Abstract
Rationale
Stem cell therapies to regenerate damaged cardiac tissue represent a novel approach to treat heart disease. However, the majority of adoptively transferred stem cells delivered to damaged myocardium do not survive long enough to impart protective benefits, resulting in modest functional improvements. Strategies to improve survival and proliferation of stem cells show promise for significantly enhancing cardiac function and regeneration.
Objective
Determine if injected cardiac progenitor cells (CPCs) genetically modified to overexpress nuclear Akt (CPCeA) increase structural and functional benefits to infarcted myocardium relative to control CPCs.
Methods and Results
CPCeA exhibit significantly increased proliferation and secretion of paracrine factors compared to CPCs. However, CPCeA exhibit impaired capacity for lineage commitment in vitro. Infarcted hearts receiving intramyocardial injection of CPCeA have increased recruitment of endogenous c-kit cells compared to CPCs, but neither population provides long-term functional and structural improvements compared to saline injected controls. Pharmacologic inhibition of Akt alleviated blockade of lineage commitment in CPCeA.
Conclusions
Although overexpression of nuclear Akt promotes rapid proliferation and secretion of protective paracrine factors, the inability of CPCeA to undergo lineage commitment hinders their capacity to provide functional or structural benefits to infarcted hearts. Despite enhanced recruitment of endogenous CPCs, lack of functional improvement in CPCeA treated hearts demonstrates CPC lineage commitment is essential to the regenerative response. Effective stem cell therapies must promote cellular survival and proliferation without inhibiting lineage commitment. Since CPCeA exhibit remarkable proliferative potential, an inducible system mediating nuclear Akt expression could be useful to augment cell therapy approaches.
Keywords: Cardiac Progenitor Cell, Proliferation, Differentiation, Akt
Introduction
Stem cell therapies are being explored as a novel way to treat heart failure1-5. Unfortunately to date, relatively modest improvements in cardiac structure and function have been observed due in part to poor stem cell proliferation and viability after delivery. To improve benefits of stem cell therapy, mechanisms promoting proliferation and survival of the stem cell population without inhibiting lineage commitment have become an area of intense research focus. Enhanced efficacy of genetically modified stem cells mediating myocardial regeneration following infarction has been demonstrated using Pim-1, a cell survival and proliferation kinase downstream of Akt/PKB6.
Akt/PKB is a pivotal regulatory kinase with various roles regarding growth, metabolism, and survival7-18. In the heart, Akt is one of the most well studied cardioprotective kinases with well-documented capacity to prevent cardiomyopathic injury6, 19-26. Activation of Akt is initiated by growth factor dependent stimulation of receptor tyrosine kinases, which in turn stimulate a cascade of signaling events beginning with the activation of PI3 kinase (PI3K) at the plasma membrane. Subsequent activation of phosphoinositide-dependent kinase-1/2 (PDK1/2) phosphorylates and activates Akt. Downstream targets of Akt are numerous and include pro-proliferative and anti-apoptotic substrates10, 14, 18, 20, 27-29.
To investigate mechanisms governing cardioprotective effects of Akt, a variety of systems including cardiac specific overexpression and viral infections have been employed. Numerous studies attribute short-term Akt activation to the profound protective effects seen in post-ischemic injury models, whereby Akt induces secretion of paracrine factors, drastically increases cell cycle and inhibits apoptosis in cardiomyocytes14, 19-22, 24-26, 30. Additionally, Akt activation stimulates neoangiogenesis and vasculogenesis31-33, in part accounting for the dramatic improvements seen in pathologically challenged Akt transgenic mice. However, constitutive activation of Akt can have detrimental effects upon the myocardium, including hypertrophic growth and abnormal vascular remodeling34. However, the majority of protective effects afforded by Akt have been found to be governed through its nuclear localization14, 21, 24, 35. Previous studies by our group demonstrate cardiac specific overexpression of nuclear Akt allows for expansion of the progenitor cell pool as well as enhanced protection of the myocardium against pathological injury without induction of hypertrophic remodeling14.
While the cardioprotective effects of nuclear Akt are clear within myocytes of transgenic animals, protective effects of nuclear Akt expression on the cardiac progenitor cell population have not been investigated. The current study evaluates the ability of cardiac progenitor cells (CPCs) modified with nuclear Akt (CPCeA) to mediate cardioprotection in infarcted hearts.
Methods
Lentiviral vectors and generation of lentivirus
Bicistronic lentiviral vectors were generated as previously described6.
Cardiac Progenitor Cell Isolation, Cell culture, Lentiviral Infection
CPCs isolated from 10-12 week old male FVB mice, cultured in cardiac stem cell media and infected with lentivirus as previously described6. Cells were incubated with Akt kinase inhibitor-V (AIV) (10μmol/L) for 7 days with and without Dexamethazone (Dex) at 10−8mol/L where indicated.
Trypan blue, CyQuant Assays
Uninfected, CPCe, and CPCeA, plated in quadruplicate (10,000 cells/well) in 24 well plates. To determine proliferation rate cells were harvested at 96-hours and stained 1:10 with trypan blue. Viable cells determined by trypan blue exclusion were counted. CPCe and CPCeA, plated in quadruplicate, in 96-well plates (4000 cells/well), CyQuant (Invitrogen) reagent was added at indicated time points, incubated for 45 minutes and read at 530nm.
Immunoblots, Immunohistochemistry, Confocal Microscopy
Immunoblots, immunohistochemistry and confocal microscopy were performed as previously described6 with additional details in the supplemental methods.
C-kit+ Cell Quantification
Sections from CPCe or CPCeA injected animals were immunolabeled with antibodies against c-kit, GFP, and Tropomyosin. Tropomyosin was used to measure infarct area using Leica confocal software. Total c-kit+ cells, c-kit+GFP+ and c-kit+GFP− CPCs were quantified in the infarct area.
Myocardial Infarction, Injections, Echocardiography, and Hemodynamics
Infarctions, echocardiography, and hemodynamics were performed as previously described6, with additional details provided in supplemental methods. Animals were injected at 5 sites surrounding the border zone with PBS, CPCe or CPCeA (total of 100,000 cells per heart).
