Cleavage of proaugurin is required for suppression of tumor cell proliferation.
Abstract
Augurin is a secretory molecule produced in pituitary, thyroid, and esophagus and implicated in a wide array of physiological processes, from ACTH release to tumor suppression. However, the specific proaugurin-derived peptides present in various cell types are not yet known. In order to shed light on the posttranslational modifications required for biological activity, we here describe the posttranslational processing of proaugurin in AtT-20 and Lovo cells and identify proaugurin-derived products generated by convertases. In vitro cleavage of proaugurin with proprotein convertases produced multiple peptides, including a major product with a mass of 9.7 kDa by mass spectrometry. Metabolic labeling of C-terminally tagged proaugurin in AtT-20 and AtT-20/PC2 cells resulted in a major 15-kDa tagged form on SDS-PAGE, which likely corresponds to the 9.7-kDa in vitro fragment, with the added tag, its linker, and posttranslational modification(s). The secretion of neither proaugurin nor this cleavage product was stimulated by forskolin, indicating its lack of storage in regulated secretory granules and lack of cleavage by PC2. Incubation of cells with the furin inhibitor nona-d-arginine resulted in impaired cleavage of proaugurin, whereas metalloprotease inhibitors did not affect proaugurin proteolysis. These data support the idea that proaugurin is cleaved by furin and secreted via the constitutive secretory pathway. Interestingly, proaugurin was sulfated during trafficking; sulfation was completely inhibited by brefeldin A. Proliferation assays with three different tumor cell lines demonstrated that only furin-cleaved proaugurin could suppress cell proliferation, suggesting that proteolytic cleavage is a posttranslational requirement for proaugurin to suppress cell proliferation.
Augurin, also known as esophageal cancer-related gene 4 (ECRG4), is a secretory molecule highly conserved among vertebrates (1). The presence of augurin has been reported in the pituitary, brain, heart, and adrenal (1). In addition, an online gene distribution database, BioGPS (http://biogps.gnf.org), indicates that augurin mRNA expression is high in the pituitary gland, thyroid, and esophagus. The potential function of augurin has been reported in recent literature as a tumor/metastasis suppressor gene (2–4); a factor linking neural cell senescence and aging (5); and an enhancer of ACTH release from the hypothalamus (6). Another study demonstrated that cellular expression of augurin is increased upon initiation of chondrocyte differentiation and cartilage destruction (7). Although these important studies focus on the potential functions of augurin, the endogenous forms of proaugurin-derived peptides have yet to be determined.
Secretory proteins undergo a number of posttranslational modifications including: proteolytic processing, C-terminal amidation, tyrosine O-sulfation, N-/O-acylation, and N-/O-glycosylation during maturation to attain bioactive status. In addition, it has been shown that tissue-specific proteolytic processing can affect bioactivity (8–12). Thus, obtaining information about specific cleavage patterns and posttranslational modifications is critical for understanding functional properties. Prohormone convertases (PC), a family of seven convertases, are known to participate in the synthesis of many different peptide hormones by generating mature secretory proteins through cleavage at arginine-containing consensus cleavage sites (12). The neural and endocrine cell-specific convertases PC1/3 and PC2 are involved in the regulated secretory pathway and synthesize specific neuropeptides and peptide hormones (13–16). On the other hand, furin, which is ubiquitously distributed, is involved in constitutive secretory pathway processing and production of mature/intermediate forms of many secretory proteins including cell surface proteins (17–20). Furin follows a highly regulated trafficking itinerary through several trans-Golgi network /endosomal compartments to the cell surface. This itinerary enables furin to process diverse collections of proproteins in various subcellular locations, such as the trans-Golgi network and the endocytic pathway (reviewed in Ref. 19). Furin can also accomplish extracellular proteolysis, similarly to metalloproteases (18), by action while on the plasma membrane (19, 21).
In the report below, we have used endocrinological and biochemical approaches to describe posttranslational modifications of proaugurin and discuss their potential roles in the activation of proaugurin.
Results
Matrix-assisted laser desorption ionization-time of flight (MALDI-ToF) mass spectrometry reveals three proaugurin-derived products generated by in vitro PC cleavage
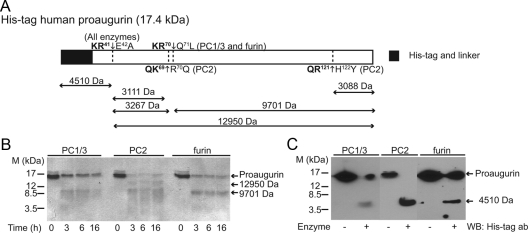
Using Neuropred, an online prediction tool for proprotein convertase cleavage sites [http://neuroproteomics.scs.illinois.edu/neuropred.html (22)], proaugurin is predicted to contain two potential cleavage sites (Fig. 1A). To determine whether these cleavage sites are actually used by PC and furin, recombinant human proaugurin was subjected to in vitro cleavage reactions with recombinant PC1/3, PC2, and furin. Figure 1B indicates that all three convertases were able to cleave recombinant proaugurin into smaller fragments. Digested proaugurin samples were subjected to MALDI-ToF mass spectrometry to determine the identity of the cleavage products. The mass spectometry results demonstrate that PC1/3 and furin cleave at two sites, LQKR41↓E42APV and RQKR70↓Q71LWD, producing peptides with masses of 9701 Da, 4511 (or 4512) Da, and 3272 (or 3269) Da (Table 1 and Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). By contrast, three cleavage sites were used by PC2, LQKR41↓E42APV, KRQK69↓R70QLW and YYQR121↓H122YDE to produce peptides with masses of 9900 Da, 4511 Da, 3117 Da, and 3087 Da (Table 1 and Supplemental Fig. 1).
Fig. 1.
