Summary
During the resolution phase of inflammation apoptotic leukocytes are efferocytosed by macrophages in a nonphlogistic fashion that results in diminished responses to bacterial moieties and production of anti-inflammatory cytokines. Complement receptor 3 (CR3) and pro-resolving lipid mediators promote the engulfment of apoptotic leukocytes by macrophages. Here, we present evidence for the emergence of pro-resolving, CD11blow macrophages in vivo during the resolution of murine peritonitis. These macrophages are distinct from the majority of peritoneal macrophages in terms of their functional protein expression profile, as well as pro-resolving properties, such as apoptotic leukocyte engulfment, indifference to TLR ligands, and emigration to lymphoid organs. Notably, we also found macrophages convert from the CD11bhigh to the CD11blow phenotype upon interaction with apoptotic cells ex vivo. In addition, we found that the pro-resolving lipid mediators resolvin (Rv) E1 and RvD1, and the glucocorticoid dexamethasone (Dex) regulated pro-resolving macrophage functions in vivo. This regulation culminated in a novel pro-resolving function, namely reducing the apoptotic leukocyte ingestion requirement for CD11blow macrophage generation. These new phenotype and molecular pathway markers define the new satiated-macrophage. Thus, we suggest that satisfying-efferocytosis generates CD11blow macrophages that are essential for complete non-phlogistic containment of inflammatory agents and the termination of acute inflammation.
Keywords: Inflammation, macrophages, phagocytosis, apoptosis, lipid mediators
Introduction
Macrophages are a highly diverse subtype of immune cells that while originating from a common precursor are also capable of metamorphosing to functionally distinct phenotypes that play key roles in acute and chronic inflammation, as well as the resolution of inflammation and fibrosis [1, 2]. During the active resolution of inflammation [3, 4] immune response elements are eliminated [5]. The leukocytes that elicited the acute inflammatory response are undergoing apoptosis [6, 7], and consequently, the apoptotic PMNs are cleared by macrophages and other phagocytic cells in a non-phlogistic fashion [8, 9]. Apoptotic cell engulfment by phagocytes is mediated by signals that are expressed on the surface of apoptotic cells and their corresponding receptors, thrombospondin-CD36 [10], milk fat globule-EGF-factor 8 (MFG-E8)- αvβ3-integrin [9], and others (reviewed in[7, 11]).
Opsonization by iC3b leads to enhances engulfment of apoptotic cells via the complement receptors CR3 (CD18/CD11b) and CR4 (CD18/CD11c) expressed on macrophages [12] . Moreover, lipoxin (LX) A4 enhances uptake of apoptotic PMN by macrophages in a CD18-dependent manner [8]. Apoptotic cells also serve as resolution cues for macrophages, as their recognition evokes distinct signaling events [13] that block the release of pro-inflammatory mediators from macrophages. This release is activated by bacterial moieties, and its blockage, which is termed immune-silencing [14, 15], is accompanied by the production of TGFβ and IL-10 [16–18], cytokines that can promote resolution and wound repair. The engulfment of apoptotic leukocytes by macrophages also leads to inhibition of inducible NO synthase (iNOS) expression and stimulates the expression of arginase-1 in the RAW 264 macrophage cell line [19], thereby preventing reactive NO production. In addition, the expression of 15-lipoxygenase (LO)-1, which is involved in the generation of pro-resolving lipid mediators [19, 20], as well as the production of angiogenic growth factors[21] by macrophages are consequent to the uptake of apoptotic cells. Pro-resolving lipid mediators, such as RvE1 and RvD1 block PMN infiltration to inflamed cavities (reviewed in[22]). RvE1 also promotes removal of apoptotic PMN by macrophages, and leukocyte emigration out of resolving inflammation sites [20]. Glucocorticoids are another set of naturally occurring pro-resolving mediators [23], that act, at least in part, through annexin-A1 release and activation of the LXA4 receptor, FPR2/ALX [24, 25].
We identified earlier a new subset of macrophages that appeared during the resolution of murine peritonitis and that expressed lower levels of CD11b than the majority of the macrophage population [26]. In the present study we found that CD11blow macrophages display a unique phenotype. CD11blow macrophages differed from CD11bhigh macrophages in the expression of functional proteins, such as iNOS, arginase-1, cyclooxygenase (COX) 2, 12/15-LO, and matrix metalloproteinase (MMP)-9. These cells engulfed significantly higher numbers of apoptotic PMN than CD11bhigh macrophages, responded poorly to activation by different TLR ligands, in terms of cytokine and chemokine secretion, lost their phagocytic potential and were prone to migrate to lymphoid organs. Of interest, exposure to apoptotic cells ex vivo was sufficient to convert CD11bhigh macrophages to their CD11blow counterparts. Moreover, in vivo introduction of pro-resolving agents substantially enhanced CD11blow macrophage emergence, despite diminished engulfment of apoptotic PMN that together define the new subpopulation as the satiated-efferocytes.
Results
CD11blow macrophages express a distinct profile of functional proteins
Whether previously noted pro-resolving, CD11blow macrophages [26] express a different set of functional proteins than CD11bhigh macrophages is not known. To define the cell populations of interest we characterized and sorted F4/80+ macrophages according to their CD11b expression. We found that at 66 hrs after peritonitis initiation 70% of the exudate cells were macrophages (supplemental Figure 1A), of which 17% expressed low levels of CD11b (Supplemental figures 1B and 2A). Expression of CD11b on CD11bhigh macrophages was 10-fold higher than its expression on CD11blow macrophages (Supplemental figure 1C) and the amount of CD11b in protein extracts of CD11blow macrophages was significantly lower than its amount in extracts from CD11bhigh macrophages (Figure 1, top panel). Consequently, analysis of some proteins that are functionally-relevant to inflammation and well characterized by their expression in classically (M1)- and alternatively (M2)- activated macrophages was performed in CD11bhigh and CD11blow macrophages. Our results (Figure 1) indicate that CD11bhigh macrophages express low levels of iNOS, moderate levels of COX-2 and MMP-9 and high levels of arginase-1, but no 12/15-LO, whereas CD11blow macrophages express low levels of COX-2 and MMP-9, moderate levels of 12/15-LO and no iNOS or arginase-1. In addition, the macrophage differentiation marker F4/80 was expressed to a higher extent on CD11bhigh macrophages (Supplemental figure 2), further indicating that these cells possess different properties in comparison to CD11blow macrophages. Of interest, CD11blow macrophages lysates contained lower levels of actin than CD11bhigh cells (Figure 1), suggesting a modulation of cytoskeletal dynamics in these cells. In contrast, another housekeeping gene – tubulin – was equally expressed in both protein extracts (Figure 1), indicating equal protein loading. Further analysis of surface receptor expression revealed increased expression of and reduced expression of CD206 and CD163 (both M2 markers) on CD11blow macrophages, in comparison to their CD11bhigh counterparts (19 and 17 fold difference, respectively; N=3, data not shown). Thus, CD11bhigh and CD11blow macrophages display different expression profiles of functional proteins and differentiation markers.
Figure 1. CD11blow macrophages differ in their protein expression signature from CD11bhigh macrophages.