SuperArray and qRT-PCRs
For quantitative real time-polymerase chain reaction (qRT-PCR), RNA was harvested as per manufacturer’s protocol (Zymo Research, R1055). cDNA was obtained using iScript cDNA synthesis kit (Bio-Rad, 170-8891). qRT-PCR was run using the iQ SYBR Green Supermix (Bio-Rad, 170-8882) since cDNA was synthesized. Cell proliferation array (SuperArray, PAMM-020) was performed as per manufacturer’s protocol. Primers for qRT-PCR were designed using Pubmed Primer-blast. Sequences provided in supplemental methods.
ELISA
Cells were plated at 3000 cells/well in 150ml of stem cell media and cell culture supernatant harvested 24-hours later. SDF-1 ELISA was run as per manufacturer’s protocol (RayBiotech, ELM-SDF1alpha-001).
Statistics
Statistics were calculated using Prism software. One-way ANOVA and two-way repeated measures ANOVA analysis for echocardiography with Tukey posthoc test were calculated. Values with p<.05 were considered statistically significant.
Animal studies
All animal studies were performed in accordance with IACUC approved protocols.
Results
Overexpression of Nuclear-Akt in Cardiac Progenitor Cells (CPCs)
cDNA from murine Akt was fused to a 3× nuclear localization sequence (NLS) targeting Akt to the nuclear compartment of the cell and a myc-tag to facilitate detection of the engineered protein. Bicistronic lentiviral vectors (Supplementary Figure IA) express enhanced green fluorescent protein alone (Lv-egfp) or in combination with nuclear Akt kinase (Lv-egfp+Akt-nuc) in order to stably deliver cDNA constructs into the genome of c-kit+ CPCs isolated from male nontransgenic FVB mice. CPC populations modified with Lv-egfp (CPCe) or with Lv-egfp+Akt-nuc (CPCeA) were subjected to immunoblot analysis to confirm stable long-term integration of the gene and overexpression of target protein. CPCeA demonstrate Akt and myc-tag protein overexpression, while both CPCeA and CPCe express eGFP protein (Supplementary Figure IB). GAPDH is shown as a loading control. Interestingly, CPCeA do not overexpress Pim-1 protein (Supplementary Figure IC) compared to CPCe or CPCs overexpressing Pim-1 (CPCeP).
CPCeA Increase Proliferation and Alter Expression of Cell Cycle Genes
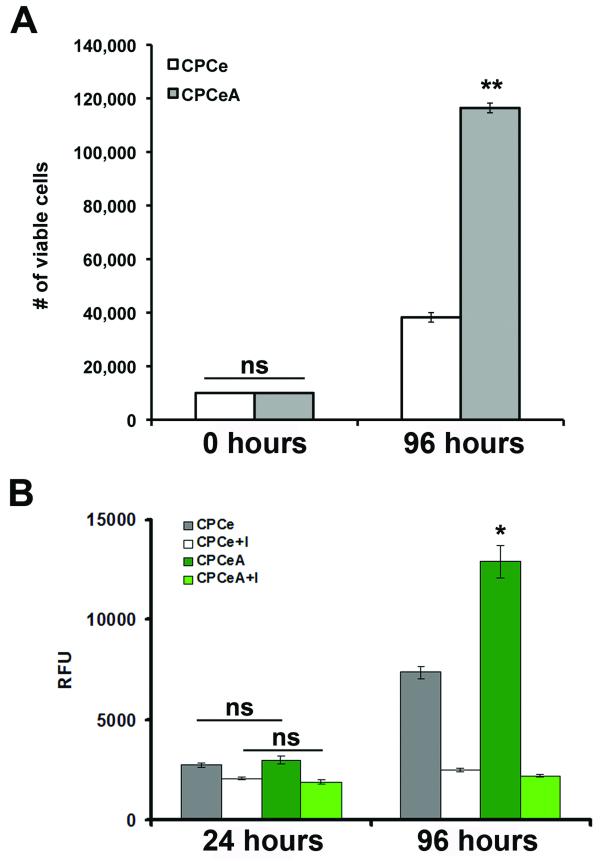
The proliferation rate of CPCeA was significantly (p<.01) increased relative to CPCe, over a 96-hour time course as determined by Trypan blue exclusion measuring total number of viable cells (Figure 1A). Additionally, CPCeA have a significant (p<.05) increase in proliferation compared to CPCe over a 96-hour time course as determined by CyQuant assay (Figure 1B). Proliferation was attenuated in CPCeA and CPCe by 96-hours with addition of AIV (Figure 1B). Altered RNA expression for several cell cycle genes was confirmed in CPCeA compared to CPCe controls by cell cycle array (Supplementary Figure IIA). Specifically, cyclin-D1 protein is markedly attenuated (Supplementary Figure IIB) and protein expression of Chk1 and CDC2 is significantly increased in CPCeA (Supplementary Figure IIC) compared to CPCe controls.
Figure 1. Overexpression of nuclear AKT increases CPC proliferation rate.
(A) Trypan blue exclusion was used to determine number of viable cells in CPCe and CPCeA over a 96-hour time course (mean ± SEM, n=4/group). (B) Proliferation rate of CPCe and CPCeA treated with and without Akt inhibitor V was determined by CyQuant assay over a 96-hour time course (mean ± SEM, n=4/group). *p<.05, **p<.01 compared to CPCe.
