnt4flox/flox mice on a C57BL/6J × 129/SvEv mixed background.
To inactivate Wnt4 in the Müllerian duct mesenchyme, Wnt4+/− mice were crossed with Amhr2Cre/+ mice to generate Amhr2Cre/+; Wnt4+/− males, which were subsequently mated with Wnt4flox/flox females to obtain Amhr2Cre/+; Wnt4flox/− males. Littermate males were used for controls.
PCR genotyping
Amhr2+/−, Mt-hAMHtg/+, Wnt4+/− and Wnt7a+/− mice were genotyped as described previously (Kobayashi et al., 2004). Mox2Cre/+ and Amhr2Cre/+; Ctnnb1flox/del mice were genotyped using the following primers: mCtnnb1-Fw12, CACAGGGACTTCCATACCAGTTGACATGGTA; mCtnnb1-Fw13, GAGAGCAAGGTAGAGTGATGAAAGTTGTT; mCtnnb1-Rv15, GGCATCCTCTCACTGCCTCCTCCTAGGTA; Cre-Fw1, GGACATGTTCAGGGATCGCCAGGC; and Cre-Rv2, CGACGATGAAGCATGTTTAGCTG, which give a 299-bp band for the Ctnnb1 wild-type allele (mCtnnb1-Fw13 and mCtnnb1-Rv15), a 402-bp band for the Ctnnb1 floxed allele (mCtnnb1-Fw13 and mCtnnb1-Rv15), a 557-bp band for the Ctnnb1 deleted allele (mCtnnb1-Fw13 and mCtnnb1-Rv15) and a 219-bp band for the Amhr2-Cre allele (Cre-Fw1 and Cre-Rv2). Wnt4 alleles were genotyped using the following primers: mWnt4-Fw10, GCAGAGAGGCCCAGCCTGCCCCTCA; and mWnt4-Rv12, CATGTGCCTGGCCCTAGAAATATCAT, which give a 239-bp band for the Wnt4 deleted allele, a 642-bp band for the Wnt4 wild-type and null allele and a 819-bp band for the Wnt4 floxed allele.
In situ hybridization
Whole-mount in situ hybridization was performed as described previously (Shawlot and Behringer, 1995). After staining, tissues were fixed in 4% paraformaldehyde at 4°C and soaked in 30% sucrose overnight at 4°C. After embedding in OCT (Sakura, 4583), cryosections were generated at 16 μm using a Microm HM560. Differential interference contrast (DIC) images were photographed using a Leica DMR fluorescence microscope.
Section in situ hybridization was performed as described previously (Wang et al., 2008). After liquid emulsion autoradiography and Hoechst staining, images were photographed using a Leica DMR fluorescence microscope.
Immunofluorescence
Immunofluorescence was performed as described previously (Chang et al., 2008; Kobayashi et al., 2008; Wang et al., 2008). Sections were incubated with primary antibodies to AMH (1:100, Santa Cruz Biotechnology, sc-6886), β-catenin (1:50, Santa Cruz Biotechnology, sc-1496; 1:1500, Thermo Scientific, RM-2101), LEF1 (1:150, Cell Signaling, 2230), cleaved caspase-3 (1:100, Cell Signaling Technology, 9661) and E-cadherin (1:200, Invitrogen, 13-1900) and detected by Alexa 488- and Alexa 568-conjugated secondary antibodies (1:250-500, Invitrogen). After mounting with VECTASHIELD containing DAPI (Vector Laboratories, H-1200) or Immu-Mount (Thermo Scientific, 9990402) following Hoechst DAPI staining, images were photographed using a Leica DMR fluorescence microscope or a Nikon D-Eclipse C1si Confocal Microscope.
RESULTS
Male-specific Wnt4 expression in the Müllerian duct mesenchyme during male sexual differentiation
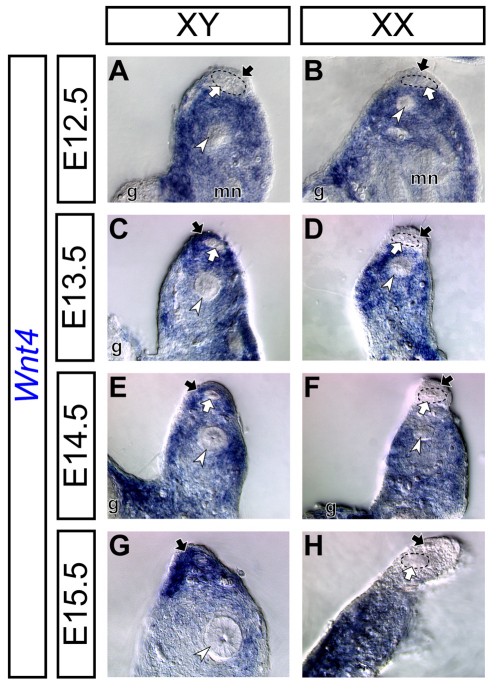
To understand the potential interactions between the AMH and WNT pathways during Müllerian duct regression, we examined the expression of a WNT ligand gene, Wnt4, during Müllerian duct regression. At E12.5, the Müllerian duct mesenchyme begins to form as a thin layer of cells between the mesonephric (coelomic) epithelium and newly formed Müllerian duct epithelium in the anterior region of the urogenital system (Guioli et al., 2007; Orvis and Behringer, 2007). At this stage, Wnt4 was not expressed in the Müllerian duct mesenchyme in both sexes (Fig. 1A,B). One day later at E13.5, the Müllerian duct mesenchyme has surrounded the lateral half of the Müllerian duct epithelium (Orvis and Behringer, 2007; Arango et al., 2008). Interestingly, Wnt4 was expressed in the Müllerian duct mesenchyme of XY males, but not XX females (Fig. 1C,D). By E15.5, most cells of the Müllerian duct epithelium start to die by apoptosis and the Müllerian duct mesenchyme surrounds the dying cells in males, whereas the Müllerian duct continues to differentiate in females at the same stage (Fig. 1E-H) (Kobayashi and Behringer, 2003; Arango et al., 2008). The male-specific Wnt4 expression in the Müllerian duct mesenchyme persisted during Müllerian duct regression (Fig. 1E-H). These data suggest that Wnt4 is expressed in the Müllerian duct mesenchyme during male sexual differentiation.
