Abstract
The Drosophila body axes are established in the oocyte during oogenesis. Oocyte polarization is initiated by Gurken, which signals from the germline through the epidermal growth factor receptor (Egfr) to the posterior follicle cells (PFCs). In response the PFCs generate an unidentified polarizing signal that regulates oocyte polarity. We have identified a loss-of-function mutation of flapwing, which encodes the catalytic subunit of Protein Phosphatase 1β (PP1β) that disrupts oocyte polarization. We show that PP1β, by regulating myosin activity, controls the generation of the polarizing signal. Excessive myosin activity in the PFCs causes oocyte mispolarization and defective Notch signaling and endocytosis in the PFCs. The integrated activation of JAK/STAT and Egfr signaling results in the sensitivity of PFCs to defective Notch. Interestingly, our results also demonstrate a role of PP1β in generating the polarizing signal independently of Notch, indicating a direct involvement of somatic myosin activity in axis formation.
Keywords: PP1β, Myosin, Oocyte polarity, Drosophila
INTRODUCTION
Cell-cell interactions between somatic and germline cells are crucial to the formation of body axes in Drosophila. During oogenesis, the anterior-posterior (AP) and dorsal-ventral (DV) axes are established in the oocyte, which, together with the nurse cells, is surrounded by a monolayer epithelium of somatically derived follicle cells. During early oogenesis, the oocyte nucleus and a microtubule organizing center (MTOC) are localized at the posterior pole of the oocyte, with microtubule (MT) plus ends extending through the ring canals into the connected nurse cells (Theurkauf et al., 1992). The TGFα-like ligand Gurken (Grk) is localized to the posterior cortex of the oocyte and signals to adjacent follicle cells through the epidermal growth factor receptor (Egfr), also known as Torpedo (Top). In response, these follicle cells adopt a posterior cell fate (Gonzalez-Reyes et al., 1995; Roth et al., 1995). At around stage 6/7 of oogenesis, these posterior follicle cells (PFCs) send an unknown signal back to the oocyte to repolarize the oocyte, resulting in the final determination of the AP axis (Gonzalez-Reyes et al., 1995; Roth et al., 1995). This polarizing signal from the PFCs causes the disassembly of the posterior MTOC and the formation of a gradient of MTs from the anterior to the posterior of the oocyte with a concentration of minus ends now emanating from the anterior and lateral cortex of the oocyte (Clark et al., 1994; Doerflinger et al., 2010; Theurkauf et al., 1992). This reorganization of the cytoskeleton subsequently leads to the localization of several important embryonic polarity determinants, such as bicoid (bcd) and oskar (osk) mRNAs, to the anterior and posterior poles, respectively (for reviews, see Poulton and Deng, 2007; Roth and Lynch, 2009). Moreover, the repolarized MTs also direct the anterior movement of the oocyte nucleus, initiating the formation of the DV axis via another round of signaling between oocyte and follicle cells (Neuman-Silberberg and Schupbach, 1993; Neuman-Silberberg and Schupbach, 1996; Roth et al., 1995; Schupbach, 1987).
The ability of the PFCs to signal to the oocyte requires proper regulation of cell proliferation and differentiation pathways (Poulton and Deng, 2007). At around stage 6, Delta ligand produced from the germline activates its receptor, Notch, in all follicle cells (Lopez-Schier and St Johnston, 2001; Ruohola et al., 1991). The activation of Notch signaling induces a switch from the mitotic cycle to the endoreplication cycle (endocycle) and promotes a transition in the gene expression pattern of the follicle cells (Deng et al., 2001; Lopez-Schier and St Johnston, 2001). In addition, the JAK (Hop – FlyBase)/STAT (Stat92E – FlyBase) pathway is activated by secretion of the ligand Unpaired (Upd; Os – FlyBase) from the polar cells, two pairs of follicle cells at the anterior and posterior poles of each chamber, resulting in the formation of two equivalent cell groups at both termini of the egg chamber (Xi et al., 2003). The anterior group develops subsequently into border cells, stretched cells and centripetal cells, whereas the posterior group receives the additional Grk-Egfr signal and adopts the PFC fate (Gonzalez-Reyes and St Johnston, 1998). Finally, the Salvador-Warts-Hippo pathway was recently shown to specifically control PFC maturation through interaction with the Notch pathway (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). Therefore, the specification of the PFCs requires the coordination of various crucial signaling events. Loss of any one of these signals leads to mis-specification of the PFCs and an absence of the posterior polarizing signal.
The nature of the polarizing signal from the PFCs and the cellular machinery directly involved in the generation and transduction of the polarizing cue still remains an open question in Drosophila polarity establishment. Here, we report a direct role of myosin activity in this process. In a genetic mosaic screen in follicle cells, we identified a lethal allele of flapwing (flw), which encodes the beta isoform of the catalytic subunit of Protein Serine/Threonine Phosphatase 1β (PP1β). We show that PP1β is required in the PFCs for axis specification in the oocyte through its function of regulating the activity of non-muscle myosin II. Strikingly, PP1β acts specifically in the PFCs to regulate endocytosis of Notch and other transmembrane proteins and, in addition, it operates as a separate and direct factor in generating the posterior polarizing signal to the oocyte. Furthermore, our results demonstrate that the particular sensitivity of the PFCs to defective Notch signaling is not caused merely by their position at the termini of the egg chamber, but results from the coordinated activation of both the JAK/STAT and the Egfr pathways. Our study establishes a novel connection between the mechanical force-generating machinery and the crucial signaling pathways controlling cell differentiation and organism development.
MATERIALS AND METHODS
Fly stocks and genetics
The flwFP41 mutation was isolated in a mosaic genetic screen in which mutations were induced by ethylmethanesulfonate (EMS) mutagenesis in y w FRT19A flies (Denef et al., 2008). Duplications and P-element lines used for mapping were obtained from the Bloomington Stock Center. Follicle cell clones were generated by e22c>Flp using the FRT/UAS-Flp/GAL4 system (Duffy et al., 1998). Eye disc clones were generated using FRT/eyFlp. Wing disc clones were generated using FRT/hsFlp. Flip-out clones were generated using y w Act<FRT yellow+ FRT>Gal4; hsFlp; UAS-GFP (Ito et al., 1997). UAS-Notch (Struhl et al., 1993), UAS-NEXT and UAS-NICD (Rebay et al., 1993) were expressed in follicle cell clones generated by the mosaic analysis with a repressible cell marker (MARCM) system (Lee and Luo, 1999). The Mbs alleles MbsT541, MbsT666 and MbsT791 were gifts from J. E. Treisman (Lee and Treisman, 2004).
The following reporter lines were used: kinesin-lacZ (Clark et al., 1994), pointed-lacZ (Gonzalez-Reyes and St Johnston, 1998), mirror-lacZ (Xi et al., 2003), expanded-lacZ (Hamaratoglu et al., 2006), cyclinE-lacZ (Jones et al., 2000) and diap1-lacZ (Hay et al., 1995). The following lines were obtained from the Bloomington Stock Center: flapwing-HA, zip1, Rhoe3.10, RhoGEF204291, UAS-Rok-CAT, UAS-Rok-CAT-KG, UAS-MYPT75D and UAS-MYPT75DF117A. Rab5-GFP (Wucherpfennig et al., 2003), Rab7-GFP (Entchev et al., 2000) and Rab11-GFP (Emery et al., 2005) were used to mark endocytic compartments. UAS-Unpaired (Harrison et al., 1998) and UAS-λtorpedo (Queenan et al., 1997) were recombined to generate UAS-Unpaired, UAS-λtorpedo.
Mapping of FP41
The lethal mutation FP41 was mapped by meiotic recombination with visible recessive markers to the region between cut (7B4) and vermilion (9F11) and between P-elements PBac(WH)CG34408f03664 (9B7) and PBac(WH)Neb-cGPf02352 (9C4). The genomic DNA from single FP41/Y larvae was isolated. PCR products covering the flapwing gene region were sequenced and compared with the sequences of the y w FRT19A control. Sequencing was repeated with three independent genomic isolates.