Increased Number of c-kit+ Cells in Hearts Receiving CPCeA
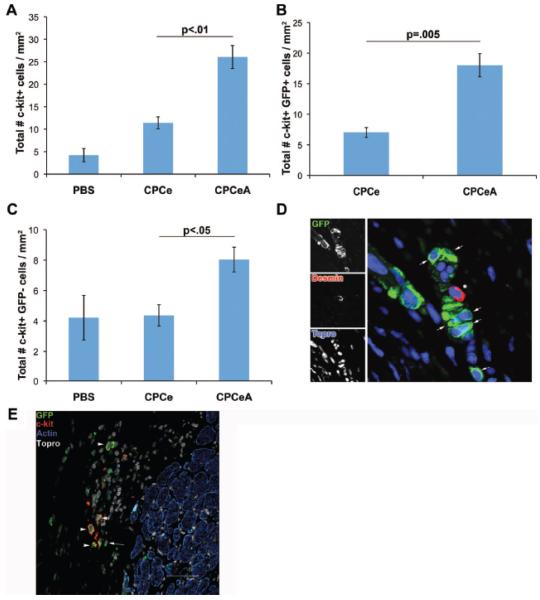
To assess whether protective benefits are gained from intramyocardial injection of CPCeA, twelve-week old female mice were subjected to infarction and CPCe or CPCeA were adoptively transferred. The number of c-kit+ cells was quantified within the infarct region of animals receiving PBS, CPCe or CPCeA. At twelve weeks, CPCeA injected hearts had a significant (p<.01) 2.3-fold increase in total c-kit+ cells compared to CPCe injected controls (Figure 2A). Additionally, CPCeA injected hearts had a significant (p=.005) 2.7-fold increase in c-kit+ eGFP+ cells (Figure 2B) and a 1.8-fold increase in c-kit+ eGFP− cells (p<.05) (Figure 2C) compared to CPCe injected controls. There was no statistical difference (p>.05) in the number of c-kit+ eGFP− cells between CPCe and saline injected hearts. Although a significant number of c-kit+ GFP+ CPCs were identified in hearts receiving CPCeA after twelve weeks, there was a noticeable lack of GFP+ CPCs expressing markers consistent with cardiac lineage commitment as evidenced by the absence of co-localization between GFP and desmin or sarcomeric α-actin (Figure 2D- E). In comparison, control CPCe acquire markers consistent with cardiogenic lineage commitment after infarction (Supplementary Figure III and previously published data6).
Figure 2. Increased c-kit+ CPCs in the infarct of CPCeA treated hearts.
Quantitation of total (A), eGFP+ (B), and eGFP− (C) c-kit+ cells in infarct region of mice injected with PBS (n=3), CPCe (n=3), or CPCeA (n=4) (mean ± SEM). (D-E) Representative immunostains of CPCeA injected heart immunolabeled with GFP (green), desmin (red), and Topro-3-iodide (blue) (D) or GFP (green), c-kit (red), actin (blue) and topro-3-iodide (white) (E). Arrows indicate GFP+ Desmin− cells, asterisk represents GFP− Desmin+ cell (D). Arrowheads indicate c-kit+ GFP+ actin- cell (E).
CPCeA Do Not Improve Function or Structure of Infarcted Myocardium
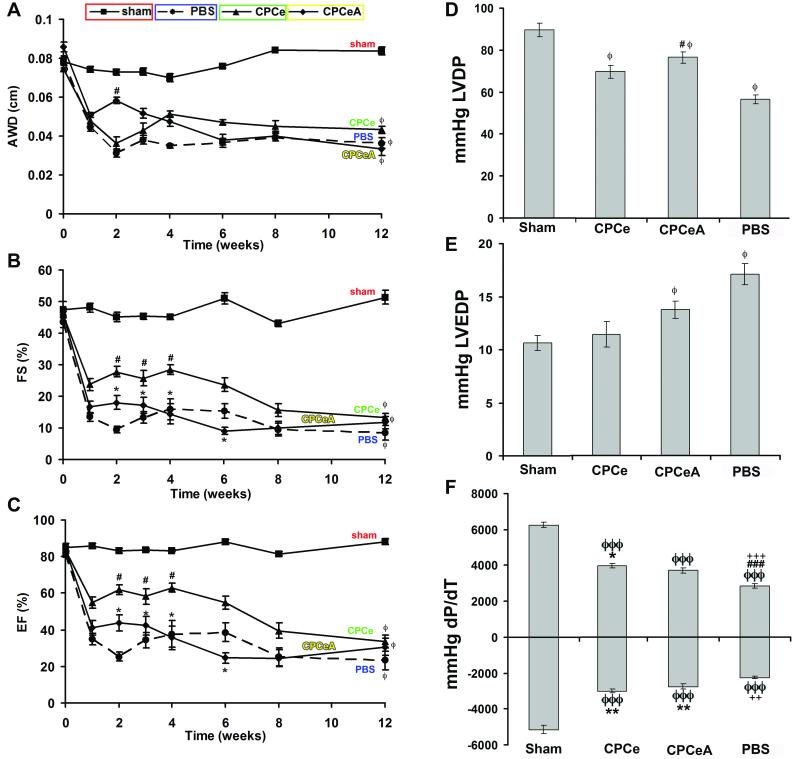
Cardiac function, after infarction and injection, was assessed by echocardiography and in vivo hemodynamics. Hearts of animals receiving CPCeA did not show a statistically significant improvement over CPCe injected hearts in anterior wall dimension (AWD, Figure 3A), fractional shortening (FS, Figure 3B), or ejection fraction (EF, Figure 3C), at twelve weeks as assessed by two-way ANOVA statistical analysis. Hemodynamic assessment further confirmed deterioration of cardiac function in CPCeA injected animals as assessed by left ventricular developed pressure (LVDP, Figure 3D), left ventricular end diastolic pressure (LVEDP, Figure 3E), and dp/dt maximum and minimum (Figure 3F). In fact, as early as four weeks post-injection, cardiac function in CPCeA injected hearts was not statistically different (p>.05) from PBS injected controls. CPCe injected hearts show a statistically significant (p<.05) improvement in cardiac function at early time points (4-weeks), but beneficial effects were not sustained and were indistinguishable from PBS injected controls by 12-weeks (Figure 3). Additionally, at 12-weeks post-infarction, CPCeA injected animals did not have a statistically significant reduction in infarct size compared to CPCe injected controls (Supplementary Figure IV).
Figure 3. Intramyocardial injection of CPCeA does not improve cardiac function.
(A-C) Electrocardiographic assessment of AWD (A), FS (B), and EF (C), in sham (□, n=4), PBS (●, n=7), CPCe (▲, n=8), and CPCeA (◆, n=7), 12-weeks post-infarction (mean ± SEM). (D-F) Cardiac function of sham (n=4), PBS (n=5), CPCe (n=6), and CPCeA (n=5) were evaluated using in vivo hemodynamic measurements of LVDP (D), LVEDP (E), and dP/dT (F) 12-weeks post-intramyocardial injection (mean ± SEM). Two-way ANOVA analysis was run for echocardiography and one-way ANOVA for hemodynamics. Where appropriate Tukey posthoc test was performed: ϕp<.05, ϕϕp<.01, ϕϕϕp<.001 compared to sham; #p<.05, ##p<.01, ###p<.001 compared to CPCe, * p<.05, **p<.01, ***p<.001 compared to PBS, +p<.05, ++p<.01, +++p<.001 compared to CPCeA.