Fig. 1.
Sexually dimorphic expression pattern of Wnt4 in the mouse Müllerian duct mesenchyme during Müllerian duct regression. (A-H) Differential interference contrast images of Wnt4 expression in the wild-type developing reproductive tract detected by in situ hybridization. The reproductive tract of XY males (A,C,E,G) and XX females (B,D,F,H) at E12.5 (A,B), E13.5 (C,D), E14.5 (E,F) and E15.5 (G,H). Black arrows, white arrows and white arrowheads indicate the Müllerian duct mesenchyme, Müllerian duct epithelium and Wolffian duct epithelium, respectively. g, gonad; mn, mesonephros.
AMH signaling regulates male-specific Wnt4 expression in the Müllerian duct mesenchyme
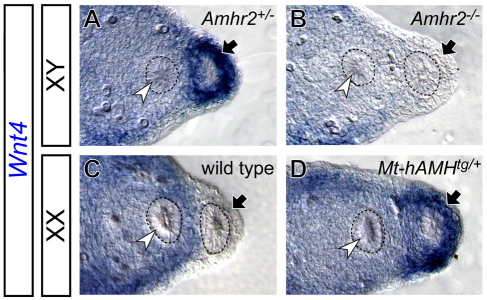
The sexually dimorphic expression pattern of Wnt4 during the period of Müllerian duct regression indicates the possibility of regulation of Wnt4 expression by AMH signaling. To test this idea, we examined Wnt4 expression in two AMH signaling mutants. In Amhr2-null males (Mishina et al., 1996), in which AMH signaling is absent, the male-specific activation of Wnt4 was not observed at E13.5 (Fig. 2A,B), suggesting that AMH signaling is required to activate Wnt4 in the Müllerian duct mesenchyme of males. Ectopic expression of human AMH under the widely expressed metallothionein 1 promoter (Mt-hAMH) activated Wnt4 ectopically in the Müllerian duct mesenchyme of females (Fig. 2C,D), suggesting that AMH signaling is sufficient to activate Wnt4 expression in the Müllerian duct mesenchyme. Taken together, our observations suggest that the AMH signaling is both required and sufficient for Wnt4 expression in the Müllerian duct mesenchyme at the onset of Müllerian duct regression.
Fig. 2.
AMH signaling regulates Wnt4 expression in the mouse Müllerian duct mesenchyme. Differential interference contrast images of Wnt4 expression in the developing reproductive tract from AMH signaling mutants detected by in situ hybridization at E13.5. (A,B) The reproductive tract of Amhr2+/− (A) and Amhr2−/− (B) XY males. (C,D) The reproductive tract of wild-type (C) and Mt-hAMHtg/+ (D) XX females. Black arrows and white arrowheads indicate the Müllerian duct mesenchyme and Wolffian duct epithelium, respectively. Dashed circles indicate the epithelium of the reproductive tracts.
Amhr2-Cre is expressed in the Müllerian duct mesenchyme during male sexual differentiation
We previously generated an Amhr2-Cre knock-in mouse line, in which Cre expression was detected in the Müllerian duct mesenchyme from E12.5 using the R26R-lacZ reporter allele (Jamin et al., 2002). However, it was reported that the Amhr2-Cre activity was detected from E15.5 using the Z/EG Cre reporter allele (Deutscher and Hung-Chang Yao, 2007). Therefore, the onset of Amhr2-Cre expression might depend on the conditional allele employed.
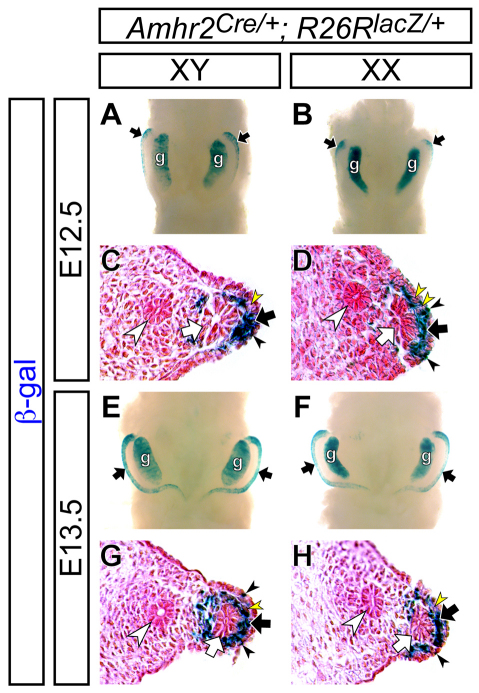
To determine the onset of Amhr2-Cre expression in the Müllerian duct mesenchyme precisely, we performed Cre reporter analysis for the Amhr2-Cre allele at the onset of Müllerian duct mesenchyme development using the R26R-lacZ reporter allele (Soriano, 1999). We detected Amhr2-Cre reporter expression in the Müllerian duct mesenchyme and gonads in both sexes from the onset of Müllerian duct mesenchyme formation (Fig. 3). At E12.5, the Müllerian duct forms in the anterior half of the urogenital ridge (Kobayashi and Behringer, 2003; Kobayashi et al., 2004; Guioli et al., 2007; Orvis and Behringer, 2007). At this stage, Amhr2-Cre reporter activity was detected in the corresponding anterior region of the urogenital ridge (Fig. 3A,B). Histological analysis of the reproductive tract organs showed that Amhr2-Cre reporter activity was detected in the Müllerian duct mesenchyme, with some mosaicism, and some cells in the mesonephric (coelomic) epithelium (Fig. 3C,D). One day later at E13.5, when the entire Müllerian duct forms along the anterior-posterior axis of the urogenital ridge (Kobayashi and Behringer, 2003; Kobayashi et al., 2004; Orvis and Behringer, 2007), Amhr2-Cre reporter activity was detected in the mesenchyme along the entire length of the Müllerian duct (Fig. 3E-H). These data suggest that the Amhr2-Cre line provides a useful tool to manipulate the Müllerian duct mesenchyme genetically throughout Müllerian duct development, including during male sexual differentiation.