Proaugurin is cleaved by PC1/3, PC2, and furin in vitro. A, Schematic model of His-tagged human proaugurin and the masses of cleavage products predicted by ProteinProspector. Cleavage sites are shown in bold. Recombinant His-tagged human proaugurin (2 μg) was cleaved by PC1/3, PC2, and furin at 37 C for the indicated time periods. Samples were separated on 18% acrylamide gels and stained with CBB (panel B) or subjected to Western blotting (panel C). ab, Antibody; WB, Western blotting.
Table 1.
Peptides identified by MALDI-TOF mass spectrometry
| Peptide | Predicted mass (Da) | Observed mass (Da) |
||
|---|---|---|---|---|
| furin | PC1/3 | PC2 | ||
| His tag-R41 | 4510 | 4511 | 4512 | 4511 |
| E42-R70 | 3267 | 3272 | 3269 | N.D. |
| E42-K69 | 3111 | N.D. | N.D. | 3117 |
| Q71-Y148 | 9701 | 9701 | N.D. | N.D. |
| R70-Y148 | 9857 | N.D. | N.D. | 9900 |
| H122-Y148 | 3088 | N.D. | N.D. | 3087 |
| E42-Y148 | 12950 | N.D. | N.D. | N.D. |
Each predicted mass was calculated using ProteinProspector. N.D. indicates not detected. The His tag-R41 fragment consists of the His-tag-linker region and augurin (N32-R41) with calculated masses of 3257 and 1271 Da, respectively; after the subtraction of 18 Da due to the formation of peptide bond between the 3257- and 1271-Da fragments, the total predicted mass of the His tag-R41 fragment is therefore 4510 Da.
Trafficking of proaugurin occurs via the constitutive secretory pathway
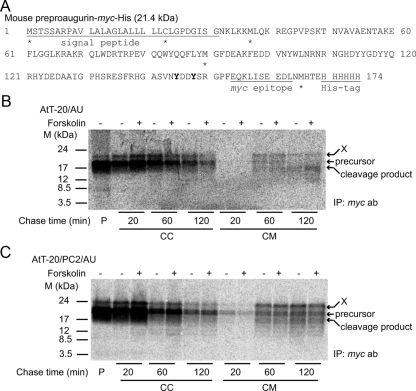
In vitro proteolysis reactions demonstrated that all convertases tested, PC1/3, PC2, and furin, can cleave recombinant proaugurin into smaller fragments (Fig. 1, B and C). However, cellular forms of proaugurin-derived peptides have not yet been determined. To elucidate the sizes of endogenously generated proaugurin-derived peptides, pulse-chase experiments using [35S]methionine/cysteine were performed using AtT-20 cells and AtT-20 cells stably expressing PC2 (AtT-20/PC2), both transiently expressing tagged proaugurin. Aside from the initiating methionine, both methionine and cysteine are present within preproaugurin at positions −23, −37, and −90, and within the C-terminal tag (Fig. 2A). AtT-20 cells are a well-studied mouse anterior pituitary tumor cell line that expresses predominantly PC1/3 and little PC2 (23), whereas AtT-20/PC2 cells represent a PC2-transfected AtT-20 cell line known to process the endogenous substrate, proopiomelanocortin, at PC2 cleavage sites (23, 24). Figure 2, panels B and C, shows that labeled proaugurin species were secreted from cells both as the intact (∼17 kDa) and as cleaved forms (∼15 kDa). Comparison of the band intensities of the various labeled augurin forms indicates that the ratio of secreted to cellular proaugurin forms increased according to the length of the chase period; 0–1% in 20-min chased media; 10–20% in 60-min chased media; and 35–50% in 120-min chased media. These data indicate that no significant degradation of proaugurin/proaugurin-derived peptides occurs during the pulse-chase experiment. It is interesting to note that the in vitro convertase-generated C-terminal peptide at YYQR121↓H122YDE was not detected in pulse-chase experiments (Fig. 2, B and C), suggesting that this cleavage does not occur physiologically. In addition, the cleavage pattern of proaugurin in both AtT-20 cells and AtT-20/PC2 cells was similar (Fig. 2, B and C), indicating that PC2 does not contribute to the cellular processing of proaugurin. Furthermore, the addition of the secretagogue forskolin did not increase the secretion of proaugurin or product peptides at any time period, supporting the idea that proaugurin-derived peptides are most likely not stored within regulated secretory granules (Fig. 2, B and C). Because we observed a radioactive band of about 20 kDa (shown as X in Fig. 2), which is larger than the predicted size of tagged proaugurin itself, we concluded that posttranslational modifications must occur on proaugurin.
Fig. 2.
Secretion of proaugurin/proaugurin-derived peptides occurs via the constitutive secretory pathway. A, Sequence of mouse preproaugurin-myc-His. Asterisks indicate the positions of methionine and cysteine in preproaugurin-myc-His. The tyrosine residues predicted as sulfation sites by the Sulfinator program are shown in bold. Mouse preproaugurin-myc-His was transiently transfected into AtT-20 (panel B) or AtT-20/PC2 (panel C) cells. After a 20-min incubation with [35S]methionine/cysteine, cells were chased with methionine/cysteine-containing medium in the presence or absence of 10 μm forskolin for the indicated time periods. P, Pulse; CC, chased cells; CM, chased medium. X indicates the likely posttranslationally modified precursor. ab, Antibody; IP, immunoprecipitation.