CPCeA Express Paracrine Factors induced by Akt Activity
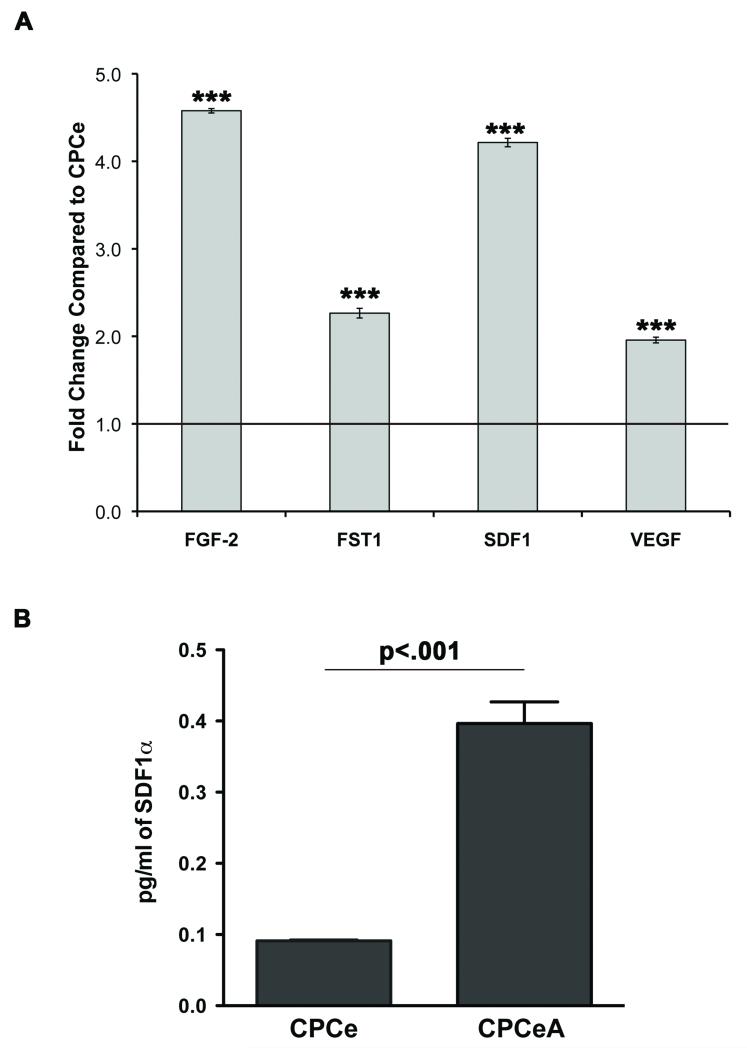
Increase numbers of endogenous (eGFP−, c-kit+) stem cells within the infarct (Figure 2C) suggests CPCeA release paracrine factors promoting recruitment of resident CPCs to the site of injury. Thus, mRNA expression of paracrine factors known to be induced by Akt activity was assessed by quantitative RT-PCR (qRT-PCR) on CPCeA in vitro. CPCeA express significantly (p<.001) more transcripts for FGF-2, FST-1, SDF-1 and VEGF (Figure 4A). CPCeA exhibit a significant 3.5-fold increase in SDF-1 protein expression compared to CPCe controls by ELISA assay (Figure 4B). Interestingly, SDF-1 is a potent chemoattractant, previously demonstrated to attract stem cells to sites of injury36, 37.
Figure 4. CPCeA express Akt inducible paracrine factors.
(A) qRT-PCR analysis of Akt inducible paracrine factors from CPCeA. Results expressed as fold change and normalized to CPCe. ***p<.001 (n=3). (B) SDF-1 ELISA performed on CPCe and CPCeA.
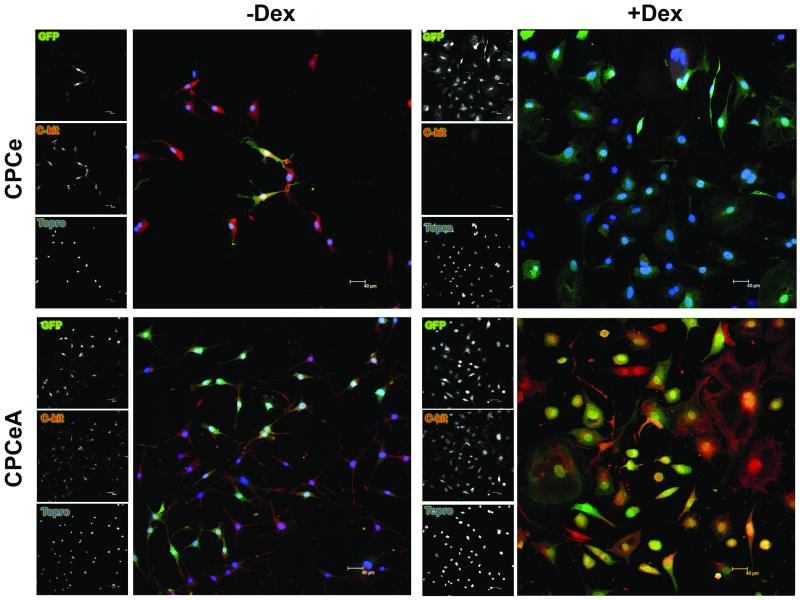
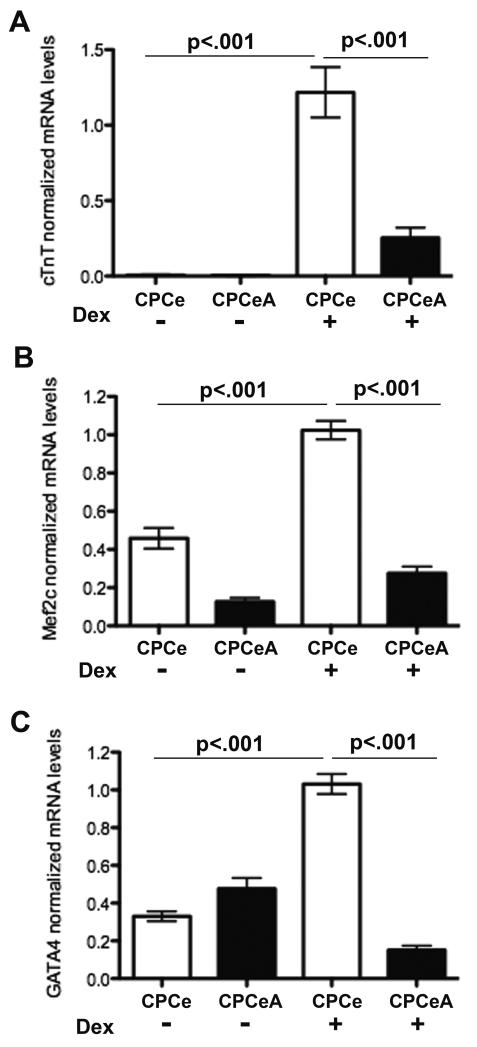
CPCeA Abrogate in vitro Differentiation
Absence of in vivo structural and functional improvement, combined with the observation that injected CPCeA did not appear to acquire markers consistent with cardiac lineage commitment, prompted assessment of CPCeA capacity for differentiation in vitro. CPCe and CPCeA were treated with dexamethasone (Dex) for seven days to induce differentiation and evaluated for c-kit protein expression by immunocytochemistry. CPCeA maintained c-kit expression after treatment with Dex in contrast to CPCe controls whereby c-kit expression was lost (Figure 5, right). Both CPCe and CPCeA expressed c-kit prior to Dex treatment (Figure 5, left). In addition to retention of c-kit expression upon differentiation