Immunostaining
Ovaries, eye discs and wing discs were dissected in PBS followed by fixation in PBS with 4% paraformaldehyde for 20 minutes at room temperature and staining using standard procedures (Denef et al., 2008). Samples were mounted in Aqua-polymount (Polysciences) and imaged using a Zeiss LSM510 microscope.
Primary antibodies used were: mouse anti-Grk (1:10), rabbit anti-Stau [1:2000 (St Johnston et al., 1991)], rabbit anti-beta-galactosidase (1:1000, Millipore), rabbit anti-phospho-Histone H3 (Ser28) (1:500, Millipore), mouse anti-Cut [2B10, 1:20, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Hindsight (1G9, 1:20, DSHB), rabbit anti-phospho-myosin light chain II (Ser19) [1:10, Cell Signaling; Ser19 in human RMLC corresponds to Ser21 in Drosophila (Jordan and Karess, 1997)], rabbit anti-Zip [1:1000 (Foe et al., 2000)], mouse anti-NICD (C17.9C6, 1:100, DSHB), rat anti-NECD [1:50 (Klueg et al., 1998)], rabbit anti-Lava [1:250 (Sisson et al., 2000)], goat anti-Egfr (dc-20, 1:100, Santa Cruz), rabbit anti-Dome [1:200 (Devergne et al., 2007)], rabbit anti-aPKC (1:1000, Santa Cruz), rabbit anti-Baz [1:500 (Wodarz et al., 1999)], rabbit anti-Patj [1:500, (Bhat et al., 1999)], mouse anti-Arm (N2 7A1, 1:50, DSHB), rat anti-DE-Cad (DCAD2, 1:20, DSHB) and mouse anti-Dlg [1:500 (Woods and Bryant, 1991)]. Secondary antibodies used were Alexa Fluor 488-, 568- or 647-conjugated (1:1000, Invitrogen). F-actin and DNA were stained with Phalloidin conjugates and Hoechst (1:1000, Invitrogen), respectively.
RESULTS
Oocyte polarity is disrupted when FP41 mutant follicle cells are present at the posterior of the egg chamber
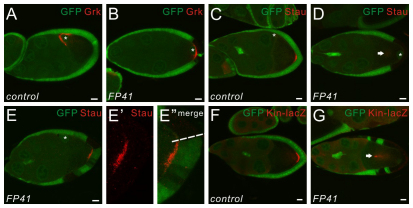
In a genetic mosaic screen performed to identify genes regulating the morphogenesis of follicle cells (Denef et al., 2008), a lethal mutation named FP41 was isolated in which oocyte mispolarization was frequently observed in the presence of posterior mutant follicle cell clones. In wild-type egg chambers after stage 7, the oocyte nucleus and Gurken (Grk) RNA and protein relocalize from the posterior pole to a dorsal-anterior corner of the oocyte in response to the polarizing signal from the PFCs (Fig. 1A). When the PFCs are homozygous mutant for FP41 (as identified by the absence of GFP), the oocyte nucleus and Grk often failed to relocalize and remained at the posterior pole of the oocyte (Fig. 1B; 50%, n=105). To characterize further the oocyte polarity defect in FP41, we examined the position of Staufen (Stau), an RNA-binding protein required for the localization of oskar (osk) mRNA to the posterior pole of the oocyte (St Johnston et al., 1991). In stage 9 wild-type egg chambers, Stau is localized as a posterior crescent in the oocyte (Fig. 1C). When all PFCs were mutant for FP41, Stau was dispersed or mislocalized at the center of the oocyte (Fig. 1D; data not shown; 76%, n=153). Interestingly, when only a portion of the PFCs were mutant for FP41, Stau was localized as a crescent precisely adjacent to the wild-type PFCs, but was missing in the region underneath the mutant cells (Fig. 1E-E″), suggesting a very local effect of FP41 in disrupting oocyte polarity.
Fig. 1.
Oocyte polarity is disrupted in the presence of FP41 mutant PFCs. (A,B) Drosophila stage 9 egg chambers stained for Grk (red). The oocyte nucleus is marked by the white asterisks. Wild-type follicle cells express GFP (green); FP41 mutant cells are marked by the absence of GFP. In wild-type egg chambers, the oocyte nucleus and Grk protein are localized to the dorsal anterior corner of the oocyte at stage 9 (A). With the presence of FP41 mutant clones at the posterior, the oocyte nucleus and Grk are mislocalized at the posterior of the oocyte (B). Note that in this egg chamber the polar cells are wild type, as marked by GFP expression. (C-E″) Stage 9 egg chambers stained for Stau (red). The oocyte nucleus is marked by the white asterisks. In wild-type egg chambers, Stau protein is localized in a crescent at the posterior pole of the oocyte at stage 9 (C). When all the PFCs are mutant for FP41, Stau (arrow) is dispersed in the middle of the oocyte (D). When a portion of the PFCs are mutant for FP41, Stau is localized as a crescent in the region of the oocyte precisely underneath the wild-type PFCs (E). E′ and E″ are higher magnifications of E with a focus on the PFCs. The boundary between the mutant and the wild-type clones is marked by a dashed line. (F,G) Stage 9 egg chambers expressing the microtubule (MT) plus-end marker kin-lacZ and stained for β-galactosidase (red). In wild-type egg chambers, β-galactosidase is localized in a crescent at the posterior pole of the oocyte at stage 9 (F). With the PFCs mutant for FP41, β-galactosidase (arrow) is mislocalized in the middle of the oocyte, showing that the oocyte MT cytoskeleton is mispolarized (G). Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
We also examined the position of Kinesin-β-galactosidase (Kin-βgal) (Clark et al., 1994), an MT plus-end marker, which is normally localized at the posterior pole of the oocyte at stage 9 of oogenesis (Fig. 1F). Similar to observations of Stau, Kin-βgal accumulated at the center of the oocyte or in a diffuse pattern when all PFCs were mutant for FP41 (Fig. 1G; data not shown), and was missing from the region in the oocyte beneath the clones when only some of PFCs were mutant for FP41 (data not shown). Therefore, the MT polarity in the oocyte was clearly perturbed by the presence of FP41 mutant PFCs.
Notably, in egg chambers with wild-type PFCs but anterior or lateral FP41 mutant clones, the oocyte polarity was unaffected (data not shown). In addition, polar cells were not sufficient for oocyte polarization, because the oocyte was still mispolarized in cases where mutant PFCs surrounded wild-type polar cells (Fig. 1B). Taken together, our data demonstrate that the gene product disrupted by FP41 is required specifically in the PFCs to generate the polarizing signal for MT-dependent axis determination of the oocyte.
Proliferation and differentiation of the posterior follicle cells are affected by FP41
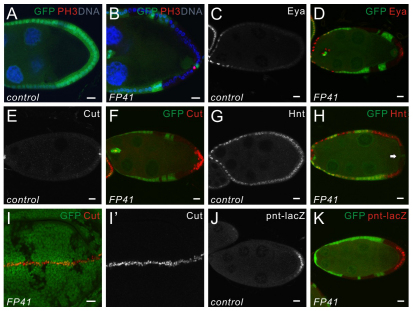
The ability of PFCs to repolarize the oocyte requires the correct specification of PFC fate as well as the production of the polarizing signal induced by Grk/Egfr signaling. When examining the morphology of FP41 mutant follicle cells, we found that although most mutant cells formed a monolayered epithelium as observed in wild type (Fig. 2A), in some clones smaller nuclei and, occasionally, multiple cell layers were present at the posterior (Fig. 2B,F). Such an overproliferation phenotype is very rare in mutant clones at the anterior and is never observed in clones at the lateral site of the epithelium. This might imply a defect in the mitotic-to-endocycle switch in FP41 mutant PFCs, which normally occurs at stage 6 (Deng et al., 2001). As a consequence, the mitotic marker Phosphorylated Histone H3 (PH3; His3 – FlyBase) cannot be observed after stage 6 in wild-type follicle cells (Fig. 2A). By contrast, PH3-positive cells were still occasionally detected in the posterior FP41 mutant clones after that stage (Fig. 2B), indicating a PFC-specific overproliferation defect.