Fig. 3.
Cre reporter analysis for Amhr2-Cre allele during Müllerian duct development. To examine Cre activity in the Amhr2-Cre mice, the R26R-lacZ Cre reporter allele (Soriano, 1999) was crossed with the Amhr2-Cre allele. (A-H) X-gal stained (blue) reproductive systems of Amhr2Cre/+; R26RlacZ/+ XY males (A,C,E,G) and XX females (B,D,F,H) at E12.5 (A-D) and E13.5 (E-H). Ventral whole-mount view (A,B,E,F) and cross-sections counterstained with Eosin (pink) (C,D,G,H) are shown. β-Galactosidase (β-gal) activity was observed in the Müllerian duct mesenchyme (black arrows), gonad (g) and some cells of the coelomic epithelium (black arrowheads). Some mosaicism of β-gal activity was seen within the Müllerian duct mesenchyme (yellow arrowheads). Black arrows, white arrows and white arrowheads indicate the Müllerian duct mesenchyme, Müllerian duct epithelium and Wolffian duct epithelium, respectively.
Loss of β-catenin activity causes retention of ectopic female reproductive tract organs in males
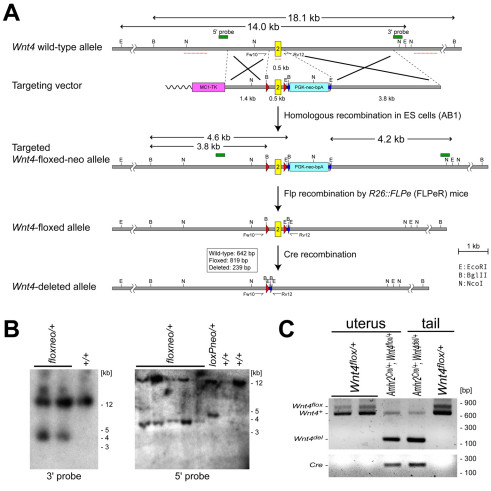
To understand the function of the WNT pathway during Müllerian duct regression, we inactivated WNT signaling components in the Müllerian duct mesenchyme. The AMH signaling-regulated expression of Wnt4 indicates that Wnt4 might be involved in Müllerian duct regression. In Wnt4-null mutants, the Müllerian duct fails to extend posteriorly in both sexes at E11.5 (Vainio et al., 1999; Kobayashi and Behringer, 2003), which prevents studies of Wnt4 function during later stages of Müllerian duct regression. Therefore, we generated a conditional Wnt4-null (Wnt4-floxed) allele in the mouse (Fig. 4A,B). Using Mox2-Cre (More-Cre) mice (Tallquist and Soriano, 2000), we validated that Wnt4del/del mice showed identical abnormalities to the Wnt4-null (Wnt4−/−) mutants (Stark et al., 1994; Vainio et al., 1999) whereas Wnt4flox/flox mice were normal (data not shown). To examine Wnt4 function in the Müllerian duct mesenchyme, we inactivated Wnt4 function using the Amhr2-Cre allele. We found that Müllerian duct regression occurred normally in Amhr2Cre/+; Wnt4flox/− males (Fig. 5), although the Amhr2-Cre allele efficiently recombined the Wnt4flox allele in the female reproductive tract (Fig. 4C). These data indicate that Wnt4 is not required for Müllerian duct regression.
Fig. 4.
Generation of a conditional null allele for Wnt4. (A) Schematic illustration of targeting strategy for a Wnt4 conditional null (floxed) allele. The genomic loci around exon 2 (yellow box) of the Wnt4 gene in different alleles are shown. Red triangles and green arrows indicate loxP and FRT sites, respectively. Red dashed lines indicate conserved regions between mouse and human. Fw10 and Rv12 indicate forward and reverse primers for PCR genotyping, respectively. (B) Confirmation of the targeted Wnt4 floxed allele by Southern hybridization. In total, five correctly targeted ES cell clones were obtained. Wnt4flox/flox mice were phenotypically normal. Wnt4 deleted (Wnt4flox) allele was generated from the Wnt4flox allele using ubiquitous Cre recombination in the embryonic tissue using the Mox2-Cre (More) allele (Tallquist and Soriano, 2000). After intercrossing, the resulting Wnt4del/del mice had identical phenotypes to those in Wnt4-null (Wnt4−/−) mice (Stark et al., 1994; Vainio et al., 1999) in the kidney, gonad and reproductive tract at birth, confirming that the Wnt4 floxed allele generates a null allele after Cre recombination (data not shown). (C) Recombination of the Wnt4 floxed allele by the Amhr2-Cre allele in the female reproductive tract. PCR genotyping with genomic DNA from the uterus of Wnt4flox/+ and Amhr2Cre/+; Wnt4flox/+ females at E18.5. Genomic DNA from the tail of Amhr2Cre/+;Wnt4del/+ and Wnt4flox/+ mice were used as controls.
Fig. 5.
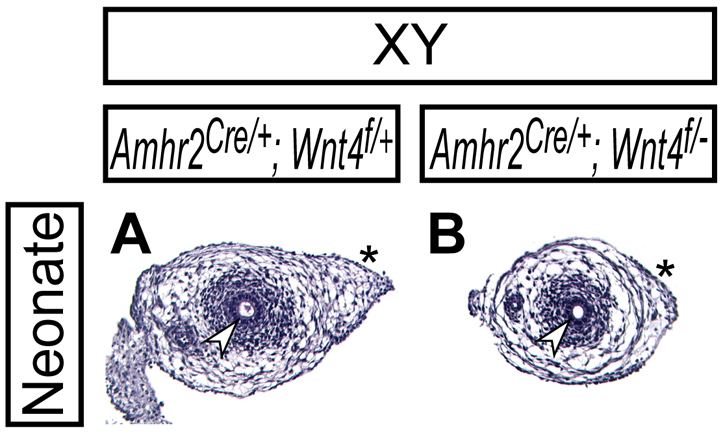
Normal Müllerian duct regression in Amhr2Cre/+; Wnt4flox/del male mice. Histological analysis of reproductive tract organs by Hematoxylin and Eosin staining in Amhr2Cre/+; Wnt4flox/+ (A) and Amhr2Cre/+; Wnt4flox/del (B) neonates. Arrowheads and asterisks indicate the Wolffian duct epithelium and the region where the Müllerian duct regressed, respectively.