Resolving peritoneal exudates were recovered 66 hrs after zymosan A (1 mg) injection into mice, the cells were immuno-stained for Ly-6G, F4/80, and CD11b, and the Ly-6G−/F4/80+ macrophages were sorted to CD11bhigh and CD11blow populations. The recovered macrophages were lysed and their protein content was analyzed by SDS-PAGE and Western blotting for the indicated proteins. Results are representative blots (A) and mean ± SE of three independent experiments (B).Fold difference in expression was calculated according to the following formula: Densitometric units (DU) of CD11bhigh extract/DU of CD11blow extracts from three experiments. Significant differences by Student's t test between CD11bhigh and CD11blow extracts (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
CD11blow macrophages engulfed more apoptotic PMN than CD11bhigh macrophages
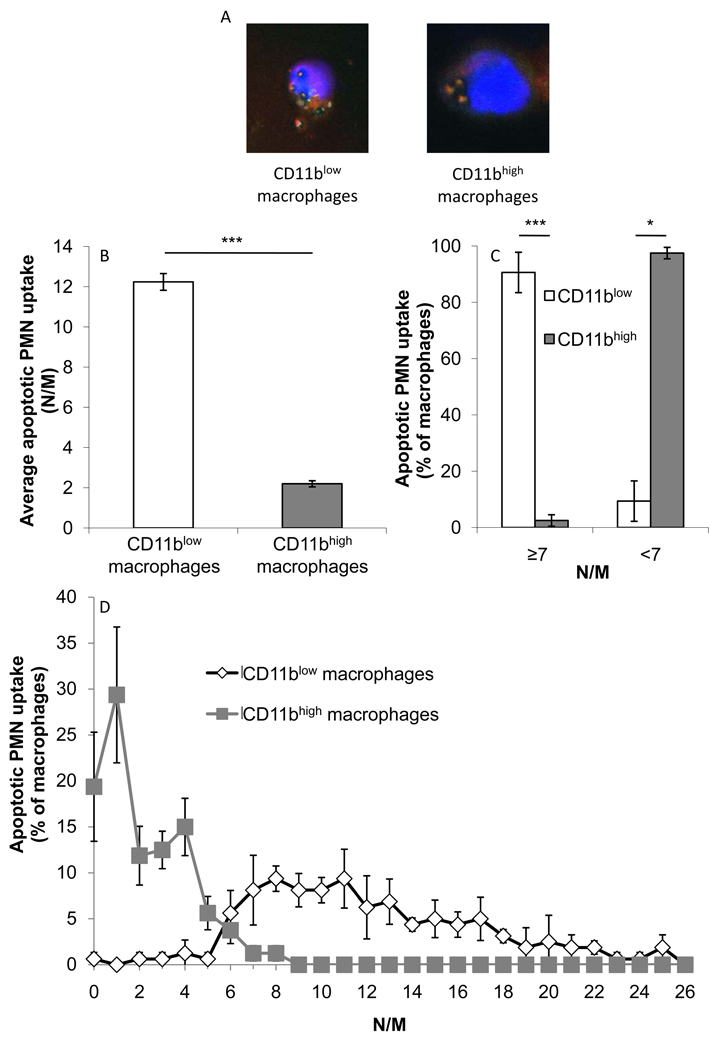
Clearance of apoptotic PMN from resolving inflammation sites is paramount for resolution and return to homeostasis [27]. Therefore, we examined whether CD11blow macrophages differ in their efferocytosis capacity from CD11bhigh cells. The results in Figure 2A–B indicate that CD11blow macrophages engulfed significantly more PMN on average than CD11bhigh macrophages (12.2 ± 0.2 neutrophils/macrophage (N/M) and 2.2 ± 0.2 N/M, respectively). LysoTracker staining (Fig. 2A and Supplemental figure 3) indicates that the apoptotic PMN visualized in macrophages are indeed phagocytosed and not merely attached to the macrophage surface. In fact, in this experimental setting CD11bhigh and CD11blow macrophages were distinguished by an engulfment threshold of seven PMN (Figure 2C): 90.6 ± 4.2% of the CD11blow macrophages engulfed seven or more PMN, whereas 97.5 ± 1.2% of the CD11bhigh macrophages engulfed less than seven PMN. The results in Figure 2D indicate that the differences between CD11bhigh and CD11blow macrophages in terms of engulfment of apoptotic PMN are distinct not only when engulfment thresholds are depicted but rather that there is very little overlap between the populations, meaning that the threshold of engulfment is enforced in a very narrow range and that in this experimental setting only macrophages that engulfed 6 PMN have the same tendency to be either CD11bhigh or CD11blow macrophages. Thus, CD11blow macrophages exhibited distinguishable pro-resolving properties, such as greater engulfment of apoptotic PMN.
Figure 2. CD11blow macrophages engulf higher numbers of apoptotic neutrophils than CD11bhigh macrophages.
Sorted CD11bhigh and CD11blow macrophages were stained with Hoechst and LysoTracker, analyzed by confocal microscopy and photographed as indicated (A; merged pictures). Similar preparations were enumerated for apoptotic PMN uptake and analyzed according to average (B) neutrophils engulfed per macrophage (N/M), and percentage of cells reaching engulfment threshold (C) or engulfing the indicated number of apoptotic PMN (D). Results are mean ± SE (B, C) or representative (A, D) from three experiments. Significant differences by Student's t test between CD11bhigh and CD11blow macrophages (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
CD11blow macrophages display reduced responsiveness to TLR ligands
Apoptotic cell engulfment by macrophages leads to their immune-silencing [14, 15, 28], which prevents unwanted excessive inflammatory responses during the resolution phase and catabasis. To determine whether CD11blow macrophages are immune-silent, we activated sorted macrophages with LPS, and determined the secretion levels of TNFα, IL-1β, IL-10, and TGFβ by CD11bhigh and CD11blow macrophages. The results (Figure 3A) indicate that CD11bhigh macrophages secreted twice as much TNFα as CD11blow macrophages, in the absence of LPS. Moreover, stimulation with LPS at 500 ng/ml resulted in a significantly elevated and maximal secretion of TNFα by CD11bhigh macrophages, whereas CD11blow macrophages did not increase their TNFα secretion. Similar results were obtained when the secretion of the pro-inflammatory cytokines and chemokines IL-1β, CCL2, CCL3, and CCL5 was determined (Figures 3B, 3E–G). Surprisingly, the secretion of IL-10, an anti-inflammatory cytokine, was also increased in CD11bhigh macrophages stimulated with LPS (Figure 3C). Of interest, the secretion of the resolution-promoting cytokine TGFβ was higher in CD11blow macrophages, but did not increase following activation with LPS (Fig. 3D).
Figure 3. CD11blow macrophages secret lower levels of pro-inflammatory cytokines and chemokines, but higher levels of TGFβ, in comparison to CD11bhigh ones.