Fig. 2.
The FP41 mutation disrupts cell differentiation and Notch signaling in PFCs. (A-H) Stage 9 egg chambers stained for PH3 (A,B), Eya (C,D), Cut (E,F) or Hnt (G,H). FP41 mutant follicle cell clones are marked by the absence of GFP (green). (A,B) In wild-type egg chambers (A), the follicle cells stop dividing and switch from mitotic cycles to endocycles upon activation of Notch signaling. No mitotic cells labeled by PH3 (red) can be observed after stage 6. PH3-postive cells were still detected in FP41 mutant PFCs at stage 9 (B), indicating a failure of these cells to switch from mitosis into endocycle. Cell nuclei are stained with Hoechst (blue). (C,D) Eya expression is restricted to the anterior follicle cells at stage 9 in wild-type egg chambers (C). Eya (red) is still expressed in FP41 mutant PFCs (D), showing that cell differentiation is affected. (E,F) In wild-type egg chambers (E), Cut is normally downregulated in follicle cells at stage 7 by Notch signaling and only expressed in polar cells afterwards. In egg chambers containing FP41 mutant follicle cells at stage 9 (F), Cut (red) is still expressed but is restricted to the posterior mutant clones. (G,H) In wild type (G), Hnt expression is induced in all the follicle cells when Notch signaling is activated at stage 7. Hnt (red) fails to be upregulated exclusively in FP41 mutant PFCs (H, arrow) whereas other mutant follicle cells express Hnt normally. (I,I′) A wing disc containing FP41 mutant clones marked by the absence of GFP (green) and stained for Cut (red). The expression pattern of Cut is not affected in FP41 mutant cells in wing discs. (J,K) Stage 9 egg chambers expressing the posterior cell fate marker pnt-lacZ, and stained for β-galactosidase (red). Upon activation of the Egfr pathway, β-galactosidase staining reveals that pnt-lacZ is expressed in the induced PFCs in wild-type controls (J). FP41 mutant PFCs, marked by the absence of GFP (green), are able to express pnt-lacZ and respond to Egfr signaling (K). Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
The failure of FP41 mutant PFCs to undergo the mitotic-to-endocycle switch suggests disrupted Notch signaling, which normally promotes this switch as well as a change in the transcriptional program of all follicle cells (FCs) (Deng et al., 2001; Lopez-Schier and St Johnston, 2001; Ruohola et al., 1991). We found that a marker of less mature follicle cells, eyes absent (eya), which is normally downregulated in the posterior upon the mitotic-to-endocycle switch (Fig. 2C), was still present at a high level in FP41 mutant PFCs (Fig. 2D). The homeodomain protein Cut is downregulated by Notch at stage 6 in all the follicle cells except for the two pairs of polar cells (Fig. 2E) (Sun and Deng, 2005). However, Cut was still expressed in FP41 mutant PFCs after stage 6 (Fig. 2F). In wild-type egg chambers, the role of Notch signaling in repressing Cut expression and cell proliferation is mediated by the zinc-finger transcription factor Hindsight (Hnt; Peb – FlyBase), which is upregulated by Notch at stage 6 in the entire follicular epithelium except for the polar cells and the stalk cells (Fig. 2G) (Sun and Deng, 2007). We observed that Hnt upregulation did not occur in FP41 mutant PFCs, suggesting that Notch signaling was indeed disrupted (Fig. 2H). However, we did not observe defective Notch signaling in FP41 clones in wing or eye imaginal discs as assayed by Cut expression (Fig. 2I,I′; data not shown). Importantly, in the follicle cells, we found that the misexpression of Cut and Hnt was completely restricted to the PFCs but did not occur in lateral or anterior follicle cell clones. This is different from mutant clones involving components in the Notch pathway, where defects are observed in all follicle cells (Sun and Deng, 2005; Sun and Deng, 2007; Yan et al., 2009).
We subsequently investigated whether regulatory pathways other than Notch are affected by the loss of FP41 from PFCs. At stage 6/7, Grk signals from the oocyte posterior to the adjacent follicle cells via Egfr and the Ras/MAPK signaling pathway resulting in the expression of the target reporter pointed-lacZ (pnt-lacZ) in the PFCs (Fig. 2J). This was still the case in FP41 mutant PFCs (Fig. 2K). In addition, another downstream target reporter of Egfr signaling, kekkon-lacZ (kek-lacZ), could also be properly induced in FP41 mutant PFCs even in cases where the oocyte nucleus was mislocalized at the posterior (see Fig. S1B in the supplementary material). These results strongly suggest that the PFCs are competent to respond to Egfr signaling and to adopt the posterior cell fate. Similarly, the expression pattern of the JAK/STAT reporter, mirror-lacZ (mirr-lacZ), was unaffected in FP41 mutant follicle cells (see Fig. S1C-D′ in the supplementary material). Finally, the Hippo pathway also appeared to function normally in the mutant cells, as shown by the proper expression of the Hippo reporters, expanded-lacZ (ex-lacZ), cyclin E-lacZ (CycE-lacZ) and diap1-lacZ (see Fig. S1E-F′ in the supplementary material and data not shown). Together, these data indicate that signaling downstream of Egfr, JAK/STAT and Hippo occurs normally in FP41 mutant follicle cells.
In conclusion, FP41 mutant PFCs are defective specifically in Notch signaling. The defects are restricted to the PFCs, which are nevertheless still responsive to Egfr, JAK/STAT and Hippo signaling.
FP41 is a loss-of-function mutation of PP1β
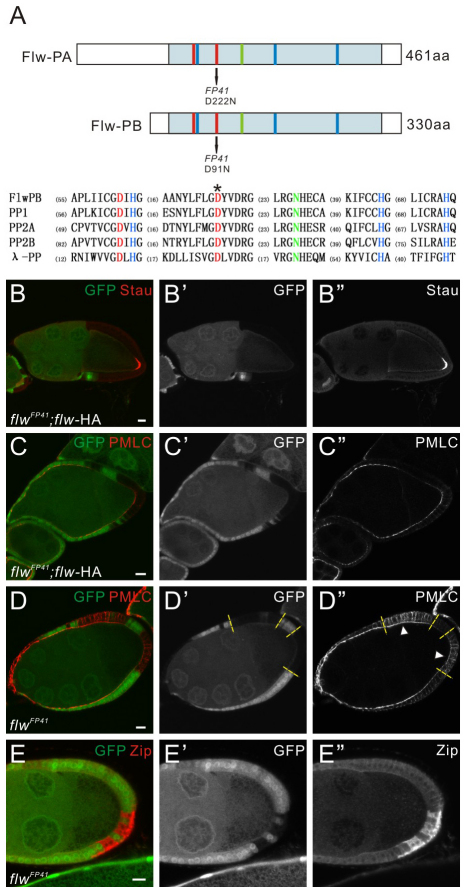
We mapped the lethal mutation of FP41 to the genomic region between 9B7 and 9C4 using P-elements. Sequencing the candidate genes in this region identified a point mutation in the coding region of the gene flapwing (flw), which encodes the catalytic subunit of the beta isoform of type 1 serine/threonine protein phosphatase (PP1β9C) (Raghavan et al., 2000).
PP1β belongs to a highly conserved family of serine/threonine phosphatases (Fig. 3A) (Shi, 2009). The gene flw (PP1β9C) encodes two isoforms of the catalytic subunit of Drosophila PP1β: flw-PA (461 aa) and flw-PB (330 aa) (Fig. 3A). Both isoforms contain the same catalytic domain, but flw-PA has a longer N terminus than flw-PB owing to alternative splicing of an extra exon. FP41 is a mis-sense mutation changing one of the six highly conserved residues in the catalytic domain, (D222N in flw-PA and D91N in flw-PB) (Fig. 3A). Earlier biochemical studies on λ phosphatase, a homolog of PP1β, showed that substitution of the corresponding Asp residue to Asn leads to a dramatic decrease in metal binding and consequent reduction of the catalytic activity (Zhuo et al., 1994), predicting that the FP41 mutation should be a strong allele of PP1β. Consistent with this prediction, we observed that FP41 homozygotes died in early larval stages, in contrast to previously described semiviable flw alleles. Confirming that the phenotypes in FP41 are caused by loss of PP1β function, an HA-tagged Flw cDNA transgene (flw-HA) expressed in FP41 mutant follicle cells using the GAL4-UAS system was able to rescue the oocyte polarity defect (Fig. 3B, Table 1).