The canonical WNT pathway is mediated by β-catenin, loss of which results in lethality during gastrulation (Haegel et al., 1995). We next inactivated β-catenin specifically in the Müllerian duct mesenchyme to examine the possible roles of the WNT/β-catenin pathway for Müllerian duct regression. When β-catenin was inactivated in the Müllerian duct mesenchyme using the Amhr2-Cre allele, we found that β-catenin mutant newborn males had both male and female reproductive tract organs (Fig. 6A,B,E,F). Histological analysis revealed an ectopic female reproductive tract lateral to the male reproductive tract in the β-catenin mutant males (Fig. 6I,J). All β-catenin mutant males examined (n=7) retained the complete female reproductive tract. These data suggest that β-catenin function in the Müllerian duct mesenchyme is required for Müllerian duct regression.
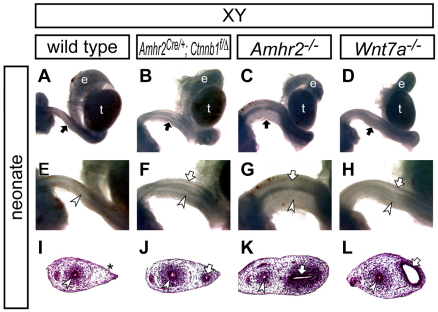
Fig. 6.
β-catenin is required for Müllerian duct regression. (A-L) Reproductive organs from wild-type (A,E,I), Amhr2Cre/+; Ctnnb1flox/del (B,F,J), Amhr2−/− (C,G,K) and Wnt7a−/− (D,H,L) XY males at birth. A-D show whole-mount view of male reproductive organs; E-H are higher magnification images of the reproductive tract indicated by black arrows in A-D, respectively. I-L show histological analysis of the reproductive tract by Hematoxylin and Eosin staining. The asterisk in I indicates the region where the Müllerian duct regressed. Black arrows, white arrows and white arrowheads indicate the reproductive tract, Müllerian duct epithelium and Wolffian duct epithelium, respectively. e, epididymis; t, testis.
β-Catenin function in the Müllerian duct mesenchyme is required for prenatal Müllerian duct differentiation
We also compared the ectopic female reproductive tract organs of AMH signaling mutants. The ectopic female reproductive tracts in the β-catenin mutant males were different from those in Amhr2-null males, in which AMH signaling was absent (Fig. 6J,K). In the absence of β-catenin, the Müllerian duct mesenchyme was less condensed, indicating an additional requirement of β-catenin for Müllerian duct differentiation during embryogenesis, as reported previously in females (Deutscher and Hung-Chang Yao, 2007; Arango et al., 2008). Wnt7a is required for both Müllerian duct regression and differentiation (Fig. 6D,H) (Miller and Sassoon, 1998; Parr and McMahon, 1998). The ectopic female reproductive tracts in the β-catenin mutant males were also different from those of Wnt7a-null males, in which the lumen of the Müllerian duct was expanded and the Müllerian duct mesenchyme was more condensed than the conditional β-catenin mutants, although the Müllerian duct mesenchyme condensation in Wnt7a-null mice was slightly reduced than that of Amhr2-null mice (Fig. 4L), indicating an involvement of other WNT ligand(s) for Müllerian duct differentiation during embryogenesis (Arango et al., 2008).
AMH signaling is activated in the Müllerian duct mesenchyme in the absence of β-catenin function
Because Amhr2-Cre is expressed in both the testis and Müllerian duct mesenchyme (Fig. 3) (Jamin et al., 2002), it is possible that loss of β-catenin function using the Amhr2-Cre allele might disrupt normal production of AMH in the testis and/or its receptor expression in the Müllerian duct mesenchyme. To exclude these possibilities, we examined the expression of AMH and Amhr2 in β-catenin mutant males. At E13.5, AMH was normally expressed in Sertoli cells of the testis in the conditional β-catenin mutant males (Fig. 7A,B). At the same stage, the AMH type II receptor, Amhr2, was also normally expressed in the Müllerian duct mesenchyme and Sertoli cells in the testes of the conditional β-catenin mutant males (Fig. 7C,D).
Fig. 7.
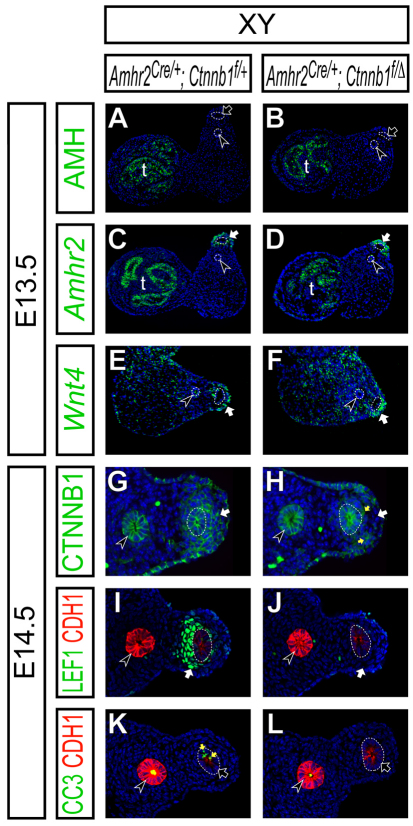
AMH signaling is activated normally in the mouse Müllerian duct mesenchyme in the absence of β-catenin activity. (A-L) Molecular marker analysis in the reproductive organs at E13.5 (A-F) and reproductive tracts at E14.5 (G-L) in Amhr2Cre/+; Ctnnb1flox/+ (A,C,E,G,I,K) and Amhr2Cre/+; Ctnnb1flox/del (B,D,F,H,J,L) XY male embryos. (A,B) Epifluorescence immunostaining of AMH (green) with DAPI staining (blue). (C-F) Radioactive section in situ hybridization for Amhr2 (green in C,D) and Wnt4 (green in E,F) with Hoechst staining (blue). (G-L) Confocal immunofluorescence. (G,H) β-catenin (Ctnnb1, green) with DAPI (blue) staining. (I,J) LEF1 (green), E-cadherin (CDH1, red) with Hoechst (blue) staining. (K,L) Cleaved caspase-3 (CC3, green), E-cadherin (CDH1, red) with Hoechst (blue) staining. E-cadherin is highly expressed in the Wolffian duct epithelium (black arrowheads) and weakly in the Müllerian duct epithelium (dotted line) in I-L. White arrows, black arrows, black arrowheads and yellow arrows indicate the Müllerian duct mesenchyme, Müllerian duct epithelium, Wolffian duct epithelium and cleaved caspase-3 positive apoptotic cells, respectively. t, testis.