Furin is the natural cellular protease generating proaugurin-derived peptides
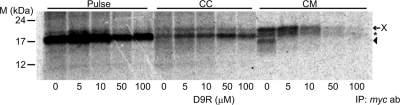
If augurin is secreted via the constitutive secretory pathway, the proteolysis reaction that generates proaugurin-derived peptides is likely to occur by the action of furin rather than by PC1/3 and PC2. Previous studies have shown that AtT-20 cells do contain sufficient furin to efficiently cleave the endogenous substrates 7B2 and prosomatostatin at known furin cleavage sites (25, 26). To confirm whether furin is involved in the cellular cleavage of proaugurin, the furin inhibitor non-d-arginine (D9R) was used. The addition of D9R to medium resulted in the disappearance of any proaugurin-derived proteolytic products, in a dose-dependent manner (Fig. 3); control cells incubated in the absence of D9R clearly showed proaugurin cleavage (Fig. 3). Interestingly, the secretion of proaugurin was significantly decreased in the cells treated with a high concentration of D9R. In addition, Fig. 3 also shows that proaugurin accumulated in the D9R-treated cells and was not degraded. This effect of D9R was not due to cellular toxicity, because no effects on cell numbers or morphology were observed even at the highest dose (data not shown). We have also previously observed that no toxicity is apparent using a concentration of D9R as high as 250 μm in a mouse leukemic monocyte macrophage cell line, RAW 264.7 cells (27). Furthermore, preproaugurin cDNA transfection of Lovo cells, a furin-deficient cell line that predominantly expresses PACE4 [paired basic amino acid cleaving enzyme 4, a related convertase involved in constitutive secretion (28)], did not result in a cleavage product (Fig. 4). This result supports the idea that PACE4 is not involved in the cellular processing of proaugurin. By contrast, Lovo cells transiently expressing both furin and preproaugurin were able to process proaugurin to the indicated cleavage product with kinetics such that 55% of secreted precursor was cleaved at the 60-min chase point, and 75% after 120 min of chase (Fig. 4). Taken together, these data strongly support the idea that furin indeed represents the natural cellular enzyme that processes proaugurin. In addition, because no cleaved products were detected in either pulsed or chased cells, furin-mediated proteolysis might occur during or immediately after secretion rather than within cells (Fig. 2, B and C, and Fig. 3). Furin, which contains a transmembrane domain, is known to carry out many proteolytic reactions at the cell surface (19, 29, 30).
Fig. 3.
The furin inhibitor D9R blocks proaugurin cleavage. Mouse preproaugurin-myc-His was transiently transfected into AtT-20/PC2 cells. Cells were incubated with the indicated concentrations of D9R. An asterisk and an arrow indicate the position of proaugurin and the major cleavage product, respectively. CC, Chased cells; CM, chased medium. X indicates the likely posttranslationally modified precursor. ab, Antibody; IP, immunoprecipitation.
Fig. 4.
Processing of proaugurin occurs only in furin-transfected Lovo cells. Mouse preproaugurin-myc-His was transiently transfected into Lovo cells with/without cDNA encoding furin. After a 20-min pulse incubation, cells were chased for indicated time periods at 37 C. CC, Chased cells; CM, chased medium. An asterisk and an arrow indicate the positions of proaugurin and major cleavage product, respectively. X indicates the likely posttranslationally modified precursor. ab, Antibody; IP, immunoprecipitation.
Sulfation of proaugurin occurs within the secretory pathway
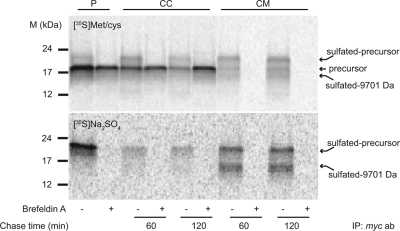
Pulse-chase experiments demonstrated an initial radioactive band around 20 kDa (indicated as X in Fig. 2, B and C), somewhat larger than the predicted size of tagged proaugurin itself. Additional posttranslational modifications could explain the increased mass of cellular proaugurin. There are no other predicted posttranslational modifications such as N-glycosylation and phosphorylation, but proaugurin does contain multiple tyrosine residues flanked by aspartate and glutamate, known to represent likely O-tyrosine sulfation sites by tyrosylprotein sulfotransferases 1 and 2 (8). An online prediction tool for O-tyrosine sulfation sites, Sulfinator, (http://expasy.org/tools/sulfinator/), confirmed that Y114, Y115, Y118, Y123, Y145, and Y148 (out of 11 tyrosine residues; shown in Fig. 2A in bold) represent potential O-sulfation sites. Additionally, these predicted sulfation sites are highly conserved among many species.
To confirm whether sulfation indeed occurs within proaugurin, pulse-chase experiments were carried out using both [35S]Met/Cys and [35S]Na2SO4. Figure 5 shows that the larger band appeared in both [35S]Met/Cys and [35S]Na2SO4 incubations. By contrast, this band disappeared in the presence of brefeldin A, an inhibitor of protein transport from the endoplasmic reticulum to the Golgi apparatus (Fig. 5). These data indicate that proaugurin is sulfated within the Golgi apparatus during its transport through the secretory pathway. Met/Cys labeling demonstrated that 30% and 45% of newly synthesized proaugurin forms were sulfated in 60- and 120-min chase media, respectively (Fig. 5, upper panel). In addition, the secretion level of sulfated proaugurin was about 65 and about 80% of the newly synthesized proaugurin in 60- and 120-min chase media, respectively (Fig. 5). It is interesting to note that sulfated rather than unsulfated proaugurin may represent the preferred target for proteolytic processing, because two sulfated bands around 20 and 15 kDa are observed in Fig. 5. Note that no tyrosines are present in the myc-His-tag and its linker (Fig. 2A); thus all sulfation must occur within the proaugurin sequence. This 15-kDa band likely corresponds to the 9701-Da fragment observed during in vitro cleavage (in Fig. 1B and Table 1); an increase in mass is expected both from the presence of the 3-kDa C-terminal myc-His-tag and from the anionic sulfation modification. The presence of both the tag and the sulfation modification would be predicted to increase the apparent molecular masses of proaugurin and the cleavage product (9701-Da fragment) during SDS-PAGE by about 6 kDa (Fig. 5). Because no larger band was observed in the presence of brefeldin A, and due to the lack of consensus sequences, N-linked glycosylation in the endoplasmic reticulum is highly unlikely.