Fig. 3.
The defects in FP41 are caused by a loss of function mutation of PP1β. (A) Schematic representation of the protein products encoded by flw (PP1β9C) and an alignment of the catalytic domains of members of the Serine/Threonine phosphatase family, including Drosophila PP1β, human PP1, PP2A, PP2B and bacteriophage phage λ protein phosphatase (λ-PP). flw encodes two isoforms of the catalytic subunit of PP1β. The catalytic domain is represented by the pale blue box in each protein. The highly conserved amino acid residues directly involved in metal binding and/or the catalytic activity are denoted by colors corresponding to those in the sequence alignment. The FP41 mutation causes a change of a conserved Asp to Asn in the catalytic domain, indicated by an asterisk in the alignment. The accession numbers for the depicted proteins are as follows: Drosophila Flw-PA, NP_727418; Drosophila Flw-PB, NP_524738; human PP1α, P62136; human PP2Aα, P67775; human PP2Bα, Q08209; λ-PP, P03772. (B-C″) Egg chambers containing flwFP41 mutant clones, expressing a flw-HA transgene, and stained for Stau (B, red) or phosphorylated myosin light chain (PMLC; C, red). Stau is correctly localized in the oocyte with the presence of posterior flwFP41 mutant clones, and the pattern of PMLC is restored in the mutant follicle cells as in the wild-type cells (compare with D below). (D-E″) Egg chambers containing flwFP41 mutant clones marked by the absence of GFP (green) and stained for PMLC (D, red) or Zip (E, red). (D-D″) Phosphorylated myosin II light chain is enriched in flwFP41 mutant follicle cells. The boundaries between the mutant and the wild-type clones are marked by dashed lines. (E-E″) The level of myosin II heavy chain encoded by zip is also elevated in flwFP41 mutant follicle cells. Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
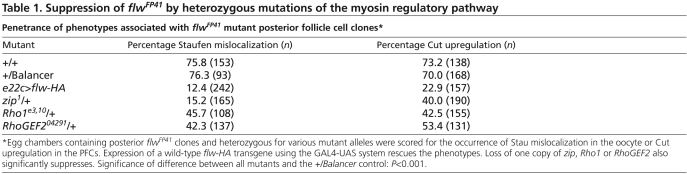
Table 1.
Suppression of flwFP41 by heterozygous mutations of the myosin regulatory pathway
The essential role of PP1β in Drosophila is to dephosphorylate and inactivate the non-muscle myosin II light chain (MLC) encoded by spaghetti squash (sqh) (Vereshchagina et al., 2004). Using an antibody recognizing the phosphorylated form of Sqh, we observed increased levels of phosphorylated myosin light chain (PMLC) in flwFP41 mutant follicle cells (Fig. 3D). We also found the levels of the myosin II heavy chain, Zipper (Zip), to be elevated in the mutant cells (Fig. 3E). Consistent with other weaker alleles of flw (Vereshchagina et al., 2004), these phenotypes were observed in mutant clones generated throughout the follicular epithelium and were not restricted to PFC clones. Expression of the flw-HA transgene restored the normal level of active myosin in the mutant clones (Fig. 3C). Together these results show that the ovarian defects in FP41 are indeed caused by a loss of PP1β function.
Oocyte polarity is disrupted by myosin hyperactivation in flwFP41 mutant PFCs
PP1, a crucial regulator of a wide range of cellular processes, consists of a catalytic subunit and a regulatory subunit that targets its cellular function to specific substrates (Shi, 2009). Drosophila has two isoforms of the catalytic subunit of PP1, α and β (PP1β9C). PP1β9C, in association with its myosin phosphatase targeting subunit MYPT, dephosphorylates MLC (Sqh), inactivating myosin (Vereshchagina et al., 2004). By contrast, Rho associated-kinase (Rok) phosphorylates both MYPT and MLC (Sqh), inhibiting the function of PP1β and activating myosin (Amano et al., 1996; Bresnick, 1999; Kimura et al., 1996; Winter et al., 2001).
To test whether the oocyte polarity defect associated with flwFP41 mutant PFCs results directly from loss of the inhibitive function of PP1β in the myosin regulatory pathway, we tested whether reducing the amount of the activating regulators would decrease myosin activity, antagonize the effect of the loss of PP1β function and hence suppress the flwFP41 phenotypes. Using Stau localization as a readout of oocyte polarity, we found that reducing the amount of myosin heavy chain protein by heterozygosity for a zip loss-of-function allele did indeed restore normal Stau localization in the oocyte in 85% (n=165) of the egg chambers with flwFP41 mutant PFCs (Table 1). Similarly, heterozygosity for mutations in the gene Rho1 (rho – FlyBase), which activates Rok to phosphorylate myosin, and in the gene RhoGEF2, which facilitates the function of Rho1, also suppressed the oocyte mispolarization caused by PFCs mutant for flwFP41, with proper Stau localization in 54% (n=108) and 58% (n=137) of the egg chambers in these backgrounds, respectively.
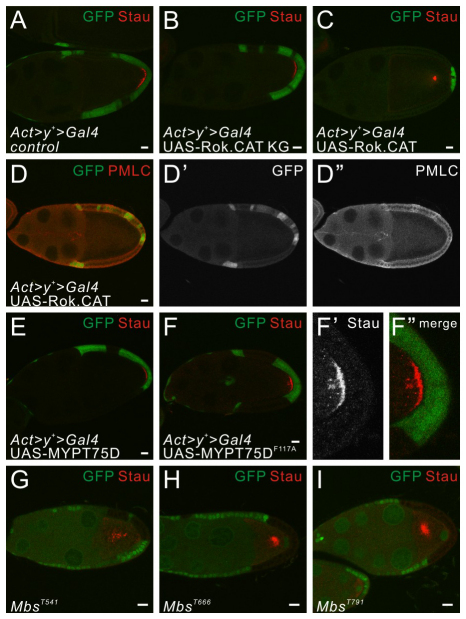
Conversely, if myosin regulation is indeed crucial for generation of the polarizing signaling from the PFCs, enhancing the activity of the activating components of the pathway, or blocking the catalytic capability of PP1β in wild-type PFCs, both of which result in myosin hyperactivation, should produce an oocyte polarity defect similar to that observed in flwFP41. Indeed, when a constitutively active form of Rok, Rok.CAT (Winter et al., 2001), was expressed in the wild-type PFC by the flip-out technique (Ito et al., 1997), we observed elevated levels of MLC phosphorylation and moderate disruption of oocyte polarity. This was not observed when expressing a kinase-dead form of Rok, Rok.CAT KG (Winter et al., 2001) (Fig. 4A-D″; data not shown). Furthermore, there are two MYPTs in Drosophila that mediate the function of PP1β in regulating myosin: Drosophila Myosin binding subunit (Mbs), which binds to both PP1α and PP1β, and MYPT-75D, the PP1β-specific myosin regulatory subunit (Vereshchagina et al., 2004). We tested three nonsense mutants of Mbs (Lee and Treisman, 2004) and observed mislocalization of Stau protein and the oocyte nucleus with the presence of posterior Mbs mutant clones (Fig. 4G-I). Moreover, expression of a mutant form of the PP1β-specific subunit MYPT-75DF117A (Vereshchagina et al., 2004) that fails to bind to PP1β causes mild oocyte polarity defects represented by a partial mislocalization of Stau (Fig. 4E-F″).
Fig. 4.