Our findings regarding the AMH-dependent Wnt4 expression indicate that Wnt4 can be used as a molecular marker for AMH signaling activation in the Müllerian duct mesenchyme. Therefore, we examined Wnt4 expression in the conditional β-catenin mutant males to determine whether AMH signaling is normally activated in the Müllerian duct mesenchyme. We observed normal activation of Wnt4 expression in the Müllerian duct mesenchyme of the conditional β-catenin mutant males (Fig. 7E,F), suggesting that the AMH signaling was activated in the Müllerian duct mesenchyme in the absence of β-catenin function.
To document inactivation of β-catenin in the Müllerian duct mesenchyme by the Amhr2-Cre allele, we examined β-catenin expression at E14.5. At this stage, cytoplasmic accumulation of β-catenin was observed in the Müllerian duct mesenchyme of control males (Fig. 7G) (Allard et al., 2000). In the conditional β-catenin mutant males, cytoplasmic β-catenin accumulation was greatly reduced whereas β-catenin expression on the cell-surface of the Müllerian duct epithelium was intact (Fig. 7H). There were some β-catenin-expressing cells in the Müllerian duct mesenchyme of the conditional β-catenin mutant males (yellow arrows in Fig. 7H) probably owing to incomplete recombination by the Amhr2-Cre allele and/or contribution from cells with a non-recombined β-catenin allele, including the coelomic epithelial cells, to the Müllerian duct mesenchyme (Guioli et al., 2007). Taken together, these data suggest that β-catenin is specifically inactivated in the Müllerian duct mesenchyme of the conditional mutants by E14.5.
We examined expression of a canonical WNT target molecule, LEF1, in the absence of β-catenin function at E14.5. At this stage, E-cadherin (CDH1) is detected strongly in the Wolffian duct epithelium and weakly in the Müllerian duct epithelium (Fig. 7I-L). We found that LEF1 upregulation in the Müllerian duct mesenchyme was not observed in the β-catenin mutant males (Fig. 7I,J), suggesting that the activation of the canonical WNT pathway in the Müllerian duct mesenchyme was abolished in the conditional β-catenin mutant males.
Lastly, we examined the expression of an apoptotic marker, cleaved caspase-3, in the Müllerian duct epithelium of the β-catenin mutant males. Because apoptosis occurs in a wave manner along the anterior-posterior axis of the Müllerian duct epithelium (Allard et al., 2000), we examined serial sections of the entire Müllerian duct and counted sections with the cleaved caspase-3 positive Müllerian duct epithelium. In control males, we observed cleaved caspase-3 positive cells in the Müllerian duct epithelium in 66.7% ± 15.7% (n=2) of sections from the entire Müllerian duct (Fig. 7K; see Fig. S1 in the supplementary material). When β-catenin was inactivated by the Amhr2-Cre allele in males, sections with the cleaved caspase-3 positive Müllerian duct epithelium were significantly reduced (26.1% ± 1.2%, n=2) (Fig. 7L), indicating suppression of apoptosis in the Müllerian duct epithelium in the absence of β-catenin activity in the Müllerian duct mesenchyme. Taken together, these observations indicate that the canonical WNT/β-catenin pathway in the Müllerian duct mesenchyme is dispensable for AMH signaling activation in the Müllerian duct mesenchyme, but required to mediate AMH signaling in the Müllerian duct mesenchyme to induce apoptosis in the Müllerian duct epithelium. Thus, AMH signaling acts upstream of β-catenin, which mediates Müllerian duct regression during male sexual differentiation (Fig. 8).
Fig. 8.
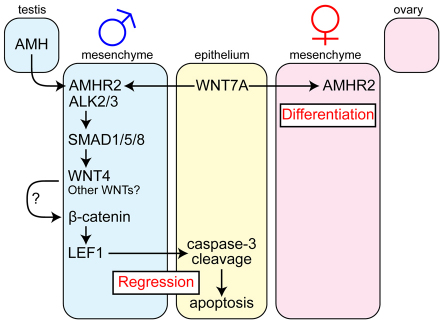
A model for genetic pathways for Müllerian duct regression during male sexual differentiation. WNT7a in the Müllerian duct epithelium is required for expression of the AMH type II receptor AMHR2 in the Müllerian duct mesenchyme in both sexes. In males, the testis secretes a TGF-β signaling molecule, AMH, which binds to the type II receptor AMHR2 and type I receptors ALK2 and ALK3 in the Müllerian duct mesenchyme. The activation of the AMH signaling is mediated by three receptor SMADs, SMAD1, SMAD5 and SMAD8 (SMAD1/5/8), which directly or indirectly activate male-specific WNT4 expression in the Müllerian duct mesenchyme. WNT4, possibly with other WNT ligands, might act as an autocrine signal to stabilize β-catenin in the Müllerian duct mesenchyme. The activation of β-catenin is transmitted by unidentified molecular signal(s) to the Müllerian duct epithelium, where apoptosis is induced, at least partially, by caspase-3 cleavage, leading to Müllerian duct regression. In females, there is no production of AMH, which permits Müllerian duct differentiation into female reproductive tract organs.