Sorted CD11bhigh and CD11blow macrophages were activated with the indicated concentrations (A–B) or 1 µg/ml (C–G) of LPS, and evaluated for secreted TNFα (A), IL-1β (B) IL-10 (C), TGFβ (D), CCL2 (E), CCL3 (F), and CCL5 (G) using standard ELISA. N.A.- not available. Results are mean ± SE of four replicates from a representative of three experiments. Significant differences by Student's t test between CD11bhigh and CD11blow macrophages or between vehicle and LPS-stimulated macrophages (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
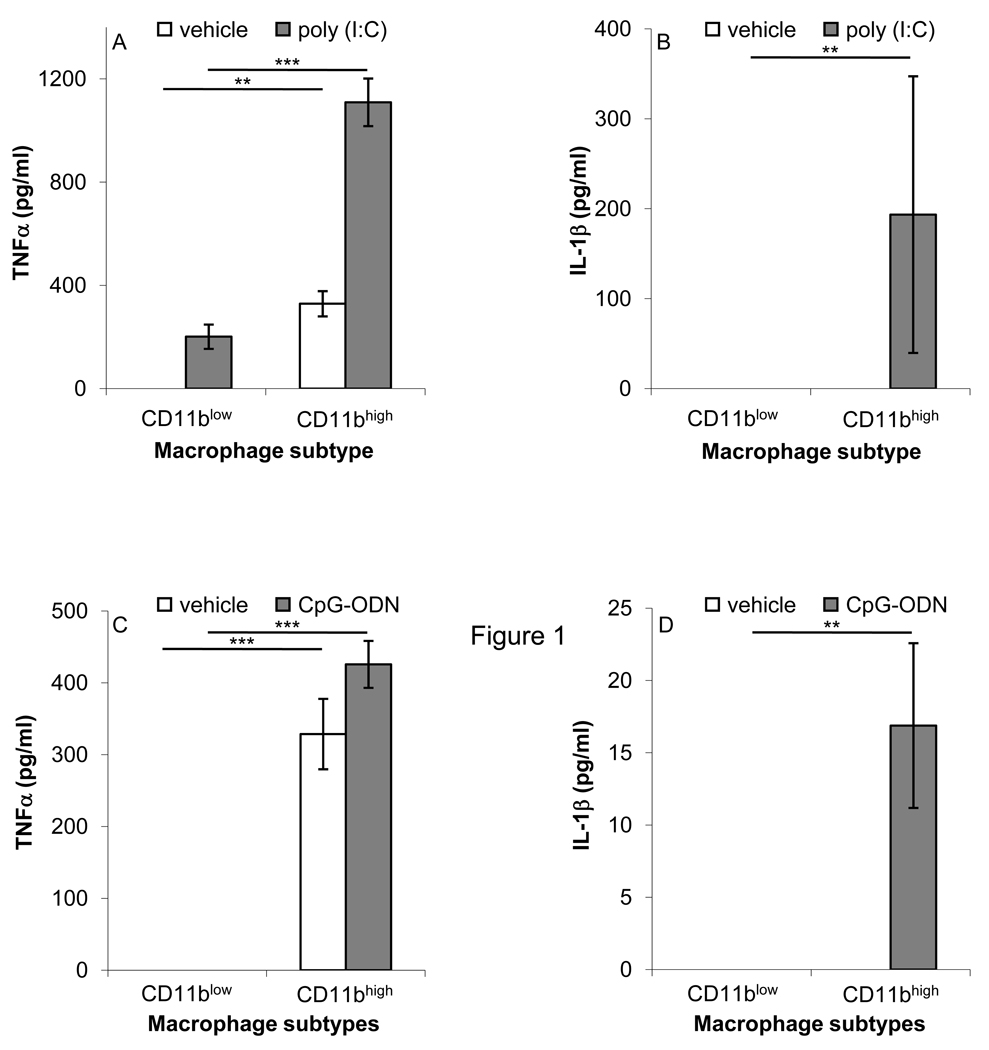
Immune-silent macrophages respond to a lower extent to different TLR ligands in terms of pro-inflammatory cytokine production [14, 29]. To determine whether CD11blow macrophages are indeed immune-silent, we stimulated CD11blow and CD11bhigh macrophages with the TLR3 and TLR9 ligands poly (I:C) and CpG-ODN, respectively (Figure 4), and determined the secretion of TNFα and IL-1β. The results indicate that both TNFα and IL-1β secretion were increased following exposure to poly (I:C) and CpG-ODN of CD11bhigh macrophages, whereas the secretion of these cytokines by CD11blow macrophages treated in the same manner was significantly lower. Of note, no significant reduction in the expression of TLR3, 4, and 9 was found in CD11blow macrophages, in comparison to their CD11bhigh counterparts (N=3, data not shown). Thus, CD11blow macrophages are poorer responders to different TLR ligands in terms of cytokine and chemokine secretion, and therefore are immune-silent.
Figure 4. CD11blow macrophages secret lower levels of pro-inflammatory cytokines in response to various TLR ligands.
Sorted CD11bhigh and CD11blow macrophages were activated with poly (I:C) (4 µg/ml; A–B), or CpG-ODN (1 µM; C–D) and evaluated for secreted TNFα (A,C), IL-1β (B,D) using standard ELISA. Results are mean ± SE of four replicates from a representative of three experiments. Significant differences by Student's t test between CD11bhigh and CD11blow macrophages or between macrophages stimulated with vehicle, poly (I:C), or CpG-ODN (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
CD11blow macrophages are "satiated"
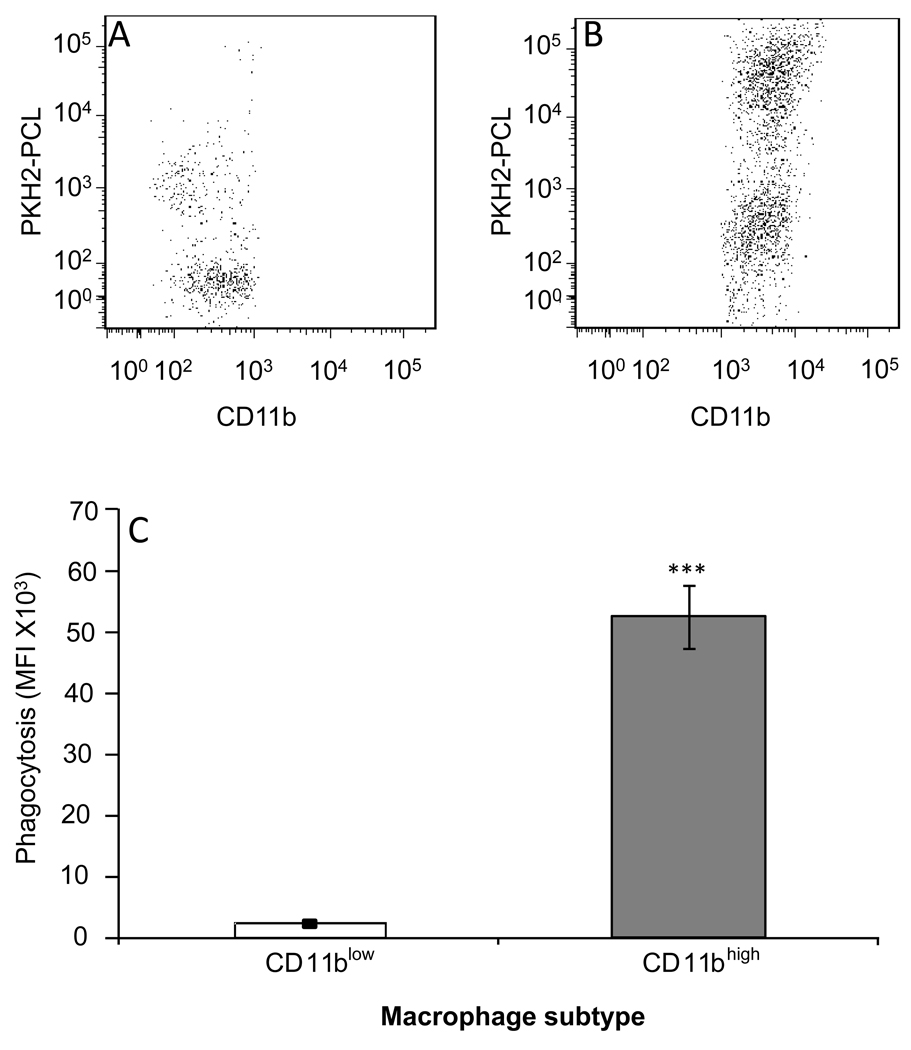
Since CD11b was found to be essential for the engulfment of iC3b-opsonized apoptotic cells by macrophages [12] , its reduced expression in macrophages that engulfed high number of apoptotic cells suggests CD11blow macrophages have lost their phagocytic function. To determine whether CD11blow and CD11bhigh macrophages differ in their ability to phagocytose external particles, the phagocyte-specific dye PKH2-PCL green was injected I.P. to mice undergoing peritonitis for 48 hrs and 4 hrs later the peritoneal cells were recovered, immune-stained, and analyzed for PKH2-PCL acquisition. The results in Figure 5 indicate that most CD11blow macrophages did not acquire PKH2-PCL (Fig. 5A), whereas the majority of CD11bhigh macrophages acquired higher amounts of PKH2-PCL (Fig. 5B). As a result the mean fluorescence intensity of PKH2-PCL was >10 fold higher in CD11bhigh macrophages (Fig. 5C). Of interest, a small portion of the CD11blow macrophages were labeled with PKH2 to a low extent, suggesting there is some residual phagocytic activity exerted by these cells, or that these macrophages converted from the CD11bhigh to the CD11blow phenotype during the assay period, and therefore lost the phagocytic capacity after acquiring a low amount of PKH2-PCL. Of interest, macrophage-PMN conjugates identified by FACS analysis as Ly-6G+F4/80+ doublets expressed higher levels of CD11b and F4/80 than CD11blow macrophages (N=8, data not shown) indicating that CD11bhigh macrophages are actively engulfing apoptotic PMN, while CD11blow macrophages are not. Thus, CD11blow macrophages are "satiated", meaning they lost their phagocytic potential upon meeting the apoptotic PMN engulfment threshold and reducing their CD11b expression.
Figure 5. CD11blow macrophages are satiated.
PKH2-PCL-green was injected I.P. to mice 48 hrs post peritonitis initiation. After 4 hrs, the peritoneal cells were recovered, immune-stained for F4/80 and CD11b and analyzed using FACSCalibur. Results are representative dot plot for CD11blow (A) and CD11bhigh macrophages (B), or mean MFI values± SE of 5 experiments (C). Significant differences by Student's t test between CD11bhigh and CD11blow macrophages (*** P value< 0.001) are indicated.
CD11blow macrophages are prone to emigrate to lymphoid organs