Excessive myosin activity in the PFCs causes the oocyte polarity defect. (A-D″) Egg chambers containing flip-out follicle cell clones positively marked by GFP (green) and stained for Stau (red), with the expression of a kinase-dead form of Rok, Rok.CAT KG (B) or a constitutively active Rho kinase, Rok.CAT (C-D″) in the clones. With the posterior flip-out clones alone (A), or with the posterior clones expressing Rok.CAT KG (B), the oocyte polarity manifested by Stau localization is normal. By contrast, with the posterior clones expressing active Rok.CAT (C), Stau is mislocalized in the middle of the oocyte. The level of PMLC (red) is elevated in the clones expressing Rok.CAT (D-D″), showing excessive myosin activity. (E-F″) Egg chambers containing flip-out follicle cell clones positively marked by GFP (green) and stained for Stau (red), with the expression of a wild-type construct of the PP1β-specific myosin targeting subunit MYPT-75D (E), or a mutant form MYPT-75DF117A (F-F″) in the clones. With the posterior clones expressing wild-type MYPT-75D, Stau is correctly localized, whereas the posterior clones expressing MYPT-75DF117A result in a partial mislocalization of Stau. (G-I) Egg chambers containing follicle cells clones mutant for MbsT541 (G), MbsT666 (H) and MbsT791 (I) marked by the absence of GFP (green) and stained for Stau (red). Stau is mislocalized in the middle of the oocyte when the PFCs lose the function of the general myosin targeting subunit MBS. Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
Compared with flwFP41, the oocyte polarity defects generated by Rok.CAT and MYPT-75DF117A are weaker in terms of both the penetrance and the expressivity of the phenotype. As for Rok, it is possible that the overexpressed, constitutively active Rok cannot fully antagonize wild-type PP1β. Similarly, the mutant form of MYPT-75D might only mildly disrupt myosin dephosphorylation, owing to the presence of wild-type protein and Mbs, another myosin targeting subunit. Interestingly, in these genetic backgrounds, in spite of the oocyte polarity defects, we did not observe a significant Cut or Hnt misregulation in the PFCs expressing these constructs (data not shown). There are two possibilities that could account for this result. PP1β might regulate Notch signaling in the follicle cells by a different pathway and not through its effects on myosin activity. Alternatively, constitutively active Rok or mutant MYPT-75D might not disrupt the myosin organization severely enough to produce an obvious Notch phenotype. To distinguish between these two scenarios, we measured the occurrence of Cut upregulation in flwFP41 mutant PFCs in combination with heterozygous mutations of zip, Rho1 and RhoGEF2. We found a significant reduction of penetrance from ~70% in flwFP41 to 40-50% after loss of a single copy of the factors that activate myosin (Table 1). Therefore, the presence of specific mutations in the myosin pathway is able to suppress Cut upregulation caused by loss of PP1β function, suggesting that PP1β interacts with the Notch pathway at least partially through its function in regulating myosin activity. In addition, our results also suggest that the oocyte polarity defect can be produced in the absence of defective Notch signaling by mutations that affect myosin activation in the PFCs.
In summary, these data demonstrate that correct regulation of myosin activity is crucial for the production of the polarizing signal in the PFCs. In addition, increasing myosin phosphorylation can result in oocyte mispolarization without overtly disrupting the Notch pathway.
The Notch intracellular domain (NICD) can rescue the Notch signaling phenotype, but not the oocyte polarity phenotype of flwFP41 mutant PFCs
Our results left us with the open question of whether the oocyte polarity phenotype observed in flwFP41 is simply a secondary consequence of disrupted Notch signaling or whether it is also a direct result of myosin hyperactivation. In addition, we wanted to determine how the regulation of myosin activity affects Notch signaling. Therefore, we analyzed whether overexpression of various Notch constructs in flwFP41 mutant PFCs using the MARCM system (Lee and Luo, 1999) would be able to rescue the Notch and the oocyte polarity defects. In wild-type egg chambers, in response to Delta binding Notch undergoes a set of sequential cleavages, in particular an extracellular (S2) cleavage by ADAM-family metalloproteases and an intramembranous (S3) cleavage by γ-secretase to produce the membrane-bound NEXT (Notch extracellular truncation) and the intracellular domain NICD, respectively (Fortini, 2009). NICD is then released and translocates into the nucleus to regulate the transcription of downstream genes (Rebay et al., 1993; Struhl et al., 1993). When posterior flwFP41 mutant clones were generated by the MARCM system, as expected we observed Cut upregulation in 74% of the mutant PFCs (n=69) (Fig. 5A). Cut upregulation was also observed in 77% of posterior flwFP41 clones overexpressing full-length Notch (n=71) and in 78% overexpressing NEXT (n=59). By contrast, only 19% of clones with NICD overexpression showed upregulation of Cut (n=57) (Fig. 5B-D), suggesting that only NICD, and not the full-length or the S2 cleavage product NEXT, was able to restore normal Notch signaling. However, none of these constructs fully rescued the oocyte polarity defect. We observed Stau mislocalization in 74% of the cases with posterior flwFP41 MARCM clones (n=84), 70% with full-length Notch co-expressed (n=92), 74% with NEXT (n=96) and 53% with NICD (n=99). Whereas Cut was properly downregulated with NICD expressed in the PFCs, Stau was still observed to be mislocalized at the center of the oocyte (Fig. 5E,E′). The oocyte polarity phenotype was not caused by NICD on its own, because Stau mislocalization was not observed when NICD was overexpressed in posterior MARCM clones in a wild-type background (n=21, data not shown). Therefore, although NICD expression can mildly suppress the oocyte polarity phenotype, it is not as effective as its rescue of the transcriptional output of Notch signaling. Considered together with the effects of constitutively active Rok and mutant MYTP-75D, our results strongly suggest that the observed oocyte polarity defect in flwFP41 is not merely a side effect of Notch disruption, but that PP1β might additionally play an independent role in the generation of the polarizing signal through its regulation of myosin activity.
Fig. 5.
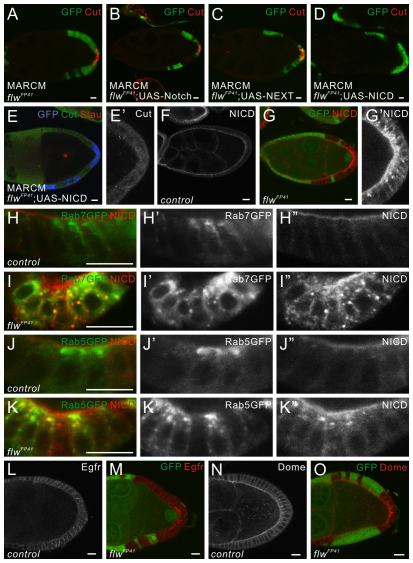
Notch protein and other transmembrane proteins accumulate in intracellular compartments in flwFP41 mutant PFCs. (A-D) Egg chambers containing flwFP41 mutant clones positively marked by GFP (green) and stained for Cut (red), with the expression of full-length Notch (B), NEXT (C) or NICD (D) in the clones. In flwFP41 clones alone, or in flwFP41 clones expressing full-length Notch or NEXT, Cut is upregulated in the mutant PFCs. Expression of NICD in posterior flwFP41 mutant clones restores the normal regulation of Cut. (E,E′) Egg chambers containing flwFP41 mutant clones positively marked by GFP (blue) and double stained for Cut (green) and Stau (red), with the expression of NICD in the clones. Stau is still mislocalized when Cut upregulation is rescued by the expression of NICD in the PFCs. Cut in the polar cells is not visible here because it is in a different optical section from that of Stau. (F-G′) Egg chambers containing flwFP41 mutant clones marked by the absence of GFP (green) and stained for NICD (red). In wild-type egg chambers, NICD is localized on the apical membrane of the follicle cells (F). flwFP41 mutant PFCs contain accumulation of NICD both on the apical surface and in cytoplasmic punctae (G-G'). (H-K″) Wild-type PFCs or flwFP41 mutant PFCs marked by the absence of GFP, expressing Rab7-GFP (H,I) or Rab5-GFP (J,K) and stained for NICD (red). Notch accumulates in intracellular compartments marked by Rab7-GFP and Rab5-GFP in flwFP41 mutant PFCs (I,K) but not in the wild-type PFCs (H,J). Here, egg chambers are oriented with the posterior side to the bottom, and the apical side of the PFCs is towards the top of the images. (L-O) Egg chambers containing flwFP41 mutant follicle cell clones marked by the absence of GFP (green) and stained for Egfr (L,M, red) or Dome (N,O, red). The transmembrane proteins Egfr and Dome accumulate in intracellular punctae in flwFP41 mutant PFCs. Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right unless otherwise stated.