Fig. 5.
Proaugurin is sulfated within the secretory pathway. Preproaugurin-myc-His was transiently transfected into AtT-20/PC2 cells. The cells were incubated with either 0.5 mCi/well [35S]Met/Cys (upper panel) or 0.25 mCi/well [35S]Na2SO4 (lower panel) for the indicated time periods at 37 C in the presence or absence of 10 μg/ml brefeldin A. P, Pulse; CC, chased cells; CM, chased medium. ab, Antibody; IP, immunoprecipitation.
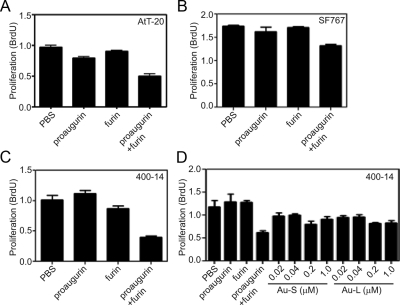
Processing of proaugurin is required to suppress cell proliferation
To clarify the functional role of posttranslational modification in augurin bioactivity, we performed cell proliferation assays with proaugurin and furin-cleaved proaugurin using AtT-20, SF767, and 400–14 cells. Furin-cleaved proaugurin reduced the proliferation rate of all cell lines (∼50% for AtT-20 cells; ∼30% for SF 767 cells; and ∼60% for 400–14 cells) relative to the control cells treated with PBS (Fig. 6). By contrast, neither proaugurin nor individual HPLC-purified proaugurin- derived peptides suppressed cell proliferation (Fig. 6 and Supplemental Fig. 2). These results support the idea that processing of proaugurin is necessary for suppression of cell proliferation, and that the 4510-Da and 9701-Da fragments alone cannot induce this bioactivity.
Fig. 6.
Cell proliferation is suppressed by furin-cleaved proaugurin. AtT-20 cells (1 × 104 cells per well; panel A), SF767 cells (2.5 × 104 cells per well; panel B), and 400–14 cells (0.25 × 104 cells per well; panels C and D) were incubated with the samples as indicated. Au-S and Au-L indicate the HPLC-purified proaugurin-derived peptides in fraction no. 48–52 and no. 58–62 in Supplemental Fig. 2, respectively. After 48 h of incubation, BrdU labeling reagent was added, and the cells were again incubated for 2 h at 37 C. BrdU incorporation was then measured by ELISA. Samples were assayed in quadruplicate, and the mean and standard deviation are shown.
Discussion
The peptide precursor proaugurin, recently reported as a potential secretory signaling molecule using bioinformatics (1), was originally identified using an mRNA differential display between cancerous and normal cells and named ECRG4 (31). Recent studies on this gene have shown that its expression is highly restricted by promoter hypermethylation in many types of cancer cells, such as esophageal, prostate, glioma, and colorectal cancers (2, 32–34). Furthermore, proaugurin has been shown to exert a suppressive effect on cell proliferation in many human tumor cell lines (2, 32, 33). This information suggests that augurin plays a suppressive role in tumor pathogenesis.
Although recent studies have shown that secreted augurin can exist as multiple molecular forms (2, 5), the major forms present in various tissues have not yet been clearly identified. Knowing the precise molecular forms of peptides generated within a given tissue is essential to understanding peptide functions. Hence, we have here described the role of posttranslational modifications of augurin in suppressing tumor cell proliferation.
Many secretory molecules require processing events for activation during transport through the secretory pathway. One of the most common processing events is endoproteolytic cleavage by a proprotein convertase during cellular trafficking through either the regulated or the constitutive secretory pathway. Our in vitro proteolytic reactions showed that PC1/3 and furin cleaved proaugurin at the same sites, whereas PC2 cleaved this precursor at different sites and at higher rates and efficiency. Therefore, we initially speculated that PC2 might represent the natural cellular enzyme cleaving proaugurin, especially because a function for augurin has recently been reported in tissues expressing this convertase (6). Surprisingly, however, our metabolic labeling and forskolin stimulation experiments with AtT-20/PC2 cells clearly show that proaugurin-derived peptides are neither cleaved nor stored within regulated secretory granules, suggesting that proaugurin must traffic through the constitutive secretory pathway. This conclusion is supported by our furin inhibition experiments showing effective inhibition by the furin inhibitor D9R (27). These results indicate that the secretion and processing of proaugurin are facilitated by furin within the constitutive secretory pathway and do not support our in vitro proteolytic reaction results demonstrating efficient proteolysis of proaugurin by PC2. This discrepancy can be explained if PC2 and proaugurin do not actually encounter each other intracellularly due to different trafficking itineraries. Because it is currently impossible to predict whether a given secretory protein will use the regulated or the constitutive secretory pathway, our results highlight the importance of using cellular processing tests to confirm in vitro convertase specificity results.
Furin is involved in the processing of a large number of secretory proteins in the constitutive pathway (19, 20). Because furin activates metalloprotease zymogens (18, 19), and metalloproteases are known to cleave secreted proteins within the extracellular matrix, these proteases might be considered as potential endogenous proteolytic enzymes for proaugurin cleavage. However, the addition of the metalloprotease inhibitors did not inhibit proaugurin cleavage, discounting this possibility (data not shown). Because the conditioned medium for the metabolic labeling assay contained high concentrations of the serine proteinase inhibitor, aprotinin, other possible serine protease cleavages, such as by thrombin and urokinase, are also deemed unlikely. These results again strongly support the idea that furin represents the natural protease that carries out proaugurin processing within cells.