DISCUSSION
A requirement of β-catenin for Müllerian duct regression
Our data suggest that β-catenin mediates AMH signaling in the Müllerian duct mesenchyme and is required for Müllerian duct regression in male fetuses as inactivation of β-catenin in the Müllerian duct mesenchyme resulted in retention of ectopic female reproductive tract organs in males. By contrast, when β-catenin was inactivated in the epithelium of the Müllerian duct using the Wnt7a-Cre allele, the Müllerian duct regressed normally in mutant males (C.A.S. and R.R.B., unpublished data), suggesting that β-catenin in the Müllerian duct epithelium is dispensable for Müllerian duct regression. Thus, β-catenin function is required specifically in the mesenchyme of the Müllerian duct for Müllerian duct regression in males.
β-catenin has two different functions: one in the protein complex at adherens junctions (AJs) of epithelial cells and the other in the canonical WNT signaling pathway. Because β-catenin activity is required in the mesenchyme, but not the epithelium, of the Müllerian duct during Müllerian duct regression, it is unlikely that β-catenin functions through AJs. Furthermore, β-catenin was observed mostly in the cytoplasm and nucleus of the Müllerian duct mesenchyme only in males, whereas β-catenin was detected at the cell surface in the Müllerian duct epithelium in both sexes (Allard et al., 2000), indicating that β-catenin regulates the canonical WNT signaling pathway required for Müllerian duct regression during male sexual differentiation.
WNT ligands for Müllerian duct regression
It still remains unclear which WNT ligand(s) mediates AMH signaling during Müllerian duct regression. Wnt7a expressed in the Müllerian duct epithelium is essential for Müllerian duct regression during male sexual differentiation (Parr and McMahon, 1998). Because Amhr2 expression in the Müllerian duct mesenchyme depends on Wnt7a activity in both sexes, the Müllerian duct lacking Wnt7a activity cannot respond to AMH. Because β-catenin accumulates in the nucleus of Müllerian duct mesenchyme cells in males but not in females (Allard et al., 2000), and we showed that the AMH signaling is activated through normal Amhr2 expression without β-catenin function in the Müllerian duct mesenchyme, we can conclude that WNT7A is not the WNT ligand that regulates β-catenin during Müllerian duct regression in males.
WNT4 is a good candidate as a WNT ligand necessary for Müllerian duct regression as AMH signaling regulates the male-specific expression of Wnt4 in the Müllerian duct mesenchyme. Interestingly, Wnt4 signaling is mediated by the canonical WNT/β-catenin pathway during kidney development (Park et al., 2007). However, our results of normal regression of the Müllerian duct in Amhr2Cre/+; Wnt4flox/del males suggest that Wnt4 is dispensable for Müllerian duct regression. We observed Amhr2-Cre reporter expression in the Müllerian duct mesenchyme from the onset of its formation at E12.5, and one day later at E13.5 detected the male-specific Wnt4 expression in the Müllerian duct mesenchyme. Thus, timing of Cre activation is probably not the issue for Wnt4 inactivation in the Müllerian duct mesenchyme.
Another consideration before excluding Wnt4 involvement in Müllerian duct regression is that the Amhr2-Cre allele might not have sufficiently high Cre recombinase activity in the Müllerian duct mesenchyme, especially during early stages of Müllerian duct development (Deutscher and Hung-Chang Yao, 2007). We observed some mosaicism of Cre recombination in the Müllerian duct mesenchyme from the Amhr2-Cre allele even with the R26R-lacZ allele, which can be recombined more efficiently than other Cre reporter alleles (Long and Rossi, 2009). Considering that Wnt4 encodes a secreted molecule, more complete recombination might be required for total inactivation of Wnt4 function. Alternatively, another WNT ligand(s) might redundantly activate β-catenin with WNT4 during Müllerian duct regression.
Dual roles of β-catenin for Müllerian duct development
Our data suggest that β-catenin has dual roles in the Müllerian duct mesenchyme: one for Müllerian duct regression in males and the other for Müllerian duct differentiation in females. It was proposed that Wnt7a activates the canonical WNT/β-catenin pathway in the Müllerian duct (Deutscher and Hung-Chang Yao, 2007). Although our observations might support this view, we found that the ectopic female reproductive tracts in the conditional β-catenin mutants are different from those in Wnt7a mutants, suggesting involvement of another WNT ligand for female reproductive tract differentiation. Our observations are consistent with a previous report that loss of β-catenin in the Müllerian duct mesenchyme results in trans-differentiation of the myometrium to adipose tissues in adults, which is not observed in Wnt7a mutants (Arango et al., 2005). Interestingly, Wnt5a plays important roles for Müllerian duct differentiation during embryogenesis (Mericskay et al., 2004), suggesting that it might also act redundantly with Wnt7a in Müllerian duct differentiation.
The mechanism for β-catenin exerting the two distinct functions for Müllerian duct development remains to be studied. One explanation might be stage-specific functions of β-catenin during Müllerian duct regression and differentiation. It is known that the competence or regressive response of the Müllerian duct to AMH signaling occurs in a narrow window of time (Picon, 1969; Tsuji et al., 1992; Allard et al., 2000). During Müllerian duct regression (e.g. E13.5-15.5), β-catenin mediates AMH signaling pathways. Subsequently (from E15.5 to birth), β-catenin might promote Müllerian duct differentiation. Consistent with this idea, the Müllerian duct remained small and round in the neonate without β-catenin (Fig. 6J).
Relationships between the AMH and WNT/β-catenin signaling pathways
Cross talk between TGF-β/BMP and WNT/β-catenin signaling pathways has been observed during limb development, when the BMP receptor IA acts upstream of β-catenin for formation of the apical ectodermal ridge (AER) (Soshnikova et al., 2003). Bmp2 also regulates β-catenin signaling pathway during bone formation (Chen et al., 2007; Zhang et al., 2009). Regulation of the canonical WNT/β-catenin pathway by TGF-β/BMP signaling is an important regulatory mechanism for a number of biological processes.