The Notch receptor and other transmembrane proteins accumulate in flwFP41 mutant PFCs
Although the Notch protein is downregulated after stage 7 in wild-type PFCs (Fig. 5F), we observed ectopic accumulation of Notch both on the apical surface and in cytoplasmic punctae of flwFP41 mutant PFCs, but not in anterior or lateral clones (Fig. 5G,G′). Using appropriate antibodies we found that both the intracellular and the extracellular domain of Notch are present in these punctae (data not shown). Consistent with the restriction of the Notch defect to the PFCs, we did not observe a similar Notch accumulation in flwFP41 mutant cells in eye discs (data not shown).
When we analyzed the colocalization of the ectopic Notch with various endocytic markers, we observed no significant overlap between the Notch punctae and the Golgi marker Lava Lamp (Lva) (Sisson et al., 2000) or the recycling endosomal marker Rab11-GFP (Emery et al., 2005) (data not shown). However, we observed colocalization of Notch in flwFP41 mutant PFCs with the late endosomal marker Rab7-GFP (Fig. 5I-I″) and the early endosomal marker Rab5-GFP (Wucherpfennig et al., 2003) (Fig. 5K-K″), but not in the wild-type cells (Fig. 5H,J), suggesting that defective Notch endocytosis accounts for the observed ectopic accumulation of Notch protein in the mutant PFCs.
In addition, we observed a similar accumulation of other transmembrane proteins such as Egfr and Domeless (Dome) as cytoplasmic punctae in the PFCs (Fig. 5L-O), indicating that loss of PP1β function does not specifically disrupt Notch protein localization but rather causes a general problem of membrane protein trafficking.
PP1β regulates the membrane levels of apical complexes in the PFCs
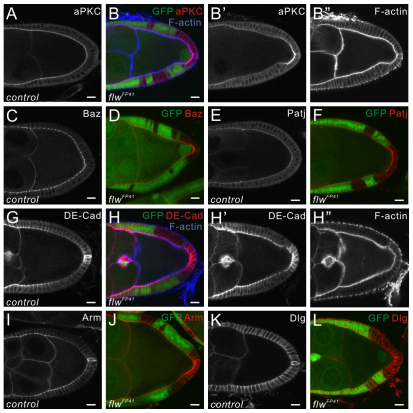
To probe further the role of PP1β in regulating the polarizing signal from the PFCs, we examined the apical-basal polarity of the flwFP41 mutant cells. By analyzing the distribution of major components of epithelial polarity complexes, we found that the overall apical-basal polarity of the flwFP41 mutant cells is normal (Fig. 6). However, we detected significantly increased levels of the apical complexes, such as Atypical Protein Kinase C (aPKC), Bazooka (Baz) and Patj on the apical membrane of the flwFP41 mutant PFCs (Fig. 6A-F). The levels of adherens junction components DE-cadherin (DE-cad; Shg – FlyBase) and Armadillo (Arm) were also elevated at the apical apex of the mutant PFCs (Fig. 6G-J). Notably, these increases were only observed in the posterior mutant clones. However, we found that the level of cortical F-actin in the mutant PFCs, which is also present at the apical membrane, remained the same as observed in the neighboring wild-type cells (Fig. 6B″,H″), suggesting that the concentrated apical markers in the mutant PFCs were not simply due to apical cortex contraction caused by excessive myosin activity. By contrast, the basolateral component Discs Large (Dlg) maintained its lateral distribution and protein level as in the wild-type cells (Fig. 6K,L). Therefore, our results suggest that PP1β regulates the membrane levels of the apical proteins in the PFCs. As this phenotype is not observed in mutants of the Notch pathway, it might account for one aspect of the Notch-independent function of PP1β in the regulation of the posterior polarizing signal.
Fig. 6.
PP1β regulates the membrane levels of apical complexes in the PFCs. (A-L) Egg chambers stained for aPKC (A,B), Baz (C,D), Patj (E,F), DE-Cad (G,H), Arm (I,J), Dlg (K,L) or F-actin (B,H). flwFP41 mutant PFCs, marked by the absence of GFP (green), contain increased membrane levels of the apical proteins aPKC, Baz, Patj and the adherens junction components Arm and DE-Cad, whereas the level of apical F-actin stained by Phalloidin in the mutant PFCs remains the same as in the neighboring wild-type cells. The distribution and protein levels of the basolateral protein Dlg are indistinguishable from wild type. Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
Integration of multiple signaling pathways renders PFCs sensitive to myosin misregulation
A striking feature of the ovarian phenotypes of flwFP41 is that the Notch defects represented by target gene misregulation and ectopic accumulation of Notch protein can only be detected in the PFCs. To determine what renders the PFC especially sensitive to myosin hyperactivation, we attempted to transform the anterior and the lateral follicle cells into PFCs and to test whether such a cell fate transformation would be able to generate the PFC restricted phenotypes in other locations in the follicular epithelium.
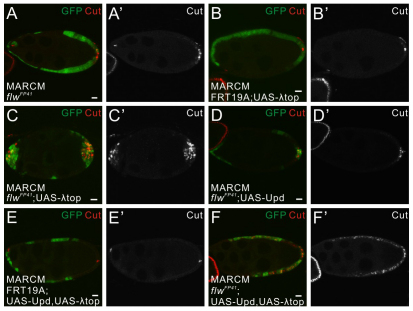
We expressed a constitutively active form of Egfr, λ-torpedo (λ-top) (Queenan et al., 1997), in clones of flwFP41 using the MARCM system, and observed a significant overproliferation and Cut upregulation in the transformed cells at the anterior but not in the lateral clones (Fig. 7C,C′). Expression of λ-top also elevated the level of cell overproliferation in the mutant PFCs compared with flwFP41 clones alone (Fig. 7A,A′). Expression of λ-top in a wild-type background did not have such an effect (Fig. 7B,B′), indicating that the observed phenotypes at the anterior do not merely result from constitutively active Egfr signaling but arise in combination with loss of PP1β function. By contrast, expression of a ligand of the JAK/STAT pathway, Unpaired (Upd) (Harrison et al., 1998), did not produce the Notch defects either in the anterior or in the lateral clones of flwFP41 (Fig. 7D,D′). However, when λ-top and Upd were co-expressed, a combination that is able to transform all the follicle cells into the posterior fate (Xi et al., 2003), Cut upregulation and mild overproliferation were found even in the lateral flwFP41 clones (Fig. 7F,F′).
Fig. 7.
The PFC restricted Notch defects in flwFP41 are caused by the integrated activity of JAK/STAT and Egfr Signaling. (A-F′) Egg chambers containing wild-type follicle cell clones or flwFP41 mutant clones positively marked by GFP (green), expressing a constitutively active form of Egfr, λ-top (B-C′), or the ligand of the JAK/STAT pathway Upd (D,D′), or both (E-F′), and stained for Cut (red). In flwFP41 clones alone (A,A′) or in flwFP41 clones expressing Upd (D,D′), Cut is upregulated only in the mutant PFCs. In flwFP41 clones expressing λ-top (C,C′), Cut is upregulated in both the anterior and the posterior mutant clones and severe cell overproliferation is observed. In flwFP41 clones expressing both λ-top and Upd (F,F′), Cut staining is visible in all the mutant cells regardless of clone positions. Expression of these constructs in wild-type follicle cells does not produce abnormal Notch signaling (B,B′,E,E′). Scale bars: 10 μm. Egg chambers are oriented with the posterior side to the right.