stimulation, CPCeA also fail to induce cardiac troponin T (cTnT), Mef2C, or Gata4 transcripts, markers consistent with cardiogenic lineage commitment (Figure 6A-C). In contrast, CPCeP express significantly (p<.001) more Mef2C and Gata4 transcript than CPCeA when treated with Dex (Supplementary Figure V).
Figure 5. CPCeA are refractory to in vitro differentiation.
C-kit protein expression in CPCe and CPCeA treated with and without Dex for 7 days. GFP (green), c-kit (red), and nuclear stain Topro (blue).
Figure 6. Overexpression of nuclear Akt abrogates lineage commitment.
qRT-PCR quantitation of (A) cTnT (B) Mef2C and (C) Gata4 transcript levels in CPCe and CPCeA treated with or without Dex. Values normalized to CPCe treated with Dex (mean ± SEM, n=3).
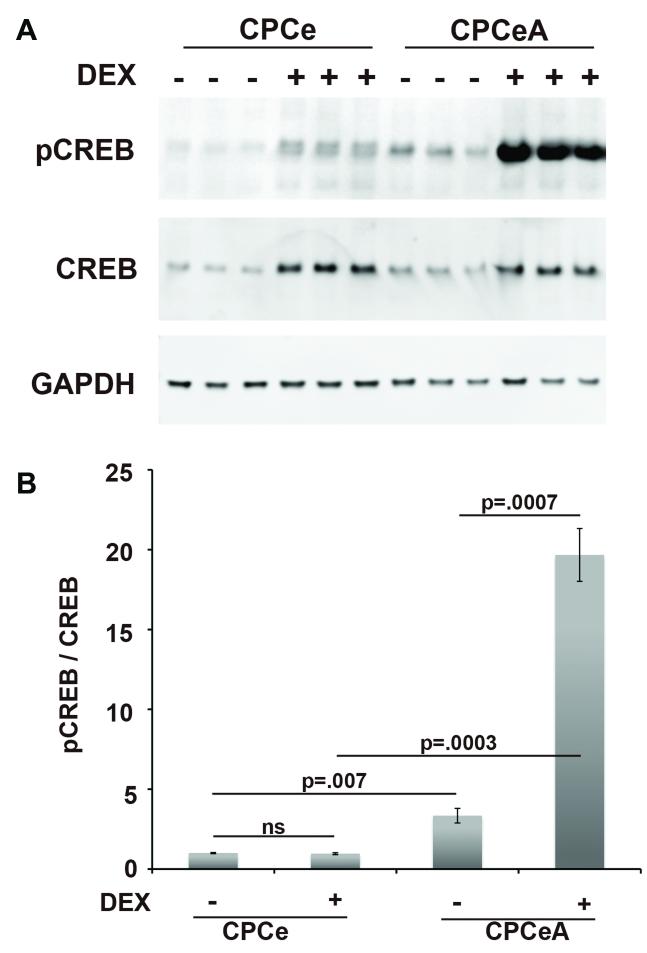
Elevated Levels of Phosphorylated CREB in CPCeA
Cyclic AMP response element binding protein (CREB) is a downstream target of Akt and has been previously demonstrated to promote progenitor cell proliferation12, 38. CPCe and CPCeA were treated with and without Dex for seven days and immunoblotted to assess CREB phosphorylation status. Undifferentiated CPCeA had a statistically significant (p=.007) 3.3-fold increase in the level of phospho-CREB compared to CPCe (Figure 7A-B). The differential in CREB phosphorylation was even greater after differentiation, with Dex treated CPCeA showing a 19-fold significant (p=.0003) increase in the level of phospho-CREB compared to Dex treated CPCe controls (Figure 7B). By comparison, increases in phospho-CREB after differentiation were not observed in CPCeP (Supplementary Figure VI).
Figure 7. Differentiated CPCeA increase phosphorylated CREB levels.
Immunoblot analysis (A) and quantitation (B) of phospho-CREB, total-CREB, and GAPDH in CPCe and CPCeA treated with and without Dex (mean ± SEM, n=3/group).