Furthermore, tyrosine O-sulfation of proteins is an important regulatory mechanism that affects many protein-protein interactions (35–37), and, in certain cases, protein secretion (38). Numerous secretory proteins, including peptide hormones, membrane proteins, and receptor proteins, undergo enzymatic tyrosine sulfation, mediated by the highly conserved enzymes tyrosylprotein sulfotransferases 1 and 2 (9, 39, 40). For example, sulfation of a tyrosine residue in cholecystokinin (CCK) is essential for its biological activity at the CCK A or CCK 1 receptor (35–37, 41). In addition, site-directed mutagenesis studies of pro-CCK tyrosine residues have shown that CCK sulfation is important for cellular trafficking and secretion decisions. Previous studies have also reported that sulfation is also important for enhancing the proteolytic efficacy of thrombin on Factors V and VIII, cofactors in the blood coagulation cascade (42, 43). Because our data also support the idea that sulfation of proaugurin results in increased processing efficiency, we hypothesize that sulfation might affect the conformation of proaugurin to better expose the furin cleavage site, although the sulfation sites predicted by the Sulfinator program are not closely flanked by furin cleavage sites. Further studies are required to clarify the molecular mechanism of augurin sulfation and to determine whether sulfation can contribute to biological function. We also ruled out the possibility that the increase in mass of 35S-sulfated proaugurin is due, in part, to the addition of O-glycosylation, a Golgi-specific event, to proaugurin, because O-glycosylated sugars can be sulfated. However, we could not detect any O-glycosylation within proaugurin, although we were able to detect this modification within proopiomelanocortin (data not shown). This result indicates that tyrosine sulfation, rather than O-glycosylation/addition of sulfated sugars, is responsible for the increase in the mass of proaugurin shortly after synthesis.
Recent studies using material obtained from augurin-overexpressing cells have shown that augurin possesses a variety of different biological functions (2–7). Although it has been shown that conditioned medium from augurin-transfected cells contains multiple forms of augurin-derived peptides (2, 5), prior studies have not addressed the identification of the cleavage sites. Our proliferation assays using different forms of augurin show that processing of proaugurin at KMLQKR41↓E42APV and/or LKRQKR70↓Q71LWD is necessary to suppress cell proliferation. Because furin action has been linked to the proteolysis of many endogenous substrates at unconventional cleavage sites lacking P4/P6 arginines (44–48), we believe the KMLQKR41↓E42APV sequence represents a reliable furin cleavage site. Our results are consistent with observations from other studies on tumor cell proliferation that employed conditioned medium from augurin-overexpressing cells (2, 4, 5) in which proaugurin was also shown to be processed (at unspecified sites). In addition, we found that suppressive effects on cell proliferation were dependent on the cell line used, suggesting cell-type sensitivity of unknown origin. Interestingly, individual proaugurin-derived peptides separated by reverse-phase HPLC did not suppress cell proliferation. We note that we obtained a very low recovery of the 3267-Da peptide in these experiments; thus we are presently unable to say whether this peptide is responsible for the suppression of cell proliferation. Further experiments to determine the source of bioactivity will require the use of much larger amounts of cleaved proaugurin-derived peptides and optimization of the recovery of all fragments.
In summary, we have here described how posttranslational modification of proaugurin is carried out during trafficking through the secretory pathway: this precursor is first sulfated in the Golgi and is then cleaved by furin, either just before or immediately after secretion. We have further demonstrated that proaugurin processing by furin is required for suppression of tumor cell proliferation. These studies provide a strong biochemical basis for further functional investigations of this interesting peptide, e.g. identification of its potential binding partner(s) and/or receptors, and determination of the signaling pathways involved in suppressing proliferation.
Materials and Methods
Materials
The preparation of mouse PC1/3, PC2, and soluble human furin from Chinese hamster ovary cell-conditioned medium has been described previously (27, 49, 50). The purity of recombinant enzymes was estimated as greater than 99% using SDS-PAGE stained with Coomassie brilliant blue (CBB).
Transient expression of preproaugurin
The coding region of mouse preproaugurin was amplified by PCR from the plasmid pOT-mouse preproaugurin (ThermoFisher Scientific, Waltham, MA) and inserted into the pcDNA3.1/myc-His vector (Invitrogen, Carlsbad, CA). pcDNA3.1-mouse preproaugurin-myc-His plasmid (2 μg) was then transfected into either AtT-20 cells (a mouse pituitary adenoma cell line) or AtT-20 cells overexpressing PC2 [a kind gift of Dr. Richard Mains (23)] using Fugene HD (Roche, Indianapolis, IN). For Lovo cells (a colon carcinoma cell line), 1 μg of pcDNA3.1-mouse preproaugurin-myc-His plasmid was transfected with/without 1 μg pRC-CMV plasmid encoding human furin, a kind gift of Dr. Gary Thomas. After 24 h of incubation, cells were subjected to metabolic labeling and immunoprecipitation as described below.
Metabolic labeling and immunoprecipitation
Cells transiently expressing mouse proaugurin in 12-well plates were labeled with 0.5 mCi/well [35S]methionine and cysteine, Transmix (MP Biomedicals, Solon, OH) in RPMI-1680, methionine- and cysteine-free medium (Invitrogen) for 20 min and chased for appropriate time periods in Opti-MEM (Invitrogen) containing 10 μg/ml aprotinin. In certain experiments, the secretagogue forskolin and the furin inhibitor D9R (27, 51) were used. For sulfation experiments, labeling was performed with 0.25 mCi/well [35S]Na2SO4 (PerkinElmer, Waltham, MA) in MEM Joklik's sulfate-free medium (Sigma Chemical Co., St. Louis, MO) for 60 min, and labeled proteins were then chased within Opti-MEM in the presence or absence of 10 μg/ml brefeldin A. Labeled proteins were extracted with 1 m acetic acid and clarified by centrifugation. The cell extracts were lyophilized and reconstituted with immunoprecipitation buffer containing 100 mm sodium phosphate buffer (pH 7.4), 150 mm NaCl, 0.1% Triton X-100, and 0.5% Nonidet P-40. Immunoprecipitations of clarified extracts were performed using c-myc tag antibody (Cell Signaling Technology, Inc., Danvers, MA) and protein A beads (GE Healthcare, Piscataway, NJ). Immunoprecipitates were subjected to SDS-PAGE on 18% Tris-HCl acrylamide gels. Gels were exposed to phosphorimaging plates overnight (in some cases for 2–3 d), and labeled proteins were visualized using a Storm phosphorimaging system (GE Healthcare). To confirm the ratio of cleaved to intact proaugurin in the conditioned medium, the density of radiolabeled proaugurin and proaugurin-derived products was quantified using ImageQuant software (GE Healthcare). After subtraction of backgrounds for each radioactive band (taken from a nonradioactive portion of the scan), the densities of all bands were determined.