In summary, our results demonstrate that the integrated activity of multiple signaling pathways is responsible for the sensitivity of Notch signaling in the PFCs to loss of PP1β function.
DISCUSSION
The AP body axis of Drosophila is established during oogenesis through intracellular communication between the oocyte and the somatic follicle cells (Poulton and Deng, 2007). Correct oocyte polarity requires a polarizing signal generated by the PFCs, in response to an earlier signal (Gurken) that is secreted from the oocyte and received by the PFCs via Egfr. Previous studies have shown that genes regulating PFC proliferation, differentiation and epithelial polarity must function normally to render the PFC competent to signal back to the oocyte; however, the nature of this polarizing signal is still unknown, neither is it clear how the signal is produced or transmitted from the PFCs to the germline. Here, we report a direct role of Drosophila PP1β in the production of the polarizing signal. We found that loss of PP1β in the PFCs due to the flwFP41 mutation causes a disruption of the oocyte MT polarity and the mislocalization of determinants of embryonic AP polarity indicative of a defect in the polarizing signal. This oocyte polarity defect was not observed with anterior or lateral follicle cell clones mutant for flwFP41, demonstrating that the activity of PP1β is required in the PFCs to repolarize the oocyte. We have also shown that heterozygous mutants of positive regulators of myosin activity suppress the oocyte polarity defect, whereas constitutive activation of Rok or expression of a mutant myosin targeting subunit in the PFCs induces a similar oocyte polarity phenotype. This supports our conclusion that myosin activity controls the polarizing signal in the PFCs.
The fact that elevated myosin activity in the PFCs interferes with the production of the polarizing signal raises the question of the specific function of myosin in this process. We found that there are two separable effects of elevated myosin activity in the PFCs: an effect on Notch signaling and a Notch-independent effect. Loss of Notch signaling in the follicle cells inhibits the developmental progress of the PFCs and results in the disruption of the formation of the AP polarity in the oocyte (Gonzalez-Reyes and St Johnston, 1998; Lopez-Schier and St Johnston, 2001). In flwFP41 PFC clones, the cells are still responsive to the patterning signals of Egfr and the JAK/STAT pathway and the mutant PFCs are able to adopt the posterior fate as indicated by the expression of pnt-lacZ. Therefore, the major problem in the generation of the polarizing event by loss of PP1β is not cell specification or cell survival. Instead, we propose that loss of Notch signaling directly affects the production of the polarizing signal, and that myosin activity is further required for the proper generation of this signal independently of its effects on Notch signaling, as discussed below.
We have shown that defective Notch signaling in flwFP41 mutant PFCs can be rescued by expression of NICD, but not by full-length Notch or NEXT. This indicates that myosin hyperactivation through loss of PP1β disrupts Notch signaling probably at the level of the final Notch cleavage. This cleavage, which is γ-secretase dependent and generates the functional NICD, is subject to regulation at the level of endosomal trafficking (Fortini and Bilder, 2009). In mutants that disrupt entry of the receptor into early endosomes, Notch accumulates at the cell surface or below the plasma membrane with significantly reduced signaling activity (Lu and Bilder, 2005; Vaccari et al., 2008). In mutants affecting the function of the Vacuolar ATPase, Notch signaling is also blocked at the step of the third cleavage, indicating that this cleavage requires an endosomal environment (Yan et al., 2009; Vaccari et al., 2010). We observed an elevated level of Notch protein at the cell surface and in early and late endosomal compartments in the subapical cell cortex in the flwFP41 mutant PFCs. It is therefore likely that the defective Notch activity in flwFP41 is caused by a failure of the receptor to efficiently enter early endosomes and subsequent sorting compartments. Such a defect in endosomal trafficking might be a direct consequence of abnormal myosin activity. The regulation of the actin cytoskeleton and of actin motor proteins plays an important role in the endocytic pathway in yeast and mammalian cells (Girao et al., 2008). In Drosophila embryos, cortical actin regulates endocytic dynamics at early cellularization (Sokac and Wieschaus, 2008a; Sokac and Wieschaus, 2008b). In addition, studies in mammalian cell culture have shown that Rho, Rok and myosin II directly regulate phagocytosis (Araki, 2006; Olazabal et al., 2002), revealing important roles of myosin II in the process of endocytosis. However, loss of PP1β does not cause a significant block in endocytosis in all cell types. We found that flwFP41 clones in the eye discs allow apparently normal Notch signaling to occur and do not show ectopic Notch accumulation. We also did not detect an overt endocytic defect in mutant eye disc cells by performing a trafficking assay (data not shown). In addition, mutant clones in anterior and lateral follicle cells did not show a defect in Notch signaling. This indicates a particular sensitivity of the PFCs to problems in Notch endocytosis and Notch activation, which is due to the coordinated activities of JAK/STAT and Egfr signaling, as shown by our results.
Our data strongly suggest that PP1β has an independent role in axis formation apart from its effects on regulating Notch cleavage and activation. Excessive myosin activity resulting from constitutive Rok activity, or from expression of a mutant myosin targeting subunit in the PFCs, disrupts Stau localization without inducing a measurable Notch phenotype. Additionally, expression of NICD only marginally suppresses Stau mislocalization caused in the flwFP41 mutant cells, whereas it strongly rescues the Notch signaling defect. Therefore, we observe oocyte polarity defects by myosin misregulation even in the presence of normal Notch signaling.
The effects of excessive myosin activity are also different from those of the Hippo pathway, which is also specifically required in the PFCs for axis formation (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). Similar to flwFP41, hippo mutant PFCs are defective in Notch signaling and result in oocyte mispolarization, and these defects are restricted to PFCs. However, previous studies demonstrate that the effects of the Hippo pathway are mediated solely by its effects on Notch (Polesello and Tapon, 2007; Yu et al., 2008). Hippo signaling itself appears to occur normally in the flwFP41 mutant follicle cells.
The abnormal accumulation of membrane proteins suggests a general membrane trafficking problem associated with myosin hyperactivation. It raises the possibility that PP1β regulates the polarizing signal, which might be a membrane associated protein, by controlling its intracellular trafficking as it is trafficked to the cell surface. However, hyperactive myosin caused by loss of PP1β function might also directly impede the interaction between the PFCs and the oocyte, possibly by affecting the function of cellular structures, such as microvilli, required for the presentation of the polarizing signal on the apical surface of the PFCs to the oocyte. We observed higher levels of components of apical membrane complexes as well as of the adherens junction proteins on the apical surface, which might result from changes in the underlying actin cytoskeleton caused by excessive myosin activity. Consequently, changes in the membrane properties, especially on the apical side that contacts the germline, might also change cell surface protein interactions between the PFCs and the oocyte, which might then affect the transmission of the polarizing signal. We observe a very local effect on oocyte polarity when a subset of PFCs are mutant for flwFP41, where Staufen protein is still localized correctly in the oocyte underneath the wild-type cells, but is absent from the region underneath the mutant cells. This strongly suggests that the polarizing signal is not freely diffusing over longer distances, and points to local interactions between the PFCs and the oocyte.
One very puzzling aspect of the flwFP41 phenotype is the fact that the phenotypes of defective Notch signaling and cell overproliferation are restricted to the PFCs. Position-dependent phenotypes have been observed in mutants disrupting the epithelial integrity of the follicle cells, such as dlg1 and crb mutants (Goode and Perrimon, 1997; Tanentzapf et al., 2000). There, defects of the epithelial architecture, such as multilayering, are mostly observed at the poles of the egg chamber. In mutants of the Hippo pathway, dramatic Notch defects are observed in PFC clones but only modest ones in clones at other sites of the epithelium (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). Such position-dependent responses might be due to the special terminal positions of the cells at the poles where they could experience more mechanical stress than the lateral cells. Excessive myosin activity caused by loss of PP1β function might exacerbate the mechanical forces experienced by the PFCs, leading to posterior-restricted phenotypes. Alternatively, signaling events specific to subpopulations of follicle cells might cause the cells to react differentially to the loss of common gene products. Strikingly, we found that the hyperactive myosin can lead to loss of Notch signaling and overproliferation when the Egfr pathway is activated in anterior follicle cells where JAK/STAT activity is normally present. Even the lateral cells produced these phenotypes when subject to the combined activity of JAK/STAT and Egfr signaling. Therefore, whereas loss of PP1β function elevates myosin activity in all the mutant cells independent of cell position, the coordinated activation of JAK/STAT and Egfr signaling creates a sensitized intracellular environment in the PFCs and renders them particularly susceptible to phenotypes such as defects in protein trafficking due to myosin misregulation. It is likely that particular targets of the combined activity of Egfr and JAK/STAT enhance the defects generated by the elevated myosin activity; however, it is presently unknown what these target proteins might be.