Most of the macrophages that are differentiating from monocytes in tissues during inflammation are emigrating to the draining lymph nodes and spleen [20, 30]. Our results indicate that CD11blow macrophages are not phagocytic (Fig. 5). Therefore, it is probable they are no longer required for resolution at the inflammation site, and might play a role in communicating the resolving state in a systemic manner. Hence, we sought to determine whether CD11blow macrophages are prone to depart resolving inflammation sites and emigrate to lymphoid organs. To this end, we determined the relative distribution of CD11bhigh and CD11blow macrophages 66 hrs post peritonitis initiation at the peritoneum, inguinal lymph node, and spleen. Our results indicate that CD11bhigh macrophages are the predominant macrophage subtype in the peritoneum during late resolution, whereas, at the same time CD11blow macrophages are the predominant macrophage subtype at the LN and spleen (Figure 6A). To examine whether CD11blow macrophages at the LN and spleen originated in the peritoneum, adoptive transfer experiments were performed in which peritoneal macrophages were isolated and labeled fluorescently, and then transferred to the peritoneum of mice undergoing peritonitis at the same period (48 hrs). The results in Figure 5B indicate that the distribution of labeled CD11bhigh and CD11blow macrophages at the peritoneum, inguinal LN, and spleen 18 hrs after transfer (Figure 6B) was similar to the distribution of unlabeled macrophages (Figure 6A). Thus, during the late phase of resolution peritoneal CD11blow macrophages are prone to emigrate to lymphoid organs and possibly transfer resolution-phase signals to the acquired immune system.
Figure 6. CD11blow macrophages are prone to migrate to lymphoid organs.
(A) Cells were recovered from the peritoneum, inguinal LN and spleen of mice undergoing peritonitis for 66 hrs. The cells were stained as above and CD11b expression on macrophages was determined by flow cytometry.
(B) Macrophages were recovered from peritoneal exudates 48 hrs post peritonitis initiation, labeled with CFSE, and transferred to recipient mice undergoing peritonitis for the same period. After 18 hrs, the cells from the peritoneum, inguinal LN, and spleen were recovered and the expression of CD11b on transferred macrophages was determined as above. Results presented in (A) and (B) are mean ± SE from three experiments, 5 mice per experiment. Significant differences by Student's t test between CD11bhigh and CD11blow macrophages (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
Interaction with apoptotic cells converts macrophages from the CD11bhigh to CD11blow phenotype
The results in Figures 2 and 6 suggest that CD11bhigh macrophages convert to CD11blow ones as they interact with apoptotic PMN and engulf them. To determine whether interaction with apoptotic cells is sufficient to reduce CD11b expression on macrophages, sorted CD11bhigh and CD11blow macrophages were incubated with or without apoptotic Jurkat cells and the changes in surface expression of CD11b were determined. The results in Figure 7 indicate that CD11b expression on the surface of both CD11bhigh and CD11blow macrophages was significantly reduced following their incubation with apoptotic cells. Of note, the level of expression of CD11b on both macrophage populations following interaction with apoptotic cells (AC) was lower than its level of expression on CD11blow macrophages recovered from peritoneal exudates, and therefore we designated the ex vivo-generated population CD11b− macrophages (Figure 7C). The detection of CD11b− macrophages ex vivo, but not in vivo, suggests that the initial reduction in CD11b expression detected in vivo might be sufficient to trigger macrophage departure of resolving tissues, and therefore CD11b− macrophages are not present in peritoneal cavities. On the other hand, the absence of exit routes in cell cultures allows CD11b down-regulation to reach significantly lower levels of CD11b expression. Interestingly, some reduction in CD11b expression on both CD11bhigh and CD11blow macrophages occurred following ex vivo culture in the absence of apoptotic cells, possibly due to the delayed effects of intracellular signals downstream of apoptotic cell recognition in vivo. Importantly, no significant reduction in surface expression of F4/80 or CD11b was observed following macrophage incubation with LB or IgG-opsonized LB (Supplemental Fig. 4A). Of interest, the impact of apopototic cells was partially mimicked by CD11b ligation with monoclonal antibodies (Supplemental Fig. 4A), which resulted in a decrease in CD11b, but not F4/80, surface expression, thus suggesting CD11b is involved in the signaling cascade that leads to its down-regulation. Notably, CD206 and CD163 surface expression was not modulated by AC ex vivo, but was reduced by anti-CD11b antibodies (Supplemental Fig. 4A). In addition, exposure to zymosan A, TGFβ, or live cells did not result in a significant reduction in the surface expression of CD11b (N=2, data not shown). Thus, the reduction in macrophage CD11b expression ex vivo is specific for interaction with apoptotic cells, and could not be achieve by treatment with other phagocytic targets, prototypic activating bacterial moieties, or pro-resolving cytokines, and is not due to the cell type of the apoptotic cells.