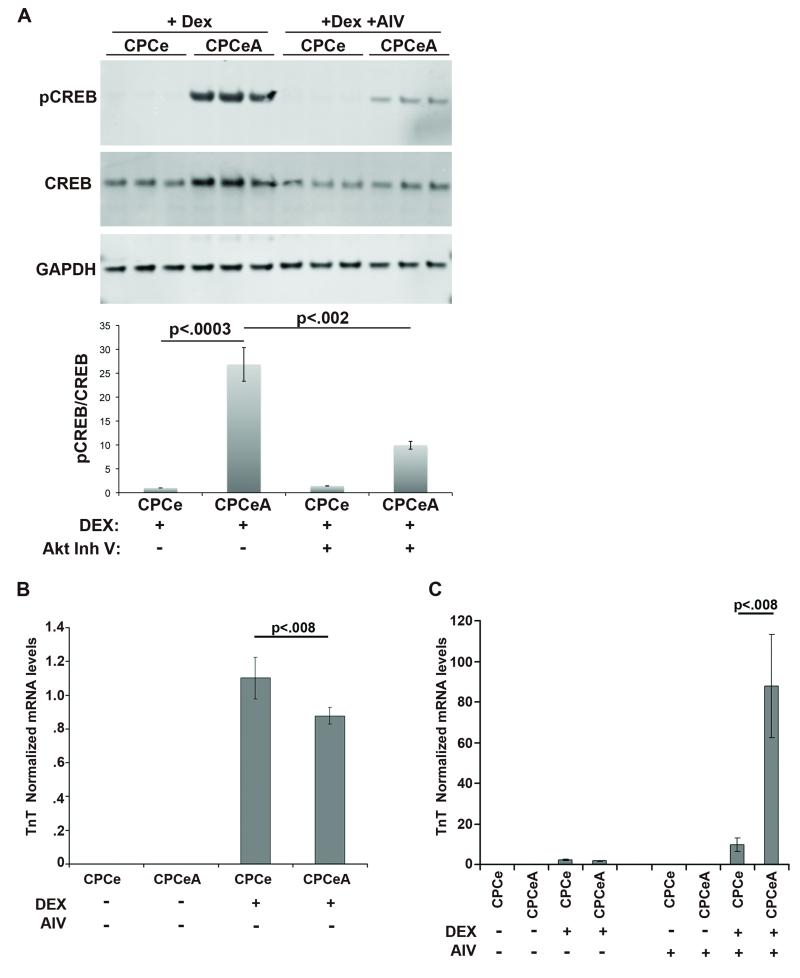
Attenuation of Akt Activity Increases Cardiac Lineage Commitment
CPCe and CPCeA were treated in vitro with inhibitor (AIV) to inhibit Akt kinase activity and subjected to Dex induced differentiation. CPCeA treated with AIV and Dex show a statistically significant (p<.002) 2.7-fold reduction in phospho-CREB protein expression (Figure 8A) compared to Dex treated CPCeA without AIV treatment. Presumably nuclear Akt activity blocks the capacity of CPCeA to undergo lineage commitment upon exposure to Dex. Therefore CPCeA and CPCe were treated with AIV and assessed for transcript levels of cTnT either with or without Dex exposure. CPCe and CPCeA do not express cTnT prior to Dex-induced differentiation (Figure 8B) as detected by qRT-PCR. In contrast, upon induction of differentiation CPCeA express low levels of cTnT, although at significantly (p<.008) reduced levels compared to CPCe controls (Figure 8B). Next, treatment of CPCeA and CPCe with AIV prior to Dex-induced differentiation was performed to confirm overexpression of nuclear Akt in CPCeA abrogates cardiac lineage commitment. Indeed, CPCeA treated with AIV and Dex had a significant reduction in phospho-CREB protein levels (Figure 8A) as well as a statistically significant (p<.008) increase in cTnT transcript (Figure 8C), compared to CPCeA treated with Dex alone. CPCe also had significant increases in TnT transcript after treatment with AIV and Dex, compared to CPCe treated with Dex alone (Figure 8C), although the difference was not as dramatic as in CPCeA.
Figure 8. Nuclear Akt Attenuation increases lineage commitment.
(A) Immunoblot and quantitation of Dex treated CPCe and CPCeA, incubated with and without Akt inhibitor. qRT-PCR quantitation of TnT transcript levels in CPCe and CPCeA treated with or without Dex treatment and no AIV (B), or with AIV (C). Values were normalized to CPCe treated with Dex (mean ± SEM, n=3).
DISCUSSION
For years treatment of the damaged myocardium has suffered the major limitation of being unable to regenerate functional cardiac tissue. Pharmaceutical treatments prolong the life of many patients, but ultimately fail as a permanent “fix” for treatment of heart failure. Recently, the advent of stem cell research and tissue regeneration presents a potential long-term solution for the repair of damaged myocardium. Clinical trials whereby stem cells are delivered to the damaged myocardium are underway; however, results generally offer modest short-term improvements in cardiac function. A plausible biological explanation for the underwhelming outcomes of such adoptive transfer studies is that only a minority of delivered stem cells survives in damaged myocardium. These observations led to the hypothesis that increasing the ability of adoptively transferred stem cells to survive and proliferate will significantly improve efficacy of stem cell regeneration in the heart.
Genetic modification of stem cells with survival kinases, in particular Akt and associated downstream targets, improves the ability of progenitor cells to mitigate cardiac damage and improve regeneration6, 26, 39. While constitutive activation of Akt leads to hypertrophic growth and abnormal vascular remodeling, a plethora of studies have shown short-term Akt activation, as well as nuclear localized Akt, imparts protective benefits including growth, inhibition of cell death and increased angiogenesis to the pathologically challenged heart. However, successful modification of stem cells requires the ability to increase proliferation and survival without inhibiting lineage commitment upon appropriate environmental stimulation. The vast majority of experiments involving Akt overexpression in stem cells do not assess for the amount or for the duration of this stimulation within the progenitor cell pool19, 22, 26, 40. While protective benefits have been gained through Akt activation, several studies also demonstrate sustained overexpression can inhibit lineage commitment and terminal differentiation in various progenitor cell populations12, 41.
Overexpression of nuclear Akt dramatically increases CPC proliferation and significantly expands the cell population in vitro (Figure 1), likely resulting from regulation of various cell cycle genes (Supplementary Figure II). Although we previously reported activation of Pim-1 downstream of nuclear Akt in myocytes20, Pim-1 expression in CPCs was not increased in CPCeA when compared to controls (Supplementary Figure IC). The basis for this differential action of nuclear Akt accumulation upon Pim-1 expression is unknown at present, but presumably is tied to context-dependent cross-talk between nuclear Akt and Pim-1 depending upon the cell type and possibly proliferation status.