Overall our study has shown that the regulation of myosin activity by PP1β is crucial in the posterior follicle cells where overactive myosin interferes with intracellular trafficking and with the generation of the posterior polarizing signal. This demonstrates the importance of the general cellular physiology in both signal transduction as well as signal generation, and adds a layer of complexity to the analysis of developmental signals important for cell specification.
Supplementary Material
Acknowledgements
We thank S. Artavanis-Tsakonas, E. Bach, H. Bellen, D. Harrison, K. Irvine, V. Riechmann, R. Karess, G. Struhl, J. E. Treisman, J. Zallen, the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for providing flies and antibodies; G. Barcelo for technical help; J. Goodhouse for assisting with confocal microscopy; and members of the Schüpbach and Wieschaus laboratories for feedback and advice. We also thank E. Gavis, O. Grimm and A. Martin for helpful comments on the manuscript. This work was supported by the Howard Hughes Medical Institute and US Public Health Service Grant RO1 GM077620. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.062190/-/DC1
References
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246-20249 [DOI] [PubMed] [Google Scholar]
- Araki N. (2006). Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front. Biosci. 11, 1479-1490 [DOI] [PubMed] [Google Scholar]
- Bresnick A. R. (1999). Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26-33 [DOI] [PubMed] [Google Scholar]
- Clark I., Giniger E., Ruohola-Baker H., Jan L. Y., Jan Y. N. (1994). Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr. Biol. 4, 289-300 [DOI] [PubMed] [Google Scholar]
- Denef N., Chen Y., Weeks S. D., Barcelo G., Schupbach T. (2008). Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev. Cell 14, 354-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. M., Althauser C., Ruohola-Baker H. (2001). Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737-4746 [DOI] [PubMed] [Google Scholar]
- Doerflinger H., Vogt N., Torres I. L., Mirouse V., Koch I., Nusslein-Volhard C., St Johnston D. (2010). Bazooka is required for polarisation of the Drosophila anterior-posterior axis. Development 137, 1765-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. B., Harrison D. A., Perrimon N. (1998). Identifying loci required for follicular patterning using directed mosaics. Development 125, 2263-2271 [DOI] [PubMed] [Google Scholar]
- Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M. G., Knoblich J. A. (2005). Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763-773 [DOI] [PubMed] [Google Scholar]
- Fortini M. E. (2009). Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16, 633-647 [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Bilder D. (2009). Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao H., Geli M. I., Idrissi F. Z. (2008). Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 582, 2112-2119 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A., St Johnston D. (1998). Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development 125, 2837-2846 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A., Elliott H., St Johnston D. (1995). Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654-658 [DOI] [PubMed] [Google Scholar]
- Goode S., Perrimon N. (1997). Inhibition of patterned cell shape change and cell invasion by Discs large during Drosophila oogenesis. Genes Dev. 11, 2532-2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. A., McCoon P. E., Binari R., Gilman M., Perrimon N. (1998). Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 12, 3252-3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y., Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761-771 [DOI] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., et al. (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245-248 [DOI] [PubMed] [Google Scholar]
- Lee A., Treisman J. E. (2004). Excessive Myosin activity in mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Mol. Biol. Cell 15, 3285-3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461 [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H., St Johnston D. (2001). Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232-1239 [DOI] [PubMed] [Google Scholar]
- Meignin C., Alvarez-Garcia I., Davis I., Palacios I. M. (2007). The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17, 1871-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schupbach T. (1993). The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell 75, 165-174 [PubMed] [Google Scholar]
- Neuman-Silberberg F. S., Schupbach T. (1996). The Drosophila TGF-alpha-like protein Gurken: expression and cellular localization during Drosophila oogenesis. Mech. Dev. 59, 105-113 [DOI] [PubMed] [Google Scholar]
- Olazabal I. M., Caron E., May R. C., Schilling K., Knecht D. A., Machesky L. M. (2002). Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. 12, 1413-1418 [DOI] [PubMed] [Google Scholar]
- Polesello C., Tapon N. (2007). Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864-1870 [DOI] [PubMed] [Google Scholar]
- Poulton J. S., Deng W. M. (2007). Cell-cell communication and axis specification in the Drosophila oocyte. Dev. Biol. 311, 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan A. M., Ghabrial A., Schupbach T. (1997). Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development 124, 3871-3880 [DOI] [PubMed] [Google Scholar]
- Raghavan S., Williams I., Aslam H., Thomas D., Szoor B., Morgan G., Gross S., Turner J., Fernandes J., VijayRaghavan K., et al. (2000). Protein phosphatase 1beta is required for the maintenance of muscle attachments. Curr. Biol. 10, 269-272 [DOI] [PubMed] [Google Scholar]
- Rebay I., Fehon R. G., Artavanis-Tsakonas S. (1993). Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 74, 319-329 [DOI] [PubMed] [Google Scholar]
- Roth S., Lynch J. A. (2009). Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 1, a001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., Neuman-Silberberg F. S., Barcelo G., Schupbach T. (1995). cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81, 967-978 [DOI] [PubMed] [Google Scholar]
- Ruohola H., Bremer K. A., Baker D., Swedlow J. R., Jan L. Y., Jan Y. N. (1991). Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66, 433-449 [DOI] [PubMed] [Google Scholar]
- Schupbach T. (1987). Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell 49, 699-707 [DOI] [PubMed] [Google Scholar]
- Shi Y. (2009). Serine/threonine phosphatases: mechanism through structure. Cell 139, 468-484 [DOI] [PubMed] [Google Scholar]
- Sisson J. C., Field C., Ventura R., Royou A., Sullivan W. (2000). Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J. Cell Biol. 151, 905-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac A. M., Wieschaus E. (2008a). Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization. Dev. Cell 14, 775-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac A. M., Wieschaus E. (2008b). Zygotically controlled F-actin establishes cortical compartments to stabilize furrows during Drosophila cellularization. J. Cell Sci. 121, 1815-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nusslein-Volhard C. (1991). Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66, 51-63 [DOI] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K., Greenwald I. (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331-345 [DOI] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2005). Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299-4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Smith C., McGlade J., Tepass U. (2000). Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151, 891-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Smiley S., Wong M. L., Alberts B. M. (1992). Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115, 923-936 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M. E., Bilder D. (2008). Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Duchi S., Cortese K., Tacchetti C., Bilder D. (2010). The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 137, 1825-1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereshchagina N., Bennett D., Szoor B., Kirchner J., Gross S., Vissi E., White-Cooper H., Alphey L. (2004). The essential role of PP1beta in Drosophila is to regulate nonmuscle myosin. Mol. Biol. Cell 15, 4395-4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Wang B., Ballew A., Royou A., Karess R., Axelrod J. D., Luo L. (2001). Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81-91 [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Brauninger M., Gonzalez-Gaitan M. (2003). Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R., McGregor J. R., Harrison D. A. (2003). A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 4, 167-177 [DOI] [PubMed] [Google Scholar]
- Yan Y., Denef N., Schupbach T. (2009). The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev. Cell 17, 387-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Poulton J., Huang Y. C., Deng W. M. (2008). The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE 3, e1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo S., Clemens J. C., Stone R. L., Dixon J. E. (1994). Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J. Biol. Chem. 269, 26234-26238 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.