Figure 7. Interaction with apoptotic leukocytes converts CD11bhigh macrophages to CD11blow ones.
Sorted CD11bhigh (A) or CD11blow (B) macrophages were immune stained for CD11b immediately (A–B, dotted line), or incubated for 20 hrs without cells (A–B, hashed line) or with apoptotic Jurkat cells (1:5 ratio; A–B, continuous line). Then the unbound apoptotic cells were washed and the macrophages were recovered and immuno-stained for CD11b expression on their surface. Results are representative histograms (A, B; depicted macrophage populations indicated) or average MFI values ± SE (C) of 3 experiments. Significant differences by Student's t test between CD11bhigh and CD11blow macrophages (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
(D). Peritoneal macrophages were recovered 66 hrs post peritonitis initiation and incubated with apoptotic Jurkat cells or without cells as indicated. After 20 hrs the macrophages were recovered and lysed. The protein extracts were run by SDS-PAGE and analyzed by Western blot for the indicated proteins. Results are a representative set of blots from 3 experiments.
To determine whether macrophage interaction with apoptotic cells also triggers the major differences in protein expression distinguishing CD11bhigh and CD11blow macrophages that were indicated in Figure 1, peritoneal macrophages were incubated with apoptotic cells and changes in the cytoplasmic content of CD11b, arginase-1, 12/15-LO, and actin were determined. The results (Figure 7D) show that macrophages incubated with apoptotic cells expressed reduced levels of CD11b and arginase-1, and increased levels of 12/15-LO, as expected of conversion from the CD11bhigh to the CD11blow phenotype. Interestingly, the levels of detergent-soluble actin in macrophages were also reduced following incubation with apoptotic cells. These findings are consistent with the findings in Fig. 1 that showed reduced actin levels in lysates of CD11blow macrophages, in comparison to their CD11bhigh counterparts. These differences are probably due to the increased numbers of actin-associated phagosomes formed in these cells. Importantly, ex vivo exposure of macrophages to senescent neutrophils, but not to latex beads (LB) or IgG-opsonized LB, resulted in a significant reduction in CD11b and arginase-1 expression (Supplemental Fig. 4B), whereas 12/15-LO expression was increased in this setting by all phagocytic targets. Together, these results indicate that interaction with apoptotic cells is sufficient to drive the conversion of CD11bhigh to CD11blow macrophages.
RvD1, RvE1, and Dex reduce the PMN engulfment threshold required for macrophage immune-silencing
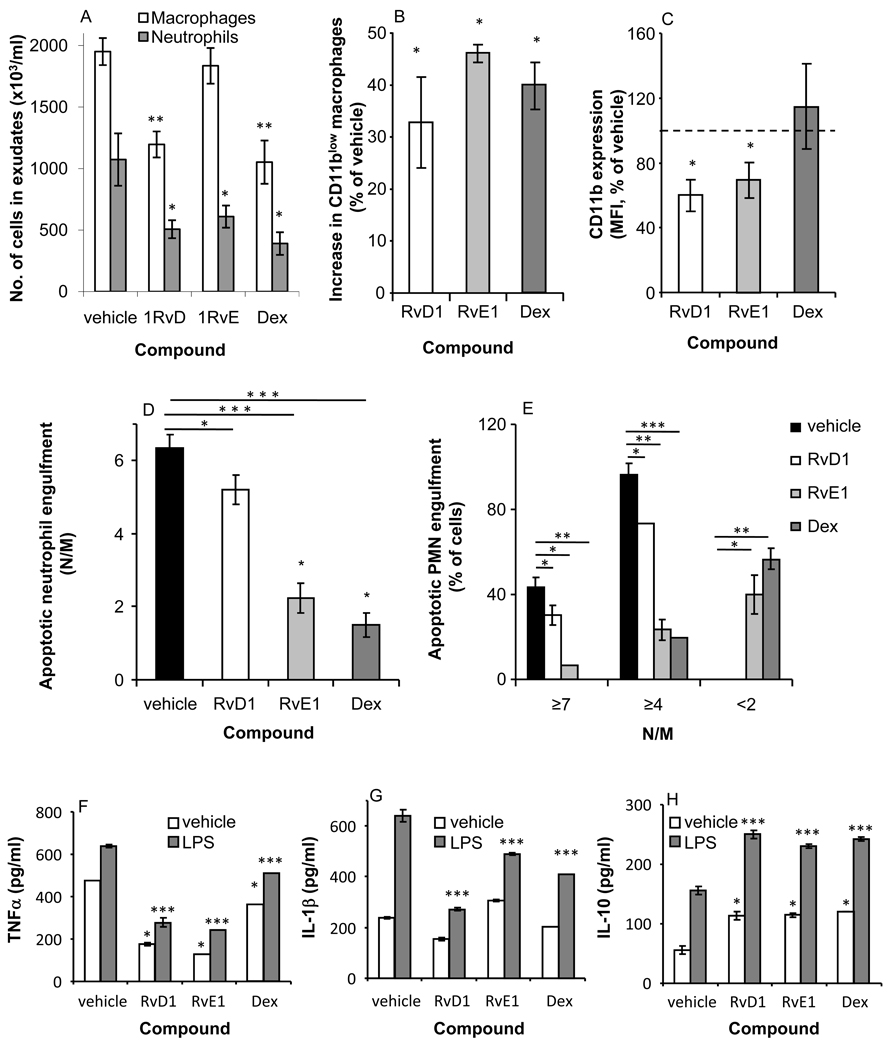
Resolvins are resolution-phase-generated mediators derived from ω-3 polyunsaturated fatty acids; they were found to promote resolution by acting on PMN and macrophages [20, 31, 32]. Similar properties were attributed to glucocorticoids, such as Dex, that inhibit leukocyte infiltration to inflammation sites and promote the clearance of apoptotic PMN by macrophages (reviewed in [33]). To determine whether pro-resolving mediators regulate emergence of CD11blow macrophages and thereby promote resolution, RvD1, RvE1, or Dex were introduced into peritonitis-affected mice, and the recovered leukocytes were collected and analyzed for macrophage and neutrophil numbers, macrophage CD11b expression, engulfment of PMN, and responsiveness to LPS. Our results indicate (Figure 8A) that RvD1, RvE1 and Dex induced a reduction in neutrophil numbers, whereas RvD1 and Dex, but not RvE1 also reduced the number of macrophages in peritoneal cavities. In addition, RvD1, RvE1 and Dex enhanced (Fig. 8B) the appearance of CD11blow macrophages (32.8 ± 8.8%, 46.2 ± 1.8%, and 39.9 ± 4.6% increases over vehicle treatment, for RvD1, RvE1, and Dex, respectively)in pertineal exudates. Of interest, RvD1 and RvE1, but not Dex, also reduced CD11b expression on CD11bhigh macrophages (Figure 8C). To determine whether RvD1, RvE1, and Dex regulate apoptotic PMN engulfment by macrophages during resolution, exudate cells were enumerated for PMN engulfment and analyzed as in Fig. 2. Surprisingly, the results in Figure 8D indicate that RvD1 and, to a greater extent, RvE1 and Dex, reduced the numbers of apoptotic PMN engulfed by macrophages present in the peritoneum. A detailed analysis of engulfment according to thresholds (Figure 8E) indicated that RvE1 and Dex, but not RvD1, induced the appearance in the peritoneum of low-engulfing macrophages, designate “inexperienced” since they phagocytosed less than 2 N/M.