Expression and purification of recombinant His-tagged human proaugurin
The coding region of human proaugurin (lacking the signal peptide) was amplified by PCR from pINCY-human preproaugurin (ThermoFisher Scientific) and inserted into the pProEX vector (Invitrogen). Recombinant protein was expressed as His-tagged protein and then purified on Ni-resin (GE Healthcare). Protein was eluted with a buffer containing 20 mm HEPES (pH 7.4), 150 mm NaCl, and 500 mm imidazole. The protein eluted from the Ni-resin was further purified by reverse-phase chromatography on a C4 column (Grace Vydac, Hesperia, CA) with an acetonitrile gradient in 0.1% trifluoroacetic acid. The purified protein was then lyophilized, resuspended in 5 mm acetic acid, and stored at −80 C until used.
Cell proliferation assays
Cell growth was evaluated by performing 5-bromo-2-deoxyuridine (BrdU) assays using AtT-20, SF 767 (human glioblastoma), or 400–14 (human breast cancer) cells. Each cell line was plated in quadruplicate in 96-well plates (AtT-20, 2.5 × 104 cells per well; SF 767, 1 × 104; and 400–14 cells 0.25 × 104 cells) and incubated overnight. The cells were then starved in medium (DMEM for AtT-20 and 400–14 cells; and MEM for SF 767 cells) containing 1 mg/ml heated fatty acid-free BSA, 1 mm glutamine, and 1% penicillin-streptomycin for 24 h. After starvation, the cells were treated with 1 μm of either proaugurin or furin-cleaved augurin, in the presence of 10% fetal bovine serum for 48 h. Cell growth was measured using BrdU assay kit (Cell proliferation ELISA, BrdU colorimetric; Roche). Absorbance at 370 nm was measured in a SpectraMax M2e plate reader (Molecular Devices, Sunnyvale, CA).
In vitro proteolysis reactions using PCs
Recombinant His-tagged human proaugurin (2 μg) was incubated with 2 U of either PC1/3, PC2, or furin in 50-μl reactions containing 100 mm sodium acetate (pH 5.5) for PC1/3; [for PC2, pH 5.0; for furin, 100 mm HEPES (pH 7.0) was used]; and 5 mm CaCl2 at 37 C for the time periods indicated. The reactions were then subjected to SDS-PAGE using 18% Tris-HCl acrylamide gels, and the gels were stained with CBB. Western blotting was also performed with the samples having been incubated for 16 h using His-tag antiserum (Cell Signaling Technology). One unit of PC activity is equal to the amount of the enzyme that is required to cleave 1 pmol/min of the pRTKR-aminomethyl coumarin (Peptides International, Louisville, KY) fluorogenic substrate. To determine the cleavage products generated by each convertase, 500 μg of His-tagged human proaugurin were cleaved by the various enzymes overnight at 37 C in 1-ml reactions using the reaction conditions described above. The reactions were purified by reverse-phase chromatography on a C4 column (Grace Vydac) with an acetonitrile gradient in 0.1% trifluoroacetic acid. Peak fractions were pooled and lyophilized and then subjected to MALDI-ToF mass spectrometry (Kratos; Shimadzu, Colombia, MD). The predicted masses for each peptide were calculated using ProteinProspector.
Preparation of furin-cleaved proaugurin and furin-derived fragments for cell proliferation assays
His-tagged human proaugurin (1 mg) was incubated with 125 μg of soluble human furin overnight at 37 C in a 2-ml reaction using the reaction conditions described above (lacking detergent). The cleaved peptides were applied to a Sep-pak C18 cartridge (Waters Corp., Milford, MA) and eluted with 60% isopropanol containing 0.1% trifluoroacetic acid. Heated fatty acid-free BSA (1 mg) was then added to the eluent, and the eluent was lyophilized. The lyophilized sample was resuspended in PBS, adjusted to a concentration to 100 μΜ, and stored at −80 C until used. A control sample was also prepared using the same procedure as described above with 125 μg of furin. To separate proaugurin-derived fragments generated by furin, 2 mg of His-tagged human proaugurin were cleaved with furin as described above. The reaction was acidified by the addition of trifluoroacetic acid (0.1% as final concentration), and the fragments generated by furin were purified by reverse-phase chromatography on a C18 column (Grace Vydac) with an acetonitrile gradient in 0.1% trifluoroacetic acid. Peak fractions were pooled and lyophilized, dissolved in PBS, and then used for proliferation assays, as described above.
Acknowledgments
We thank J. F. Miceli (University of Maryland-Baltimore, Baltimore, MD) for help with the construction of plasmids and the purification of recombinant proaugurin.
This work was supported by National Institutes of Health Grant R21 DK084481.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine (5-bromo-2-deoxyuridine)
- CBB
- Coomassie brilliant blue
- CCK
- cholecystokinin
- D9R
- non-d-arginine
- ECRG4
- esophageal cancer-related gene 4
- MALDI-ToF
- matrix-assisted laser desorption ionization-time of flight
- PC
- prohormone convertase.