During kidney development, the WNT9b/β-catenin signaling pathway activates the WNT4/β-catenin signaling pathway in precursor cells of the nephron (Park et al., 2007). Inactivation of Wnt4 alone blocks nephron formation (Stark et al., 1994; Kobayashi et al., 2005; Perantoni et al., 2005), indicating the significance of an additional signaling pathway for signaling amplification mechanisms. Thus, we propose that, like other family members, the WNT/β-catenin signaling might amplify AMH signaling to ensure complete Müllerian duct regression in males. Given these findings, mutations in WNT signaling component genes should be investigated in persistent Müllerian duct syndrome (PMDS) human patients.
Supplementary Material
Acknowledgements
We thank Allan Bradley for AB1 ES cells; Rolf Kemler for conditional Ctnnb1-null mice; Andrew McMahon for Wnt4 and Wnt7a null mice and a Wnt4 in situ probe; and Philippe Soriano for Mox2-Cre mice. A.K. was supported by Research Fellowships from the National Kidney Foundation, Peter Tsacoyeanes Pediatric Renal Research Award from National Kidney Foundation serving New England, Carl W. Gottschalk Research Scholar Grant from American Society of Nephrology, and Seed Grant and Junior Faculty Grant from Harvard Stem Cell Institute. C.A.S. was supported by the Odyssey Program, M.D. Anderson Cancer Center. S.P.J. was supported by the Lalor Foundation. These studies were supported by a grant from the National Institutes of Health (NIH) HD30284, the Ben F. Love Chair, and the Kleberg Foundation to R.R.B. Veterinary animal care was supported by the NIH Cancer Center Support Grant CA16672. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.056143/-/DC1
References
- Allard S., Adin P., Gouedard L., di Clemente N., Josso N., Orgebin-Crist M. C., Picard J. Y., Xavier F. (2000). Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development 127, 3349-3360 [DOI] [PubMed] [Google Scholar]
- Arango N., Szotek P., Manganaro T., Oliva E., Donahoe P., Teixeira J. (2005). Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 288, 276-283 [DOI] [PubMed] [Google Scholar]
- Arango N. A., Kobayashi A., Wang Y., Jamin S. P., Lee H. H., Orvis G. D., Behringer R. R. (2008). A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol. Reprod. Dev. 75, 1154-1162 [DOI] [PubMed] [Google Scholar]
- Baarends W. M., van Helmond M. J., Post M., van der Schoot P. J., Hoogerbrugge J. W., de Winter J. P., Uilenbroek J. T., Karels B., Wilming L. G., Meijers J. H., et al. (1994). A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development 120, 189-197 [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Cate R. L., Froelick G. J., Palmiter R. D., Brinster R. L. (1990). Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature 345, 167-170 [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Finegold M. J., Cate R. L. (1994). Mullerian-inhibiting substance function during mammalian sexual development. Cell 79, 415-425 [DOI] [PubMed] [Google Scholar]
- Belville C., Josso N., Picard J. Y. (1999). Persistence of Mullerian derivatives in males. Am. J. Med. Genet. 89, 218-223 [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264 [DOI] [PubMed] [Google Scholar]
- Chang H., Gao F., Guillou F., Taketo M. M., Huff V., Behringer R. R. (2008). Wt1 negatively regulates beta-catenin signaling during testis development. Development 135, 1875-1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Whetstone H. C., Youn A., Nadesan P., Chow E. C., Lin A. C., Alman B. A. (2007). Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 282, 526-533 [DOI] [PubMed] [Google Scholar]
- Cox S., Smith L., Bogani D., Cheeseman M., Siggers P., Greenfield A. (2006). Sexually dimorphic expression of secreted frizzled-related (SFRP) genes in the developing mouse Mullerian duct. Mol. Reprod. Dev. 73, 1008-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T., Capel B. (2009). Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu. Rev. Cell Dev. Biol. 25, 457-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher E., Hung-Chang Yao H. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. 307, 227-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Clemente N., Wilson C., Faure E., Boussin L., Carmillo P., Tizard R., Picard J. Y., Vigier B., Josso N., Cate R. (1994). Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol. Endocrinol. 8, 1006-1020 [DOI] [PubMed] [Google Scholar]
- di Clemente N., Josso N., Gouedard L., Belville C. (2003). Components of the anti-Mullerian hormone signaling pathway in gonads. Mol. Cell. Endocrinol. 211, 9-14 [DOI] [PubMed] [Google Scholar]
- DiNapoli L., Capel B. (2008). SRY and the standoff in sex determination. Mol. Endocrinol. 22, 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley F. W., Soriano P., Steffen L. S., Dymecki S. M. (2000). Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106-110 [PubMed] [Google Scholar]
- Filali M., Cheng N., Abbott D., Leontiev V., Engelhardt J. F. (2002). Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J. Biol. Chem. 277, 33398-33410 [DOI] [PubMed] [Google Scholar]
- Guioli S., Sekido R., Lovell-Badge R. (2007). The origin of the Mullerian duct in chick and mouse. Dev. Biol. 302, 389-398 [DOI] [PubMed] [Google Scholar]
- Haegel H., Larue L., Ohsugi M., Fedorov L., Herrenknecht K., Kemler R. (1995). Lack of beta-catenin affects mouse development at gastrulation. Development 121, 3529-3537 [DOI] [PubMed] [Google Scholar]
- Jamin S. P., Arango N. A., Mishina Y., Hanks M. C., Behringer R. R. (2002). Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat. Genet. 32, 408-410 [DOI] [PubMed] [Google Scholar]
- Josso N., Picard J. Y., Rey R., di Clemente N. (2006). Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr. Endocrinol. Rev. 3, 347-358 [PubMed] [Google Scholar]