Figure 8. RvD1, RvE1, and Dex modulate CD11b expression, apoptotic PMN engulfment, and cytokine secretion by macrophages.
Mice undergoing peritonitis were injected I.P. with RvD1, RvE1 (100 ng/mouse), Dex (25 µg/mouse) or vehicle 48 hrs after peritonitis initiation. At 66 hrs, the peritoneal exudate cells from all treatments were recovered, enumerated and immuno-stained for Ly-6G, F4/80, and CD11b, as above, and analyzed by flow cytometry to determine the number of macrophages and neutrophils (A), percentage of CD11blow macrophages (B) and the level of CD11b expression on CD11bhigh macrophages (C). Some of the exudate cells were also stained with Hoechst and apoptotic PMN engulfment by macrophages was determined (D–E). In addition, macrophages were isolated from all treatments and stimulated ex vivo with LPS (500 ng/ml). After 24 hrs, the secretion of TNFα (F), IL-1β (G), and IL-10 (H) from the macrophages was determined as above. Results are mean ± SE of four replicates from a representative of three experiments. Significant differences by ANOVA between leukocytes and macrophages from mice treated with vehicle and macrophages treated with RvD1, RvE1, or Dex (* P value< 0.05, ** P value< 0.005, *** P value< 0.001) are indicated.
To validate the improvement in immune-silencing of macrophages that followed treatment with RvD1, RvE1 and Dex, macrophages were activated with LPS, and cytokine secretion was determined. The results in Figure 8F indicate that RvD1 and RvE1, and, to a lesser extent, Dex, inhibited the secretion of TNFα from unstimulated and LPS-stimulated macrophages. A similar response was observed (Figure 8G) with RvD1, RvE1 and Dex when IL-1β secretion from LPS-stimulated macrophages was determined. Of interest, the secretion of IL-10, a pro-resolving cytokine generated following the ingestion of apoptotic cells [17], was upregulated by each of the pro-resolving mediators, in unstimulated and LPS-stimulated macrophages (Figure 8H). Thus, treatment with RvD1, RvE1, and Dex, promoted macrophage immune-silencing, as well as the secretion of pro-resolving cytokines from these cells.
Discussion
Macrophages are currently believed to display two functionally distinct phenotypes: classically- and alternatively- activated macrophages, termed M1 and M2, respectively. M2 macrophages are also subdivided into M2a, M2b, and M2c, based on different profiles of responses following exposure to different macrophage-polarizing mediators, such as immune complexes, TH2 cytokines, or glucocorticoids [1, 2]. The encounter of macrophages with apoptotic cells was proposed as an additional polarizing trigger that evokes TH2-like responses, as well as the expression of arginase-1, and 12/15-LO [19]. A seminal study from Bystrom and colleagues [29] has identified hybrid resolving-phase macrophages (rMs) that exert properties of M1 macrophages, such as the expression of iNOS and COX 2, and at the same time express the mannose receptor and arginase-1 and secret lower levels of inflammatory cytokines, but high levels of IL-10, which are properties of M2 [2]. In addition, rMs were found to possess a greater propensity to stay in the peritoneum and promote homeostatic responses at the resolving tissue. Here, we describe a distinct subtype of macrophages that appear to populate the peritoneal cavity simultaneously to rMs. However, these CD11blow macrophages express neither iNOS nor arginase-1, while expressing lower levels of COX 2 and MMP9 (M1 enzymes) than their CD11bhigh counterparts (Figure 1). Of note, CD11blow, but not CD11bhigh macrophages express the lipid converting enzyme 12/15-LO, which is involved in the production of pro-resolving lipid mediators, such as 15-hydroxydocosahexaenoate (HETE), lipoxins, protectins, and resolvins [3, 19, 34, 35].
Although the clearance of apoptotic cells by macrophages and its consequent immune-silencing of macrophages has been extensively described [14, 17, 36, 37], no identification of a nonphlogistic macrophage population in vivo has been provided. Moreover, evidence for a quantitative requirement for macrophages engulfment of apoptotic PMN to result in their immune-silencing in vivo is still lacking. CD11b was found to be involved in complement-mediated engulfment of apoptotic cells by macrophages [12], and recent studies indicate its binding to different ligands results in a significant immune-suppression in macrophages [38, 39]. In addition, CD11b expression on immature dendritic cells was downregulated following the iC3b-mediated ingestion of apoptotic cells [40]. Our findings determine that CD11blow macrophages that appear in resolving inflammatory sites during the late resolution phase [26] engulf more apoptotic PMN than CD11bhigh macrophages (Figure 2) and secret lower amount of pro-inflammatory cytokines and chemokines, but not TGFβ in response to TLR ligands (Figures 3 and 4), thereby fulfilling the designation of pro-resolving macrophages. Of interest, CD11blow macrophages did not produce IL-10, in accord with earlier studies that examined the responses of macrophages to apoptotic cell instillation in vivo [14].
Notably, CD11blow macrophages were found to be the prominent F4/80+ macrophage subtype in the LN and spleen, in particular in the population that originated in the peritoneum (Figure 6), where the CD11bhigh macrophages were the prevalent macrophage subtype, indicating a conversion from the CD11bhigh to CD11blow phenotype is required for the emigration of macrophages to lymphoid organs during the resolution of inflammation. Indeed, we also found that incubation of CD11bhigh macrophages with apoptotic cells ex vivo resulted in a significant enhancement of CD11b down-regulation (Figure 7). Moreover, this reduction in CD11b expression is associated with changes in the protein expression signature characteristic of the CD11bhigh to CD11blow conversion, thereby indicating a complete phenotype change driven by satisfying macrophage interaction with apoptotic cells. Overall, our results show that CD11bhigh macrophages seem to display mixed properties of M1 and M2, while CD11blow macrophages, although originating in the CD11bhigh population, express a unique phenotype. Putting our current study in perspective with previous works that characterized macrophages during resolution [20, 29] and following their interaction with apoptotic cells [19], it is tempting to suggest that the phenotype displayed by the macrophage depends on the number of apoptotic PMN that it engulfed, as well as the environment it is operating in. Hence, the functional scheme presented in figure 9A–C takes place.