CPCeA injected hearts also retain significantly more c-kit+ GFP+ cells after infarction compared to CPCe controls, indicating CPCeA had increased proliferation in vivo (Figure 2A,B,E). Numerous studies also demonstrate modification of progenitor cells with Akt, induces secretion of paracrine factors23, 30, 42-44, promoting survival of endogenous myocardium as well as the adoptively transferred population. Similarly, CPCeA secrete paracrine factors, most notably SDF-1 (Figure 4A-B), a chemoattractant that promotes recruitment of stem cells to sites of injury. SDF-1 production by CPCeA may account in part for the observed increase of endogenous c-kit+ GFP− cells to the infarct. Although the majority of the c-kit+GFP− population are presumably endogenous CPCs at the time of assessment, we cannot exclude the possibility that SDF-1 secretion also leads to increased homing of bone marrow stem cells to the infarct in CPCeA injected hearts (Figure 2C) that may include mast cells. Regardless of cell origin the increase in endogenous c-kit+ cells observed in CPCeA injected animals, whether CPCs, bone marrow, mast cells or a combination, does not mediate salutary action in the infarcted myocardium (Figure 3).
Although CPCeA persistence after adoptive transfer into infarcted hearts was significantly improved compared to CPCe controls there was no concurrent benefit for myocardial function (Figure 3) or structure (Figure 2B,D,E and Supplementary Figure IV), since overexpression of nuclear Akt antagonizes cardiogenic lineage commitment (Figures 2D-E, 5, 6 and 8B-C). In contrast to CPCeA, CPCs overexpressing Pim-1, a downstream target of Akt, retain the capacity for cardiogenic commitment as demonstrated by increased gene expression of Gata4 and Mef2C when compared to CPCeA (Supplementary Figure V). These effects are likely the consequence of Akt-mediated deregulation of cell cycle control and overriding normal differentiation programming. These results help to explain, at least in part, the stark contrast in protective benefits afforded by CPCeP6 but not from CPCeA when delivered to the infarcted heart.
The transcription factor CREB, is phosphorylated and activated by Akt on serine 13312, 45. Phosphorylation of CREB induces proliferation and elevated levels of phosphorylated CREB are found in several forms of cancer46. Chondrocyte progenitor cells overexpressing Akt and phospho-CREB were highly proliferative but also refractory to differentiation12, consistent with our observations of CPCeA. Furthermore, terminal differentiation of chondrocyte progenitor cells was observed only after inhibition of Akt activity12. Similarly in our studies, inhibition of Akt activity in CPCeA reduced phospho-CREB levels 2.7-fold (Figure 8A) and resulted in increased expression of cardiac TnT transcript, a marker consistent with cardiogenic differentiation (Figure 8B, C). In contrast to CPCeA, CPCeP do not overexpress phospho-CREB after differentiation (Supplementary Figure VI) and are capable of cardiogenic lineage commitment6 (Supplementary Figure V). Collectively, our findings are consistent with previous studies demonstrating high levels of Akt expression promote rapid proliferation of progenitor cells that must be attenuated in order to initiate lineage commitment41.
Thus it seems evident that despite long-term persistence and enhanced proliferation, the successful differentiation of adoptively transferred stem cell populations is essential for amelioration of damage after pathological injury. While transient overexpression of cardioprotective genes would provide short-term rapid expansion of the progenitor cell pool and increased survival within the damaged myocardium, sustained protective benefits necessary for clinical implementation would likely be lost. Our results suggest fostering stable cell proliferation and survival may come at the expense of efficient differentiation. Possible solutions to achieve the best possible outcome could employ regulated gene expression to control progenitor cell biology after adoptive transfer, allowing for temporal reactivation of the cardioprotective gene as well as increased control over the activation of pro-proliferative and pro-survival genes. Alternatively, an episomal vector strategy such as adeno-associated virus or minicircle delivery systems that allow temporally limited but persistent expression could provide sufficient beneficial signaling during the critical time window of days to weeks after adoptive transfer. Episomal genetic modifications obviate concerns related to oncogenic transformation resulting from random integration into the genome as well as eventual diminution of expression over time and ongoing cellular proliferation. Studies are currently being pursued to examine the potential utility of inducible or episomal delivery systems to augment survival and proliferative signaling in CPCs.
Short-term studies consistently demonstrate delivery of paracrine factors facilitate minor improvements in structure and function of the damaged myocardium22, 42-44, 47-50. However, despite secretion of paracrine factors from CPCeA that likely contribute to early minor improvements in myocardial function (Figure 3A-C) long-term benefits from paracrine signaling were not evident. Recently, our group published a study demonstrating CPCs modified with Pim-1, a downstream target of Akt, were able to promote regeneration and impart drastic improvements to damaged myocardium. Significantly, the major difference between the two studies was that CPCeP were able to transdifferentiate6 (Supplementary Figure V). Although CPCeA delivery enhances endogenous CPC recruitment that presumably are capable of lineage commitment6, the increase in endogenous CPCs fails to provide functional improvements in the damaged myocardium. Thus, improving endogenous repair responses may modestly increase short-term benefits to infarcted myocardium but ultimately fail to bring about the magnitude of a response necessary for meaningful regeneration. Collectively, results presented here help to reconcile the argument that while paracrine factor delivery may provide minor benefits, it is not sufficient for long-term improvements in structure and function of the damaged myocardium.
Genetic modification of stem cell populations with cardioprotective genes has now been demonstrated in numerous studies as a legitimate approach to foster repair and regeneration in the pathologically damaged heart. Importantly, this study demonstrates that while paracrine factors may mitigate damage in early stages, CPC commitment is essential to the long-term regenerative response. Therefore, effective cardiac stem cell therapies must promote cellular survival and proliferation, as well as long-term engraftment and successful lineage commitment of the donated cell population.
NOVELTY and SIGNIFICANCE.
What is known
Cell mediated cardiac regeneration is a novel therapeutic modality for the treatment of heart disease.