References
- 1. Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, Birney E, Rosenthal N, Gross C. 2007. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res 17:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Götze S, Feldhaus V, Traska T, Wolter M, Reifenberger G, Tannapfel A, Kuhnen C, Martin D, Müller O, Sievers S. 2009. ECRG4 is a candidate tumor suppressor gene frequently hypermethylated in colorectal carcinoma and glioma. BMC Cancer 9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li LW, Yu XY, Yang Y, Zhang CP, Guo LP, Lu SH. 2009. Expression of esophageal cancer related gene 4 (ECRG4), a novel tumor suppressor gene, in esophageal cancer and its inhibitory effect on the tumor growth in vitro and in vivo. Int J Cancer 125:1505–1513 [DOI] [PubMed] [Google Scholar]
- 4. Li W, Liu X, Zhang B, Qi D, Zhang L, Jin Y, Yang H. 2010. Overexpression of candidate tumor suppressor ECRG4 inhibits glioma proliferation and invasion. J Exp Clin Cancer Res 29:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kujuro Y, Suzuki N, Kondo T. 2010. Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells. Proc Natl Acad Sci USA 107:8259–8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tadross JA, Patterson M, Suzuki K, Beale KE, Boughton CK, Smith KL, Moore S, Ghatei MA, Bloom SR. 2010. Augurin stimulates the hypothalamo-pituitary-adrenal axis via the release of corticotrophin-releasing factor in rats. Br J Pharmacol 159:1663–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huh YH, Ryu JH, Shin S, Lee DU, Yang S, Oh KS, Chun CH, Choi JK, Song WK, Chun JS. 2009. Esophageal cancer related gene 4 (ECRG4) is a marker of articular chondrocyte differentiation and cartilage destruction. Gene 448:7–15 [DOI] [PubMed] [Google Scholar]
- 8. Moore KL. 2003. The biology and enzymology of protein tyrosine O-sulfation. J Biol Chem 278:24243–24246 [DOI] [PubMed] [Google Scholar]
- 9. Moore KL. 2009. Protein tyrosine sulfation: a critical posttranslation modification in plants and animals. Proc Natl Acad Sci USA 106:14741–14742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murthy AS, Mains RE, Eipper BA. 1986. Purification and characterization of peptidylglycine α-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem 261:1815–1822 [PubMed] [Google Scholar]
- 11. Barnea A, Cho G. 1983. Acetylation of adrenocorticotropin and β-endorphin by hypothalamic and pituitary acetyltransferases. Neuroendocrinology 37:434–439 [DOI] [PubMed] [Google Scholar]
- 12. Steiner DF. 1998. The proprotein convertases. Curr Opin Chem Biol 2:31–39 [DOI] [PubMed] [Google Scholar]
- 13. Nillni EA, Friedman TC, Todd RB, Birch NP, Loh YP, Jackson IM. 1995. Pro-thyrotropin-releasing hormone processing by recombinant PC1. J Neurochem 65:2462–2472 [DOI] [PubMed] [Google Scholar]
- 14. Friedman TC, Loh YP, Cawley NX, Birch NP, Huang SS, Jackson IM, Nillni EA. 1995. Processing of prothyrotropin-releasing hormone (Pro-TRH) by bovine intermediate lobe secretory vesicle membrane PC1 and PC2 enzymes. Endocrinology 136:4462–4472 [DOI] [PubMed] [Google Scholar]
- 15. Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA. 2004. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114:357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peinado JR, Li H, Johanning K, Lindberg I. 2003. Cleavage of recombinant proenkephalin and blockade mutants by prohormone convertases 1 and 2: an in vitro specificity study. J Neurochem 87:868–878 [DOI] [PubMed] [Google Scholar]
- 17. Nakayama K. 1997. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J 327:625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kessenbrock K, Plaks V, Werb Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3:753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. 2002. Precursor processing by kex2/furin proteases. Chem Rev 102:4525–4548 [DOI] [PubMed] [Google Scholar]
- 21. Molloy SS, Anderson ED, Jean F, Thomas G. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol 9:28–35 [DOI] [PubMed] [Google Scholar]
- 22. Southey BR, Amare A, Zimmerman TA, Rodriguez-Zas SL, Sweedler JV. 2006. NeuroPred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res 34:W267–W272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou A, Mains RE. 1994. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem 269:17440–17447 [PubMed] [Google Scholar]
- 24. Paquet L, Zhou A, Chang EY, Mains RE. 1996. Peptide biosynthetic processing: distinguishing prohormone convertases PC1 and PC2. Mol Cell Endocrinol 120:161–168 [DOI] [PubMed] [Google Scholar]
- 25. Paquet L, Bergeron F, Boudreault A, Seidah NG, Chrétien M, Mbikay M, Lazure C. 1994. The neuroendocrine precursor 7B2 is a sulfated protein proteolytically processed by a ubiquitous furin-like convertase. J Biol Chem 269:19279–19285 [PubMed] [Google Scholar]
- 26. Galanopoulou AS, Kent G, Rabbani SN, Seidah NG, Patel YC. 1993. Heterologous processing of prosomatostatin in constitutive and regulated secretory pathways. Putative role of the endoproteases furin, PC1, and PC2. J Biol Chem 268:6041–6049 [PubMed] [Google Scholar]
- 27. Kacprzak MM, Peinado JR, Than ME, Appel J, Henrich S, Lipkind G, Houghten RA, Bode W, Lindberg I. 2004. Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-D-arginine. J Biol Chem 279:36788–36794 [DOI] [PubMed] [Google Scholar]
- 28. Seidah NG, Chrétien M, Day R. 1994. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie 76:197–209 [DOI] [PubMed] [Google Scholar]