Figure 9. A functional scheme for macrophage phenotype conversions during resolution-phase efferocytosis.
A macrophage that infiltrated an inflamed tissue adopts an M1-like phenotype (including expression of inflammatory cytokines and chemokines, iNOS, and COX 2 [1]) previous to encounter with apoptotic PMNs (A). Once it encounters apoptotic PMN and starts to engulf them, the macrophage switches to an M2-like phenotype that is involved in tissue repair and return to homeostasis. This switch is accompanied by an increase in the expression of arginase-1 and IL-10, whereas a decrease in the expression of pro-inflammatory cytokines, as well as COX-2, and iNOS also takes place (B). Reports documenting a role for M2 cytokines in the resolution of inflammation and efferocytosis [42, 43] seem to support this part of our scheme. As the engulfment of apoptotic PMN by the macrophage continues and reaches a threshold level (satiating efferocytosis) the macrophage undergoes another switch to the CD11blow phenotype (C) that secretes neither pro-inflammatory cytokines and chemokines nor IL-10, but does secret higher levels of TGFβ. CD11blow macrophages also do not express iNOS or arginase-1, and express only low levels of COX-2. Interestingly, this switch leads to the expression of 12/15-LO, which is exclusively expressed by CD11blow macrophages and was previously reported to be expressed in a TGFβ-dependent manner following the interaction of macrophages with apoptotic cells [19]. The second phenotype switch also seems to stop CD18/CD11b-mediated apoptotic PMN engulfment [8, 12] thereby allowing rapid macrophage departure of the resolving tissue and emigration to lymphoid organs. At these target organs CD11blow macrophages presumably produce 12/15-LO-derived pro-resolving lipid mediators, and deliver homeostatic signals to antigen presenting cells and lymphocytes [34, 44]. Satiating efferocytosis can be modulated by pro-resolving mediators, such as RvD1, RvE1, and Dex (D). This modulation enhances the immune-silencing and departure of pro-resolving CD11blow macrophages to the lymphatics, where they can contribute to the termination of the acquired immune response. RvE1 and Dex also promote the infiltration of new macrophages to the resolving tissue to continue the necessary clearance of apoptotic PMN and return to homeostasis.
As the engulfment of apoptotic PMN is not a synchronized event and macrophages continue to infiltrate into the resolving inflammation site during resolution, the macrophage population in the peritoneum at any given time is probably heterogeneous. Integrating the results from Bystrom et al. with our study we conclude that the majority of the macrophages present at the peritoneum during late resolution (rMs or CD11bhigh macrophages) are either M1-like or M2-like, although a hybrid or M1-to-M2 transition phenotype cannot be excluded. These macrophages are distinct from CD11blow macrophages that are apparently more adept at migration to lymphoid tissues and delivery of resolution cues to the acquired immune system.
Importantly, CD11blow macrophages were also found to lose their phagocytic activity (Figure 5) upon conversion from CD11bhigh ones, and therefore were termed "satiated macrophages". This is, to the best of our knowledge, the first indication that the engulfment of apoptotic PMN by macrophages is self-limiting, and therefore serves as a feedback mechanism that can modulate macrophage pro-resolving functions and site of action (the resolving tissue or the lymphatics). Therefore, once a macrophage reaches the engulfment threshold for apoptotic PMN uptake it is undergoing immune-silencing and satiation. Consequently, the macrophage departs the resolving tissue through the lymphatics, as it can make no further contribution to resolution on site.
RvD1 and RvE1 exhibit a range of anti-inflammatory and pro-resolving actions (reviewed in [22]). Dex is also a potent regulator of acute immune responses, and mediates pro-resolving functions, primarily through the generation of annexin A1 peptides, which act through the LXA4 receptor [24]. Notably, both Dex and LXA4, as well as RvE1 promote the engulfment of apoptotic PMN by macrophages during the resolution of inflammation [8, 20, 25]. The present report reveals a novel pro-resolving function for RvE1, RvD1 and Dex, namely, reducing the number of engulfment-related events required for immune-silencing of macrophages, illustrated in Fig. 9. In fact, RvE1 and Dex reduced the number of macrophages that engulfed seven or more apoptotic PMN by 84.6% and 100%, respectively, while increasing the number of CD11blow macrophages by 46.2% and 39.9%, respectively (Fig. 8). This function enables enhanced immune-silencing of macrophages at resolving sites, and might promote their departure from resolving sites, as was previously reported for RvE1 [20]. Moreover, the emergence of “inexperienced” macrophages that engulfed less than 2 PMNs following the introduction of RvE1 and Dex at resolving cavities (Fig. 8E), suggests these compounds increase turnover of macrophages at the resolving site to “compensate” for the reduced engulfment by each macrophage and thereby to enhance apoptotic cell clearance. Therefore, the reduction in apoptotic PMN found in peritoneal macrophages during resolution after treatment with RvE1 and Dex presumably stems from the cumulative actions of each of these compounds that include induction of infiltration of additional macrophages to the peritoneum, as well as promotion of the departure of satiated macrophages following their immune-silencing. Of note, RvE1 and RvD1 inhibited LPS-stimulated TNFα production from peritoneal macrophages to a higher extent than Dex, despite being used at a 250 times lower dose, which suggests that resolvins will exhibit improved actions over glucocorticoids in treatment of pathologies associated with hampered resolution.
In sum, our study revealed a new population of macrophages that display pro-resolving properties important for completing the resolution sequel and for communicating the return to a homeostatic state at lymphoid organs during resolution of the acute inflammatory response. In addition, we uncovered novel actions for resolvins and glucocorticoids on macrophages during the resolution of inflammation, which could indicate new therapeutic benefits in acute inflammatory disorders.
Materials and Methods
Reagents
ELISA kits for mouse TNFα, IL-1β, IL-10, TGFβ, CCL2, CCL3, and CCL5 were obtained from R&D Systems. Carboxyfluorescein succinimidyl ester (CFSE), staurosporine (STS), LPS (from e.coli, clone 055:B5), PKH2-PCL green fluorescence linker kit, and Dex from Sigma. Poly (I:C) and CpG-oligodeoxynucleotides (CpG-ODN) from InvivoGen. RvE1 (5S,12R,18R-trihydroxy-4Z,8E,10E,14Z,16E-eicosapentaenoic acid) and RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) were obtained from Cayman Chemicals and the synthetic resolvins were matched according to previously published biological and physical material [32, 41].
Murine peritonitis
Briefly, male C57BL/6 mice (6–8 wk; protocol approved by the Committee of Ethics, University of Haifa, authorization no. 088/07) were injected I.P. with zymosan A (1 mg). After 66 hrs, peritoneal exudates were collected and exudate cells were stained with FITC-conjugated rat anti-Ly-6G, PE-conjugated rat anti-F4/80, and PerCP-conjugated rat anti-mouse CD11b (Biolegend) and analyzed by FACSCalibur (Beckton-Dickinson). In some experiments the macrophages were sorted to CD11bhigh and CD11blow populations of >95% purity, using FACSaria (Beckton-Dickinson), and the separate populations were used for microscopic analysis and for ex vivo stimulation. In some experiments, PKH2-PCL green (0.25 µM, 0.5 ml) was injected I.P. at 48 hrs and peritoneal cells were recovered 4 hrs later, immuno-stained for F4/80 and CD11b as above and analyzed for fluorescence intensity of different macrophage populations. In relevant experiments, vehicle, RvD1, RvE1 (100 ng/mouse each), or Dex (25 µg/mouse) were introduced to the peritoneum 48 hrs after peritonitis initiation, and the exudates were recovered after 66 hrs and analyzed as before. For detailed methodologies please see "supporting information".