- Kitisin K., Saha T., Blake T., Golestaneh N., Deng M., Kim C., Tang Y., Shetty K., Mishra B., Mishra L. (2007). Tgf-Beta signaling in development. Sci. STKE 2007, cm1 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Behringer R. (2003). Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 4, 969-980 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Shawlot W., Kania A., Behringer R. (2004). Requirement of Lim1 for female reproductive tract development. Development 131, 539-549 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K., Carroll T., McMahon A., Mendelsohn C., Behringer R. (2005). Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132, 2809-2823 [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Valerius M. T., Mugford J. W., Carroll T. J., Self M., Oliver G., McMahon A. P. (2008). Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. A., Rossi F. M. (2009). Silencing inhibits Cre-mediated recombination of the Z/AP and Z/EG reporters in adult cells. PLoS One 4, e5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. (2009). Endocrine disruptors in female reproductive tract development and carcinogenesis. Trends Endocrinol. Metab. 20, 357-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J., Seoane J., Wotton D. (2005). Smad transcription factors. Genes Dev. 19, 2783-2810 [DOI] [PubMed] [Google Scholar]
- Masse J., Watrin T., Laurent A., Deschamps S., Guerrier D., Pellerin I. (2009). The developing female genital tract: from genetics to epigenetics. Int. J. Dev. Biol. 53, 411-424 [DOI] [PubMed] [Google Scholar]
- Mericskay M., Kitajewski J., Sassoon D. (2004). Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131, 2061-2072 [DOI] [PubMed] [Google Scholar]
- Miller C., Sassoon D. A. (1998). Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125, 3201-3211 [DOI] [PubMed] [Google Scholar]
- Mishina Y., Rey R., Finegold M. J., Matzuk M. M., Josso N., Cate R. L., Behringer R. R. (1996). Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 10, 2577-2587 [DOI] [PubMed] [Google Scholar]
- Mishina Y., Whitworth D. J., Racine C., Behringer R. R. (1999). High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology 140, 2084-2088 [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. (2009). The regulation of TGFbeta signal transduction. Development 136, 3699-3714 [DOI] [PubMed] [Google Scholar]
- Munsterberg A., Lovell-Badge R. (1991). Expression of the mouse anti-mullerian hormone gene suggests a role in both male and female sexual differentiation. Development 113, 613-624 [DOI] [PubMed] [Google Scholar]
- Nef S., Parada L. F. (2000). Hormones in male sexual development. Genes Dev. 14, 3075-3086 [DOI] [PubMed] [Google Scholar]
- Orvis G. D., Behringer R. R. (2007). Cellular mechanisms of Mullerian duct formation in the mouse. Dev. Biol. 306, 493-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvis G. D., Jamin S. P., Kwan K. M., Mishina Y., Kaartinen V. M., Huang S., Roberts A. B., Umans L., Huylebroeck D., Zwijsen A., et al. (2008). Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Mullerian hormone-induced Mullerian duct regression in the mouse. Biol. Reprod. 78, 994-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Valerius M., McMahon A. (2007). Wnt/{beta}-catenin signaling regulates nephron induction during mouse kidney development. Development 134, 2533-2539 [DOI] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. (1995). Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374, 350-353 [DOI] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. (1998). Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395, 707-710 [DOI] [PubMed] [Google Scholar]
- Perantoni A., Timofeeva O., Naillat F., Richman C., Pajni-Underwood S., Wilson C., Vainio S., Dove L., Lewandoski M. (2005). Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859-3871 [DOI] [PubMed] [Google Scholar]
- Picon R. (1969). Action of the fetal testis on the development in vitro of the Mullerian ducts in the rat (French). Arch. Anat. Microsc. Morphol. Exp. 58, 1-19 [PubMed] [Google Scholar]
- Polanco J. C., Koopman P. (2007). Sry and the hesitant beginnings of male development. Dev. Biol. 302, 13-24 [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Visser J. A., Ingraham H. A. (2002). Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development 129, 1487-1496 [DOI] [PubMed] [Google Scholar]
- Sekido R., Lovell-Badge R. (2009). Sex determination and SRY: down to a wink and a nudge? Trends Genet. 25, 19-29 [DOI] [PubMed] [Google Scholar]
- Shawlot W., Behringer R. (1995). Requirement for Lim1 in head-organizer function. Nature 374, 425-430 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 [DOI] [PubMed] [Google Scholar]
- Soshnikova N., Zechner D., Huelsken J., Mishina Y., Behringer R. R., Taketo M. M., Crenshaw E. B., 3rd, Birchmeier W. (2003). Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 17, 1963-1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A. P. (1994). Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679-683 [DOI] [PubMed] [Google Scholar]
- Tallquist M. D., Soriano P. (2000). Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26, 113-115 [DOI] [PubMed] [Google Scholar]
- Teixeira J., Maheswaran S., Donahoe P. K. (2001). Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr. Rev. 22, 657-674 [DOI] [PubMed] [Google Scholar]
- Tsuji M., Shima H., Yonemura C. Y., Brody J., Donahoe P. K., Cunha G. R. (1992). Effect of human recombinant mullerian inhibiting substance on isolated epithelial and mesenchymal cells during mullerian duct regression in the rat. Endocrinology 131, 1481-1488 [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405-409 [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. (2009). Towards an integrated view of Wnt signaling in development. Development 136, 3205-3214 [DOI] [PubMed] [Google Scholar]
- Verheyen E. M., Gottardi C. J. (2010). Regulation of Wnt/beta-catenin signaling by protein kinases. Dev. Dyn. 239, 34-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cheon D. J., Lu Z., Cunningham S. L., Chen C. M., Luo R. Z., Xing D., Orsulic S., Bast R. C., Jr, Behringer R. R. (2008). MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation 76, 1081-1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters P. D., Wallis M. C., Marshall Graves J. A. (2007). Mammalian sex-Origin and evolution of the Y chromosome and SRY. Semin. Cell Dev. Biol. 18, 389-400 [DOI] [PubMed] [Google Scholar]
- Zhan Y., Fujino A., MacLaughlin D. T., Manganaro T. F., Szotek P. P., Arango N. A., Teixeira J., Donahoe P. K. (2006). Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development 133, 2359-2369 [DOI] [PubMed] [Google Scholar]
- Zhang M., Yan Y., Lim Y. B., Tang D., Xie R., Chen A., Tai P., Harris S. E., Xing L., Qin Y. X., et al. (2009). BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. J. Cell. Biochem 108, 896-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.