- 29. Klimpel KR, Molloy SS, Thomas G, Leppla SH. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA 89:10277–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh Y, Chaudhary VK, Leppla SH. 1989. A deleted variant of Bacillus anthracis protective antigen is non-toxic and blocks anthrax toxin action in vivo. J Biol Chem 264:19103–19107 [PubMed] [Google Scholar]
- 31. Su T, Liu H, Lu S. 1998. [Cloning and identification of cDNA fragments related to human esophageal cancer]. Zhonghua Zhong Liu Za Zhi 20:254–257 [PubMed] [Google Scholar]
- 32. Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, Tindall DJ, Cantor CR, Young CY. 2009. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest 27:549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mori Y, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Kurehara H, Mori R, Tomoda K, Ogawa R, Katada T, Harata K, Fujii Y. 2007. Expression of ECRG4 is an independent prognostic factor for poor survival in patients with esophageal squamous cell carcinoma. Oncol Rep 18:981–985 [PubMed] [Google Scholar]
- 34. Yue CM, Deng DJ, Bi MX, Guo LP, Lu SH. 2003. Expression of ECRG4, a novel esophageal cancer-related gene, downregulated by CpG island hypermethylation in human esophageal squamous cell carcinoma. World J Gastroenterol 9:1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang SC, Yu DH, Wank SA, Mantey S, Gardner JD, Jensen RT. 1989. Importance of sulfation of gastrin or cholecystokinin (CCK) on affinity for gastrin and CCK receptors. Peptides 10:785–789 [DOI] [PubMed] [Google Scholar]
- 36. Gigoux V, Escrieut C, Silvente-Poirot S, Maigret B, Gouilleux L, Fehrentz JA, Gully D, Moroder L, Vaysse N, Fourmy D. 1998. Met-195 of the cholecystokinin-A receptor interacts with the sulfated tyrosine of cholecystokinin and is crucial for receptor transition to high affinity state. J Biol Chem 273:14380–14386 [DOI] [PubMed] [Google Scholar]
- 37. Gigoux V, Maigret B, Escrieut C, Silvente-Poirot S, Bouisson M, Fehrentz JA, Moroder L, Gully D, Martinez J, Vaysse N, Fourmy AD. 1999. Arginine 197 of the cholecystokinin-A receptor binding site interacts with the sulfate of the peptide agonist cholecystokinin. Protein Sci 8:2347–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vishnuvardhan D, Beinfeld MC. 2000. Role of tyrosine sulfation and serine phosphorylation in the processing of procholecystokinin to amidated cholecystokinin and its secretion in transfected AtT-20 cells. Biochemistry 39:13825–13830 [DOI] [PubMed] [Google Scholar]
- 39. Ouyang YB, Moore KL. 1998. Molecular cloning and expression of human and mouse tyrosylprotein sulfotransferase-2 and a tyrosylprotein sulfotransferase homologue in Caenorhabditis elegans. J Biol Chem 273:24770–24774 [DOI] [PubMed] [Google Scholar]
- 40. Ouyang Y, Lane WS, Moore KL. 1998. Tyrosylprotein sulfotransferase: purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci USA 95:2896–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beisswanger R, Corbeil D, Vannier C, Thiele C, Dohrmann U, Kellner R, Ashman K, Niehrs C, Huttner WB. 1998. Existence of distinct tyrosylprotein sulfotransferase genes: molecular characterization of tyrosylprotein sulfotransferase-2. Proc Natl Acad Sci USA 95:11134–11139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michnick DA, Pittman DD, Wise RJ, Kaufman RJ. 1994. Identification of individual tyrosine sulfation sites within factor VIII required for optimal activity and efficient thrombin cleavage. J Biol Chem 269:20095–20102 [PubMed] [Google Scholar]
- 43. Pittman DD, Tomkinson KN, Michnick D, Selighsohn U, Kaufman RJ. 1994. Posttranslational sulfation of factor V is required for efficient thrombin cleavage and activation and for full procoagulant activity. Biochemistry 33:6952–6959 [DOI] [PubMed] [Google Scholar]
- 44. Bergeron E, Basak A, Decroly E, Seidah NG. 2003. Processing of α4 integrin by the proprotein convertases: histidine at position P6 regulates cleavage. Biochem J 373:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang T, Nagase H, Pei D. 2002. Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network. Cancer Res 62:675–681 [PubMed] [Google Scholar]
- 46. Viale A, Ortola C, Hervieu G, Furuta M, Barbero P, Steiner DF, Seidah NG, Nahon JL. 1999. Cellular localization and role of prohormone convertases in the processing of pro-melanin concentrating hormone in mammals. J Biol Chem 274:6536–6545 [DOI] [PubMed] [Google Scholar]
- 47. Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chrétien M, Seidah NG, Khatib AM. 2003. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest 111:1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McColl BK, Paavonen K, Karnezis T, Harris NC, Davydova N, Rothacker J, Nice EC, Harder KW, Roufail S, Hibbs ML, Rogers PA, Alitalo K, Stacker SA, Achen MG. 2007. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J 21:1088–1098 [DOI] [PubMed] [Google Scholar]
- 49. Lamango NS, Zhu X, Lindberg I. 1996. Purification and enzymatic characterization of recombinant prohormone convertase 2: stabilization of activity by 21 kDa 7B2. Arch Biochem Biophys 330:238–250 [DOI] [PubMed] [Google Scholar]
- 50. Zhou Y, Lindberg I. 1993. Purification and characterization of the prohormone convertase PC1(PC3). J Biol Chem 268:5615–5623 [PubMed] [Google Scholar]
- 51. Sarac MS, Cameron A, Lindberg I. 2002. The furin inhibitor hexa-D-arginine blocks the activation of Pseudomonas aeruginosa exotoxin A in vivo. Infect Immun 70:7136–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]