Western blot analysis
Protein extracts of sorted populations (>95% purity) of CD11bhigh and CD11blow macrophages were run using 10% SDS-PAGE (5 µg/lane), transferred to nitrocellulose membranes and immuno-blotted with either goat anti-mouse CD11b (SantaCruz biotechnology), rabbit anti-mouse iNOS (Abcam), goat anti-mouse araginase-1 (Abcam) Rabbit anti-mouse COX-2 (Cayman chemicals), rabbit anti-mouse 15-lipoxygenase-1 (Cayman chemical), goat anti-mouse MMP-9 (R&D systems), goat anti-mouse β-actin (SantaCruz biotechnology) or goat anti mouse tubulin (SantaCruz biotechnology). Then, the membranes were washed, and incubated with the appropriate HRP-conjugated secondary antibody. The blots were washed and developed using EZ-ECL (Biological Industries).
Macrophage transfer
Macrophages were isolated from peritoneal exudates 48 hrs post peritonitis initiation, stained with CFSE (1 µM) and injected into the peritoneum of mice that underwent peritonitis for 48 hrs. After an additional 18 hrs, cells from peritoneal exudates, inguinal lymph nodes and spleen were recovered from the recipient mice, immuno-stained as above, and the percentage of CD11bhigh and CD11blow macrophages in each site was determined.
Confocal microscopy
Sorted CD11bhigh and CD11blow macrophages were isolated and loaded with 50 nM LysoTracker Red DND 99 dye (Molecular Probes) for 2h at 37 °C in RPMI. Cells were then fixed with 2% paraformaldehyde at room temperature and stained with Hoechst (Molecular Probes) and FITC-conjugated anti-mouse Ly-6G. Mounted slides were kept in the dark at 4°C until analyzed by confocal microscopy. Confocal images were acquired using Z-sections of 1 µm thickness. The images were processed with Zeiss LSM Image Browser software.
Apoptotic PMN engulfment enumeration
Exudate cells or sorted CD11bhigh and CD11blow macrophages were stained with Hoechst, and enumerated under a fluorescent microscope (Zeiss). Two areas of two cover slips, each containing at least 50 (overall 200) macrophages were analyzed, and the average number of PMN engulfed per macrophage, as well as the number of macrophages with cutoff numbers of engulfed PMN were calculated. In sorted cells, F4/80+/Ly-6G+ entities (identified as macrophages that are attached to but did not fully engulf a PMN by forward vs. side scatter analysis) were excluded from the samples to avoid counting of attached PMNs.
TLR-mediated responsiveness ex vivo
Exudate macrophages were sorted or separated using PE selection magnetic beads (StemCell Technologies) and incubated (1 × 106 cells in 0.5 ml of culture media) with LPS (0–1000 ng/ml), poly (I:C) (4 µg/ml), or CpG-ODN (1 µM). After 16 hrs the supernatants were collected and their TNFα, IL-1β, IL-10, TGFβ, CCL2, CCL3, and CCL5 contents were determined by standard ELISA.
Regulation of macrophage phenotype by apoptotic cells ex-vivo
Jurkat cells were treated with 1 µM staurosporine (4 hrs, Sigma) to induce apoptosis, and washed. Then, peritoneal macrophages or sorted subpopulations thereof were incubated in the presence or absence of apoptotic Jurkat cells (1:5 macrophage to apoptotic cell ratio). At the beginning of the incubation and after 20 hrs, macrophages were immuno-stained for CD11b and analyzed by FACS analysis. Alternatively, protein extracts were prepared from the macrophages after the incubation period and run by SDS-PAGE followed by Western blot analysis for CD11b, arginase-1, 12/15-LO, actin, and tubulin, as above.
Statistical analysis
Ex vivo and in vivo experiments were performed at least 3 times with at least 4 replicates. Results were analyzed by one-way analysis of variance (for multiple groups) or Student’s t test (for comparison between 2 groups) with p values < 0.05 considered as statistically significant. Results are expressed as means ± SEM.
Supplementary Material
Acknowledgements
This work was supported by grants from the Israel Science Foundation, the Nutricia Research Foundation, and the Marc Rich Foundation (to A.A.) and the National Institute of Health (P50 DE016191 to C.N.S.). A.A. is a recipient of the young scientist award from Teva Pharmaceuticals Ltd.
Abbreviations
- CR3
complement receptor 3
- Rv
resolvin
- Dex
dexamethasone
- MFG-E8
milk fat globule-EGF-factor 8
- LO
lipoxygenase
Footnotes
Conflict of interest: CNS is inventor on resolvins as biotemplates for novel therapeutic development assigned to Brigham and Women’s Hospital, Partners Healthcare and are licensed for clinical development. The other authors declare no other competing financial interests.
References
- 1.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 3.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 5.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. New York: Oxford University Press; 2004. [Google Scholar]
- 6.Rossi AG, Haslett C. Inflammation, cell injury, and apoptosis. In: Said SI, editor. pro-inflammatory and anti-inflammatory peptides. 1998. pp. 9–24. [Google Scholar]
- 7.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 8.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 9.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 10.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 12.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel VA, Longacre A, Hsiao K, Fan H, Meng F, Mitchell JE, Rauch J, Ucker DS, Levine JS. Apoptotic cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant over those triggered by necrotic cells: Implications for the delayed clearance model of autoimmunity. J Biol Chem. 2006;281:4663–4670. doi: 10.1074/jbc.M508342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 19.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006 doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 20.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. Faseb J. 2004;18:1716–1718. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 22.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Gilroy DW, Perretti M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr Opin Pharmacol. 2005;5:405–411. doi: 10.1016/j.coph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. Faseb J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- 26.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 27.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 28.Tassiulas I, Park-Min KH, Hu Y, Kellerman L, Mevorach D, Ivashkiv LB. Apoptotic cells inhibit LPS-induced cytokine and chemokine production and IFN responses in macrophages. Hum Immunol. 2007;68:156–164. doi: 10.1016/j.humimm.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 31.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 33.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 34.Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The Docosatriene Protectin D1 Is Produced by TH2 Skewing and Promotes Human T Cell Apoptosis via Lipid Raft Clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 35.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. Faseb J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 38.Amarilyo G, Verbovetski I, Atallah M, Grau A, Wiser G, Gil O, Ben-Neriah Y, Mevorach D. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur J Immunol. 2010;40:699–709. doi: 10.1002/eji.200838951. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, Arthur JS, Kalliolias GD, Ivashkiv LB. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity. 2010;32:518–530. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, Kutikov I, Gill O, Mevorach D. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS One. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.