Current stem cell therapies provide only modest structural and functional benefits to the damaged myocardium.
Genetic modification of CPCs could potentially increase their therapeutic efficacy.
What new information does this article contribute?
Nuclear Akt modification of CPCs inhibits lineage commitment.
Paracrine factor secretion improves homing but does not contribute to long-term protective benefits in the structure or the function of the infarcted myocardium.
CPC lineage commitment is essential for long-term structural and functional recovery in the pathologically-challenged myocardium.
Cell mediated cardiac regeneration has withstood concerns related to safety and is now undergoing scrutiny for efficacy and durability. Unfortunately, present stem cell therapies provide only modest functional and structural improvements to the damaged heart, lagging far behind the desired benefits necessary for justifiable widespread clinical implementation. To enhance the regenerative process, genetically-altered stem cells capable of enhanced proliferation and survival have been shown to drastically improve structural and functional benefits to the pathologically-challenged myocardium. This study demonstrates that increased proliferation and survival of CPCs is ineffective for enhancing reparative processes if lineage commitment is inhibited. CPCs modified with nuclear-targeted Akt kinase, a mediator of cell survival and proliferation, show enhanced expansion and persistence upon adoptive transfer to the infarcted myocardium with increased secretion of protective paracrine factors. Despite short-term benefits to the infarcted heart, long-term cardiac repair fails because nuclear Akt-modified CPCs are incapable of cardiac lineage commitment. Therefore, to realize the full potential of CPC-mediated regeneration, cell-based therapy will require enhancement of cellular survival, proliferation, and long-term engraftment, together with successful lineage commitment of the donated cell population.
Supplementary Material
Acknowledgements
The authors would like to thank all members of the Sussman laboratory for helpful discussions and technical support.
Sources of Funding K.M.F. is supported by the Rees-Stealy Foundation, ARCS fellowship, and Inamori fellowship. M.A.S. is supported by NIH grants 2R01HL067245, 1R37HL091102-01, RC1HL100891-02, 1R21HL102714-01, P01HL085577-05, R01HL105759-01, 1R21HL104544-01. M.H.K. is supported by Deutsche Forchungsgemeinschaft (DFG grant KO 3900/1-1).
Non-Standard Abbreviations and Acronyms
- AIV
Akt Inhibitor V
- AWD
Anterior Wall Dimension
- CPC
Cardiac Progenitor Cell
- CPCe
EGFP positive Cardiac Progenitor Cell
- CPCeA
Cardiac Progenitor Cell expressing egfp and nuclear Akt
- CPCeP
Cardiac Progenitor Cell expressing eGFP and Pm-1
- dP/dt
Change in Pressure over Change in Time
- EF
Ejection Fraction
- FS
Fractional Shortening
- Lv+Akt-nuc
Egfp and nuclear Akt lentivirus
- Lv+egfp
Egfp Lentivirus
- LVDP
Left Ventricular Developed Pressure
- LVEDP
Left Ventricular End Diastolic Pressure
- TBST
Tris Buffered Saline and Tween
- TN
Tris NaCl
- TNB
Tris NaCl Blocking Buffer
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 3.Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sussman MA. Mitochondrial integrity: preservation through Akt/Pim-1 kinase signaling in the cardiomyocyte. Expert Rev Cardiovasc Ther. 2009;7:929–938. doi: 10.1586/erc.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio M, Avitabile D, Fischer K, Emmanuel G, Gude N, Miyamoto S, Mishra S, Schaefer EM, Brown JH, Sussman MA. Cardioprotective stimuli mediate phosphoinositide 3-kinase and phosphoinositide dependent kinase 1 nuclear accumulation in cardiomyocytes. J Mol Cell Cardiol. 2009;47:96–103. doi: 10.1016/j.yjmcc.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto M, Iwase A, Harata T, Takigawa S, Suzuki K, Manabe S, Kikkawa F. IGF1-induced AKT phosphorylation and cell proliferation are suppressed with the increase in PTEN during luteinization in human granulosa cells. Reproduction. 2009;137:835–842. doi: 10.1530/REP-08-0315. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D’Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sussman M. “AKT”ing lessons for stem cells: regulation of cardiac myocyte and progenitor cell proliferation. Trends Cardiovasc Med. 2007;17:235–240. doi: 10.1016/j.tcm.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 13.Choudhari SR, Khan MA, Harris G, Picker D, Jacob GS, Block T, Shailubhai K. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther. 2007;6:112–121. doi: 10.1158/1535-7163.MCT-06-0561. [DOI] [PubMed] [Google Scholar]
- 14.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 15.Catalucci D, Condorelli G. Effects of Akt on cardiac myocytes: location counts. Circ Res. 2006;99:339–341. doi: 10.1161/01.RES.0000239409.90634.a9. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard C, Marquardt J, Bras A, Medema RH, Eilers M. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J. 2004;23:2830–2840. doi: 10.1038/sj.emboj.7600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 18.Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644–5657. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 21.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 24.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 25.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 26.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 27.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–325. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- 28.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA. Activation of Notch-mediated protective signaling in the myocardium. Circ Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 34.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 35.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 36.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 37.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 38.Dworkin S, Malaterre J, Hollande F, Darcy PK, Ramsay RG, Mantamadiotis T. cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells. 2009;27:1347–1357. doi: 10.1002/stem.56. [DOI] [PubMed] [Google Scholar]
- 39.Koc ON, Gerson SL. Akt helps stem cells heal the heart. Nat Med. 2003;9:1109–1110. doi: 10.1038/nm0903-1109. [DOI] [PubMed] [Google Scholar]
- 40.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Kita K, Kimura T, Nakamura N, Yoshikawa H, Nakano T. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells. 2008;13:839–850. doi: 10.1111/j.1365-2443.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 42.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 43.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 44.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 45.Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem Biophys Res Commun. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 46.Xiao X, Li BX, Mitton B, Ikeda A, Sakamoto KM. Targeting CREB for cancer therapy: friend or foe. Curr Cancer Drug Targets. 10:384, 391. doi: 10.2174/156800910791208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Tang J, Wang J, Kong X, Yang J, Guo L, Zheng F, Zhang L, Huang Y, Wan Y. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, Zhang L, Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.